Abstract
We tested 32 Candida isolates recovered in the early 1990s from the bloodstreams of patients with candidemia for in vitro susceptibility to fluconazole and determined if MIC and/or the daily dose of fluconazole/MIC ratio correlated with the response to therapy. This is a unique data set since 87.5% (28/32) of patients were treated with fluconazole doses now considered to be inadequate (≤200 mg), which contributed to high therapeutic failure rates (53% [17/32]). The geometric mean MIC and dose/MIC ratio for isolates associated with therapeutic failure (11.55 μg/ml and 14.3, respectively) differed significantly from values associated with therapeutic success (0.95 μg/ml and 219.36 [P = 0.0009 and 0.0004, respectively]). The therapeutic success rates among patients infected with susceptible (MIC ≤ 8 μg/ml), susceptible-dose dependent (S-DD) (MIC = 16 or 32 μg/ml), and resistant (MIC ≥ 64 μg/ml) isolates were 67% (14/21), 20% (1/5), and 0% (0/6), respectively. A dose/MIC ratio >50 was associated with a success rate of 74% (14/19), compared to 8% (1/13) for a dose/MIC ratio ≤50 (P = 0.0003). Our data suggest that both fluconazole MIC and dose/MIC ratio correlate with the therapeutic response to fluconazole among patients with candidemia. In clinical practice, dose/MIC ratio might prove easier to interpret than breakpoint MICs, since it quantitates the effects of increasing fluconazole doses that are alluded to in the S-DD designation.
Candida spp. emerged as common causes of mucosal and systemic diseases with the onset of the AIDS epidemic and increasing populations of immunosuppressed individuals (18). The introduction of fluconazole revolutionized the therapy of candidal infections in the 1990s by offering a well-tolerated alternative to amphotericin B, the long-standing and significantly toxic antifungal of choice (23). Despite the recent development of newer antifungal drugs, fluconazole remains an attractive front-line agent against candidiasis because of its excellent oral bioavailability and overall clinical efficacy.
The increasing importance of candidal infections created the need for reliable methods of testing clinical isolates for susceptibility to fluconazole and other antifungal agents. After 15 years of collaborative research, the National Committee for Clinical Laboratory Standards (NCCLS) approved a standardized reference method for testing of yeasts that demonstrated excellent intra- and interlaboratory reproducibility (17). With this method, the NCCLS proposed interpretive breakpoint MICs of fluconazole that correlated with the response to therapy in vivo, largely based upon the experience in treating human immunodeficiency virus (HIV)-infected patients with oropharyngeal candidiasis caused by Candida albicans (27). Since their publication, the NCCLS interpretative breakpoints have been supported by further data on oropharyngeal candidiasis (5-7, 21, 22, 25, 30, 34). A correlation between in vitro susceptibility and the response to therapy of nonmucosal candidiasis has been demonstrated in some studies (11, 12) but not others (26).
The NCCLS breakpoints included a novel interpretive category called susceptible-dose dependent (S-DD) (fluconazole MICs of 16 and 32 μg/ml) (27). In devising this category, the NCCLS recognized that higher dosages of fluconazole might be necessary to successfully treat infections caused by isolates that are less susceptible to the drug. Indeed, the S-DD designation implicitly acknowledges the major limitation of MIC as a predictor of therapeutic response. Although MIC accurately expresses the efficacy of an antimicrobial under defined conditions in vitro, it does not consider other factors that contribute to the outcome in vivo, such as serum or tissue concentrations. For this reason, there has been interest in applying pharmacodynamic parameters that relate serum concentration of fluconazole, which depends upon dose, adsorption, distribution, and elimination, to its therapeutic effects (28).
Elegant animal studies have demonstrated that the ratio of the area under the exposure curve (AUC) to the MIC (AUC/MIC) best predicts the response to therapy with fluconazole (4, 13). This observation has potential clinical relevance, given the well-characterized linear pharmacokinetics of fluconazole in humans (10, 14). The AUC of fluconazole in healthy adults, expressed as milligrams per hour per liter, is virtually identical to the daily dose in milligrams, a relationship that holds over a range of dosages up to 2,000 mg (13, 14). In reassessing the oropharyngeal candidiasis data originally used to establish the NCCLS breakpoint MICs, Rex et al. demonstrated that a fluconazole dose/48-h MIC ratio of <25 correlated with an increased likelihood of therapeutic failure (28). The ability to extrapolate these findings to the settings of candidemia or other types of nonmucosal candidiasis is limited by the paucity of clinical data (11, 12, 26, 27). In particular, data evaluating a range of drug dosages to outcome for infections due to isolates with elevated MICs are lacking (28).
In this regard, we have access to a potentially unique set of 32 clinical isolates collected from patients enrolled during the early 1990s in a prospective, multicenter, observational study of candidemia (18, 19). These isolates were obtained from 15 patients who responded to fluconazole therapy, 4 patients who failed fluconazole therapy, and 13 patients who developed breakthrough candidemia while receiving fluconazole for empirical therapy. The patients were treated with a wide range of fluconazole dosages, the majority of which would now be considered inadequate. Furthermore, a large percentage of patients were critically ill, neutropenic, or otherwise immunosuppressed. As such, we hypothesized that these isolates might be well suited to demonstrate a correlation between the results of in vitro susceptibility testing and therapeutic outcome. We used the NCCLS macrobroth reference method to determine fluconazole MICs for clinical isolates and determined if MIC and/or dose/MIC ratio correlated with the therapeutic response to fluconazole.
