Abstract
We suggest a novel approach to enhancing antimicrobial drug action by utilizing engineered peptide conjugates. Our most potent conjugates, [fMLF]PMBN and [fMLF]PMEN, are nonapeptides derived from polymyxin B's (PMB's) cyclic moiety (Thr-Dab-cyclo[Dab-Dab-d-Phe-Leu-Dab-Dab-Thr], where Dab is 2,4-diaminobutyric acid) and polymyxin E's (PME's) cyclic moiety (Thr-Dab-cyclo[Dab-Dab-d-Leu-Leu-Dab-Dab-Thr]), respectively, attached to a linear tail comprised of formyl-Met-Leu-Phe (fMLF). The cyclic part binds to gram-negative lipopolysaccharides, rendering the bacterial outer membrane permeable to hydrophobic antibiotics. The tail confers chemotactic and opsonic activities upon the conjugates. These two activities appear to be the basis for the conjugates' antibacterial activities. The conjugates are 8 to 10 times less toxic than the parent PMB or PME antibiotics. Fourteen of 18 mice lethally challenged with erythromycin-resistant Klebsiella pneumoniae survived following intraperitoneal administration of erythromycin and [fMLF]PMBN, whereas erythromycin or the peptide conjugate alone had no effect. Moreover, the clearance of Klebsiella from blood was markedly enhanced by intravenous injection of the [fMLF]PMEN peptide conjugate compared to the clearance of the organism from the mice treated with buffer alone as a control and was similar to that achieved by the PME antibiotic. Blood clearance was also significantly enhanced by administration of PMEN either alone or in a mixture with fMLF, although the effect was less than that produced by the peptide conjugate. Since resistance to polymyxins, the parent molecules of the synthetic cyclic peptides, is rare, the emergence of bacteria resistant to the antimicrobial properties of the peptide conjugates may be precluded as well.
Blood infections caused by gram-negative bacteria are one of the major challenges facing modern medicine, despite treatment with the available conventional antibiotics (22). Mortality rates in the range of 20 to 80% for septicemia caused by gram-negative bacteria have been reported. Antibiotic treatment is often administered when the disease reaches an advanced stage, usually when symptoms appear, by which point insufficient time remains for the antibiotic to kill the pathogen before the onset of irreversible tissue damage. Moreover, in many cases the antibiotic is given before sensitivity tests have been performed to identify an effective treatment. The emergence of bacterial strains resistant to conventional antibiotics, the lack of a rapid means of diagnosing the infection, and the generally unknown antibiotic sensitivity pattern of the infecting bacteria are probably among the major causes of inefficient therapy and high mortality rates (26).
Most of these blood infections are caused by opportunistic pathogens that are not usually capable of initiating bacteremia in otherwise healthy individuals. Host defense mechanisms of the innate, nonclonal immune system serve as the principal pathway for effective elimination of pathogens (11). Potent components of innate immunity are the macrophages and polymorphonuclear leukocytes (PMNs) that mediate the early clearance of bacteria by the phagocytic process (1, 23). Agents that enhance phagocytosis may promote clearance of the pathogen and termination of the infectious process. In some cases the phagocytotic process is mediated by adhesins expressed on the bacterial surface, in a process termed nonopsonic phagocytosis (18). However, the interaction of many bacteria with phagocytic cells is greatly facilitated by opsonins, which act as a bridge between the surfaces of these two types of cells (5, 8). The development of agents that can function as opsonins may provide a useful new approach to terminating the infectious process by enhancing bacterial attachment to phagocytic cells, followed by ingestion and digestion of the pathogen. For an agent to function as an opsonin, it must contain a moiety that recognizes a specific target molecule on the bacterial surface and another that recognizes specific receptors on phagocytic cells. Moreover, its toxicity must be relatively low, and most importantly, there should be little likelihood for the emergence of strains resistant to its action.
In the present study, we describe the synthesis of antimicrobial peptide conjugates that act both as opsonins to enhance destruction by phagocytic cells and as agents that permeabilize the bacterial membrane to enhance eradication by hydrophobic antibiotics and other antimicrobial agents. The peptides are derived from polymyxin B (PMB) or polymyxin E (PME) covalently linked to a short chemotactic peptide. Early studies have established that although polymyxin-based antibiotics are relatively toxic, they can be rendered 10 to 15 times less toxic by cleaving the lipid moiety from the molecule (7, 10). These polymyxin-based peptides lack direct bactericidal activity but retain their ability to bind to lipopolysaccharides (LPSs) on the bacterial surface and to permeabilize the outer membrane (OM) to hydrophobic antibiotics that cannot otherwise penetrate the bacteria, as well as to other bactericidal agents, such as the complement (33). Since resistance to the parent polymyxins is rare (25), it is expected that resistance to these polymyxin-derived peptides will be rare as well. Indeed, we did not find strains resistant to the permeabilizing activity of a polymyxin B (PMB) nonapeptide (PMBN) among 59 PMB-sensitive strains tested (21). Moreover, administration of the nonapeptide in combination with erythromycin protected mice against a lethal dose of an erythromycin-resistant strain of Klebsiella pneumoniae (19). More recently, we showed that the polymyxin B nonapeptide binds to LPS, which is consistent with previous studies showing that the parent PMB and PME molecules bind to lipid A of LPS on bacterial surfaces (16, 25, 31).
Encouraged by these results, we reasoned that conjugation of PMBN or PMEN to a moiety that recognizes specific receptors on phagocytic cells may confer opsonic activity on the molecule. We chose to use formyl methionine-leucine-phenylalanine (fMLF), a chemotactic peptide, as our conjugant because it has been studied extensively for its property of binding to specific receptors on phagocytic cells (17). It will be shown that conjugates consisting of polymyxin peptides covalently linked to fMLF enhance the bactericidal activities of conventional antibiotics, act as opsonins in promoting the phagocytic destruction of bacteria in vitro, and protect mice from lethal infections and bacteremia in vivo.
MATERIALS AND METHODS
Conjugate peptide synthesis.
