Abstract
PPI-0903M is a novel N-phosphono-type cephalosporin active against oxacillin-resistant staphylococci and many other gram-positive organisms. This study evaluated the in vitro activity and spectrum of PPI-0903M against 1,478 recent clinical isolates collected from 80 medical centers (22 countries). PPI-0903M demonstrated broader in vitro activity against gram-positive bacteria, particularly against multidrug-resistant staphylococci and streptococci of current clinical concern, than currently available extended-spectrum cephalosporins while maintaining similar activity against gram-negative pathogens.
Gram-positive bacterial pathogens have revealed an extraordinary ability to develop resistance to antimicrobial agents in the last two decades. Methicillin- and glycopeptide-resistant staphylococci, glycopeptide-resistant enterococci, and penicillin- and macrolide-resistant Streptococcus pneumoniae and viridans group streptococci have forced clinicians to seek alternative treatments for patients with serious gram-positive infections (9). Although a number of new agents have recently become available to treat infections caused by resistant gram-positive organisms, all these agents (including daptomycin, linezolid, and quinupristin-dalfopristin) lack activity against common gram-negative pathogens, necessitating combination therapy for empirical treatment of many serious infections. There is a clinical need for a new broad-spectrum antimicrobial agent that covers both resistant gram-positive and gram-negative pathogens.
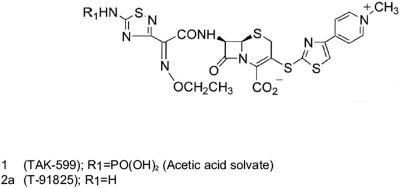
PPI-0903 (formerly TAK-599) is a novel N-phosphono-type prodrug cephalosporin administered intravenously. Its active form, PPI-0903M, is released in vivo upon hydrolysis of the phosphonate group (Fig. 1). Preliminary in vitro studies have indicated that this compound has a high affinity for PBP2′ or PBP2A and shows a potent in vitro activity against MRSA and many other gram-positive organisms (6, 7). In this report, we summarize the results of testing PPI-0903M and numerous comparator agents against contemporary clinical isolates (2003).
FIG. 1.
Chemical structure of PPI-0903 (TAK-599) and PPI-0903M (T-91825).
A total of 1,478 strains derived from numerous laboratories worldwide were processed in the study. These strains included well-characterized subsets of gram-positive organisms with specific resistance phenotypes. Only nonduplicate isolates judged to be clinically significant by local criteria were included in the study. All isolates were collected in 2003 except some isolates of the multidrug-resistant groups, which were cultured over the last 5 years (16).
The PPI-0903M reagent-grade compound was manufactured by Takeda Pharmaceutical, Inc. (Osaka, Japan). Comparator agents were purchased from Sigma Chemical Co. (St. Louis, MO) or obtained from their respective manufacturers in the United States. A total of 25 comparators were tested depending upon the species evaluated.
PPI-0903M MICs were determined by reference broth microdilution methods according to procedures published by the Clinical and Laboratory Standards Institute (CLSI) (formerly the National Committee for Clinical Laboratory Standards [NCCLS]) (3, 11, 12). Oxacillin was used in the susceptibility test to categorize the staphylococcal isolates as methicillin susceptible or resistant. Supplement 2 to 5% lysed horse blood was added for testing fastidious Streptococcus spp., and Haemophilus Test Medium was utilized for testing Haemophilus influenzae. The MICs were interpreted according to CLSI/NCCLS criteria (3). Quality control was monitored using the following organisms: S. pneumoniae ATCC 49619, Enterococcus faecalis ATCC 29212, Staphylococcus aureus ATCC 29213, Escherichia coli ATCC 25923, and Pseudomonas aeruginosa ATCC 27853. All quality control results were within published guidelines (3).
The in vitro activities of PPI-0903M in comparison to those of selected antimicrobial agents against gram-positive pathogens are summarized in Table 1. Among all cephalosporins tested, PPI-0903M shows the greatest activity against gram-positive organisms, including resistant isolates. PPI-0903M was very active against S. aureus, including methicillin-resistant (MRSA) strains. PPI-0903M was 8- to >16-fold more potent than cefepime and 16- to >32-fold more active than ceftriaxone against MRSA strains. PPI-0903M was also active against hetero-vancomycin-intermediate S. aureus (100 strains), with MIC results ranging from 0.25 to 4 μg/ml (MIC at which 50% of strains were inhibited [MIC50], 1 μg/ml; MIC at which 90% of strains were inhibited [MIC90], 2 μg/ml). Furthermore, 91 and 93% of these isolates were resistant to cefepime and ceftriaxone, respectively. Against 11 quinupristin-dalfopristin-non-susceptible strains, PPI-0903M MIC results were similar in activity to that described for MRSA (MIC50 and MIC90, 1 μg/ml).
TABLE 1.
In vitro activities of PPI-0903M compared to those of selected antimicrobial agents when tested against gram-positive bacteria
| Bacterium group or antimicrobial agent (no. of isolates tested) | MIC (μg/ml)
|
% of isolates by category
|
|||
|---|---|---|---|---|---|
| 50% | 90% | Range | Susceptible | Resistant | |
| Staphylococcus aureus | |||||
| Methicillin susceptible (73)a | |||||
| PPI-0903M | 0.25 | 0.25 | 0.03-0.5 | —b | — |
