Abstract
Studies of early bactericidal activity provide a fast and economic way to evaluate the clinical efficacy of potential agents for the treatment of tuberculosis. Based on good early bactericidal activity data, ciprofloxacin entered further studies and is now recommended as part of treatment for multidrug-resistant tuberculosis. We examined the relationship between ciprofloxacin bactericidal activity and the emergence of resistance in an in vitro pharmacodynamic infection model in which we exposed Mycobacterium tuberculosis to simulated free-drug ciprofloxacin serum concentration-time profiles that mimic those encountered in humans treated with ciprofloxacin orally for 2 weeks. Mycobacterium tuberculosis cultures were sampled during the experiment in order to determine the effect of therapy on the total microbial population as well as the drug-resistant population. The ciprofloxacin regimen, which achieved a ratio of the area under the concentration time curve from 0 to 24 h to MIC of 80.4, resulted in a rapid microbial kill similar to that encountered in humans during studies of early bactericidal activity. However, despite this impressive bactericidal activity, resistance emerged quickly. By the end of the first week, most of the microbial population had been replaced by a ciprofloxacin-resistant population. Given the MICs encountered in clinical isolates of M. tuberculosis, we estimate that most clinically tolerable doses of ciprofloxacin will lead to emergence of resistance, especially when used as the only effective component of regimens given for treatment of multidrug-resistant tuberculosis. One of the explanations for why early bactericidal activity fails to predict sterilization may be the emergence of a resistant subpopulation, which only becomes ≥1% at the end of the early bactericidal activity studies.
Tuberculosis (TB) is one of the most common infectious causes of death on earth (22). The increasing global burden of the disease and the escalating problem of multidrug-resistant TB (MDR-TB) have led to increased pressure to test new anti-TB drugs. Several decades ago, it was observed that isoniazid monotherapy resulted in a rapid decline of Mycobacterium tuberculosis density in sputum, but the effect lasted for only 2 days (11). This led to the development of an index, termed early bactericidal activity (EBA), which provided a rapid and economical way to evaluate new drugs (18). The EBA is calculated as the average rate of decline in the density of M. tuberculosis in sputum per day for the first 2 to 5 days of treatment. Studies of EBA are now commonly used to evaluate the efficacy of candidate anti-TB agents (5). Agents identified to have a high EBA are isoniazid, moxifloxacin, and ciprofloxacin (8, 12, 16). For ciprofloxacin, the highest EBA was demonstrated with oral doses of at least 500 mg twice a day (19). Ciprofloxacin has been studied further and is now a central part of second-line anti-TB regimens. Unfortunately, EBA has been noted to be a poor predictor of overall success of drug at the time of completion of therapy (2, 15). The reason may be the coexistence of metabolic populations of M. tuberculosis with reduced drug susceptibility. Another reason may be the emergence of resistance. The relationship between emergence of resistance and EBA has not been examined, since it has always been assumed that the best way to prevent M. tuberculosis resistance to one drug is to introduce a second drug. However, we have demonstrated for the quinolone moxifloxacin that doses can be established that will minimize the probability that a drug will select for resistance to itself during therapy (10). There is, therefore, a need to examine the relationship between ciprofloxacin microbial kill, EBA, and resistance.
The microbial population of exponential-phase-growth M. tuberculosis in pulmonary cavities is often between 107 and 109 bacilli (3). Since the mutation frequency of ciprofloxacin resistance for M. tuberculosis in exponential-phase cultures is 1 × 10−7 to 1 × 10−6 (20), ciprofloxacin-resistant mutants are expected to exist in cavitary pulmonary lesions prior to therapy. Unfortunately, measuring the emergence of resistance is not part of the criteria used to evaluate EBA. It has been assumed that patients do not develop drug resistance during studies of EBA. Yet, despite its excellent EBA, the frequent use of ciprofloxacin for the treatment of TB has led to an alarming increase in the prevalence of M. tuberculosis resistant to quinolones (9). A dramatic example is from the Philippines, where ciprofloxacin resistance increased from 10.3% from 1989 to 1994 to 51.4% from 1995 to 2000, mainly because ciprofloxacin was often the only effective drug in the regimen used for treatment of MDR-TB (9). In the current study, we examined both the role of ciprofloxacin's bactericidal activity and emergence of ciprofloxacin resistance during the ciprofloxacin therapy in determining efficacy in an in vitro pharmacodynamic infection model of TB.