The conjugates [fMLF]PMBN, [fMLF]PMBO, and [fMLF]PMEN are derived from PMBN (H-Thr-Dab-cyclo[Dab-Dab-d-Phe-Leu-Dab-Dab-Thr], where Dab is 2,4-diaminobutyric acid), polymyxin B octapeptide (PMBO; H-Dab-cyclo[Dab-Dab-d-Phe-Leu-Dab-Dab-Thr]), and polymyxin E nonapeptide (PMEN; H-Thr-Dab-cyclo[Dab-Dab-d-Leu-Leu-Dab-Dab-Thr]), respectively, each of which is attached to the C terminus of fMLF. The conjugates [nfMLF]PMBN and [nfMLF]PMBO are derived from PMBN and PMBO, respectively, and nonformylated MLF [nfMLF]. All conjugates were prepared by using solid-phase peptide synthesis (27). Briefly, linear peptide chains were assembled by conventional solid-phase synthesis with an AMS-422 automated solid-phase multiple-peptide synthesizer (ABIMED, Langenfeld, Germany). The 9-fluorenylmethoxy carbonyl (Fmoc) strategy was employed throughout peptide chain assembly, following the company's protocol (19). Synthesis was initiated by using Fmoc-Thr(tBu)-Wang resin (0.4 mmol/g) and was performed on a 25-μmol scale. The side chain amino-protecting groups for Dab were tert-butyloxycarbonyl and benzyloxycarbonyl (Cbz). Fmoc-Met-OH was employed as the final building unit. Coupling was achieved by using 4 equivalents of benzotriazole-1-yl-oxy-Tris-pyrolidino-phosphonium hexafluorophosphate (PyBOP) as a coupling agent and 8 equivalents of 4-methylmorpholine (NMM), all dissolved in dimethylformamide (DMF). The fully protected peptide-bound resin was treated with piperidine (20% in DMF) for 20 min and washed (with DMF), and the free N-terminus amino moiety was reacted with 4 equivalents of Cbz-OSu or 2,4,5-trichlorophenyl formate and 4 equivalents of N,N-diisopropylethylamine in DMF for 3 h. The fully protected peptide-bound resin was treated with a mixture of trifluoroacetic acid (TFA), water, and triethylsilane (95:2.5:2.5; vol/vol/vol) for 1 h at room temperature (RT) and filtered. The solution containing the cleavage mixture was cooled to 4°C, and the partially protected linear peptide was precipitated with ice-cold di-tert-butyl methyl ether and petroleum ether (30 to 40°C) (1:3; vol/vol) and centrifuged. The pellet was washed with the same mixture, dissolved in water and acetonitrile (2:3; vol/vol), and lyophilized. Cyclization was then performed in DMF at a peptide concentration of 1 mM by using PyBOP, 1-hydroxybenzotriazole, and NMM (4:4:8; equivalents) as reagents for 2 h at RT (yield, >95%, according to analytical high-pressure liquid chromatography [HPLC]). The reaction mixture was concentrated under high vacuum, and the cyclic peptidic product was precipitated by treatment with water. Final deprotection, i.e., removal of Cbz, was achieved by catalytic (Pd/C) hydrogenation in acetic acid, methanol, and water (5:4:1; vol/vol/vol). The product that was obtained was purified to homogeneity (>98%) by HPLC and characterized by amino acid analysis and mass spectrometry. A control [Lys2,4,7,8PMBN nonapeptide, in which the amino acids at positions 2, 4, 7, and 8 were replaced by lysine residues, was prepared as described above (27). Overall, the peptides prepared and tested in this study were PMBN and its formyl and nonformyl conjugates, PMBO and its formyl and nonformyl conjugates, and PMEN and its formyl conjugate. These peptides are henceforth referred to as the “test peptides,” and their sequences are given in Table 1, which also gives the sequence for the [Lys2,4,7,8]PMBN control.
TABLE 1.
Peptide sequences
| Peptide | Sequence |
|---|---|
| PMB | MOAa-Dab-Thr-Dab-cyclo[Dab-Dab-d-Phe-Leu-Dab-Dab-Thr] |
| PMBN | H-Thr-Dab-cyclo[Dab-Dab-d-Phe-Leu-Dab-Dab-Thr] |
| [fMLF]PMBN | Formyl-Met-Leu-Phe-Thr-Dab-cyclo[Dab-Dab-d-Phe-Leu-Dab-Dab-Thr] |
| [nfMLF]PMBN | H-Met-Leu-Phe-Thr-Dab-cyclo[Dab-Dab-d-Phe-Leu-Dab-Dab-Thr] |
| PMBO | H-Dab-cyclo[Dab-Dab-d-Phe-Leu-Dab-Dab-Thr] |
| [fMLF]PMBO | Formyl-Met-Leu-Phe-Dab-cyclo[Dab-Dab-d-Phe-Leu-Dab-Dab-Thr] |
| [nfMLF]PMBO | H-Met-Leu-Phe-Dab-cyclo[Dab-Dab-d-Phe-Leu-Dab-Dab-Thr] |
| PME | MOA-Dab-Thr-Dab-cyclo[Dab-Dab-d-Leu-Leu-Dab-Dab-Thr] |
| PMEN | H-Thr-Dab-cyclo[Dab-Dab-d-Leu-Leu-Dab-Dab-Thr] |
| [fMLF]PMEN | Formyl-Met-Leu-Phe-Thr-Dab-cyclo[Dab-Dab-d-Leu-Leu-Dab-Dab-Thr] |
| [Lys2,4,7,8]PMBNb | H-Thr-Lys-cyclo[Dab-Lys-d-Phe-Leu-Lys-Lys-Thr] |
MOA, 6-methyl octanoic acid.
The [Lys2,4,7,8]PMBN conjugate was prepared as a control.
Reversed-phase HPLC purification and analyses.