| Cefepime | 2 | 4 | 0.25-16 | 98.6 | 0.0 |
| Ceftriaxone | 4 | 4 | 0.5-16 | 98.6 | 0.0 |
| Piperacillin-tazobactam | 1 | 2 | ≤0.12-4 | 100.0 | 0.0 |
| Imipenem | ≤0.5 | ≤0.5 | ≤0.5 | 100.0 | 0.0 |
| Levofloxacin | 0.12 | 0.5 | 0.06->4 | 93.2 | 4.1 |
| Vancomycin | 1 | 1 | 0.5-2 | 100.0 | 0.0 |
| Linezolid | 2 | 2 | 0.5-2 | 100.0 | — |
| Methicillin resistant (102)a | |||||
| PPI-0903M | 1 | 2 | 0.12-2 | — | — |
| Cefepime | >16 | >16 | 1->16 | (26.5)c | (60.8)c |
| Ceftriaxone | >32 | >32 | 4->32 | (11.8)c | (60.8)c |
| Piperacillin-tazobactam | 64 | 256 | 0.5->256 | (20.6)c | (79.4)c |
| Imipenem | 2 | >8 | ≤0.5->8 | (63.7)c | (30.4)c |
| Levofloxacin | >4 | >4 | 0.12->4 | 10.8 | 87.3 |
| Vancomycin | 1 | 1 | 0.5-4 | 100.0 | 0.0 |
| Linezolid | 2 | 2 | 0.25-2 | 100.0 | — |
| h-VISA and VISA (100)d | |||||
| PPI-0903M | 1 | 2 | 0.25-4 | — | — |
| Cefepime | >16 | >16 | 4->16 | 7.0 | 91.0 |
| Ceftriaxone | >32 | >32 | 4->32 | 4.0 | 93.0 |
| Oxacillin | >2 | >2 | ≤0.25->2 | 12.0 | 88.0 |
| Piperacillin-tazobactam | 128 | 256 | 1->256 | 5.0 | 95.0 |
| Imipenem | >8 | >8 | ≤0.5->8 | 12.0 | 80.0 |
| Levofloxacin | >4 | >4 | 0.12->4 | 1.0 | 94.0 |
| Linezolid | 1 | 1 | 0.5->8 | 99.0 | — |
| Quinupristin-dalfopristin nonsusceptible (11) | |||||
| PPI-0903M | 1 | 1 | 0.25-1 | — | — |
| Cefepime | >16 | >16 | 2->16 | 18.2 | 63.6 |
| Ceftriaxone | >32 | >32 | 8->32 | 9.1 | 72.7 |
| Oxacillin | >8 | >8 | 8->8 | 0.0 | 100.0 |
| Piperacillin-tazobactam | >64 | >64 | 4->64 | 16.7 | 83.3 |
| Imipenem | >8 | >8 | ≤0.06->8 | 36.4 | 63.6 |
| Levofloxacin | 4 | >4 | 1->4 | 9.1 | 90.9 |
| Vancomycin | 2 | 2 | 1-2 | 100.0 | 0.0 |
| Linezolid | 1 | 2 | 0.5-2 | 100.0 | — |
| CoNS | |||||
| Methicillin susceptible (50)a | |||||
| PPI-0903M | 0.06 | 0.25 | ≤0.016-0.5 | — | — |
| Cefepime | 1 | 2 | 0.25-2 | 100.0 | 0.0 |
| Ceftriaxone | 2 | 4 | 0.5-8 | 100.0 | 0.0 |
| Piperacillin-tazobactam | 0.25 | 1 | ≤0.12-4 | 100.0 | 0.0 |
| Imipenem | ≤0.5 | ≤0.5 | ≤0.5 | 100.0 | 0.0 |
| Levofloxacin | 0.25 | 1 | 0.06->4 | 90.0 | 8.0 |
| Vancomycin | 1 | 2 | 0.5-4 | 100.0 | 0.0 |
| Linezolid | 1 | 1 | 0.5-2 | 100.0 | — |
| Methicillin resistant (80)a | |||||
| PPI-0903M | 0.25 | 0.5 | ≤0.016-2 | — | — |
| Cefepime | 4 | 8 | 1->16 | (92.5)c | (6.3)c |
| Ceftriaxone | 8 | 32 | 4->32 | (55.0)c | (5.0)c |
| Piperacillin-tazobactam | 1 | 4 | 0.25-256 | (96.3)c | (3.7)c |
| Imipenem | ≤0.5 | 2 | ≤0.5->8 | (92.5)c | (6.3)c |
| Levofloxacin | 4 | >4 | 0.06->4 | 37.5 | 55.0 |
| Vancomycin | 2 | 2 | ≤0.12-2 | 100.0 | 0.0 |
| Linezolid | 1 | 1 | 0.25-2 | 100.0 | — |
| h-VIS (51)e | |||||
| PPI-0903M | 1 | 2 | ≤0.016-2 | — | — |
| Cefepime | >16 | >16 | 0.5->16 | 31.4 | 62.7 |
| Ceftriaxone | >32 | >32 | 1->32 | 15.7 | 66.7 |
| Oxacillin | >8 | >8 | ≤0.25->8 | 9.8 | 90.2 |
| Piperacillin-tazobactam | 64 | >64 | ≤0.5->64 | 28.6 | 71.4 |
| Imipenem | >8 | >8 | ≤0.5->8 | 43.1 | 56.9 |
| Levofloxacin | 4 | >4 | 0.12->4 | 22.5 | 77.5 |
| Linezolid | 1 | 2 | 0.5-2 | 100.0 | — |
| Quinupristin-dalfopristin nonsusceptible (15) | |||||
| PPI-0903M | 0.5 | 1 | 0.12-2 | — | — |
| Cefepime | 8 | >16 | 1->16 | (60.0)c | (26.7)c |
| Ceftriaxone | 32 | >32 | 8->32 | (33.3)c | (40.0)c |
| Oxacillin | >8 | >8 | 0.5->8 | 0.0 | 100.0 |
| Piperacillin-tazobactam | 4 | >64 | 1->64 | (53.8)c | (46.2)c |
| Imipenem | 1 | >8 | ≤0.5->8 | (73.3)c | (26.7)c |
| Levofloxacin | >4 | >4 | 1->4 | 9.1 | 72.7 |
| Vancomycin | 2 | 2 | 0.5-2 | 100.0 | 0.0 |
| Linezolid | 1 | 2 | 1-2 | 100.0 | — |
| Streptococcus pneumoniae | |||||
| Penicillin susceptible (33) | |||||
| PPI-0903M | ≤0.016 | ≤0.016 | ≤0.016-0.06 | — | — |
| Cefepimef | ≤0.06 | 0.25 | ≤0.06-0.5 | 100.0 | 0.0 |
| Ceftriaxonef | 0.016 | 0.06 | 0.016-0.5 | 100.0 | 0.0 |
| Meropenem | 0.016 | 0.03 | ≤0.008-0.03 | 100.0 | 0.0 |
| Levofloxacin | 1 | 1 | 0.5-1 | 100.0 | 0.0 |
| Clarithromycin | ≤0.25 | 8 | ≤0.25->32 | 81.8 | 15.2 |
| Erythromycin | ≤0.25 | 8 | ≤0.25->32 | 81.8 | 12.1 |
| Vancomycin | 0.25 | 0.5 | ≤0.06-0.5 | 100.0 | — |
| Penicillin intermediate (53) | |||||
| PPI-0903M | 0.03 | 0.06 | ≤0.016-0.12 | — | — |
| Cefepimef | 0.25 | 1 | ≤0.06-4 | 95.9 | 2.0 |
| Ceftriaxonef | 0.25 | 0.5 | 0.03-1 | 100.0 | 0.0 |
| Meropenem | 0.12 | 0.5 | ≤0.008-0.5 | 82.2 | 0.0 |
| Levofloxacin | 1 | 1 | 0.12-2 | 100.0 | 0.0 |
| Clarithromycin | 1 | >32 | ≤0.25->32 | 36.0 | 64.0 |
| Erythromycin | 2 | >32 | ≤0.25->32 | 36.0 | 64.0 |
| Vancomycin | 0.25 | 0.5 | 0.12-2 | 98.0 | — |
| Penicillin resistant (50) | |||||
| PPI-0903M | 0.12 | 0.25 | 0.06-0.5 | — | — |
| Cefepimef | 1 | 2 | 0.25-4 | 78.0 | 2.0 |
| Ceftriaxonef | 1 | 2 | ≤0.008-8 | 82.0 | 8.0 |
| Meropenem | 1 | 1 | 0.25-2 | 2.1 | 55.3 |
| Levofloxacin | 1 | 1 | 0.5-1 | 100.0 | 0.0 |
| Clarithromycin | 8 | >32 | ≤0.25->32 | 12.0 | 86.0 |
| Erythromycin | 8 | >32 | ≤0.25->32 | 12.0 | 88.0 |
| Vancomycin | 0.25 | 0.5 | 0.12-0.5 | 100.0 | — |
| Levofloxacin nonsusceptible (22) | |||||
| PPI-0903M | ≤0.016 | 0.12 | ≤0.016-0.12 | — | — |
| Cefepime | ≤0.06 | 0.5 | ≤0.06-1 | 100.0 | 0.0 |
| Ceftriaxone | 0.03 | 0.5 | ≤0.008-1 | 100.0 | 0.0 |
| Penicillin | ≤0.03 | 1 | ≤0.03-2 | 68.2 | 9.1 |
| Clarithromycin | 2 | >32 | ≤0.25->32 | 40.9 | 59.1 |
| Erythromycin | 4 | >32 | ≤0.25->32 | 40.9 | 59.1 |
| Vancomycin | 0.25 | 0.5 | 0.12-0.5 | 100.0 | — |
| Multidrug resistant (23)g | |||||
| PPI-0903M | 0.12 | 0.25 | 0.12-0.5 | — | — |
| Cefepimef | 1 | 2 | 0.5-4 | 73.9 | 4.3 |
