Abstract
The mitochondrial rRNA of the tapeworm species Echinococcus multilocularis carries an adenine at sequence position 2058 (numbering according to that for Escherichia coli) of the large-subunit rRNA (lsrRNA), while the nucleus-encoded rRNA, as determined in this study, is characterized by 2058G. This indicates a dichotomy in the drug susceptibilities of ribosomes: cytoplasmic ribosomes are predicted to be resistant to macrolide antibiotics, while mitochondrial ribosomes lack the most common chromosomal resistance determinant, lsrRNA 2058G. Upon incubation with the macrolide clarithromycin, the formation of vesicles from metacestode tissue was reduced in a dose-dependent manner. Electron microscopy revealed distinct morphological alterations both of the mitochondria and of the vesicle wall (e.g., loss of microtriches) in drug-treated vesicles. Adult worms lost their motility and displayed morphological changes (shortening and constriction of proglottids and the presence of vacuoles) upon incubation with clarithromycin. Our findings demonstrate that macrolides have distinct in vitro effects on E. multilocularis, endorsing the use of sequence-based in silico approaches for exploitation of available ribosomal drugs as anthelmintic agents.
Echinococcosis is a severe human disease caused by the larval (metacestode) stages of tapeworms belonging to the genus Echinococcus. Depending on the parasite species involved, different forms of echinococcosis are found, namely, cystic echinococcosis (causative agent Echinococcus granulosus), alveolar echinococcosis (causative agent Echinococcus multilocularis), and polycystic echinococcosis (causative agents Echinococcus vogeli and Echinococcus oligarthrus). Metacestodes develop in a tumor-like manner in different sites of the human body from oncospheres liberated from accidentally ingested worm eggs, which are shed in the feces of various species of carnivores that can act as definitive hosts for the adult, intestinal tapeworms. Cystic echinococcosis and alveolar echinococcosis are of special medical importance, with the former occurring worldwide and with the latter being restricted to the northern hemisphere (9). Treatment of echinococcosis involves a variety of options, including surgery and chemotherapy. The benzimidazole derivatives mebendazole and albendazole, which interfere with tubulin polymerization, are widely being used as antiparasitic drugs, with good efficacies. However, benzimidazoles do not seem to be parasiticidal in vivo, and the relapse rate after cessation of chemotherapy is relatively high (1, 9, 18). Hence, novel treatment options are needed,and the recently reported growth inhibition of metacestodes in vitro by amphotericin B, a fungicide that interferes with the sterols in cell membranes, albeit only at high concentrations of the drug (26), and particularly the good efficacy of nitazoxanide, whose mode of action is unknown, against murine alveolar echinococcosis (33) are important steps toward this goal.
Ribosomes are important targets for antibacterial agents, and different classes of drugs interfere with essential steps of protein synthesis. It is only recently that the principles governing the selectivity and toxicity of protein synthesis inhibitors are being understood. Macrolides (e.g., erythromycin and clarithromycin) are antibacterial agents which inhibit prokaryotic protein synthesis by binding to the nascent peptide exit tunnel near the peptidyltransferase center of large-subunit rRNA (lsrRNA) (27). A single nucleotide polymorphism directs macrolide susceptibility, as shown by the experimental mutation of lsrRNA position 2058 (31). Higher eukaryotes carry a guanine at lsrRNA position 2058 of both the cytoplasmic and the mitochondrial rRNAs that accounts for natural drug resistance (4).
Eukaryotic protozoa have been shown to be susceptible to macrolides both in vitro and in vivo (2, 8, 20, 21, 25, 29, 34, 35). In particular, the plastid (“apicoplast”) ribosome in apicomplexan protozoa emerged as the target for antibiotics that inhibit protein synthesis (3, 6, 7, 12, 22, 28). In contrast to the cytoplasmic ribosome, which is eukaryotic and thus largely precludes selective toxicity, the mitochondrial and plastid ribosomes are related to bacterial ribosomes. Little is known concerning the mitochondrial ribosome in eukaryotic protozoa as a drug target (13, 19). Analyses of the mitochondrial sequences of Toxoplasma gondii and Plasmodium falciparum suggested that the corresponding ribosomes are resistant to macrolides (3). In contrast, the mitochondrial lsrRNA of the amoebal species Acanthamoeba castellanii carries 2058A and was recently shown to be the target for macrolide antibiotics (21).