The crude synthetic test peptides were purified with a prepacked LichroCart RP-18 column (250 by 10 mm; 7-μm bead size; E. Merck, Darmstadt, Federal Republic of Germany) by employing a binary gradient formed from 0.1% TFA in water (solution A) and 0.1% TFA in 75% acetonitrile in water (solution B). The column was eluted at time zero with 0% solution B and at 48 min with 60% solution B at a flow rate of 5 ml/min. For purity evaluation, analytical reversed-phase HPLC was performed with a prepacked Lichrospher-100 RP-18 column (250 by 4 mm; 5-μm bead size; E. Merck) by employing the following binary gradient at a flow rate of 0.8 ml/min: at time zero, 10% solution B; at 40 min, 60% solution B; and at 50 min, 100% solution B. The separations were performed with a Spectra-Physics SP8800 liquid chromatography system equipped with an Applied Biosystems 757 variable-wavelength absorbance detector. The column effluents were monitored by determination of the UV absorbance at 220 nm. The corresponding fractions were collected, lyophilized, and analyzed and then subjected to exhaustive acid hydrolysis and precolumn reaction with 6-aminoquinolyl-N-hydroxysuccinimidyl carbamate to ascertain the amino acid composition (2690 separations module; Waters, Milford, MA). Mass spectrum analyses were performed to determine the molecular weights (VG-platform-II electrospray single quadropole mass spectrometer; Micro Mass, United Kingdom). The purities of the test peptides were >98% (with yields of 35 to 40%).
Polymyxin B preparations of PMBNs and PMBOs by enzymatic cleavage.
The PMB antibiotics (the sequences are given in Table 1) were purchased from Sigma Chemical Co., St. Louis, MO. For certain experiments, PMB (Sigma Chemical Co.) was used to prepare PMBNs and PMBOs by proteolysis of PMB with papain and ficin, respectively, as described elsewhere (9, 15). The crude products were purified (>98%) by HPLC, analyzed, and characterized as described above (see Table 1 for the peptide sequences).
Determination of MIC.
Clinical isolates of Escherichia coli (EC1), Klebsiella pneumoniae (K2), and Pseudomonas aeruginosa (33347) were obtained as described elsewhere (19). The gram-negative bacteria were grown on nutrient agar plates (Difco Laboratories, Detroit, Mich.) and kept at 4°C. Lyophilized aliquots of the test peptides (2 mg; determined by weight and ascertained by amino acid composition analysis) were dissolved in sterile, double-distilled water and filtered by using a 0.2-μm-pore-size Acrodisc. The number of CFU in an overnight culture of bacteria in Isotonic Sensitest Broth (ISB; Oxoid) was adjusted to 1 × 105 CFU/ml and inoculated onto microtiter plate wells, each of which contained 100 μl of a serial twofold dilution (1,000 to 0.5 μg/ml) of the tested antibiotics or peptides in ISB. The MIC was defined as the lowest peptide concentration at which there was no visible bacterial growth after incubation for 20 h at 37°C. The results (Table 2) are reported for three to five separate tests, which varied by no more than 1 dilution.
TABLE 2.
Effects of peptides and peptide conjugates on sensitivities of gram-negative bacteria to novobiocin and erythromycina
| Peptide | MIC (μg/ml)
|
||||
|---|---|---|---|---|---|
| Novobiocin
|
Erythromycin
|
||||
| EC | KP | PA | EC | KP | |
| None | >125 | >250 | >1,000 | >250 | >250 |
| PMBN | 1 | 4 | ND | 8 | 8 |
| PMBNs | 2 | 2 | 125 | 16 | 16 |
| [fMLF]PMBN | 0.12 | 0.8 | 125 | 1 | 2 |
| [nfMLF]PMBN | 0.5 | 0.8 | ND | ND | ND |
| PMEN | 2 | 32 | 250 | 8 | 8 |
| [fMLF]PMEN | 0.5 | 1 | 250 | 0.5 | 1 |
| PMBO | 2 | 4 | ND | 4 | 4 |
| PMBOs | 4 | 4 | 62 | 8 | 8 |
| [nfMLF]PMBO | 0.25 | 1 | 62 | ND | ND |
| [Lys2,4,7,8]PMBNb | >125 | >250 | ND | >250 | >250 |
The MICs of novobiocin and erythromycin were determined in the absence (none) or presence of 30 μg/ml (∼25 × 10−6 M) of the indicated peptide for E. coli and K. pneumoniae and 16 μg/ml for P. aeruginosa, as described in the text. Values are the means of at least six determinations, and in no case did the values exceed 1 dilution. Abbreviations: EC, E. coli; KP, K. pneumoniae; PA, P. aeruginosa; ND, not determined.
A control peptide consisting of a PMBN analog with lysine substitution has a reduced affinity for LPS (28).
Permeabilizing activity.
The ability of the test peptides to permeabilize the membranes of the gram-negative bacteria was determined as described elsewhere (19). Briefly, a bacterial suspension (10 μl; 1 × 105 CFU) was inoculated onto microtiter plate wells containing 100 μl of a serial twofold dilution (1,000 to 0.5 μg/ml) of novobiocin or erythromycin (Sigma Chemical Co.) in ISB. To each well, 10 μl of test peptide was added, to achieve a final test peptide concentration of 50 μg/ml. The extent to which the MIC of novobiocin or erythromycin decreased between wells, in the presence or the absence of the test peptides, was calculated and was designated the peptide's permeabilizing activity.
LPS binding assay.
The binding of the test peptides to bacterial LPS and the latter's affinity for the peptides were determined as described previously (27). Briefly, the fluorescence of dansyl-PMBN bound to E. coli LPS was measured with an MC200 monochromator (SLM AMINCO; SLM Instruments, Inc.) set at an excitation wavelength of 340 nm and at an emission wavelength of 485 nm. Binding affinity was evaluated by determining the concentration of the test peptide required to displace dansyl-labeled PMBN from LPS. To a quartz cuvette containing LPS solution (2 ml; 3 μg/ml; ∼2 × 10−7 M) in HEPES buffer (5 mM; pH 7.2), dansyl-PMBN (0.55 μM) was added and was allowed to equilibrate at RT for 10 to 15 min. Subsequently, small portions (5 to 10 μl) of test peptide solution (1 × 10−5 to 1 × 10−3 M) were added. The inhibition of fluorescence was measured 5 min after each addition. The percent inhibition was plotted as a function of the peptide concentration, and 50% inhibitory concentrations were calculated from the maximal specific displacement.
Preparation of phagocytic cells.
Two types of phagocytic cells were employed. Human PMN suspensions (106 cells/ml) were prepared as described previously (14, 20), and a suspension of mouse peritoneal macrophages (106 cells/ml) was prepared as described elsewhere (2, 21).
Chemotactic activity.