| Ceftriaxonef | 1 | 4 | ≤0.008-8 | 73.9 | 13.0 |
| Meropenem | 1 | 1 | 0.5-1 | 0.0 | 65.2 |
| Levofloxacin | 1 | 1 | 0.5-4 | 95.7 | 0.0 |
| Vancomycin | 0.25 | 0.5 | 0.12-0.5 | 100.0 | 0.0 |
| β-Haemolytic group A streptococci (22) | |||||
| PPI-0903M | ≤0.016 | ≤0.016 | ≤0.016 | — | — |
| Cefepime | ≤0.12 | ≤0.12 | ≤0.12 | 100.0 | 0.0 |
| Ceftriaxone | ≤0.25 | ≤0.25 | ≤0.25 | 100.0 | 0.0 |
| Penicillin | ≤0.016 | ≤0.016 | ≤0.016-0.03 | 100.0 | 0.0 |
| Meropenem | ≤0.008 | ≤0.008 | ≤0.008 | 100.0 | — |
| Ertapenem | ≤0.06 | ≤0.06 | ≤0.06-0.12 | 100.0 | — |
| Levofloxacin | 0.5 | 1 | 0.25-1 | 100.0 | 0.0 |
| Erythromycin | ≤0.06 | ≤0.06 | ≤0.06->8 | 90.5 | 9.5 |
| Vancomycin | 0.25 | 0.5 | 0.25-0.5 | 100.0 | — |
| β-Haemolytic group B streptococci (26) | |||||
| PPI-0903M | ≤0.016 | ≤0.016 | ≤0.016 | — | — |
| Cefepime | ≤0.12 | ≤0.12 | ≤0.12 | 100.0 | 0.0 |
| Ceftriaxone | ≤0.25 | ≤0.25 | ≤0.25 | 100.0 | 0.0 |
| Penicillin | 0.06 | 0.12 | 0.03-0.12 | 100.0 | 0.0 |
| Meropenem | 0.06 | 0.06 | 0.03-0.12 | 100.0 | — |
| Ertapenem | ≤0.06 | ≤0.06 | ≤0.06-0.12 | 100.0 | — |
| Levofloxacin | 0.5 | 1 | 0.25-1 | 100.0 | 0.0 |
| Erythromycin | ≤0.06 | 2 | ≤0.06->8 | 73.1 | 26.9 |
| Vancomycin | 0.5 | 0.5 | 0.25-0.5 | 100.0 | — |
| Other β-haemolytic streptococci (20) | |||||
| PPI-0903M | ≤0.016 | ≤0.016 | ≤0.016-0.03 | — | — |
| Cefepime | ≤0.12 | 0.5 | ≤0.12-0.5 | 100.0 | 0.0 |
| Ceftriaxone | ≤0.25 | ≤0.25 | ≤0.25 | 100.0 | 0.0 |
| Penicillin | ≤0.016 | 0.06 | ≤0.016-0.06 | 100.0 | 0.0 |
| Meropenem | 0.016 | 0.12 | ≤0.008-0.12 | 100.0 | — |
| Ertapenem | ≤0.06 | 0.25 | ≤0.06-0.5 | 100.0 | — |
| Levofloxacin | 0.5 | 0.5 | 0.25-1 | 100.0 | 0.0 |
| Erythromycin | ≤0.06 | ≤0.06 | ≤0.06-2 | 95.0 | 5.0 |
| Vancomycin | 0.25 | 0.5 | 0.25-1 | 100.0 | — |
| Levofloxacin-resistant β-haemolytic streptococci (10)h | |||||
| PPI-0903M | ≤0.016 | ≤0.016 | ≤0.016-0.03 | — | — |
| Cefepime | ≤0.12 | 0.25 | ≤0.12-0.25 | 100.0 | 0.0 |
| Ceftriaxone | ≤0.25 | ≤0.25 | ≤0.25 | 100.0 | 0.0 |
| Penicillin | 0.03 | 0.06 | ≤0.016-0.12 | 100.0 | 0.0 |
| Erythromycin | ≤0.06 | >8 | ≤0.06->8 | 80.0 | 20.0 |
| Vancomycin | 0.5 | 0.5 | 0.25-1 | 100.0 | — |
| Viridans group streptococci | |||||
| Penicillin susceptible (32) | |||||
| PPI-0903M | ≤0.016 | 0.03 | ≤0.016-1 | — | — |
| Cefepime | ≤0.12 | 0.25 | ≤0.12-0.5 | 100.0 | 0.0 |
| Ceftriaxone | ≤0.25 | ≤0.25 | ≤0.25-0.5 | 100.0 | 0.0 |
| Meropenem | 0.03 | 0.06 | ≤0.008-0.12 | 100.0 | — |
| Ertapenem | ≤0.06 | 0.12 | ≤0.06-0.5 | 100.0 | — |
| Levofloxacin | 0.5 | 1 | 0.06-2 | 100.0 | 0.0 |
| Erythromycin | ≤0.06 | 1 | ≤0.06->8 | 78.1 | 18.7 |
| Vancomycin | 0.5 | 1 | 0.25-1 | 100.0 | — |
| Penicillin intermediate (53) | |||||
| PPI-0903M | 0.03 | 0.12 | ≤0.016-0.5 | — | — |
| Cefepime | 0.5 | 1 | ≤0.12-4 | 90.6 | 1.9 |
| Ceftriaxone | 0.5 | 2 | ≤0.25-4 | 90.6 | 1.9 |
| Meropenem | 0.12 | 1 | 0.06-1 | 88.0 | — |
| Ertapenem | 0.25 | 2 | ≤0.06-2 | 88.5 | — |
| Levofloxacin | 1 | >4 | 0.5->4 | 85.5 | 10.9 |
| Erythromycin | 1 | 4 | ≤0.06->8 | 35.8 | 52.8 |
| Vancomycin | 0.5 | 0.5 | 0.25-1 | 100.0 | — |
| Penicillin resistant (52) | |||||
| PPI-0903M | 0.25 | 1 | 0.03-8 | — | — |
| Cefepime | 4 | 8 | 1->16 | 21.2 | 53.8 |
| Ceftriaxone | 4 | 16 | 1->32 | 9.8 | 58.8 |
| Meropenem | 2 | 4 | 0.5-4 | 16.7 | — |
| Ertapenem | 4 | 8 | 1-8 | 11.1 | — |
| Levofloxacin | 1 | 2 | 0.5->4 | 94.2 | 3.8 |
| Erythromycin | 2 | >8 | ≤0.06->8 | 11.5 | 78.8 |
| Vancomycin | 0.5 | 1 | 0.25-1 | 100.0 | — |
| Levofloxacin nonsusceptible (20) | |||||
| PPI-0903M | 0.06 | 0.25 | ≤0.016-1 | — | — |
| Cefepime | 0.25 | 4 | ≤0.12-8 | 75.0 | 15.0 |
| Ceftriaxone | ≤0.25 | 2 | ≤0.25-16 | 75.0 | 6.2 |
| Penicillin | 0.06 | 4 | ≤0.03-16 | 55.0 | 15.0 |
| Erythromycin | 0.5 | 4 | ≤0.06->32 | 45.0 | 40.0 |
| Vancomycin | 0.5 | 1 | 0.25-1 | 100.0 | — |
| Quinupristin-dalfopristin nonsusceptible streptococci (6)i | |||||
| PPI-0903M | ≤0.016 | — | ≤0.016-8 | — | — |
| Cefepime | ≤0.12 | — | ≤0.12->16 | 50.0 | 50.0 |
| Ceftriaxone | 32 | — | ≤0.25->32 | 40.0 | 60.0 |
| Penicillin | 0.12 | — | ≤0.016->32 | 50.0 | 50.0 |
| Levofloxacin | 1 | — | 1->4 | 83.3 | 16.7 |
| Erythromycin | >8 | — | >8 | 0.0 | 100.0 |
| Vancomycin | 0.5 | — | 0.25-0.5 | 100.0 | — |
| Enterococcus faecalis | |||||
| Vancomycin-susceptible (22) | |||||
| PPI-0903M | 2 | 16 | 0.5->32 | — | — |
| Ceftriaxone | >32 | >32 | >32 | — | — |
| Ampicillin | ≤2 | ≤2 | ≤2-8 | 100.0 | 0.0 |
| Imipenem | 1 | 4 | ≤0.5-8 | — | — |
| Levofloxacin | 2 | >4 | 0.5->4 | 54.5 | 45.5 |
| Teicoplanin | ≤2 | ≤2 | ≤2 | 100.0 | 0.0 |
| Linezolid | 2 | 2 | 0.5->8 | 86.4 | 13.6 |
| Vancomycin resistant (22) | |||||
| PPI-0903M | 4 | 8 | 1-16 | — | — |
| Ceftriaxone | >32 | >32 | >32 | — | — |
| Ampicillin | ≤2 | 4 | ≤2->16 | 95.5 | 4.5 |
| Imipenem | 2 | 4 | 1->8 | — | — |
| Levofloxacin | >4 | >4 | >4 | 0.0 | 100.0 |
| Teicoplanin | 8 | >16 | ≤2->16 | 50.0 | 45.5 |
| Linezolid | 1 | 2 | 1->8 | 95.5 | 4.5 |
| Enterococcus faecium | |||||
| Vancomycin-susceptible (11) | |||||
| PPI-0903M | 16 | >32 | 0.5->32 | — | — |
| Ceftriaxone | >32 | >32 | 16->32 | — | — |
| Ampicillin | >16 | >16 | ≤2->16 | 27.3 | 72.7 |
| Imipenem | >8 | >8 | 2->8 | — | — |
| Levofloxacin | >4 | >4 | 1->4 | 18.2 | 0.0 |
| Teicoplanin | ≤2 | ≤2 | ≤2 | 100.0 | 0.0 |
| Linezolid | 1 | 2 | 1-2 | 100.0 | 0.0 |
| Vancomycin resistant (vanA) (13) | |||||