MATERIALS AND METHODS
Bacterial strain.
Mycobacterium tuberculosis H37Ra (ATCC 25177; American Type Culture Collection, Manassas, VA) was used for this study. Prior to use, a stock of M. tuberculosis was thawed from a storage temperature of −80°C. In order to achieve exponential-phase growth, the cultures were incubated at 37°C and 5% CO2 atmosphere for 4 days in Middlebrook 7H9 broth.
Antibiotic agent.
Ciprofloxacin hydrochloride was donated by Bayer Pharmaceuticals (West Haven, CT). The drug was dissolved in sterile water and diluted to the desired concentration in Middlebrook 7H9 broth.
Susceptibility testing and mutation frequency.
The MIC of ciprofloxacin was determined by methods recommended by the NCCLS (14). Mutation frequency of the M. tuberculosis strain was determined by plating 1 ml of 106 CFU/ml M. tuberculosis onto each of 10 Middlebrook 7H10 plates that had been supplemented with ciprofloxacin at four times the MIC. This ciprofloxacin concentration was chosen because a single-step mutation in gyrA of M. tuberculosis results in a ≥4-fold change in the ciprofloxacin MIC (13).
In vitro experiment in a pharmacodynamic model of tuberculosis.
Our in vitro pharmacodynamic infection model of TB utilizes a hollow-fiber system, as described in detail in a prior publication (10). Briefly, the hollow-fiber system allows M. tuberculosis to grow in the peripheral compartment of a hollow-fiber cartridge (Fig. 1). The peripheral compartment is separated from the central compartment by semipermeable hollow fibers (surface area, 2100 cm2), with pore sizes that are large enough to allow nutrients, drugs, and bacterial metabolites to freely transverse into and out of the peripheral compartment but too small for bacteria to leave the peripheral compartment (molecular mass cutoff, 20 kDa). The high surface-area-to-volume ratio of 140 cm2/ml guarantees that drug levels in the peripheral compartment rapidly approximate the concentrations in the central compartment as confirmed in a preliminary study (data not shown). On the fourth day of exponential-phase growth, 15 ml of 106 CFU/ml of M. tuberculosis was inoculated into the peripheral compartment of each of two hollow-fiber systems (Fibercell Systems, Frederick, MD) that had been preconditioned with Middlebrook 7H9 broth for 72 h. Throughout the experiment, the hollow-fiber systems were maintained in an incubator under a 5% CO2 atmosphere and at 37°C. Starting 24 h later, a computer-controlled syringe pump administered ciprofloxacin into the central compartment through a dosing port twice daily for 14 days. The treatment simulated the serum concentration-time profile reported in humans given 500 mg of ciprofloxacin orally twice a day (1). Since it is the free or non-protein-bound drug that is pharmacologically active, free-drug exposures were simulated (protein binding of 25%; nominal free-drug area under the concentration-time curve from 0 to 24 h [AUC0-24] of 20 mg/h/liter). Fresh medium was pumped into the system while drug-containing media was isovolumetrically removed from the system at rates programmed to simulate a nominal ciprofloxacin half-life of 4 h (1), as occurs in human patients with normal renal function. The central compartment of the in vitro pharmacodynamic system was sampled at 1.5-, 4-, 7-, 11-, 13.5-, 22-, 24-, 25.5-, 29-, 31-, 35-, 37-, 45-, and 48-h time points in order to validate that the intended ciprofloxacin concentrations were actually achieved. The peripheral compartments of both hollow-fiber systems, where M. tuberculosis was growing, were sampled just prior to infusion of the first ciprofloxacin dose on day 0 and just prior to infusion of the dose on days 3, 7, 10, and 13. The samples were washed twice to remove drug carryover and then plated onto antibiotic-free Middlebrook 7H10 agar plates in order to determine the effect of therapy on the density of total bacterial populations at each time point. In order to determine the density of the drug-resistant population, the samples were plated on Middlebrook 7H10 agar that had been supplemented with ciprofloxacin at four times the MIC. Plates were incubated for 3 weeks at 37°C under 5% CO2, at which point the number of colonies growing on agar plates was counted.
FIG. 1.