MATERIALS AND METHODS
Candida isolates were obtained from the bloodstreams of unique patients enrolled in a prospective multicenter study of candidemia (18, 19). To be eligible for enrollment, patients had to have any vascular catheters that were in place at the time of the first positive blood culture removed. Among enrolled patients treated with fluconazole for at least 3 days for whom complete clinical and drug dosing data were available, 32 isolates were retrievable from −80°C stock solutions. Prior to testing, the isolates were subcultured onto Sabouraud dextrose agar plates, grown at 35°C for 24 to 48 h, and subcultured again for 24 h.
Antifungal susceptibility testing against fluconazole was performed using the broth macrodilution technique proposed by the NCCLS (M27-A) (17). The concentrations tested ranged from 0.125 to 64 μg/ml. Four Candida reference strains (C. albicans ATCC 90028 and ATCC 90029, C. parapsilosis ATCC 90018, and C. glabrata ATCC 90030) were incorporated into each set of experiments as quality controls. The MIC was defined as the lowest concentration of fluconazole causing an 80% decrease in turbidity compared to the growth of a control well.
Definitions.
Therapeutic failure was defined as either persistence of Candida in the bloodstream despite 3 days of therapy with fluconazole or development of breakthrough candidemia while receiving fluconazole for ≥3 days as empirical therapy. In all other cases, the response to therapy was defined as therapeutic success. Neutropenia was defined as an absolute neutrophil count of <1,000 cells/mm3. Critical illness was defined as a Pitt bacteremia score of ≥4 (18).
Statistical analysis.
MIC and dose/MIC ratio data were transformed to log10 to approximate a normal distribution prior to statistical analysis. Statistical analysis was performed using GraphPad InStat. The statistical difference between the geometric mean MIC and dose/MIC ratio of isolates recovered from patients experiencing therapeutic failure and those recovered from patients experiencing therapeutic success was determined using the Mann-Whitney test. Univariate analysis of contingency data was performed using Fisher's exact test.
RESULTS
The demographics and clinical data for the 32 patients with candidemia are presented in Tables 1 and 2. The patients were enrolled during the period of January 1991 to May 1994 (18). The infecting strains are listed by species as well as ranges of MICs and dose/MIC ratios in Table 3. Underlying diseases and clinical characteristics of the patients included hematologic malignancy (59% [19/32]), neutropenia (47% [15/32]), receipt of corticosteroids (47% [15/32]), critical illness (31% [10/32]), bone marrow transplant (19% [6/32]), solid organ malignancy (19% [6/32]), and solid organ transplant (3% [1/32]). In order of frequency, the daily dosages of fluconazole used to treat patients were as follows: 200 mg (21 patients), 100 mg (n = 6), 400 mg (n = 4), and 50 mg (n = 1).
TABLE 1.
Clinical data for patients with candidemia who responded to therapy with fluconazole
| Patient | Age (yr) | Critically illa | Cancer | Trans- plant | Neutro- peniab | Candida sp. | 24-h MIC (μg/ml) | 48-h MIC (μg/ml) | Daily fluconazole dosage (mg) | 24-h dose/ MIC ratio | 48-h dose/ MIC ratio |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 5 | 33 | No | None | No | No | C. albicans | 0.25 | 0.5 | 400 | 1,600 | 800 |
| 9 | 73 | No | Solid | No | No | C. albicans | 0.25 | 0.5 | 200 | 800 | 400 |
| 15 | 55 | No | AMLc | No | Yes | C. tropicalis | 0.25 | 0.5 | 200 | 800 | 400 |
| 2 | 50 | No | Ovary | No | No | C. albicans | 0.25 | 0.25 | 200 | 800 | 800 |
| 10 | 18 | Yes | None | No | No | C. albicans | 0.25 | 0.25 | 200 | 800 | 800 |
| 1 | 66 | No | AML | No | Yes | C. albicans | 0.5 | 0.5 | 200 | 400 | 400 |
| 8 | 67 | No | Solid | No | No | C. lusitaniae | 0.25 | 0.25 | 100 | 400 | 400 |
| 12 | 25 | No | None | No | No | C. albicans | 0.5 | 0.5 | 200 | 400 | 400 |
| 13 | 76 | Yes | None | No | No | C. parapsilosis | 1 | 2 | 200 | 200 | 100 |
| 3 | 65 | No | Colon | No | No | C. albicans | 0.5 | 0.5 | 100 | 200 | 200 |
| 7 | 47 | Yes | None | No | No | C. albicans | 1 | 1 | 200 | 200 | 200 |
| 4 | 64 | No | Multiple myeloma | No | No | C. tropicalis | 4 | 4 | 400 | 100 | 100 |
| 11 | 30 | No | AML | No | Yes | C. parapsilosis | 4 | 4 | 400 | 100 | 100 |
| 14 | 25 | No | Neuroblastoma | No | No | C. glabrata | 2 | 2 | 200 | 100 | 100 |
| 6 | 70 | Yes | Lymphoma | No | No | C. glabrata | 8 | 32 | 200 | 25 | 6.25 |
Critically ill, Pitt bacteremia score ≥4 (18).
Neutropenia, absolute neutrophil count <1,000 cells/mm3.
AML, acute myelogenous leukemia.
TABLE 2.