| PPI-0903M | >32 | >32 | 16->32 | — | — |
| Ceftriaxone | >32 | >32 | >32 | — | — |
| Ampicillin | >16 | >16 | >16 | 0.0 | 100.0 |
| Imipenem | >8 | >8 | >8 | — | — |
| Levofloxacin | >4 | >4 | 2->4 | 7.7 | 92.3 |
| Linezolid | 1 | 2 | 1-2 | 100.0 | 0.0 |
| Linezolid-resistant enterococci (10)j | |||||
| PPI-0903M | 16 | >32 | 1->32 | — | — |
| Ceftriaxone | >32 | >32 | >32 | — | — |
| Ampicillin | >16 | >16 | ≤2->16 | 40.0 | 60.0 |
| Imipenem | >8 | >8 | 0.5->8 | — | — |
| Levofloxacin | >4 | >4 | 1->4 | 10.0 | 90.0 |
| Vancomycin | 2 | >16 | 0.5->16 | 50.0 | 40.0 |
| Teicoplanin | ≤2 | >16 | ≤2->16 | 60.0 | 40.0 |
| Bacillus spp. (20)k | |||||
| PPI-0903M | 4 | 8 | 0.06-32 | — | — |
| Cefepime | >16 | >16 | 2->16 | — | — |
| Ceftriaxone | 32 | >32 | ≤0.25->32 | — | — |
| Penicillin | 32 | >32 | ≤0.016->32 | — | — |
| Ampicillin | 16 | >16 | ≤2->16 | — | — |
| Imipenem | ≤0.5 | 4 | ≤0.5-8 | — | — |
| Levofloxacin | 0.12 | 0.25 | 0.06->4 | — | — |
| Vancomycin | 1 | 1 | ≤0.12-1 | — | — |
Oxacillin was used in the susceptibility test to categorize staphylococcal isolates as methicillin susceptible or resistant.
—, no breakpoints have been established by the CLSI/NCCLS (3).
Oxacillin-resistant S. aureus should be considered resistant to all β-lactams according to CLSI/NCCLS (3) recommendations.
Includes 19 well-characterized vancomycin-intermediate S. aureus (VISA) strains and 81 hetero (h)-VISA strains, using the PAP method and results reported in an evaluation of AZD2563, an oxazolidinone (16).
Isolates with vancomycin MIC at 4 μg/ml.
Breakpoints for nonmeningitis isolates were applied (3).
Resistant to penicillin, erythromycin, clarithromycin, clindamycin, tetracycline, and trimethoprim/sulfamethoxazole.
Includes one group A, five group B, one group C, two group G, and one ungrouped β-haemolytic strain.
Includes four S. mitis and two S. bovis strains.
Includes four E. faecalis strains and six E. faecium strains. Resistances confirmed as having G2576U ribosomal target mutations.
Includes 11 Bacillus cereus strains, one B. circulans strain, and eight Bacillus spp. strains.
PPI-0903M was slightly more potent against coagulase-negative staphylococci (CoNS) than against S. aureus. Against methicillin-resistant CoNS (80 strains), the PPI-0903M MIC50 and MIC90 results were 0.25 and 0.5 μg/ml, respectively. PPI-0903M (MICs, ≤0.016 to 2 μg/ml) was also active against CoNS (51 strains) having reduced susceptibility to vancomycin (hVIS; MIC, 4 μg/ml). Those hVIS strains were resistant to other β-lactam antibiotics, including imipenem. PPI-0903M was also active against 15 tested quinupristin-dalfopristin-nonsusceptible strains (MIC90, 1 μg/ml). Although all methicillin-resistant staphylococci should be considered resistant to all β-lactams by the CLSI/NCCLS interpretive criteria (3, 12), the activities of PPI-0903M against these staphylococci would imply potential clinical utility against MRSA depending upon the dosing and pharmacokinetics of the molecule. The susceptibility rates of these isolates to PPI-0903M awaits breakpoint determination by regulatory and consensus organizations.
As with other β-lactams, PPI-0903M in vitro activity against S. pneumoniae (178 strains; Table 1) varied according to penicillin susceptibility. Penicillin-resistant strains (50 strains; MIC90, 0.25 μg/ml) showed the most elevated PPI-0903M MICs (0.06 to 0.5 μg/ml), but they remained low, and PPI-0903M potency was generally eightfold greater than that of either ceftriaxone or cefepime. Resistance to levofloxacin did not affect PPI-0903M in vitro activity, and multidrug-resistant strains (resistant to six drugs; see Table 1) were all inhibited by PPI-0903M at ≤0.5 μg/ml.
PPI-0903M was also very potent against β-hemolytic streptococci, with the vast majority of strains inhibited at ≤0.016 μg/ml. The highest PPI-0903M MIC among the β-hemolytic streptococci was only 0.03 μg/ml (two group F strains). Levofloxacin-resistant β-hemolytic streptococci were also very susceptible to PPI-0903M (MIC90, ≤0.016 μg/ml). Viridans group streptococci susceptibility to PPI-0903M fluctuated according to the penicillin susceptibility, and resistance to levofloxacin did not adversely influence the PPI-0903M in vitro activity against this pathogen. When quinupristin-dalfopristin-nonsusceptible streptococcal strains were tested, PPI-0903M MIC results varied widely from ≤0.016 μg/ml (two S. bovis strains and one S. mitis strain) to 8 μg/ml (two S. mitis strains).
PPI-0903M showed limited activity against both vancomycin-susceptible and -resistant Enterococcus faecium. In addition, vancomycin-resistant E. faecalis isolates (MIC50, 4 μg/ml) had slightly higher PPI-0903M MICs than vancomycin-susceptible E. faecalis strains (MIC50, 2 μg/ml).
PPI-0903M activity was also evaluated against a combined collection of linezolid-resistant staphylococcal and streptococcal strains, which included seven S. aureus strains (PPI-0903M MICs, 1 to 4 μg/ml), two CoNS strains (PPI-0903M MICs, 0.5 μg/ml), and one Streptococcus oralis strain (PPI-0903M MIC, ≤0.016 μg/ml). These PPI-0903M MIC ranges were consistent with those of linezolid-susceptible staphylococci (data not shown). Despite the small number of isolates tested, it is concluded that PPI-0903M retained an activity against linezolid-resistant gram-positive organisms, excluding enterococci.