In vitro pharmacodynamic infection model of Mycobacterium tuberculosis that simulates human pharmacokinetics. The central compartment of the pharmacodynamic model consists of the central reservoir, the inner lumina of the hollow-fiber capillaries, and the tubing connecting the central reservoir to the hollow fibers. The peripheral compartment, which contains the Mycobacterium tuberculosis cultures, is the enclosed space outside the hollow-fiber capillaries. (Reprinted from reference 10 with permission of the publisher.)
Drug assay.
Ciprofloxacin concentrations in the samples were measured by high-performance liquid chromatography using a modification of the methodology described previously by Wright et al. (25). Aliquots (100 μl) were mixed with 200 μl of ice-cold acetonitrile and centrifuged for 5 min at 13,000 × g. A 200-μl aliquot of supernatant fluid was withdrawn and mixed with 200 μl of 0.02 M sodium phosphate containing 0.2% triethylamine and 0.2% sodium dodecyl sulfate adjusted to pH 3 with phosphoric acid. Ciprofloxacin standards were prepared in Middlebrook 7H9 medium and then treated in the same manner as described above for the experimental samples. Analysis was performed using an Agilent 1100 series high-performance liquid chromatography system equipped with a model 1046A fluorescence detector. The mobile phase was a 60:40 mixture of the above-described buffer and acetonitrile, delivered at a rate of 1.5 ml/min. An Alltech Adsorbosphere HS C18 column (150 × 4.6 mm) served as the stationary phase. Ciprofloxacin was detected fluorometrically using excitation and emission wavelengths of 275 and 450 nm, respectively. Assay response was linear over a concentration range of 0.1 to 10 mg/liter (r2, >0.999).
Pharmacokinetic analysis.
Pharmacokinetic analysis using a single-compartment open model with first-order input and elimination was performed using ADAPT II software (4).
RESULTS
The ciprofloxacin MIC for the M. tuberculosis isolate was 0.25 mg/liter. The mutation frequency to 1 mg/liter of ciprofloxacin was 1 per 4.8 × 105 CFU. Ciprofloxacin pharmacokinetics achieved in the in vitro pharmacodynamic system were a half-life of 4.8 h and a steady-state AUC0-24 of 20.1 mg/h/liter. Since the MIC for the M. tuberculosis isolate was 0.25 mg/liter, the isolate was exposed to an AUC0-24/MIC ratio of 80.4 for each of the 14 days.
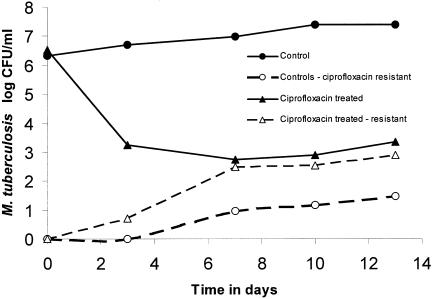
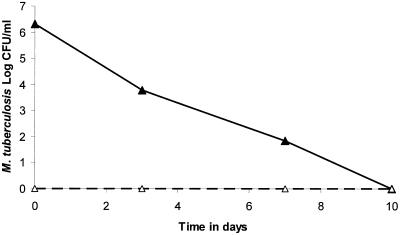
The nontreated cultures were in logarithmic-phase growth during the study (Fig. 2). The prevalence of ciprofloxacin-resistant M. tuberculosis was approximately 1 CFU per 2 × 106 CFU on day 0 and was approximately 1 CFU per 7.9 × 105 CFU on day 13, indicating little change in the size of the resistant subpopulation relative to the total population size (Fig. 2). In the treated cultures, a ciprofloxacin AUC0-24/MIC ratio of 80.4 was associated with rapid microbial kill of almost 3 log10 CFU/ml in the first 7 days, as depicted in Fig. 2. However, this impressive microbial kill was accompanied by a rapid increase in the size of the ciprofloxacin-resistant subpopulation. Just prior to the start of therapy, the resistant subpopulation comprised 0.00003% of the total population, increased to 0.27% by day 3, and had risen to 54.5% by day 7. The change in the relative proportion of the resistant subpopulation was a function of both the rate of microbial kill of the drug-sensitive population and the rate of amplification of the drug-resistant population.
FIG. 2.