In this study, we have analyzed the peptidyltransferase region of cytoplasmic and mitochondrial lsrRNAs of E. multilocularis, the causative agent of human alveolar echinococcosis, prompting us to investigate in vitro the effect of the macrolide clarithromycin against the larval (metacestode) and the adult stages.
MATERIALS AND METHODS
Parasite.
E. multilocularis (isolate IM280) was propagated intraperitoneally in jirds (Meriones unguiculatus) and field mice (Microtus arvalis) as described previously (10) in approved experiments in accordance with the Swiss legislation on animal welfare. Parasite metacestode tissue was recovered under aseptic conditions from the euthanized rodents approximately 2 months after inoculation. Adult intestinal worms were recovered (11) 63 days postinfection from experimentally infected red foxes (Vulpes vulpes) (17), which were kept under a Danish experimental animal permission and treated according to the animal ethics laws of the European Union.
In vitro experiments.
The effects of the drugs in vitro were assessed by counting the number of vesicles produced from metacestode tissue after 14 days (14) and by assessing the viability and morphology of adult worms after 2 days of drugs exposure.
Metacestode tissue was cut into small blocks, with the lengths of the edges being approximately 3 mm, and the blocks were washed twice in complete medium (minimum essential medium [MEM; Gibco, Basle, Switzerland] supplemented with 10% fetal calf serum, 2 mM glutamine, 100 U penicillin/ml, 100 μg streptomycin/ml, 0.25 μg amphotericin B/ml). Two blocks per well were placed in 12-well plates (Techno Plastic Products, Trasadingen, Switzerland) with 3 ml of culture medium per well. The culture medium consisted of a 1:1 mixture of complete MEM and fresh supernatants of human fibroblast cell (MRC-5) cultures growing in the same medium in T flasks. The plates were kept in an incubator at 37°C with 5% CO2, and 2 ml of medium per well was exchanged every 3 to 4 days. Four wells per treatment (control or drugs) were used, and these experiments were done three times.
Adult worms (approximately 20 per well) were seeded into 12-well plates and incubated as described above in 3 ml of the complete MEM supplemented with 12 mg/ml glucose. The effects of the drugs were assessed in triplicate assays in a single experiment after 2 days.
Drugs.
Clarithromycin (Klacid) was from Abbott (Baar, Switzerland); hygromycin B, mebendazole, and praziquantel were from Fluka (Buchs, Switzerland). Stock solutions (10 mg/ml) of mebendazole were prepared in dimethyl sulfoxide; those of praziquantel were prepared in ethanol.
lsrRNA sequences.
The peptidyltransferase region of the cytoplasmic lsrRNA was amplified by PCR with primers 22 and 27, which target conserved flanking regions (36), with E. multilocularis DNA isolated from metacestode material. The sequences of both strands were determined by direct sequencing with an ABI PRISM 310 sequencer (Applied Biosystems, Baar, Switzerland). The corresponding sequence of the mitochondrial lsrRNA was available (Fukunaga, unpublished data; GenBank accession no. AB018440).
Electron microscopy.
Cysts that had developed in culture for 14 days were fixed with 2.5% glutaraldehyde in 100 mM Na/K-phosphate, pH 7.4, at 4°C for 1 h, washed in Na/K-phosphate, postfixed with 2% osmium tetroxide in Na/K-phosphate at 4°C for 1 h, and embedded in Epon. Sections of 1 μm were stained with toluidine blue for light microscopy; sections of 50 to 60 nm from areas of interest were stained with uranyl acetate and lead citrate and examined in a Cm 12 electron microscope (Philips, Eindhoven, The Netherlands) equipped with a slow-scan charge-coupled-device camera (Gatan, Pleasanton, CA).