PPI-0903M was the most active cephalosporin tested against Bacillus spp. (Table 1), with MIC results ranging from 0.06 to 32 μg/ml (MIC50, 4 μg/ml, and MIC90, 8 μg/ml). Imipenem (MIC90, 4 μg/ml), levofloxacin (MIC90, 0.25 μg/ml), and vancomycin (MIC90, 1 μg/ml) also showed reasonable activity against this pathogen.
The spectrum of activity of PPI-0903M against gram-negative bacteria is similar to those of the expanded-spectrum cephalosporins. Among the Enterobacteriaceae (221 strains) (Table 2), the vast majority of Citrobacter freundii (MIC90, 2 μg/ml), non-extended-spectrum-β-lactamase (ESBL)-producing E. coli (MIC90, 0.12 μg/ml) or Klebsiella pneumoniae (MIC90, 0.5 μg/ml), Morganella morganii (MIC90, 0.12 μg/ml), Proteus mirabilis (MIC90, 0.12 μg/ml), and Serratia marcescens (MIC90, 2 μg/ml) were inhibited at ≤2 μg/ml of PPI-0903M. However, as with other expanded-spectrum cephalosporins, PPI-0903M MIC results were observed to be elevated for some Enterobacter cloacae (MIC90, 32 μg/ml), Proteus vulgaris/Providencia spp. (MIC90, >32 μg/ml), and ESBL-producing strains regardless of species.
TABLE 2.
In vitro activities of PPI-0903M compared to those of selected antimicrobial agents when tested against gram-negative organisms
| Bacterium group or antimicrobial agent (no. of isolates tested) | MIC (μg/ml)
|
% of isolates by category
|
|||
|---|---|---|---|---|---|
| 50% | 90% | Range | Susceptible | Resistant | |
| Citrobacter freundii (20) | |||||
| PPI-0903M | 0.12 | 2 | 0.06->32 | — a | |
| Cefepime | ≤0.12 | 0.25 | ≤0.12-1 | 100.0 | 0.0 |
| Ceftriaxone | ≤0.25 | 0.5 | ≤0.25-1 | 100.0 | 0.0 |
| Ceftazidime | ≤1 | 2 | ≤1-4 | 100.0 | 0.0 |
| Piperacillin-tazobactam | 2 | 16 | 1->64 | 90.0 | 10.0 |
| Imipenem | ≤0.5 | 1 | ≤0.5-1 | 100.0 | 0.0 |
| Levofloxacin | 0.06 | 0.25 | ≤0.03->4 | 95.0 | 5.0 |
| Tobramycin | 0.5 | 1 | 0.25->16 | 95.0 | 5.0 |
| Enterobacter cloacaeb | |||||
| Wild type (23) | |||||
| PPI-0903M | 0.12 | 32 | 0.03->32 | — | — |
| Cefepime | ≤0.12 | 2 | ≤0.12-4 | 100.0 | 0.0 |
| Ceftriaxone | ≤0.25 | 16 | ≤0.25->32 | 87.0b | 8.7 |
| Ceftazidime | ≤1 | >16 | ≤1->16 | 87.0b | 13.0 |
| Piperacillin-tazobactam | 2 | 128 | ≤0.5-256 | 87.0 | 13.0 |
| Imipenem | ≤0.5 | 1 | ≤0.5-2 | 100.0 | 0.0 |
| Levofloxacin | ≤0.03 | 1 | ≤0.03->4 | 91.3 | 8.7 |
| Tobramycin | 0.5 | 2 | 0.25->16 | 91.3 | 8.7 |
| ESBL-producing strains (15) | |||||
| PPI-0903M | >32 | >32 | 4->32 | — | — |
| Cefepime | 16 | >16 | 8->16 | 40.0 | 33.3 |
| Ceftriaxone | >32 | >32 | 16->32 | 0.0 | 86.7 |
| Ceftazidime | >16 | >16 | 2->16 | 33.3 | 53.3 |
| Piperacillin-tazobactam | 32 | >64 | 2->64 | 40.0 | 26.7 |
| Imipenem | ≤0.5 | ≤0.5 | ≤0.5-8 | 93.3 | 0.0 |
| Levofloxacin | >4 | >4 | ≤0.03->4 | 33.3 | 53.3 |
| Tobramycin | 8 | >16 | 0.5->16 | 26.7 | 46.7 |
| E. coli | |||||
| Wild type (20) | |||||
| PPI-0903M | 0.06 | 0.12 | ≤0.016-0.25 | — | — |
| Cefepime | ≤0.12 | ≤0.12 | ≤0.12 | 100.0 | 0.0 |
| Ceftriaxone | ≤0.25 | ≤0.25 | ≤0.25 | 100.0 | 0.0 |
| Ceftazidime | ≤1 | ≤1 | ≤1 | 100.0 | 0.0 |
| Piperacillin-tazobactam | 1 | 2 | 0.25-4 | 100.0 | 0.0 |
| Imipenem | ≤0.5 | ≤0.5 | ≤0.5 | 100.0 | 0.0 |
| Levofloxacin | ≤0.03 | >4 | ≤0.03->4 | 80.0 | 15.0 |
| Tobramycin | 0.5 | 2 | 0.25-8 | 90.0 | 0.0 |
| ESBL-producing strains (15) | |||||
| PPI-0903M | >32 | >32 | 0.5->32 | — | — |
| Cefepime | 16 | >16 | ≤0.12->16 | 46.7 | 40.0 |
| Ceftriaxone | >32 | >32 | ≤0.25->32 | 40.0 | 60.0 |
| Ceftazidime | 8 | >16 | ≤1->16 | 53.3 | 20.0 |
| Piperacillin-tazobactam | 8 | 32 | 2->64 | 86.7 | 6.7 |
| Imipenem | ≤0.5 | ≤0.5 | ≤0.5 | 100.0 | 0.0 |
| Levofloxacin | >4 | >4 | ≤0.03->4 | 26.7 | 66.7 |
| Tobramycin | 16 | >16 | 0.5->16 | 28.6 | 64.3 |
| Klebsiella pneumoniae | |||||
| Wild type (21) | |||||
| PPI-0903M | 0.06 | 0.5 | 0.03-4 | — | — |
| Cefepime | ≤0.12 | ≤0.12 | ≤0.12-0.25 | 100.0 | 0.0 |
| Ceftriaxone | ≤0.25 | ≤0.25 | ≤0.25 | 100.0 | 0.0 |
| Ceftazidime | ≤1 | ≤1 | ≤1 | 100.0 | 0.0 |
| Piperacillin-tazobactam | 2 | 4 | 0.5-256 | 95.2 | 4.8 |
| Imipenem | ≤0.5 | ≤0.5 | ≤0.5 | 100.0 | 0.0 |
| Levofloxacin | 0.06 | 0.5 | ≤0.03->4 | 90.5 | 9.5 |
| Tobramycin | 0.5 | 0.5 | ≤0.12-2 | 100.0 | 0.0 |
| ESBL-producing strains (15) | |||||
| PPI-0903M | >32 | >32 | 32->32 | — | — |
| Cefepime | 8 | >16 | 2->16 | 60.0 | 20.0 |
| Ceftriaxone | >32 | >32 | 16->32 | 0.0 | 66.7 |
| Ceftazidime | >16 | >16 | ≤1->16 | 40.0 | 53.3 |
| Piperacillin-tazobactam | 8 | >256 | 1->256 | 58.8 | 41.2 |
| Imipenem | ≤0.5 | ≤0.5 | ≤0.5 | 100.0 | 0.0 |
| Levofloxacin | 0.5 | >4 | ≤0.03->4 | 60.0 | 26.7 |
| Tobramycin | >16 | >16 | 0.5->16 | 20.0 | 80.0 |
| Morganella morganii (20) | |||||
| PPI-0903M | 0.06 | 0.12 | 0.03-0.5 | — | — |
| Cefepime | ≤0.12 | ≤0.12 | ≤0.12 | 100.0 | 0.0 |