Clinical data for patients with candidemia who failed to respond to therapy with fluconazole
| Patient/mode of failure | Age | Critically ill | Cancerb | Transplanta | Neutropenia | Candida sp. | 24-h MIC (μg/ml) | 48-h MIC (μg/ml) | Daily fluconazole dosage (mg) | 24-h dose/ MIC ratio | 48-h dose/ MIC ratio |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Failurec | |||||||||||
| 4 | 62 | Yes | AML | No | Yes | C. glabrata | 4 | 16 | 200 | 50 | 12.5 |
| 2 | 64 | Yes | Renal cell carcinoma | No | Yes | C. tropicalis | 1 | 2 | 200 | 200 | 100 |
| 14 | 42 | No | None | No | No | C. parapsilosis | 0.5 | 1 | 200 | 400 | 200 |
| 7 | 51 | Yes | Unknown solid cancer | Heart | No | C. albicans | 0.5 | 0.5 | 200 | 400 | 400 |
| Breakthroughd | |||||||||||
| 6 | 48 | No | Lymphoma | No | Yes | C. parapsilosis | 0.5 | 1 | 200 | 400 | 200 |
| 8 | 14 | No | ALL | BMT | No | C. albicans | 1 | 1 | 100 | 100 | 100 |
| 15 | 2 | No | AML | No | Yes | C. glabrata | 2 | 8 | 200 | 100 | 25 |
| 12 | 43 | No | AML | BMT | Yes | C. parapsilosis | 1 | 32 | 100 | 100 | 3.125 |
| 9 | 2 | No | None | No | Yes | C. albicans | 1 | 1 | 50 | 50 | 50 |
| 5 | 48 | Yes | ALL | BMT | No | C. glabrata | 16 | 16 | 200 | 12.5 | 12.5 |
| 10 | 51 | No | ALL | No | Yes | C. krusei | 32 | 64 | 400 | 12.5 | 6.25 |
| 17 | 71 | Yes | Myelofibrosis/ polycythemia vera | No | No | C. lusitaniae | 32 | 32 | 200 | 6.25 | 6.25 |
| 16 | 48 | No | Leukemia | BMT | Yes | C. krusei | 64 | 64 | 200 | 3.125 | 3.125 |
| 1 | 63 | Yes | Multiple Myeloma | No | Yes | C. krusei | 64 | >64 | 200 | 3.125 | 1.56 |
| 11 | 48 | No | ALL | BMT | Yes | C. krusei | 32 | 64 | 100 | 3.125 | 1.56 |
| 13 | 42 | No | Lymphoma | No | Yes | C. albicans | 64 | >64 | 200 | 3.125 | 1.56 |
| 3 | 32 | Yes | Aplastic anemia | BMT | Yes | C. glabrata | 64 | >64 | 100 | 1.56 | 0.78 |
BMT, bone marrow transplant.
AML, acute myelogenous leukemia; ALL, acute lymphocytic leukemia.
Failure, patients with persistent candidemia despite 3 days of fluconazole therapy.
Breakthrough, patients who developed candidemia while receiving at least 3 days of fluconazole for empiric therapy.
TABLE 3.
In vitro susceptibility to fluconazole at 48 h according to Candida sp.
| Candida sp. | No. of patients | MIC (μg/ml) (range) | Dose/MIC ratio (range) |
|---|---|---|---|
| C. albicans | 12 | 0.25->64 | 1.56-800 |
| C. glabrata | 6 | 2->64 | 0.78-100 |
| C. parapsilosis | 5 | 1-32 | 3.125-200 |
| C. krusei | 4 | ≥64 | 0.78-6.25 |
| C. tropicalis | 3 | 0.5-4 | 100-400 |
| C. lusitaniae | 2 | 0.25-32 | 6.25-400 |
Therapeutic failure was observed in 53% (17/32) of patients. Four patients had persistent candidemia despite at least 3 days of fluconazole therapy. Thirteen other patients had breakthrough candidemia despite usage of fluconazole for empirical therapy in a high-risk setting. Patients with neutropenia and those undergoing transplantation were significantly more likely to fail therapy than respond (80% [12/15] failure rate if neutropenic versus a 29% [5/17] failure rate if nonneutropenic [P = 0.006]; 100% [7/7] failure rate for transplant recipients versus a 40% [10/25] failure rate for nontransplant [P = 0.008]). There were no associations between therapeutic failure and age, diabetes mellitus, the presence of malignancy, or the severity of illness at the onset of candidemia.
Correlation between fluconazole MIC and therapeutic response.
The 24-h MIC at which 50% of the isolates tested are inhibited (MIC50) and MIC90 were 1 and >64 μg/ml, respectively. The 48-hour MIC50 and MIC90 were 2 and >64 μg/ml, respectively. Using the NCCLS proposed breakpoints, 75% (24/32) of isolates were susceptible at 24 h, 12.5% (4/32) were S-DD, and 12.5% (4/32) were resistant to fluconazole. At 48 h, 66% (21/32) were susceptible, 16% (5/32) were S-DD, and 19% (6/32) were resistant. The 48-hour MICs were either the same or higher than the 24-hour MICs. The agreement within twofold between the 24-hour and 48-hour MICs was 88% (28/32), and the agreement within fourfold was 97% (31/32).
The geometric mean MIC at 24 h for isolates that were associated with therapeutic failure (5.77 μg/ml) was significantly higher than the geometric mean MIC for those that were associated with therapeutic success (0.72 μg/ml; P = 0.003). Similarly, the geometric mean MIC at 48 h for isolates that were associated with therapeutic failure (11.55 μg/ml) was significantly higher than that for those associated with success (0.95 μg/ml; P = 0.0009).