Nucleotide sequence accession number.
The part of the lsrRNA gene of the cytoplasmic ribosome containing the peptidyltransferase region obtained in this study has been submitted to GenBank and can be found under accession no. AY615426.
RESULTS
Sequence analysis of large-subunit rRNA.
Part of the lsrRNA gene of the cytoplasmic ribosome containing the peptidyltransferase region was amplified by PCR and sequenced (604 bp; GenBank accession no. AY615426), and the sequence was compared to the corresponding published sequence of the mitochondrial gene (GenBank accession no. AB018440). The presence of a guanine at lsrRNA position 2058 (numbering according to that for Escherichia coli) of the nucleus-encoded rRNA is expected to provide resistance to macrolides, while mitochondrial ribosomes carry an adenine at this position, which is predictive of susceptibility.
In vitro investigations.
To study the effect of clarithromycin on E. multilocularis in vitro, metacestode tissue was incubated with the antibiotic; and vesicle formation was recorded and compared with that in the presence of two other drugs, hygromycin B and mebendazole, used as controls. Hygromycin B is a protein synthesis inhibitor with universal activity against archae, prokaryotes, and eukaryotes and is expected to inhibit both the cytoplasmic and the mitochondrial ribosomes (24). Mebendazole, a benzimidazole carbamate derivative, is an established anthelmintic compound that interferes with the microtubulus system of parasitic cells.
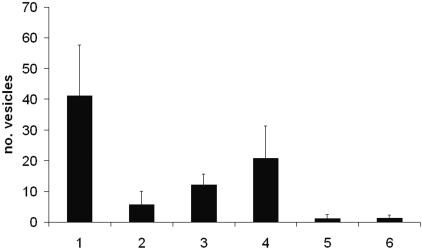
Upon exposure to clarithromycin, the vesicle formation of E. multilocularis was reduced in a dose-dependent manner (Fig. 1). Whereas 41 vesicles were produced in the absence of the drug, 6 (14.6% of control) were recorded in the presence of 100 μg/ml clarithromycin and 21 (58.5%) were recorded in the presence of 10 μg/ml clarithromycin. In cultures containing hygromycin (100 μg/ml) or mebendazole (10 μg/ml), an average of one (2.8%) vesicle was produced.
FIG. 1.
Effects of drugs on vesicle formation of E. multilocularis after 14 days of in vitro cultivation. 1, control, no drug; 2, 100 μg/ml clarithromycin; 3, 33 μg/ml clarithromycin; 4, 10 μg/ml clarithromycin; 5, hygromycin (100 μg/ml); 6, mebendazole (10 μg/ml). The mean values of the numbers of vesicles per well and the standard deviations are shown; experiments were done in quadruplicate and were repeated three times (twice with the two lower concentrations of clarithromycin). The results for all treatments were statistically significantly lower (P < 0.01, t test based on log + 1 transformed data) than those for the control treatment.
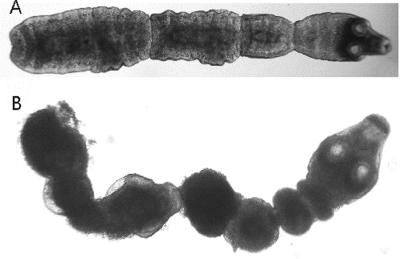
The effect of clarithromycin on adult worms was compared with those of hygromycin B and praziquantel. Praziquantel is an established anthelmintic compound that causes severe spasms and paralysis of the worm's muscles. Compared to the untreated adult worms (Fig. 2A), which had a high motility, worms incubated for 2 days in 10 μg/ml clarithromycin had a similar motility but differed morphologically by being slightly swollen. A dramatic effect was observed in worms incubated in 100 μg/ml clarithromycin: their motility was highly reduced, they appeared shortened and darker, the proglottids were deeply constricted, and vacuoles were present (Fig. 2B). Hygromycin B and praziquantel had similar, although more pronounced, effects on morphology compared to that of 100 μg/ml clarithromycin; and the worms were motionless.