| Ceftriaxone | ≤0.25 | ≤0.25 | ≤0.25-0.5 | 100.0 | 0.0 |
| Ceftazidime | ≤1 | ≤1 | ≤1 | 100.0 | 0.0 |
| Piperacillin-tazobactam | ≤0.5 | ≤0.5 | ≤0.5-2 | 100.0 | 0.0 |
| Imipenem | 2 | 4 | ≤0.5-4 | 100.0 | 0.0 |
| Levofloxacin | ≤0.03 | 0.12 | ≤0.03-1 | 100.0 | 0.0 |
| Tobramycin | 0.5 | 1 | ≤0.12-2 | 100.0 | 0.0 |
| Proteus mirabilis | |||||
| Wild type (20) | |||||
| PPI-0903M | 0.12 | 0.12 | 0.03-4 | — | — |
| Cefepime | ≤0.12 | ≤0.12 | ≤0.12 | 100.0 | 0.0 |
| Ceftriaxone | ≤0.25 | ≤0.25 | ≤0.25-0.5 | 100.0 | 0.0 |
| Ceftazidime | ≤1 | ≤1 | ≤1 | 100.0 | 0.0 |
| Piperacillin-tazobactam | ≤0.5 | 1 | ≤0.5-2 | 100.0 | 0.0 |
| Imipenem | 1 | 2 | ≤0.5-2 | 100.0 | 0.0 |
| Levofloxacin | 0.06 | >4 | ≤0.03->4 | 80.0 | 20.0 |
| Tobramycin | 1 | 2 | 0.5-8 | 90.0 | 0.0 |
| ESBL-producing strains (10) | |||||
| PPI-0903M | >32 | >32 | 4->32 | — | — |
| Cefepime | >16 | >16 | >16 | 0.0 | 100.0 |
| Ceftriaxone | >32 | >32 | 1->32 | 30.0 | 70.0 |
| Ceftazidime | 4 | 16 | ≤1->16 | 80.0 | 10.0 |
| Piperacillin-tazobactam | 1 | 2 | ≤0.5-32 | 90.0 | 0.0 |
| Imipenem | 1 | 2 | 0.25-2 | 100.0 | 0.0 |
| Levofloxacin | >4 | >4 | 2->4 | 10.0 | 80.0 |
| Tobramycin | 4 | >16 | 0.5->16 | 60.0 | 40.0 |
| Providencia spp./P. vulgaris (22)c | |||||
| PPI-0903M | 1 | >32c | 0.03->32 | — | — |
| Cefepime | ≤0.12 | 1 | ≤0.12-8 | 100.0 | 0.0 |
| Ceftriaxone | ≤0.25 | 4 | ≤0.25-32 | 90.9 | 9.1 |
| Ceftazidime | ≤1 | ≤1 | ≤1-8 | 100.0 | 0.0 |
| Piperacillin-tazobactam | ≤0.5 | 4 | ≤0.5-4 | 100.0 | 0.0 |
| Imipenem | 1 | 2 | ≤0.5-4 | 100.0 | 0.0 |
| Levofloxacin | 0.06 | >4 | ≤0.03->4 | 72.7 | 13.6 |
| Tobramycin | 1 | 4 | 0.25-16 | 90.9 | 4.5 |
| Serratia marcescens (20) | |||||
| PPI-0903M | 0.5 | 2 | 0.12-8 | — | — |
| Cefepime | ≤0.12 | 0.25 | ≤0.12-0.5 | 100.0 | 0.0 |
| Ceftriaxone | ≤0.25 | 1 | ≤0.25-8 | 100.0 | 0.0 |
| Ceftazidime | ≤1 | ≤1 | ≤1 | 100.0 | 0.0 |
| Piperacillin-tazobactam | 1 | 4 | 0.5-16 | 100.0 | 0.0 |
| Imipenem | ≤0.5 | 1 | ≤0.5-2 | 100.0 | 0.0 |
| Levofloxacin | 0.12 | 0.5 | 0.06-2 | 100.0 | 0.0 |
| Tobramycin | 2 | 4 | 0.5-16 | 90.0 | 5.0 |
| Haemophilus influenzae | |||||
| β-lactamase negative (23) | |||||
| PPI-0903M | ≤0.016 | ≤0.016 | ≤0.016 | — | — |
| Cefepime | ≤0.06 | 0.12 | ≤0.06-0.12 | 100.0 | 0.0 |
| Ceftriaxone | ≤0.008 | ≤0.008 | ≤0.008-0.016 | 100.0 | 0.0 |
| Ampicillin | ≤0.5 | 1 | ≤0.5-1 | 100.0 | 0.0 |
| Piperacillin-tazobactam | ≤0.12 | ≤0.12 | ≤0.12 | 100.0 | 0.0 |
| Meropenem | 0.03 | 0.12 | 0.016-0.25 | 100.0 | 0.0 |
| Levofloxacin | ≤0.03 | ≤0.03 | ≤0.03 | 100.0 | 0.0 |
| Azithromycin | 1 | 2 | 0.5-2 | 100.0 | — |
| β-Lactamase positive (24) | |||||
| PPI-0903M | ≤0.016 | ≤0.016 | ≤0.016-0.25 | — | — |
| Cefepime | ≤0.06 | 0.12 | ≤0.06-1 | 100.0 | 0.0 |
| Ceftriaxone | ≤0.008 | 0.016 | ≤0.008-0.06 | 100.0 | 0.0 |
| Ampicillin | >4 | >4 | 2->4 | 0.0 | 97.1 |
| Piperacillin-tazobactam | ≤0.12 | ≤0.12 | ≤0.12 | 100.0 | 0.0 |
| Meropenem | 0.03 | 0.06 | 0.016-0.12 | 100.0 | 0.0 |
| Levofloxacin | ≤0.03 | ≤0.03 | ≤0.03 | 100.0 | 0.0 |
| Azithromycin | 1 | 2 | 0.25-8 | 95.8 | —a |
| BLNAR (30)d | |||||
| PPI-0903M | ≤0.016 | 0.03 | ≤0.016-0.03 | — | — |
| Cefepime | 0.25 | 0.5 | ≤0.06-1 | 100.0 | 0.0 |
| Ceftriaxone | 0.016 | 0.6 | ≤0.008-2 | 100.0 | 0.0 |
| Piperacillin-tazobactam | ≤1 | ≤1 | ≤1 | 100.0 | 0.0 |
| Levofloxacin | ≤0.03 | ≤0.03 | ≤0.03 | 100.0 | 0.0 |
| Azithromycin | 1 | 2 | ≤0.12-4 | 100.0 | — |
| M. catarrhalis (25)e | |||||
| PPI-0903M | 0.06 | 0.12 | ≤0.016-0.12 | — | — |
| Cefepime | 0.25 | 1 | ≤0.06-2 | 100.0 | 0.0 |
| Ceftriaxone | 0.06 | 0.5 | ≤0.008-1 | 100.0 | 0.0 |
| Ampicillinf | ≤0.5 | 2 | ≤0.5->4 | 4.0 | 96.0 |
| Amoxicillin-clavulanic acid | ≤0.06 | 0.25 | ≤0.06-0.5 | 100.0 | 0.0 |
| Levofloxacin | ≤0.03 | ≤0.03 | ≤0.03 | 100.0 | 0.0 |
| Azithromycin | ≤0.5 | ≤0.5 | ≤0.5 | 100.0 | — |
| Neisseria meningitidis (10) | |||||
| PPI-0903M | ≤0.016 | ≤0.016 | ≤0.016 | — | — |
| Cefepime | ≤0.12 | ≤0.12 | ≤0.12 | — | — |
| Ceftriaxone | ≤0.25 | ≤0.25 | ≤0.25 | — | — |
| Penicillin | ≤0.016 | 0.25 | ≤0.016-0.25 | — | — |
| Ampicillin | ≤1 | ≤1 | ≤1 | — | — |
| Piperacillin-tazobactam | ≤0.5 | ≤0.5 | ≤0.5 | — | — |
| Imipenem | ≤0.5 | ≤0.5 | ≤0.5 | — | — |
| Levofloxacin | ≤0.03 | ≤0.03 | ≤0.03-0.06 | — | — |
| Acinetobacter baumannii (20) | |||||
| PPI-0903M | 16 | >32 | 2->32 | — | — |
| Cefepime | 8 | >16 | 2->16 | 55.0 | 35.0 |
| Ceftriaxone | 16 | >32 | 0.5->32 | 35.0 | 35.0 |
| Ceftazidime | 8 | >16 | 2->16 | 50.0 | 25.0 |
| Ampicillin-sulbactam | 4 | 32 | 2->32 | 70.0 | 25.0 |
| Piperacillin-tazobactam | 32 | >256 | ≤0.12->256 | 40.0 | 45.0 |
| Imipenem | ≤0.5 | 1 | ≤0.5->8 | 95.0 | 5.0 |
| Levofloxacin | 1 | >4 | 0.06->4 | 50.0 | 50.0 |
| Tobramycin | 1 | >16 | 0.5->16 | 65.0 | 20.0 |