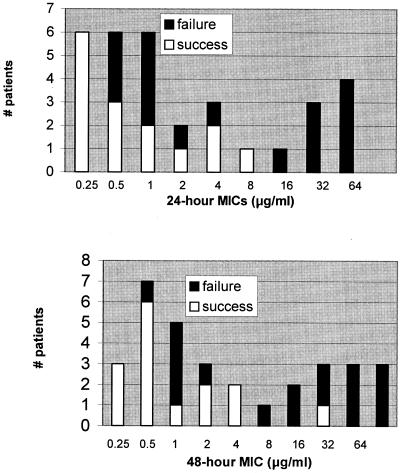
Among the patients infected with isolates for which the 24-hour and 48-hour MICs were within the fluconazole-susceptible range, the therapeutic success rates were 62.5% (15/24) and 67% (14/21), respectively (Fig. 1 and Table 4). Therapeutic failure was observed in all patients infected with isolates for which the 24-hour or 48-hour MICs were within the resistant range (4/4 and 6/6, respectively) (Fig. 1 and Table 4). Among the patients infected with isolates for which the 24-hour and 48-hour MICs were in the S-DD range, the therapeutic success rates were 0% (0/4) and 20% (1/5), respectively (Fig. 1 and Table 4). The patients infected with 24-hour S-DD strains received daily dosages of 400 mg (one patient), 200 mg (n = 2), and 100 mg (n = 1). The six patients infected with 48-hour S-DD strains who failed to respond to fluconazole received 400 mg (one patient), 200 mg (n = 4), and 100 mg (n = 1), while the patient who responded received 200 mg.
FIG. 1.
Therapeutic response stratified by 24- and 48-hour MICs (top and bottom panels, respectively).
TABLE 4.
Therapeutic response stratified by fluconazole MIC
| MIC (μg/ml) | No. of patients
|
|||
|---|---|---|---|---|
| 24-h MIC
|
48-h MIC
|
|||
| Failure | Success | Failure | Success | |
| ≤8 | 9 | 15 | 7 | 14 |
| 16-32 | 4 | 0 | 4 | 1 |
| ≥64 | 4 | 0 | 6 | 0 |
In order to overcome the diminished susceptibility of S-DD strains, it is recommended that clinicians treat patients infected with such strains with at least 400 mg/day or 6 mg/kg/day of fluconazole (27, 29). Furthermore, C. krusei is taken to be intrinsically resistant to fluconazole, regardless of MIC (27). In assessing the value of breakpoint MICs in predicting the response to therapy with fluconazole, therefore, we assumed the following: (i) that patients infected with S-DD strains would be likely to respond to 400 mg but would fail to respond to lower dosages and (ii) that all patients infected with C. krusei would be likely to fail to respond, regardless of dosage. We found that 60% (15/25) of patients predicted to respond to fluconazole by the 24-hour breakpoint MICs were successfully treated, compared to 0% (0/7) of those predicted to fail therapy (P = 0.008). Corresponding figures for 48-hour MICs were 67% (14/21) and 9% (1/11), respectively (P = 0.003). The sensitivities of the 24-hour and 48-hour MICs in identifying therapeutic failures were 41% (7/17) and 59% (10/17), respectively. Overall, the 24- and 48-hour MICs “correctly” predicted the response to therapy in 69% (22/32) and 75% (24/32) of patients, respectively.
Correlation between dose/MIC ratio and therapeutic response.
The geometric mean dose/MIC ratio determined using 24-hour and 48-hour MICs was lower for isolates that were associated with therapeutic failure (28.25 and 14.13, respectively) than the geometric mean dose/MIC ratio for isolates associated with success (289.4 and 219.36, respectively [P = 0.001 and 0.0004, respectively]).
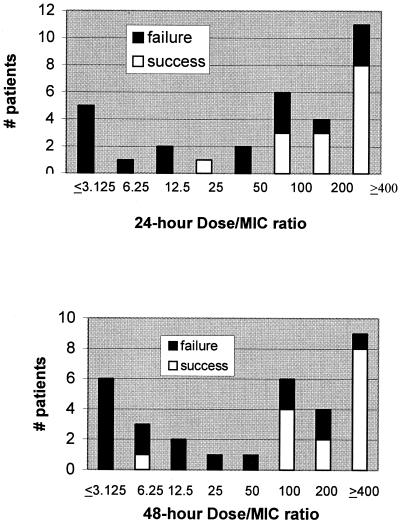
The distribution of dose/MIC ratio stratified by the therapeutic response is presented in Fig. 2. Patients for whom the ratio of fluconazole dose/MIC was >50 achieved the best response to therapy. Specifically, the therapeutic success rate of patients infected with isolates for which the 24-hour dose/MIC ratio was >50 was 67% (14/21), compared to 9% (1/11) of patients infected with isolates for which the dose/MIC ratio was ≤50 (P = 0.003). Corresponding figures at 48 h were 74% (14/19) and 8% (1/13), respectively (P = 0.0003). The sensitivity of dose/MIC ratios ≤50 by the reference method in predicting therapeutic failure was 59% (10/17) and 71% (12/17) at 24 and 48 h, respectively. Overall, the 24- and 48-hour dose/MIC ratio “correctly” predicted the response to therapy in 75% (24/32) and 81% (26/32) of patients, respectively.
FIG. 2.
Therapeutic response stratified by dose/MIC ratio using 24- and 48-hour MICs (top and bottom panels, respectively).
The 48-h dose/MIC ratios of isolates of different species, stratified by therapeutic response, are presented in Table 5.
TABLE 5.