Effect of a non-protein-bound ciprofloxacin AUC0-24/MIC ratio of 80.4 on the emergence of Mycobacterium tuberculosis resistance. Replacement of the total population by a resistant subpopulation is a function of both the microbial kill of the drug-susceptible subpopulation and amplification of the drug-resistant subpopulation.
DISCUSSION
A ciprofloxacin AUC0-24/MIC ratio of 80.4 was associated with a rapid microbial kill. The rate of M. tuberculosis density decline during the first 7 days was 0.5 log10 CFU/ml per day in our in vitro model. In human EBA studies, 1,500 mg a day of ciprofloxacin treatment led to 0.21 ± 0.14 log10 CFU/ml/sputum per day (19). The ciprofloxacin dose of 1,500 mg a day achieves a non-protein-bound AUC0-24 of 30 mg/h/liter in patients (1). Since M. tuberculosis clinical isolates have a mean ciprofloxacin MIC of 0.8 mg/liter (17), the EBA of 0.21 log10 CFU/ml/sputum per day achieved in patients (19) would have been mediated by a ciprofloxacin AUC0-24/MIC ratio of approximately 37.5 or 46.6% of that achieved in our in vitro TB model. An AUC0-24/MIC ratio of 37.5 would be expected to achieve 46.6% of the 0.5 log10 CFU/ml per day mediated by an AUC0-24/MIC ratio of 80.4 in our in vitro TB model, or 0.23 log10 CFU/ml per day. This bactericidal activity is concordant with the 0.21 log10 CFU/ml/sputum per day observed in the human EBA studies (19), indicating that the results from our in vitro TB model have clinical relevance.
The most important aspect of the current study is the demonstration that despite the impressive bactericidal activity by a ciprofloxacin AUC0-24/MIC ratio of 80.4, the total M. tuberculosis population was virtually replaced by a resistant subpopulation by the end of the first week of therapy. Our findings from the in vitro pharmacodynamic model of TB received striking confirmation from the report of a TB patient who developed ciprofloxacin resistance (resistant population >1% of total population) during the first 13 days of inadvertent ciprofloxacin and levofloxacin therapy (7). Clinically meaningful resistance occurs, by prior definition (14), when the resistant subpopulation comprises ≥1% of the total microbial population. Since EBA studies are finished in 2 to 5 days of therapy (5, 8, 19), they would fail to detect emergence of resistance at the level of 1% of the total microbial population. Moreover, since pharmacodynamic effects such as microbial kill and emergence of resistance depend almost exclusively on interaction of the microbial species and the non-protein-bound drug (6), a ciprofloxacin AUC0-24/MIC ratio of 80.4 in human patients is also expected to result in an impressive EBA as well as the accompanying emergence of resistance. This is even more likely in the clinical arena since the mean MIC of ciprofloxacin in clinical M. tuberculosis isolates is 0.8 mg/liter (17). Since ciprofloxacin doses of 1,500 mg a day result in a non-protein-bound AUC0-24/MIC ratio of approximately 37.5, they would also be likely to fail. This is of great concern, given that ciprofloxacin and its sister drug, ofloxacin, are central to the World Health Organization recommended treatment of MDR-TB (21, 23, 24) and are often the only highly effective drug in the multidrug regimen (9). When this occurs, they effectively act as monotherapeutic agents.
A potential solution may be replacement of the recommended second-line quinolones (21, 23, 24) with C-8 methoxy-substituted fluoroquinolones. Moxifloxacin is a promising C-8-substituted fluoroquinolone shown to have good bactericidal activity in murine tuberculosis (26) and in human patients (16). We recently investigated the emergence of M. tuberculosis resistance to moxifloxacin in our in vitro pharmacodynamic infection model of TB (10). Moxifloxacin doses of 600 to 800 mg were most likely to achieve AUC0-24/MIC exposures with both good bactericidal activity and the ability to minimize the emergence of M. tuberculosis resistance (Fig. 3). However, the tolerance of moxifloxacin doses of 600 to 800 mg still needs to be demonstrated. Nevertheless, the optimal effects of moxifloxacin demonstrated in monotherapy studies are expected to be maintained when it is used as part of multidrug therapy. Even though the cost of moxifloxacin may be higher than that of ciprofloxacin, and may thereby place a financial burden on developing countries, the cost of resistance, and the phenomenon of cross-resistance among quinolones, may ultimately outweigh the continued use of either ciprofloxacin or ofloxacin as a second-line drug.