The chemotactic activity of the test peptides toward PMNs was assayed as described previously (20). Briefly, a human PMN suspension of 7 × 106 cells/ml of Hanks balanced salt solution was placed in the upper compartment and a 50-μg/ml (∼3.5 × 10-5 M) solution of the test peptide was placed in the lower compartment of a 5-μm membrane filter chamber. After incubation for 3 h at 37°C, the number of PMNs per microscopic field that crossed the 5-μm membrane filter from the chamber's upper to lower compartment was enumerated under oil immersion (magnification, ×1,000) for 10 fields.
Opsonophagocytosis assays.
The binding of the bacteria to phagocytic cells and their subsequent destruction were determined by a modified version of a method described previously (2). The indicated concentrations of the test peptides in 0.05 ml RPMI 1640 or the buffer alone (control) were added to an equivalent volume (0.5 ml) of a mixture of phagocytic (2 × 106/ml) and bacterial (5 × 108/ml) cell suspensions in buffer. The mixture was rotated end over end at 25 rpm at 4°C for 30 min and washed three times with the buffer by centrifugation at 300 × g for 5 min to remove the unbound bacteria. The phagocytic cell pellet was resuspended in buffer, its temperature was raised to 37°C, and 0.1-ml samples were withdrawn after 30 min to determine the number of bacteria associated with the phagocytic cells and after 3 h to determine the destruction of the bacteria by the phagocytes. To estimate the number of bound bacteria, the samples were distributed onto glass coverslips (22 by 22 mm) and placed in small petri dishes (35 mm in diameter) at RT for 15 min to allow the phagocytic cells to adhere onto the glass surfaces. The coverslips were then washed once in buffer, removed from the petri dishes with forceps, rinsed briefly in 0.5% NaCl, mounted on slides, dried vertically, fixed with methanol, and stained with Hemacolor (E. Merck). Two hundred cells were counted to determine the average number of bacteria per phagocytic cell. To estimate the intracellular destruction of the associated bacteria, the samples were disrupted in 1 ml distilled water, diluted serially 10-fold, plated on MacConkey agar plates, and incubated overnight at 37°C. The number of CFU was then determined.
Acute toxicity.
Solutions of test peptides (0.2 ml in sterile saline, administered with a needle 0.5 by 16 mm) were intravenously (i.v.) injected into the tail veins of male CD1 mice (age, 4 to 6 weeks; weight, 24 to 26 g; n = 5 mice per group). Survival was monitored after 1 day, and the amount of test peptide per kg of mouse body weight that constituted a lethal dose for 50% of the animals (LD50) was calculated.
Mouse protection assays.
The ability of the PMBN, the PMBN-based peptide conjugates, and PMB to protect mice from a lethal dose of erythromycin-resistant K. pneumoniae was determined as described previously (19). Briefly, 0.5 ml of bacterial suspension (2 × 105 CFU/ml) was intraperitoneally (i.p.) injected into male ICR mice (weight, 18 to 20 g; n = 6 per group). Four hours after bacterial inoculation (i.e., on day 1 at 4 h), each of three groups of mice was i.v. injected with erythromycin, PMB, or PMBN alone. Also at 4 h, other experimental groups of mice received an i.v. peptide-antibiotic mixture containing PMBN, [fMLF]PMBN, or [nfMLF]PMBN (4 mg/kg of mouse body weight) together with erythromycin (10 mg/kg of mouse body weight) in 0.5 ml of phosphate-buffered saline. On the following day (day 2), each group received the same treatment given on day 1, except that the doses were split in half, with the second dose being delivered 4 h after the first one. Thus, the experimental mice received two i.v. injections of test peptide (2 mg/kg) and/or erythromycin (5 mg/kg mice), as appropriate, 4 h apart on day 2. On day 3 the mice were injected in the same manner once more. Survival was monitored daily for 7 days.
Blood clearance in mice.
The ability of PMEN, [fMLF]PMEN, and PME to enhance the clearance of bacteria from blood was determined by i.v. injection of 0.5 ml saline containing 106 CFU K. pneumoniae into the tail veins of groups of four mice. At 2.5 min, a blood sample was taken from the eye. The mice were then immediately injected with 0.5 ml saline alone (group a) or with saline containing 2 mg/kg of mouse body weight (∼40 μg/mouse) of PMEN (group b), a mixture of PMEN and fMLF (group c), [fMLF]PMEN (group d), or PME (group e). At various time intervals thereafter, a 0.02-ml blood sample was withdrawn from the eye with a disposable pipette, diluted 10-fold with distilled water, and plated on nutrient agar to determine the number of CFU/ml blood.
RESULTS
Membrane permeabilization of gram-negative bacteria by PMBN conjugates.
Previous studies have shown that PMBN and PMBO increase OM permeability for gram-negative bacteria by binding to the bacterial surface (15, 31, 32). The test peptides lacked bactericidal activity at concentrations ≥250 μg/ml (data not shown). Significantly, the MICs of the formyl peptide conjugate ([fMFL]PMBN) were 250 to 500, 500, and 250 to 125 μg/ml against E. coli, K. pneumonia, and P. aeruginosa, respectively, suggesting that the formyl peptide did not affect the MIC of the PMBN. However, at concentrations of 30 μg/ml (∼20 × 10-6 M) they rendered E. coli, K. pneumonia, and P. aeruginosa sensitive to novobiocin and rendered E. coli and K. pneumonia sensitive to erythromycin (Table 2). The sensitivity of the bacteria to the hydrophobic antibiotics increased by up to 50-fold in the presence of the formyl and nonformyl peptide conjugates. The synthetic nona- and octapeptides were as active as their counterparts prepared from Sigma PMB, which is purified from Bacillus cultures. The Lys2,4,7,8]PMBN control peptide was not effective in sensitizing the bacteria to the antibiotics.
In a separate set of experiments we determined the minimum concentration of the test peptides needed to render K. pneumoniae and E. coli sensitive to 30 μg/ml of erythromycin and novobiocin. We found that 1.5 to 0.75 μg/ml PMEN and of 0.38 to 0.19 μg/ml [fMLF]PMEN were sufficient to render the bacteria sensitive to novobiocin, while concentrations of at least 3 to 1.5 μg/ml of these peptides were required to render the bacterial species sensitive to erythromycin.