Forty-eight-hour dose/MIC ratios of isolates of different Candida species stratified by therapeutic response to fluconazole therapy
| Candida sp. | Dose/MIC ratio (no. of isolates)
|
|
|---|---|---|
| Success | Failure | |
| C. albicans | 800 (3); 400 (3); 200 (3) | 400 (1); 100 (1); 50 (1); 1.56 (1) |
| C. glabrata | 100 (1); 6.25 (1) | 25 (1); 12.5 (2); 0.78 (1) |
| C. parapsilosis | 100 (2) | 200 (2); 3.12 (1) |
| C. krusei | None | 6.25 (1); 3.12 (1); 1.56 (1); 0.78 (1) |
| C. tropicalis | 400 (1); 100 (1) | 100 (1) |
| C. lusitaniae | 400 (1) | 6.25 (1) |
DISCUSSION
In this study, we make use of a unique collection of Candida isolates recovered in the early 1990s from the bloodstreams of patients with candidemia. For our purposes, the most significant aspects of our data set are the relatively high number of isolates with elevated fluconazole MICs and the administration of relatively low doses of fluconazole to a significant number of patients. These factors, coupled with the fact that most patients were immunosuppressed and/or critically ill, contributed to an unusually high failure rate of fluconazole therapy. In these regards, this study differs from other studies of candidemia (23, 26). Indeed, with the wide range of fluconazole doses and MICs, our data more closely resemble data from previous studies of HIV-infected patients with oropharyngeal candidiasis. Notably, the oropharyngeal candidiasis data have provided the most compelling evidence for the clinical relevance of fluconazole MICs. Using a similar data set, we demonstrate that fluconazole MIC and dose/MIC ratio both correlate with the response to therapy among patients with candidemia.
The geometric mean MIC and geometric mean dose/MIC ratio against the isolates associated with the therapeutic failure of fluconazole differed significantly from those of isolates associated with therapeutic success (48-hour MIC, 11.55 μg/ml and 0.95 μg/ml, respectively [P = 0.0009]; dose/MIC ratio, 14.13 and 219.36, respectively [P = 0.0004]). Moreover, we demonstrated that breakpoint MICs and dose/MIC ratio distinguished isolates based on the likelihood of response to therapy (Fig. 1 and 2). Using the NCCLS interpretive criteria for 48-hour MICs, 67% (14/21) of patients infected with susceptible (MIC ≤ 8 μg/ml) isolates were successfully treated. Conversely, therapeutic success was noted in none of the six patients infected with resistant (MIC ≥ 64 μg/ml) isolates. A dose/48-hour MIC ratio of >50 was associated with a therapeutic success rate of 74% (14/19), compared to a rate of only 8% (1/13) for patients infected with isolates for which the dose/MIC ratio was ≤50.
Previous studies attempting to correlate in vitro fluconazole susceptibility and breakpoint MICs to the efficacy of treatment of candidemia and other types of nonmucosal candidiasis have yielded conflicting results. In a study by Lee et al. of patients treated with 400 mg of fluconazole for candidemia (21 patients) or deep-seated candidal infections (11 patients), clinical cure rates among candidemic patients (defined as resolution of signs and symptoms of disease and sterilization of blood cultures within at least 1 week of therapy and no evidence of relapse within 3 months after the completion of therapy) were 79% (11/14) for susceptible, 60% (3/5) for S-DD, and 0% (0/2) for resistant isolates (12). Using the interpretive criteria and assuming 400 mg to be an adequate dosage for S-DD isolates, patients predicted to respond to fluconazole had a cure rate of 74% (14/19), compared to 0% (0/2) for those predicted to fail. Although the data supported the validity of the breakpoint MICs, only 29% (2/7) of the patients who ultimately failed therapy were identified as likely to fail by in vitro testing. Another study evaluated 161 candidemic patients who were treated with fluconazole, of whom 1.3% (n = 21) were infected with resistant isolates (11). Attributable mortality was significantly higher for these patients (19% versus 8.6%; P < 0.01). Other endpoints, such as clinical or microbiologic response to therapy, however, were not assessed, and the utility of the S-DD breakpoints was not addressed.
Contradictory findings were noted in a large multicenter study of candidemia comparing treatment with fluconazole and amphotericin B among nonneutropenic hosts (23) which failed to demonstrate a relationship between in vitro susceptibility and outcome (26). There are several possible explanations for the disparate results in that study and ours. First, the daily dosage of fluconazole in the earlier study was 400 mg, whereas 88% (28/32) of our patients received ≤200 mg (including 22% [7/32] who received ≤100 mg). Second, fluconazole MICs of ≥32 μg/ml were present in only 4% (4/104) of the isolates in the earlier study, which greatly limited the ability to show a correlation between elevated MICs and therapeutic failure. By comparison, MICs in this range were observed for 28% (9/32) of our isolates. Overall, 34% (11/32) of our isolates were classified as S-DD or resistant. Third, the patients in our study were significantly more likely to be critically ill, neutropenic, or otherwise immunosuppressed, settings in which drug susceptibility might be a more important determinant of response to therapy. Finally, the patients in the comparative trial did not necessarily have intravenous catheters discontinued as part of therapy (23, 26). Since this was necessary for enrollment in our study, none of the therapeutic failures can be ascribed to a retained catheter. For this reason, diminished susceptibility to fluconazole might have been more important in the failure of the drug in vivo.