FIG. 3.
Effect of moxifloxacin exposures higher than the breakpoint AUC0-24/MIC ratio associated with suppression of resistance. The figure shows the effect of a non-protein-bound moxifloxacin AUC0-24/MIC ratio of 101.6 administered daily for 10 days in our in vitro pharmacodynamic model of TB. The solid line indicates the decline of the total Mycobacterium tuberculosis population, while the dashed line indicates that the resistant subpopulation remains below baseline during 10 days of therapy. (Reprinted from reference 10 with permission of the publisher.)
Acknowledgments
Financial support for this work was provided by the Charitable Leadership Foundation.
REFERENCES
- 1.Bayer Pharmaceuticals. 2004. Ciprofloxacin hydrochloride tablets, p. 839-845. In Physicians' desk reference. Thomson PDR, Montvale, N.J.
- 2.Burman, W. J. 2003. The hunt for the elusive surrogate marker of sterilizing activity in tuberculosis treatment. Am. J. Respir. Crit. Care Med. 167:1299-1301. [DOI] [PubMed] [Google Scholar]
- 3.Canetti, G. 1965. Present aspects of bacterial resistance in tuberculosis. Am. Rev. Respir. Dis. 92:687-703. [DOI] [PubMed] [Google Scholar]
- 4.D'Argenio, D. Z., and A. Schumitzky. 1997. ADAPT II. A program for simulation, identification, and optimal experimental design. User manual. Biomedical Simulations Resource, University of Southern California, Los Angeles, Calif.
- 5.Donald, P. R., F. A. Sirgel, A. Venter, D. P. Parkin, H. I. Seifart, B. W. Van de Wal, J. S. Maritz, and P. B. Fourie. 2003. Early bactericidal activity of antituberculosis agents. Expert Rev. Anti. Infect. Ther. 1:141-155. [DOI] [PubMed] [Google Scholar]
- 6.Drusano, G. L. 2004. Antimicrobial pharmacodynamics: critical interactions of ‘bug and drug.’ Nat. Rev. Microbiol. 2:289-300. [DOI] [PubMed] [Google Scholar]
- 7.Ginsburg, A. S., S. C. Woolwine, N. Hooper, W. H. Benjamin, Jr., W. R. Bishai, S. E. Dorman, and T. R. Sterling. 2003. The rapid development of fluoroquinolone resistance in M. tuberculosis. N. Engl. J. Med. 349:1977-1978. [DOI] [PubMed] [Google Scholar]
- 8.Gosling, R. D., L. Heifets, and S. H. Gillespie. 2003. A multicentre comparison of a novel surrogate marker for determining the specific potency of anti-tuberculosis drugs. J. Antimicrob. Chemother. 52:473-476. [DOI] [PubMed] [Google Scholar]
- 9.Grimaldo, E. R., T. E. Tupasi, A. B. Rivera, M. I. Quelapio, R. C. Cardano, J. O. Derilo, and V. A. Belen. 2001. Increased resistance to ciprofloxacin and ofloxacin in multidrug-resistant Mycobacterium tuberculosis isolates from patients seen at a tertiary hospital in the Philippines. Int. J. Tuberc. Lung Dis. 5:546-550. [PubMed] [Google Scholar]
- 10.Gumbo, T., A. Louie, M. R. Deziel, L. M. Parsons, M. Salfinger, and G. L. Drusano. 2004. Selection of a moxifloxacin dose that suppresses drug resistance in Mycobacterium tuberculosis, by use of an in vitro pharmacodynamic infection model and mathematical modeling. J. Infect. Dis. 190:1642-1651. [DOI] [PubMed] [Google Scholar]
- 11.Jindani, A., V. R. Aber, E. A. Edwards, and D. A. Mitchison. 1980. The early bactericidal activity of drugs in patients with pulmonary tuberculosis. Am. Rev. Respir. Dis. 121:939-949. [DOI] [PubMed] [Google Scholar]
- 12.Kennedy, N., R. Fox, G. M. Kisyombe, A. O. Saruni, L. O. Uiso, A. R. Ramsay, F. I. Ngowi, and S. H. Gillespie. 1993. Early bactericidal and sterilizing activities of ciprofloxacin in pulmonary tuberculosis. Am. Rev. Respir. Dis. 148:1547-1551. [DOI] [PubMed] [Google Scholar]