Binding of PMBN conjugates to the LPS of gram-negative bacteria.
In our previous studies we found that PMBN binds to LPS and has the ability to displace synthetic dansyl-labeled PMBN bound to LPS (27). Employing similar types of assays, we found that both PMBN and the [fMLF]PMBN conjugate were able to displace 50% of 0.55 μM dansyl-PMBN bound to E. coli LPS at concentrations of 2.5 ± 0.03 μM and 5 ± 0.1 μM, respectively (data not shown). By contrast, displacement of the dansyl derivative was not detected in the presence of as much as 25 μM of the control peptide, [Lys2,4,7,8]PMBN, which lacks the ability to permeate the OM.
Interaction with phagocytic cells. (i) Chemotaxis.
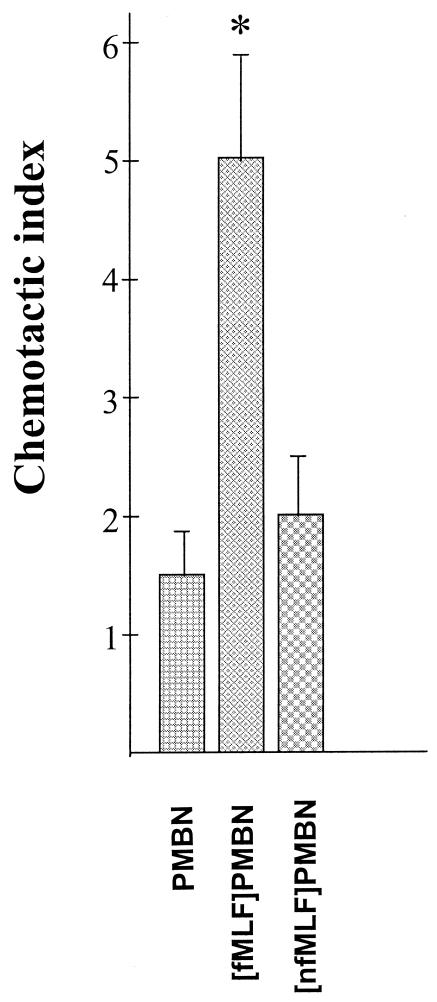
Previous studies have shown that formyl MLF peptides are potent chemotactic peptides for phagocytic cells, whereas nfMLF peptides are poor chemoattractants (4, 13). Consistent with those studies, we found that the chemotactic activity of [fMLF]PMBN conjugate at a concentration of 5 μg/ml (∼3.5 × 10-6 M) was about five times those of PMBN and [nfMLF]PMBN (Fig. 1).
FIG. 1.
Chemotactic activity of PMB peptide derivatives. The chemotactic activity of the indicated conjugates (5 μg/ml; ∼3.5 × 10-6 M) was assayed with human PMNs, as described in the text. The chemotactic activity is expressed as the number of PMNs per microscopic field that crossed the 5-μm membrane filter from the upper to the lower chamber compartment after screening of at least 10 fields. Data are presented as the means and standard deviations of three experiments. *, significantly higher (P > 0.01) values compared to those obtained in the presence of PMBN.
(ii) Opsonization.
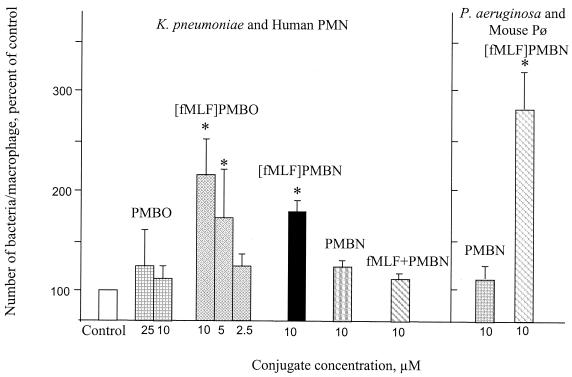
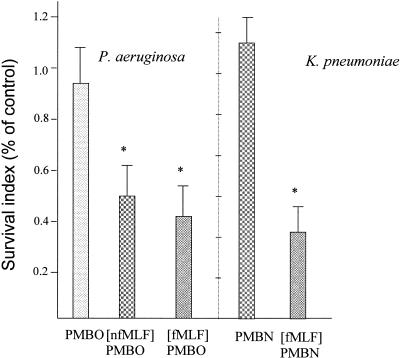
At concentrations of 8 to 20 × 10-6 M (2.5 to 10 μg/ml), the test conjugate peptides significantly enhanced the association of the tested bacteria with phagocytic cells compared to the association of their respective nona- or octapeptides or the control buffer alone (Fig. 2). The [fMLF]PMBN conjugate caused a threefold enhancement in the association of P. aeruginosa with mouse peritoneal macrophages over that achieved by PMBN. Similarly, [fMLF]PMBO markedly enhanced the association of K. pneumoniae with human PMNs over that achieved by PMBO. In contrast, the mixture of formyl peptide and PMBN was inactive. The increased association of the bacteria with phagocytic cells mediated by the formylated conjugates was followed by killing of the bacteria (Fig. 3). Reduction of bacterial viability was also enhanced by the nonformylated conjugate (Fig. 3).
FIG. 2.
Opsonic activity of polymyxin B-based derivatives. Suspensions of K. pneumoniae or P. aeruginosa in either buffer alone (control) or buffer containing the indicated peptide concentrations were mixed with suspensions of human PMNs (left panel) or mouse peritoneal macrophages (mouse Pø; right panel) for 30 min at 37°C. Nonbound bacteria were washed off by differential centrifugation. The average number of bacteria associated with a phagocytic cell was determined microscopically by counting at least 200 cells, as described in the text. The results are expressed as a percentage of the values obtained for the controls, which were 15 ± 3 and16 ± 4 bacteria per phagocytic cell for the controls of K. pneumoniae and P. aeruginosa, respectively. Data are the means and standard deviations of three experiments. *, significantly higher (P > 0.01) values than the respective control values.
FIG. 3.