Our finding that the fluconazole dose/MIC ratio correlated with the response to therapy among patients with candidemia is consistent with previous studies of human oropharyngeal candidiasis and animal models of disseminated candidiasis (4, 13, 28). Rex et al. demonstrated that a dose/48-h MIC ratio of <25 was associated with an increased likelihood of therapeutic failure among HIV-infected patients with oropharyngeal candidiasis, the overwhelming majority of whom had disease attributable to C. albicans (28). During murine disseminated candidiasis, Louie et al. found that killing of C. albicans followed a very steep dose response curve at an AUC/MIC ratio between 42.5 and 55 (13); higher ratios did not increase killing further. Using C. albicans strains with MICs of 0.5, 16, and 32 μg/ml to establish murine disseminated candidiasis, Andes and Van Ogtrop demonstrated that AUC/MIC ratios of 12 to 24 achieved a 50% maximal microbiologic effect (4). In their paper, the authors used previously published fluconazole 50% effective dose data (i.e., dosages required to achieve 50% maximal microbiologic effect) from murine and rat studies of disseminated candidiasis to calculate corresponding AUC/MIC ratios of 44, 14, and 18 (4, 13, 31, 33). In addition to human oropharyngeal candidiasis and animal disseminated candidiasis data, reassessment of the human candidemia data from the study of Lee et al. reveals a cure rate of 79% (11/14) among patients infected with isolates with a dose/MIC ratio ≥50, compared to a failure rate of 57% (4/7) for patients with disease due to isolates with a dose/MIC ratio of <50 (12).
Although we showed that a dose/MIC ratio of ≤50 was associated with increased likelihood of fluconazole failure, our data set is too small to conclusively assign a breakpoint interpretive value. The fact that the breakpoint suggested by our data was higher than that identified by Rex et al. in their study of oropharyngeal candidiasis might reflect the limited number of our isolates with dose/MIC ratios in the range of 12.5 to 50 (28). At the same time, we cannot make any definitive conclusions about whether breakpoint values for each of the non-C. albicans spp. might differ from those for C. albicans. Certainly, major differences were not suggested in our testing of limited numbers of a wide range of species commonly implicated in nonmucosal infections. The issue of breakpoint values, particularly in the setting of non-C. albicans infections, will need to be addressed using more extensive data sets.
In addition, we must acknowledge that the low doses of fluconazole used in this study somewhat limited our ability to interpret the S-DD breakpoints. Indeed, 80% (4/5) of patients infected with S-DD isolates were treated with ≤200 mg of fluconazole, which might largely account for the therapeutic success rate of only 20% (1/5). We might fairly conclude from our data that while many patients infected with susceptible isolates who receive low doses of fluconazole will respond to therapy, similar doses are clearly insufficient in the treatment of those infected with S-DD isolates. We cannot, however, definitively conclude what daily dose might be most appropriate in this setting. Our dose/MIC ratio findings would seem to suggest that therapeutic failures might be less likely if dosages of ≥800 mg/day were employed.
Interestingly, several recent reports have recommended high-dose fluconazole regimens (i.e., ≥12 mg/kg per day or 800 mg/day in a 70-kg patient) in the treatment of candidemia caused by S-DD isolates (29, 32). In a study of 20 candidemic patients with solid tumors treated with 600 to 800 mg of fluconazole, 3 were infected with isolates for which the MICs were 32 (one patient) or 64 μg/ml (n = 2) (32). The only patient in the study who failed therapy with fluconazole received 600 mg and was infected with an isolate with an MIC of 64 μg/ml (dose/MIC ratio of 9.4). The other two patients infected with S-DD or resistant isolates responded (dose/MIC ratios of 12.5 and 25), as did the remainder of patients infected with susceptible isolates (dose/MIC ratio ≥300). In another study, three candidemic patients infected with S-DD isolates (MICs = 16 μg/ml) were treated with 200 to 400 mg of fluconazole and failed to respond (dose/MIC ratios ≤25) (16). Since fluconazole is well tolerated at doses up to 2,000 mg/day and since the majority of candidemia is caused by susceptible or S-DD isolates (8, 9, 15, 16, 20, 24), our finding of a correlation between dose/MIC ratio and outcome would support the use of higher doses (at least until in vitro susceptibility data are available).
Finally, our study offers further evidence of how pharmacodynamic considerations might enhance the ability of clinicians to optimize their use of fluconazole in treating candidal infections (28). Dose/MIC ratios may prove to be easier to interpret and apply in the clinical setting than MIC alone, since it quantitates the effects of increasing fluconazole dosages that are alluded to in the S-DD breakpoint designation. As new triazole compounds and other classes of antifungal agents become available (1-3), pharmacodynamic applications have the potential to improve our understanding of how best to employ these drugs against Candida spp. and other fungal pathogens.