| Alcaligenes spp. (10)g | |||||
| PPI-0903M | >32 | >32 | 16->32 | — | — |
| Cefepime | >16 | >16 | 16->16 | — | — |
| Ceftriaxone | >32 | >32 | 16->32 | — | — |
| Ceftazidime | 4 | >16 | 2->16 | — | — |
| Piperacillin-tazobactam | 2 | >64 | ≤0.5->64 | — | — |
| Imipenem | 2 | >8 | 1->8 | — | — |
| Levofloxacin | 2 | 4 | 0.12->4 | — | — |
| Tobramycin | >16 | >16 | 8->16 | — | — |
| Pseudomonas aeruginosa (20) | |||||
| PPI-0903M | 16 | >32 | 4->32 | — | — |
| Cefepime | 2 | 8 | 1-16 | 95.0 | 0.0 |
| Ceftriaxone | >32 | >32 | 8->32 | 15.0 | 60.0 |
| Ceftazidime | 2 | 8 | ≤1-8 | 100.0 | 0.0 |
| Piperacillin-tazobactam | 4 | 16 | 2-256 | 95.0 | 5.0 |
| Imipenem | 1 | 8 | ≤0.5->8 | 85.0 | 10.0 |
| Levofloxacin | 0.5 | >4 | 0.25->4 | 75.0 | 25.0 |
| Tobramycin | 0.5 | >16 | ≤0.12->16 | 75.0 | 25.0 |
| Stenotrophomonas maltophilia (10) | |||||
| PPI-0903M | >32 | >32 | 32->32 | — | — |
| Cefepime | 16 | >16 | 4->16 | — | — |
| Ceftriaxone | >32 | >32 | 32->32 | — | — |
| Ceftazidime | 8 | >16 | ≤1->16 | — | — |
| Piperacillin-tazobactam | >256 | >256 | 8->256 | — | — |
| Imipenem | >8 | >8 | >8 | — | — |
| Levofloxacin | 1 | 2 | 0.25->4 | — | — |
| Tobramycin | >16 | >16 | 16->16 | — | — |
| Trimethoprim-sulfamethoxazole | ≤0.5 | ≤0.5 | ≤0.5 | 100.0 | 0.0 |
—, no breakpoints have been established by the CLSI/NCCLS (3).
13% of isolates produced a stably derepressed AmpC enzyme.
Two Proteus vulgaris and two Providencia stuartii isolates had PPI-0903M MICs of >32 μg/ml.
BLNAR, β-lactamase-negative ampicillin-resistant strains.
Haemophilus influenzae breakpoints were applied (3).
Interpreted by β-lactamase production or a penicillin MIC at ≤0.06 μg/ml, susceptible (one strain [4.0%] was β-lactamase test negative).
Includes eight A. xylosoxidans strains, one A. faecalis strain, and one Alcaligenes sp. strain.
PPI-0903M was highly active against H. influenzae, and its in vitro activity was not affected by β-lactamase production (MIC90 of ≤0.016 μg/ml) (Table 2). In comparison, β-lactamase ampicillin-resistant strains showed PPI-0903M MIC results that were only slightly elevated (MIC90, 0.03 μg/ml). Moraxella catarrhalis and Neisseria meningitidis strains also showed low MICs for PPI-0903M (MIC90 of 0.12 μg/ml and ≤0.016 μg/ml, respectively) and most of the comparison antimicrobial agents.
In general, PPI-0903M showed in vitro activity most similar to that of ceftriaxone and less than that of cefepime or ceftazidime against a diverse group of nonfermentative gram-negative bacilli (60 strains; Table 2). Against anaerobes, PPI-0903M had excellent activity against some gram-positive organisms and marginal activity against Clostridium difficile (MIC50, 2 μg/ml, and MIC90, 4 μg/ml). Bacteroides fragilis and Prevotella spp. showed higher PPI-0903M MIC results (MIC90, >32 μg/ml) (Table 3).
TABLE 3.
In vitro activities of PPI-0903M and comparator agents tested against anaerobes
| Bacterium group or antimicrobial agent (no. of isolates tested) | MIC (μg/ml)
|
% of isolates by category
|
|||
|---|---|---|---|---|---|
| 50% | 90% | Range | Susceptible | Resistant | |
| Bacteroides fragilis (20) | |||||
| PPI-0903M | 32 | >32 | 4->32 | —a | — |
| Meropenem | 0.25 | 1 | 0.12-2 | 100.0 | 0.0 |
| Metronidazole | 1 | 2 | 0.5-2 | 100.0 | 0.0 |
| Clindamycin | 4 | >16 | 1->16 | 35.0 | 50.0 |
| Clostridium difficile (10) | |||||
| PPI-0903M | 2 | 4 | 0.06-8 | — | — |
| Meropenem | 2 | 2 | 1-4 | 100.0 | 0.0 |
| Metronidazole | 0.5 | 0.5 | ≤0.12-1 | 100.0 | 0.0 |
| Clindamycin | 4 | 8 | 0.5-16 | 40.0 | 40.0 |
| Clostridium spp. (16)b | |||||
| PPI-0903M | 0.06 | 1 | ≤0.016-1 | — | — |
| Meropenem | ≤0.016 | 0.5 | ≤0.016-1 | 100.0 | 0.0 |
| Metronidazole | 4 | 4 | 0.5-4 | 100.0 | 0.0 |
| Clindamycin | 0.5 | 8 | ≤0.12->16 | 68.8 | 12.5 |
| Other gram-positive anaerobes (14)c | |||||
| PPI-0903M | 0.06 | 0.12 | 0.03-0.12 | — | — |
| Meropenem | 0.12 | 0.12 | ≤0.016-0.12 | 100.0 | 0.0 |
| Metronidazole | >32 | >32 | 1->32 | 7.1 | 92.9 |
| Clindamycin | 0.25 | 2 | ≤0.12->16 | 92.9 | 7.1 |
| Prevotella spp. (16)d | |||||
| PPI-0903M | 8 | >32 | 0.03->16 | — | — |
| Meropenem | 0.12 | 0.12 | 0.03-0.25 | 100.0 | 0.0 |
| Metronidazole | 2 | 8 | 1-16 | 93.8 | 0.0 |
| Clindamycin | ≤0.12 | >16 | ≤0.12->16 | 62.5 | 37.5 |
—, no breakpoints have been established by the CLSI/NCCLS (3, 12).
Includes 12 C. perfringens strains, 3 C. septicum strains, and 1 Clostridium sp. strain.
Includes two Proprionibacterium acnes strains, nine Proprionibacterium sp. strains, and three Peptostreptococcus sp. strains.
Includes six Prevotella bivia strains, one P. intermedia strain, one P. melaninogenica strain, two P. oralis strains, and six Prevotella sp. strains.