Effect of polymyxin B derivatives on the destruction of bacteria by PMNs. Mixtures of P. aeruginosa with mouse peritoneal macrophages and mixtures of K. pneumoniae with human PMNs were prepared in buffer alone (control) or in buffer containing a 10-μg/ml (8 × 10−6 M) concentration of the indicated peptides and were incubated for 3 h at 37°C. The number of viable bacteria was enumerated by lysing the phagocytic cells and plating the cells on agar to determine the number of CFU. The results are expressed as a percentage of the values obtained for the controls (survival index of 1), these being 3 ± 0.6 × 107 CFU and 4.2 × 107±0.7 CFU for K. pneumoniae and P. aeruginosa, respectively. Data are the means and standard deviations of three experiments. *, significantly higher (P > 0.01) values compared to the respective control values.
Protective effects of conjugates.
The LD50s (acute toxicity) of the PMBN-based peptides for mice were 43 mg/kg and 30 mg/kg for PMBN and [fMLF]PMBN, respectively. The LD50s of the parent PME and PMB antibiotics were 8 and 9 mg/kg, respectively, consistent with previous studies (6, 9). The toxicities of PMEN-based peptides were 90 and 95 mg/kg for PMEN and [fMLF]PMEN, respectively.
To assess the protective activity of the PMB, PMBN, and the PMBN conjugates, mice were challenged with an erythromycin-resistant strain of K. pneumonia (Table 3). All the untreated mice and all the mice treated solely with erythromycin, PMBN, or [fMLF]PMBN died 3 to 6 days after being challenged with the bacterium. By contrast, treatment with a mixture of erythromycin and PMBN protected 8 of the 18 mice challenged with K. pneumoniae (P < 0.001), while mixtures of erythromycin with either [fMLF]PMBN or [nfMLF]PMBN yielded a survival rate of 14 of 18 mice (P > 0.001). The mixtures of erythromycin with [fMLF]PMBN or [nfMLF]PMBN protected the mice from erythromycin-resistant K. pneumoniae somewhat more effectively than mixtures of the antibiotic with the parent PMBN did (P = 0.04).
TABLE 3.
Protection of mice from K. pneumonia by PMBN or its conjugates, either alone or in conjunction with erythromycin antibiotic
| Drug(s) | Amount (mg/kg) of i.v. drug administered at the indicated time after i.p. injection of K. pneumoniae
|
No. of surviving mice/total no. of mice 7 days after challenge | ||
|---|---|---|---|---|
| 4 h | 1 daya | 2 days | ||
| Erythromycin | 10 | 10 | 10 | 0/18 |
| PMBN | 4 | 4 | 2 | 0/18 |
| [fMLF]PMBN | 4 | 4 | 2 | 0/6 |
| Erythromycin + [fMLF]PMBN | 10 + 4 | 10 + 4 | 5 + 2 | 14/18 |
| Erythromycin + [nfMLF]PMBN | 10 + 4 | 10 + 4 | 5 + 2 | 14/18 |
| Erythromycin + PMBN | 10 + 4 | 10 + 4 | 5 + 2 | 8/18 |
| PMB | 4 | 4 | 2 | 14/18 |
The amounts of the indicated peptide were i.v. administered in two portions, 4 h apart, on the first day after injection with erythromycin. The survival rates for mice treated with erythromycin and a PMBN conjugate were significantly higher than those for mice treated with erythromycin and PMBN (P > 0.001).
Klebsiella clearance from the blood of mice.
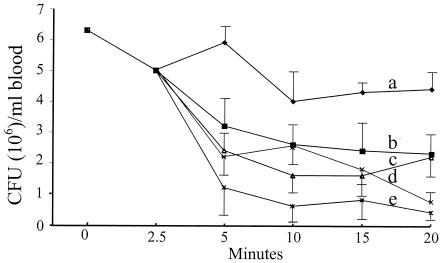
Following the intravenous injection of Klebsiella, the untreated mice developed a steady bacteremia over a period of 20 min (Fig. 4). PMEN alone or PMEN in a mixture with fMLF caused a significant drop in the number of CFU in the blood, probably by increasing the permeability of the bacterial membrane to bactericidal constituents in the blood (e.g., to the complement). However, injection of the mice with [fMLF]PMEN significantly enhanced the clearance of the bacteria from the blood of the mice, bringing the bacteria to levels similar to those obtained in mice that received the parent PME antibiotic.
FIG. 4.
Blood clearance of K. pneumoniae K2 in mice treated with PMEN or PMEN-based preparations. Mice were injected intravenously with 106 CFU of K. pneumoniae K2, and 2.5 min later, a blood sample was taken from the eye. The mice were then immediately injected with 0.5 ml saline alone (group a) or with saline containing 2 mg/kg of mouse body weight (∼40 μg/mouse) of PMEN (group b), a mixture of PMEN and fMLF (group c), [fMLF]PMEN (group d), or PME (group e). At the indicated time intervals, samples were withdrawn from the eye, diluted 10-fold, and plated on nutrient agar to determine the number of CFU/ml blood. The results are the means and standard deviations of three experiments. A Student t test was performed to determine statistical differences in the number of CFU after 20 min between the various groups of mice. Significant differences were found between groups a and d (P = 0.01), groups a and e (P = 0.02), groups a and b (P = 0.03), groups a and c (P = 0.03), groups b and d (P = 0.04), groups c and d (P = 0.04), groups b and e (P = 0.03), and groups c and e (P = 0.03).
DISCUSSION
We find that [fMLF]PMBN, [fMLF]PMEN, and [fMLF]PMBO render gram-negative bacteria sensitive to erythromycin (Table 2). This finding is significant, as it indicates that the conjugates are able to specifically bind to LPS on the OM of gram-negative bacteria and, with respect to the PMBN and PMEN-based conjugates, suggests that they retain the LPS binding properties of their nonapeptide parent molecules (31-33). In a separate set of experiments, we also found that the magnitude and the minimal amount of the hybrid peptides required to inhibit LPS-mediated stimulation of macrophages were similar to those of the corresponding PMBN peptide, suggesting that the addition of chemotactic peptide did not alter the LPS binding activity of the polymyxin peptide (data not shown). The LPS binding ability of the conjugates is probably responsible for their ability to permeabilize the bacterial OM (33). In addition, we find that the conjugates are able to bind to the fMLF receptors on phagocytic cells (Fig. 1). Together, these two properties enable fMLF-polymyxin conjugates to behave as opsonization agents and bridge between the bacterial and phagocytic cells, thus enhancing phagocytosis of the gram-negative bacteria (Fig. 2) and their subsequent destruction (Fig. 3). These findings are consistent with the findings that the mixture of PMBN and formyl peptide did not enhance the association of the bacteria with the phagocytic cells and with previous studies showing that fMLF receptors mediate the phagocytosis of fMLF-coated particles (4).