REFERENCES
- 1.Andes, D., K. Marchillo, R. Conklin, G. Krishna, F. Ezzet, A. Cacciapuoti, and D. Loebenberg. 2004. Pharmacodynamics of a new triazle, posaconazole, in a murine model of disseminated candidiasis. 48:137-142. [DOI] [PMC free article] [PubMed]
- 2.Andes, D., K. Marchillo, T. Stamstad, and R. Conklin. 2003. In vivo pharmacodynamics of a new triazole, ravuconazole, in a murine candidiasis model. Antimicrob. Agents Chemother. 47:1193-1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Andes, D., K. Marchillo, T. Stamstad, and R. Conklin. 2003. In vivo pharmacokinetics and pharmacodynamics of a new triazole, voriconazole, in a murine candidiasis model. Antimicrob. Agents Chemother. 47:3165-3169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Andes, D., and M. Van Ogtrop. 1999. Characterization and quantitation of the pharmacodynamics of fluconazole in a neutropenic murine disseminated candidiasis infection model. Antimicrob. Agents Chemother. 43:2116-2120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arikan, S., and J. H. Rex. 2000. New agents for treatment of systemic fungal infections. Emerg. Drugs 5:135-160. [DOI] [PubMed] [Google Scholar]
- 6.Cartledge, J. D., J. Midgley, M. M. Petrou, D. Shanson, and B. G. Gazzard. 1997. Unresponsive HIV-related oro-oesophageal candidosis: an evaluation of two new in-vitro azole susceptibility tests. J. Antimicrob. Chemother. 40:517-523. [DOI] [PubMed] [Google Scholar]
- 7.Dannaoui, E., S. Colin, J. Pichot, and M. A. Piens. 1997. Evaluation of the E tests for fluconazole susceptibility testing of Candida albicans isolates from oropharyngeal candidiasis. Eur. J. Clin. Microbiol. Infect. Dis. 16:228-232. [DOI] [PubMed] [Google Scholar]
- 8.Diekema, D. J., S. A. Messer, A. B. Brueggemann, S. L. Coffman, G. V. Doern, L. A. Herwaldt, and M. A. Pfaller. 2002. Epidemiology of candidemia: 3-year results from the emerging infections and the epidemiology of Iowa organisms study. J. Clin. Microbiol. 40:1298-1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Duswald, K. H., A. Pend, and L. Pittrow. 1997. High-dose fluconazole of at least 800mg daily. Mycoses 40:267-277. [DOI] [PubMed] [Google Scholar]
- 10.Grant, S. M., and S. P. Clissold. 1990. Fluconazole: a review of its pharmacodynamic and pharmacokinetic properties, and therapeutic potential in superficial and systemic mycoses. Drugs 39:877-916. [DOI] [PubMed] [Google Scholar]
- 11.Kovacicova, G., Y. Krupova, M. Lovaszova, A. Roidova, J. Trupl, A. Liskova, J. Hanzen, P. Milosovic, M. Lamosova, L. Macekova, Z. Szovenyiova, A. Purgelova, T. Obertik, J. Bille, and V. Krcmery. 2000. Antifungal susceptibility of 262 bloodstream yeast isolates from a mixed cancer and non-cancer patient population: is there a correlation between in-vitro resistance to fluconazole and the outcome of fungemia? J. Infect. Chemother. 6:216-221. [DOI] [PubMed] [Google Scholar]
- 12.Lee, S.-C., C.-P. Fung, J.-S. Huang, C.-J. Tsai, K.-S. Chen, H.-Y. Chen, N. Lee, L.-C. See, and W.-B. Shieh. 2000. Clinical correlates of antifungal macrodilution susceptibility test results for non-AIDS patients with severe Candida infections treated with fluconazole. Antimicrob. Agents Chemother. 44:2715-2718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Louie, A., G. L. Drusano, P. Banerjee, Q. Liu, W. Liu, P. Kaw, M. Shayegani, H. Taber, and M. H. Miller. 1998. Pharmacodynamics of fluconazole in a murine model of systemic candidiasis. Antimicrob. Agents Chemother. 42:1105-1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Louie, A., Q. Liu, G. L. Drusano, W. Liu, M. Mayers, E. Anaissie, and M. H. Miller. 1998. Pharmacokinetic studies of fluconazole in rabbits characterizing doses which achieve peak levels in serum and area under the concentration-time curve values which mimic those of high-dose fluconazole in humans. Antimicrob. Agents Chemother. 42:1512-1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Milefchik, E., M. Leal, R. Haubrich, S. Bozzette, J. Tilles, J. Leedon, J. A. McCutchen, and R. A. Larsen. 1997. A phase II dose escalation trial of high dose fluconazole with and without flucytosine for AIDS associated cryptococcal meningitis. 4th Conference on Retroviruses and Opportunistic Infections, Washington, D.C., abstr. 5.
- 16.Munoz, P., C. P. Fernandez-Turegano, L. Alcala, M. Rodriguez-Creixems, T. Pelaez, and E. Bouza. 2002. Frequency and clinical significance of bloodstream infections caused by C. albicans strains with reduced susceptibility to fluconazole. Diagn. Microbiol. Infect. Dis. 44:163-167. [DOI] [PubMed] [Google Scholar]
- 17.National Committee for Clinical Laboratory Standards. 1997. Reference method for broth dilution antifungal susceptibility testing of yeasts. Approved standard NCCLS document M27-A. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 18.Nguyen, M. H., J. E. Peacock, Jr., A. J. Morris, D. C. Tanner, M. L. Nguyen, D. R. Snydman, M. M. Wagener, M. G. Rinaldi, and V. L. Yu. 1996. The changing face of candidemia: emergence of non-Candida albicans species and antifungal resistance. Am. J. Med. 100:617-623. [DOI] [PubMed] [Google Scholar]
- 19.Nguyen, M. H., J. E. Peacock, D. C. Tanner, A. J. Morris, M. L. Nguyen, D. R. Snydman, M. M. Wagner, and V. L. Yu. 1995. Therapeutic approaches in patients with candidemia: evaluation in a multicenter, prospective observational study. Arch. Intern. Med. 155:2429-2435. [PubMed] [Google Scholar]