- 13.Kocagoz, T., C. J. Hackbarth, I. Unsal, E. Y. Rosenberg, H. Nikaido, and H. F. Chambers. 1996. Gyrase mutations in laboratory-selected, fluoroquinolone-resistant mutants of Mycobacterium tuberculosis H37Ra. Antimicrob. Agents Chemother. 40:1768-1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.National Committee for Clinical Laboratory Standards. 2003. Susceptibility testing of mycobacteria, nocardiae, and other aerobic actinomycetes. Approved standard M24-A. National Committee for Clinical Laboratory Standards. Wayne, Pa. [PubMed]
- 15.O'Brien, R. J. 2002. Studies of the early bactericidal activity of new drugs for tuberculosis: a help or a hindrance to antituberculosis drug development? Am. J. Respir. Crit Care Med. 166:3-4. [DOI] [PubMed] [Google Scholar]
- 16.Pletz, M. W., A. De Roux, A. Roth, K. H. Neumann, H. Mauch, and H. Lode. 2004. Early bactericidal activity of moxifloxacin in treatment of pulmonary tuberculosis: a prospective, randomized study. Antimicrob. Agents Chemother. 48:780-782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ruiz-Serrano, M. J., L. Alcala, L. Martinez, M. Diaz, M. Marin, M. J. Gonzalez-Abad, and E. Bouza. 2000. In vitro activities of six fluoroquinolones against 250 clinical isolates of Mycobacterium tuberculosis susceptible or resistant to first-line antituberculosis drugs. Antimicrob. Agents Chemother. 44:2567-2568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sirgel, F. A., F. J. Botha, D. P. Parkin, B. W. Van de Wal, P. R. Donald, P. K. Clark, and D. A. Mitchison. 1993. The early bactericidal activity of rifabutin in patients with pulmonary tuberculosis measured by sputum viable counts: a new method of drug assessment. J. Antimicrob. Chemother. 32:867-875. [DOI] [PubMed] [Google Scholar]
- 19.Sirgel, F. A., F. J. Botha, D. P. Parkin, B. W. Van de Wal, R. Schall, P. R. Donald, and D. A. Mitchison. 1997. The early bactericidal activity of ciprofloxacin in patients with pulmonary tuberculosis. Am. J. Respir. Crit. Care Med. 156:901-905. [DOI] [PubMed] [Google Scholar]
- 20.Takiff, H. E., L. Salazar, C. Guerrero, W. Philipp, W. M. Huang, B. Kreiswirth, S. T. Cole, W. R. Jacobs, Jr., and A. Telenti. 1994. Cloning and nucleotide sequence of Mycobacterium tuberculosis gyrA and gyrB genes and detection of quinolone resistance mutations. Antimicrob. Agents Chemother. 38:773-780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.World Health Organization. 1997. Guidelines for the treatment of drug resistant tuberculosis. World Health Organization, Geneva, Switzerland.
- 22.World Health Organization. 2000. Tuberculosis. Fact sheet no. 104. World Health Organization, Geneva, Switzerland.
- 23.World Health Organization. 2003. W.H.O. model list of essential drugs. World Health Organization, Geneva, Switzerland.
- 24.World Health Organization Working Group on DOTS-Plus for MDR-TB. 2000. Procurement of second-line anti-tuberculosis drugs for DOTS-Plus pilot projects. World Health Organization, Geneva, Switzerland.
- 25.Wright, D. H., V. K. Herman, F. N. Konstantinides, and J. C. Rotschafer. 1998. Determination of quinolone antibiotics in growth media by reversed-phase high-performance liquid chromatography. J. Chromatogr. B Biomed. Sci. Appl. 709:97-104. [DOI] [PubMed] [Google Scholar]
- 26.Yoshimatsu, T., E. Nuermberger, S. Tyagi, R. Chaisson, W. Bishai, and J. Grosset. 2002. Bactericidal activity of increasing daily and weekly doses of moxifloxacin in murine tuberculosis. Antimicrob. Agents Chemother. 46:1875-1879. [DOI] [PMC free article] [PubMed] [Google Scholar]