We also show that the [fMLF]PMBN conjugate, in combination with erythromycin, is able to protect mice against challenge with an otherwise lethal dose of erythromycin-resistant K. pneumonia, whereas PMBN is significantly less effective. Of all the formyl peptide conjugates tested, [fMLF]PMEN was most effective in the mouse protection assays. Moreover, [fMLF]PMEN was as active as the PME antibiotic in enhancing blood clearance. This is probably because [fMLF]PMEN increases the susceptibility of gram-negative bacteria to the host organism's own antimicrobial agents and their subsequent phagocytosis. In fact, it has been shown that in the presence of serum and PMBN, bacteria are more sensitive to both the bactericidal effects of the serum itself and the antimicrobial constituents of phagocytic cells (24). Perhaps most impressive are the results showing that [fMLF]PMEN is significantly more effective in enhancing the clearance of bacteria from the blood than a simple mixture of PMEN and fMLP is, reinforcing the notion that the conjugate is effective as an opsonin in the blood, in addition to its membrane-permeabilizing activity. Interestingly, the nonformylated peptide conjugates were also active in the protection assay, even though they were considerably less chemotactically active than the formylated petide conjugate. This can be explained by the fact that while the binding of the nonformyl peptides to their cognate receptor in solution is weak (12, 13), it is enhanced severalfold when the conjugates are immobilized on the bacterial cell surface and so are able to form multiple bonds between the bacteria and the phagocytic cells. This potential for multivalent binding opens up fresh directions for investigation in the design of new conjugates that have a higher affinity toward bacteria in solution and a stronger affinity to phagocytic cells after the conjugates have bound to the surface of the bacteria. Two features of the PMB- and PME-based peptide conjugates that we investigated are particularly noteworthy. The first is that they are 8 to 10 time less toxic than their parent PMB or PME antibiotics. Attempts to modify the structure of PMBN in order to increase its membrane-permeabilizing activity revealed that the interaction of the nonapeptide with its target LPS molecule is stereospecific (27-30). The cyclic part of the molecule efficiently perturbs the outer membrane of gram-negative bacteria and binds to bacterial LPS with high specificity. Oligoalanyl substitutions of PMBN do not affect most of PMBN′s activities, but a hydrophobic aromatic substitution generated a PMB-like molecule with high antibacterial activity and significantly reduced toxicity. These results demonstrate the significant role of PMBN′s hydrophobic segment in promoting biological activity. The other feature of these peptides is that bacteria are unlikely to develop resistance to them, since no mobile genes encoding for resistance to the parent PMB and PME antibiotics have been found during several decades of use.
All patients with bacteremia are treated with multiple antibiotics, some of which are hydrophobic, mainly because the infecting strain may be resistant to one or more of the antibiotics, especially if the antibiotic sensitivity pattern is unknown (34). In the present study we do not attempt to recommend the use of a specific antibiotic for the treatment of a specific type of infection. Rather, we demonstrate that resistance to certain hydrophobic antibiotics due to their inability to penetrate the bacterial outer membrane may be overcome by peptide polymyxin-based conjugates that simultaneously permeabilize the membrane and enhance the bactericidal activity of host-derived constituents and phagocytic cells. More studies are needed to determine which such conjugate peptides are capable of functioning alone or in combination with other conventional antibiotics against various gram-negative species in vivo.
Acknowledgments
This study was supported by a grant from the Chief Scientist's Office of the Ministry of Commerce, Israel.
REFERENCES
- 1.Agramonte-Hevia, J., A. Gonzalez-Arenas, D. Barrera, M. Velasco-Velazquez. 2002. Gram-negative bacteria and phagocytic cell interaction mediated by complement receptor 3. Immunol. Med. Microbiol. 34:255-266. [DOI] [PubMed] [Google Scholar]
- 2.Athamna, A., and I. Ofek. 1988. Enzyme-linked immunosorbent assay for quantitation of attachment and ingestion stages of bacterial phagocytosis. J. Clin. Microbiol. 26:62-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Atheron, E., and R. C. Sheppard. 1989. Solid phase peptide synthesis: a practical approach. IRL Press, Oxford, England.