- 20.Pfaller, M. A., R. N. Jones, G. V. Doern, H. S. Sader, S. A. Messer, A. Houston, S. Coffman, R. J. Hollis, and The SENTRY Participant Group. 2000. Bloodstream infections due to Candida species: SENTRY antimicrobial surveillance program in North America and Latin America, 1997-1998. Antimicrob. Agents Chemother. 44:747-751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Quereda, C., A. M. Polanco, C. Giner, A. Sanchez-Sousa, E. Pereira, E. Navas, J. Fortun, A. Guerrero, and F. Baquero. 1996. Correlation between in vitro resistance to fluconazole and clinical outcome of oropharyngeal candidiasis in HIV-related patients. Eur. J. Clin. Microbiol. Infect. Dis. 15:30-37. [DOI] [PubMed] [Google Scholar]
- 22.Revankar, S. G., O. P. Dib, W. R. Kirkpatrick, R. K. McAtee, A. W. Fothergill, M. G. Rinaldi, S. W. Redding, and T. F. Patterson. 1998. Clinical evaluation and microbiology of oropharyngeal infection due to fluconazole-resistant Candida in human immunodeficiency virus-infected patients. Clin. Infect. Dis. 26:960-963. [DOI] [PubMed] [Google Scholar]
- 23.Rex, J. H., J. E. Bennett, A. M. Sugar, P. G. Pappas, C. M. van der Horst, J. E. Edwards, R. G. Washburn, W. M. Scheld, A. W. Karchmer, A. P. Dine, M. J. Levenstein, C. D. Webb, et al. 1994. A randomized trial comparing fluconazole with amphotericin B for the treatment of candidemia in patients without neutropenia. N. Engl. J. Med. 331:1325-1330. [DOI] [PubMed] [Google Scholar]
- 24.Rex, J. H., P. G. Pappas, A. W. Karchmer, J. Sobel, J. E. Edwards, S. Hadley, C. Brass, J. A. Vazquez, S. W. Chapman, H. W. Horowitz, M. Zervos, D. McKinsey, J. Lee, T. Babinchak, R. W. Bradsher, J. D. Cleary, D. M. Cohen, L. Danziger, M. Goldman, J. Goodman, E. Hilton, N. E. Hyslop, D. H. Kett, J. Lutz, R. H. Rubin, W. M. Scheld, M. Schuster, B. Simmons, D. K. Stein, R. G. Washburn, L. Mautner, T. Chu, H. Panzer, R. B. Rosenstein, J. Booth, et al. 2003. A randomized and blinded multicenter trial of high-dose fluconazole plus placebo versus fluconazole plus amphotericin B as therapy for candidemia and its consequences in nonneutropenic subjects. Clin. Infect. Dis. 36:1221-1228. [DOI] [PubMed] [Google Scholar]
- 25.Rex, J. H., and M. A. Pfaller. 2002. Has antifungal susceptibility testing come of age? Clin. Infect. Dis. 35:982-989. [DOI] [PubMed] [Google Scholar]
- 26.Rex, J. H., M. A. Pfaller, A. L. Barry, P. W. Nelson, and C. D. Webb for the NIAID Mycoses Study Group and the Candidemia Study Group. 1995. Antifungal susceptibility testing of isolates from a randomized, multicenter trail of fluconazole versus amphotericin B as treatment of nonneutropenic patients with candidemia. Antimicrob. Agents Chemother. 39:40-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rex, J. H., M. A. Pfaller, J. N. Galgiani, M. S. Bartlett, A. Espinel-Ingroff, M. H. Ghannoum, M. Lancaster, F. C. Odds, M. G. Rinaldi, T. J. Walsh, A. L. Barry, et al. 1997. Development of interpretive breakpoints for antifungal susceptibility testing: conceptual framework and analysis of in vitro-in vivo correlation data for fluconazole, itraconazole, and Candida infections. Clin. Infect. Dis. 24:235-247. [DOI] [PubMed] [Google Scholar]
- 28.Rex, J. H., M. A. Pfaller, T. J. Walsh, V. Chaturvedi, A. Espinel-Ingroff, M. A. Ghannoum, L. L. Gosey, F. C. Odds, M. G. Rinaldi, D. J. Sheehan, and D. W. Warnock. 2001. Antifungal susceptibility testing: practical aspects and current challenges. Clin. Microbiol. Rev. 14:643-658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rex, J. H., T. J. Walsh, J. D. Sobel, S. G. Filler, P. G. Pappas, W. E. Dismukes, and J. E. Edwards. 2000. Practice guidelines for the treatment of candidiasis. Clin. Infect. Dis. 30:662-678. [DOI] [PubMed] [Google Scholar]
- 30.Rodriguez-Tudela, J. L., J. V. Martinez-Suarez, F. Dronda, F. Chaves, and E. Valencia. 1995. Correlation of in vitro susceptibility test results with in vivo response: a study of azole therapy in AIDS patients. J. Antimicrob. Chemother. 35:793-804. [DOI] [PubMed] [Google Scholar]
- 31.Rogers, T. E., and J. N. Galgiani. 1986. Activity of fluconazole (UK 49,858) and ketoconazole against Candida albicans in vitro and in vivo. Antimicrob. Agents Chemother. 30:418-422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Torres, H. A., D. P. Kontoyiannis, and K. V. I. Rolston. 2004. High-dose fluconazole therapy for cancer patients with solid tumors and candidemia: an observational, noncomparative retrospective study. Support Care Cancer 12:511-516. [DOI] [PubMed] [Google Scholar]
- 33.Van't Wout, J. W., M. Herman, and R. V. Furth. 1989. Comparison of the efficacies of amphotericin B, fluconazole, and itraconazole against a systemic Candida albicans infection in normal and neutropenic mice. Antimicrob. Agents Chemother. 33:147-151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Walmsley, S., S. King, A. McGeer, Y. Ye, and S. Richardson. 2001. Oropharyngeal candidiasis in patients with human immunodeficiency virus: correlation of clinical outcome with in vitro resistance, serum azole levels, and immunosuppression. Clin. Infect. Dis. 32:1554-1561. [DOI] [PubMed] [Google Scholar]