One of the most striking features of PPI-0903M was its in vitro activity against gram-positive organisms. Staphylococcus spp. including MRSA and oxacillin-resistant CoNS appeared to be particularly susceptible to PPI-0903M (MIC90, 0.25 to 2 μg/ml). This finding is important in light of the increasing prevalence of MRSA in community-acquired infections, including community-acquired pneumonia, and in nosocomial infections (1, 4). In addition, PPI-0903M appears to be active against vancomycin-nonsusceptible MRSA, a pathogen of concern that has been increasingly documented in recent years (9, 16).
PPI-0903M was also highly active against other key respiratory pathogens, such as S. pneumoniae and H. influenzae, which are common causes of community-acquired pneumonia, otitis media, and bacterial meningitis. In addition, these pathogens also represent an important cause of nosocomial pneumonia, especially when the onset of the disease occurs within 3 to 5 days after hospital admission (13). Mortality and suppurative complications associated with these infections decrease dramatically with the early introduction of appropriate antimicrobial therapy (17). The clinical impact of antimicrobial resistance among these pathogens, especially S. pneumoniae, varies according to the site of infection, reflecting the degree of drug penetration to that site and the ability of the host immune response to clear the infection. Thus, antimicrobial resistance has led to documented treatment failure in patients with meningitis and acute otitis media, but the impact of pneumococcal resistance on treatment of pneumonia has been more difficult to determine. High-level β-lactam or macrolide resistance has, however, been associated with increased morbidity and a longer hospital stay (10, 14). PPI-0903M demonstrated increased in vitro potency compared with currently available cephalosporins against resistant pneumococci.
Our study showed that PPI-0903M was very active against many clinically important bacterial pathogens, especially streptococci (β-hemolytic, viridans group, and pneumococci), staphylococci (S. aureus and CoNS), H. influenzae, and M. catarrhalis. In vitro activity of PPI-0903M against these pathogens was similar to or more potent than those of other described anti-MRSA cephalosporins (2, 5, 8, 15). Moreover, PPI-0903M exhibited activity against several multidrug-resistant gram-positive pathogens that may cause both community-acquired and nosocomial infections, including MRSA and penicillin-resistant S. pneumoniae.
While possessing enhanced activity against resistant gram-positive cocci, PPI-0903M retains in vitro activity against common gram-negative pathogens. This spectrum of activity distinguishes it from currently available β-lactams and suggests that PPI-0903 has potential for use in the treatment of bacterial infections in the hospital environment. The prodrug, PPI-0903, is current under the phase I clinical evaluation and has the potential to be a unique addition to the well-established, safe cephalosporin class.
Acknowledgments
We thank P. R. Rhomberg, H. (Huynh) Becker, K. L. Meyer, and M. G. Stilwell for their valuable contribution to the manuscript.
This study was funded by an educational/research grant from Peninsula Pharmaceuticals, Inc.
REFERENCES
- 1.Baba, T., F. Takeuchi, M. Kurodo, H. Yuzawa, K. Aoki, A. Oguchi, Y. Nagal, N. Iwama, K. Asano, T. Naimi, H. Kuroda, L. Cui, K. Yamamoto, and K. Hiramatsu. 2002. Genome and virulence determinants of high virulence community-acquired MRSA. Lancet 359:1819-1827. [DOI] [PubMed] [Google Scholar]
- 2.Chamberland, S., J. Blais, M. Hoang, C. Dinh, D. Cotter, E. Bond, C. Gannen, C. Park, F. Malouin, and M. N. Dudley. 2001. In vitro activities of RWJ-54428 (MC-02,479) against multiresistant gram-positive bacteria. Antimicrob. Agents Chemother. 45:1422-1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Clinical and Laboratory Standards Institute. 2005. Performance standards for antimicrobial susceptibility testing, 15th informational supplement M100-S15. Clinical and Laboratory Standards Institute, Wayne, Pa.
- 4.Frank, A. L., J. F. Marcinak, P.D. Mangat, and P. C. Schreckenberger. 1999. Increase in community-acquired methicillin-resistant Staphylococcus aureus in children. Clin. Infect. Dis. 29:935-936. [DOI] [PubMed] [Google Scholar]
- 5.Fujimura, T., Y. Yamano, I. Yoshida, J. Shimada, and S. Kuwahara. 2003. In vitro activity of S-3578, a new broad-spectrum cephalosporin active against methicillin-resistant staphylococci. Antimicrob. Agents Chemother. 47:923-931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Iizawa, Y., J. Nagai, T. Ishikawa, S. Hashiguchi, M. Nakao, and K. Okonogi. 2004. In vitro antimicrobial activity of T-91825, a novel anti-MRSA cephalosporin, and in vivo anti-MRSA activity of its prodrug, TAK-599. J. Infect. Chemother. 10:146-156. [DOI] [PubMed] [Google Scholar]
- 7.Ishikawa, T., N. Matsunaga, H. Tawada, N. Kuroda, Y. Nakayama, Y. Ishibashi, M. Tomimoto, Y. Ikeda, Y. Tagawa, Y. Iizawa, K. Okonogi, S. Hashiguchi, and A. Miyake. 2003. TAK-599, a novel N-phosphono type prodrug of anti-MRSA cephalosporin T-91825: synthesis, physicochemical and pharmacological properties. Bioorg. Med. Chem. 11:2427-2437. [DOI] [PubMed] [Google Scholar]
- 8.Jones, R. N., L. M. Deshpande, A.H. Mutnick, and D. J. Biedenbach. 2002. In vitro evaluation of BAL9141, a novel parenteral cephalosporin active against oxacillin-resistant staphylococci. J. Antimicrob. Chemother. 50:915-932. [DOI] [PubMed] [Google Scholar]
- 9.Livermore, D. M. 2003. Bacterial resistance: origins, epidemiology, and impact. Clin. Infect. Dis. 36(Suppl. 1):S11—S23. [DOI] [PubMed]
- 10.Lonks, J. R., J. Garau, L. Gomez, M. Xercavins, A. Ochoa de Echaguen, I. F. Gareen, P. T. Reiss, and A. A. Medeiros. 2002. Failure of macrolide antibiotic treatment in patients with bacteremia due to erythromycin-resistant Streptococcus pneumoniae. Clin. Infect. Dis. 35:556-564. [DOI] [PubMed] [Google Scholar]
- 11.National Committee for Clinical Laboratory Standards. 2004. Methods for antimicrobial susceptibility testing of anaerobic bacteria. Approved standard, 6th ed., M11-A6. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 12.National Committee for Clinical Laboratory Standards. 2003. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically; approved standard, 6th ed. Approved document M7-A6. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 13.Paradisi, F., G. Corti, and R. Cinelli. 2001. Streptococcus pneumoniae as an agent of nosocomial infection: treatment in the era of penicillin-resistant strains. Clin. Microbiol. Infect. 7(Suppl. 4):34-42. [DOI] [PubMed] [Google Scholar]
- 14.Rowland, K. E., and J. D. Turnidge. 2000. The impact of penicillin resistance on the outcome of invasive Streptococcus pneumoniae infection in children. Aust. N. Z. J. Med. 30:441-449. [DOI] [PubMed] [Google Scholar]
- 15.Sader, H. S., D. M. Johnson, and R. N. Jones. 2004. In vitro activities of the novel cephalosporin LB 11058 against multidrug-resistant staphylococci and streptococci. Antimicrob. Agents Chemother. 48:53-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wootton, M., R. A. Howe, R. Hillman, T. R. Walsh, P. M. Bennett, and A. P. MacGowan. 2001. A modified population analysis profile (PAP) method to detect hetero-resistance to vancomycin in Staphylococcus aureus in a UK hospital. J. Antimicrob. Chemother. 47:399-403. [DOI] [PubMed] [Google Scholar]
- 17.Ziglam, H. M., and R. G. Finch. 2002. Penicillin-resistant pneumococci: implications for management of community-acquired pneumonia and meningitis. Int. J. Infect. Dis. 6(Suppl. 1):S14-S20. [DOI] [PubMed] [Google Scholar]