- 4.Becker, E. L. 1976. Some interrelations of neutrophil chemotaxis, lysosomal enzyme secretion, and phagocytosis as revealed by synthetic peptides. Am. J. Pathol. 85:385-394. [PMC free article] [PubMed] [Google Scholar]
- 5.Borregaard, N. 1988. The human neutrophil. Function and dysfunction. Eur. J. Haematol. 41:401-413. [DOI] [PubMed] [Google Scholar]
- 6.Chihara, S., A. Ito, M. Yahata, T. Tobita, and Y. Koyama. 1973. Chemical synthesis and characterization of α-N-octanoyl and other α-N-acyl colistin nonapeptide derivatives. Agric. Biol. Chem. 37:2709-2717. [Google Scholar]
- 7.Chihara, S., A. Ito, M. Yhata, T. Tobita, and Y. Koyama. 1974. Chemical synthesis, isolation and characterization of α-N-fatty acyl colistin nonapeptide with special reference to the correlation between antimicrobial activity and carbon number of fatty acyl moiety. Agric. Biol. Chem. 38:521-529. [Google Scholar]
- 8.Cohen, M. S. 1994. Molecular events in the activation of human neutrophils for microbial killing. Clin. Infect. Dis. 18:S170-S179. [DOI] [PubMed] [Google Scholar]
- 9.Danner, R. L., K. A. Joiner, M. Rubin, W. H. Patterson, N. Johnson, K. M. Ayers, and J. E. Parriello. 1989. Purification, toxicity, and antiendotoxin activity of polymyxin B nonapeptide. Antimicrob. Agents Chemother. 33:1428-1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Duwe, A. K., C. A. Rupar, G. B. Horsman, and S. I. Vas. 1986. In vitro cytotoxicity and antibiotic active of polymyxin B nonapeptide. Antimicrob. Agents Chemother. 30:340-341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fearon, D. T. 2000. Innate immunity—beginning to fulfill its promise? Nat. Immunol. 1:102-103. [DOI] [PubMed] [Google Scholar]
- 12.Freer, R. J., A. R. Day, J. A. Padding, E. Schiffmann, S. Aswanikumar, H. J. Showell, and E. L. Becker. 1980. Further studies on the structural requirements for synthetic peptide chemoattractants. Biochemistry 19:2404-2408. [DOI] [PubMed] [Google Scholar]
- 13.Gao, J.-L., E. L. Becker, R. J. Freer, N. Muthukumaraswamy, and P. M. Murphy. 1994. A high potency nonformylated peptide agonist for the phagocyte N-formylpeptide chemotactic receptor. J. Exp. Med. 180:2191-2197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goldhar, J., M. Yavzori, Y. Keisari, and I. Ofek. 1991. 1991. Phagocytosis of Escherichia coli mediated by mannose resistant nonfimbrial haemaglutinin (NFA-1). Microb. Pathog. 11:171-178. [DOI] [PubMed] [Google Scholar]
- 15.Kimura, Y., H. Matsunaga, and M. Vaara. 1992. Polymyxin B octapeptide and polymyxin B heptapeptide are potent outer membrane permeability-increasing agents. J. Antibiot. (Tokyo) 45:742-749. [DOI] [PubMed] [Google Scholar]
- 16.Morrison, D. E., and D. M. Jacobs. 1976. Binding of polymyxin B to the lipid A portion of bacterial lipopolysaccharides. Immunochemistry 13:813-818. [DOI] [PubMed] [Google Scholar]
- 17.Niedel, J. E., and P. Cuatrecasas. 1980. Formyl peptide chemotactic receptors of leukocytes and macrophages. Curr. Top. Cell. Regul. 17:137-169. [DOI] [PubMed] [Google Scholar]
- 18.Ofek, I., Y. Goldhar, Y. Keisari, and N. Sharon. 1995. Nonopsonic phagocytosis of microorganisms. Annu. Rev. Microbiol. 49:239-276. [DOI] [PubMed] [Google Scholar]
- 19.Ofek, I., S. Cohen, R. Rahmani, K. Kabha, Y. Herzig, and E. Rubinstein. 1994. Antibacterial synergism of polymyxin B nonapeptide and hydrophobic antibiotics in experimental gram-negative infections in mice. Antimicrob. Agents Chemother. 38:374-377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ofek, I., and A. Bekierkunst. 1976. Chemotactic response of leukocytes to cord factor (trehalose-6′,6′-dimicolate). J. Natl. Cancer Inst. 57:1379-1381. [DOI] [PubMed] [Google Scholar]
- 21.Ofek, I., D. Zafriri, J. Goldhar, and B. I. Eisenstein. 1990. Inability of toxin inhibitors to neutralize enhanced toxicity caused by bacteria adherent to tissue culture cells. Infect. Immun. 56:3076-3079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Parrillo, J. E. 1993. Pathogenetic mechanisms of septic shock. N. Engl. J. Med. 328:1471-1477. [DOI] [PubMed] [Google Scholar]
- 23.Rautemaa, R., and S. Meri. 1999. Complement-resistane mechanisms of bacteria. Microbes Infect. 1:785-794. [DOI] [PubMed] [Google Scholar]
- 24.Rose, F., K. U. Heuer, U. Sibelius, S. Hombach-Klonisch, L. Kiss, W. Seeger, and F. Grimminger. 2000. Targeting lipopolysaccharides by the nontoxic polymyxin B nonapeptide sensitizes resistant Escherichia coli to the bactericidal effect of human neutrophils. J. Infect. Dis. 182:191-199. [DOI] [PubMed] [Google Scholar]
- 25.Soogard, H. 1982. The pharmacodynamics of polymyxin antibiotics with special reference to drug resistance liability. J. Vet. Pharmacol. Ther. 5:219-231. [DOI] [PubMed] [Google Scholar]
- 26.Travis, J. 1994. Reviving the antibiotic miracle? Science 264:360-362. [DOI] [PubMed] [Google Scholar]
- 27.Tsubery, H., I. Ofek, S. Cohen, and M. Fridkin. 2000. Structure-function studies of polymyxin B nonapeptide: implications to sensitization of gram-negative bacteria. J. Med. Chem. 43:3085-3092. [DOI] [PubMed] [Google Scholar]
- 28.Tsubery, H., I. Ofek, S. Cohen, and M. Fridkin. 2000. The functional association of polymyxin B with bacterial lipopolysaccharide is stereospecific: studies on polymyxin B nonapetide. Biochemistry 39:11837-11844. [DOI] [PubMed] [Google Scholar]
- 29.Tsubery, H., I. Ofek, S. Cohen, and M. Fridkin. 2001. N-terminal modifications of polymyxin B nonapeptide and their effect on antibacterial activity. Peptides 22:1675-1681. [DOI] [PubMed] [Google Scholar]
- 30.Tsubery, H., I. Ofek, S. Cohen, M. Eisenstein, and M. Fridkin. 2002. Modulation of the hydrophobic domain of polymyxin B nonapeptide: effect on outer-membrane permeabilization and lipopolysaccharide neutralization. Mol. Pharmacol. 62:1036-1042. [DOI] [PubMed] [Google Scholar]
- 31.Vaara, M., and P. Viljanen. 1985. Binding of polymyxin B nonapeptide to gram-negative bacteria. Antimicrob. Agents Chemother. 27:548-554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vaara, M., and T. Vaara. 1983. Polycations sensitize enteric bacteria to antibiotics. Antimicrob. Agents Chemother. 24:107-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vaara, M. 1992. Agents that increase the permeability of the outer membrane. Microbiol. Rev. 56:395-411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Young, L. S. 1992. Gram-negative sepsis, p. 452-475. In G. L. Mandell, R. G. Douglas, and J. E. Bennett (ed.), Principles and practice of infectious diseases, 2nd ed. John Wiley & Sons, Inc., New York, N.Y.