FIG. 2.
Effects of drugs on adult worms in vitro after 2 days. (A) Control (no drugs); (B) clarithromycin at 100 μg/ml.
Electron microscopy.
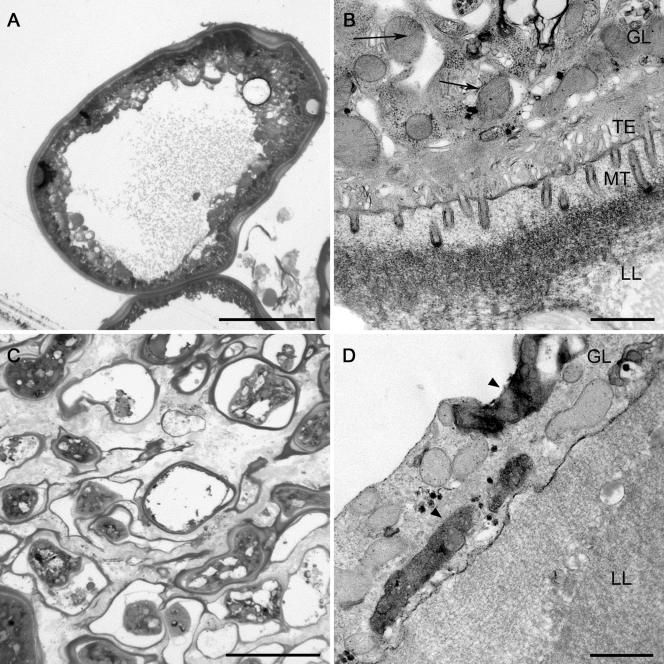
Electron microscopy was used to study alterations at the subcellular level. These studies revealed distinct morphological and ultrastructural alterations of metacestode tissue from in vitro cultures after cocultivation with clarithromycin (Fig. 3). The germinal layer of control vesicles consisted of several layers of cells containing mitochondria with distinct cristae and of a distinct tegument and numerous microtriches protruding into the acellular laminated layer (Fig. 3A and B). After incubation with clarithromycin (100 μg/ml) for 14 days, the vesicles were smaller and had laminated layers of various thicknesses (Fig. 3C). The cells lining the laminated layers were thin and often detached. They contained condensed mitochondria and mitochondria with indistinct cristae (Fig. 3C and D). Microtriches and the tegument were largely lacking (Fig. 3D).
FIG. 3.
Micrographs of E. multilocularis metacestode tissue in semithin sections (A and C) and ultrathin sections (B and D) of controls (no drugs) and after in vitro incubation with clarithromycin (100 μg ml−1) for 14 days. The vesicles of the controls (A and B) are characterized by a thick laminated layer (LL) which is lined with several layers of cells (germinal layer [GL]) and by the presence of a distinct distal tegument (TE) and numerous microtriches (MT) protruding into the acellular laminated layer. Note the mitochondria with distinct cristae (arrows, panel B). After incubation with clarithromycin (C and D), the vesicles were smaller and had laminated layers of various thicknesses (C). The cells lining the laminated layers were thin and often detached. They contained condensed mitochondria and mitochondria with indistinct cristae (D, arrowheads). Microtriches and the tegument were largely lacking (D). Bars, 50 μm (A and C) or 1 μm (B and D).
DISCUSSION
In this paper, we demonstrate for the first time that an antibacterial agent, the macrolide clarithromycin, can have a direct effect on a helminth organism, E. multilocularis. Antibacterial drugs have previously been shown to have promise for the treatment of infections with filarial nematodes, but in an indirect manner, by acting on endosymbiotic bacteria (15). In addition, the aminoglycoside antibiotic paromomycin was used to treat human intestinal infections with taeniid tapeworms (5, 30), but the mode of action against Taenia is not known.
As predicted by in silico analysis, the target of clarithromycin in E. multilocularis is the mitochondrial translational machinery. Indeed, we observed dramatic changes in the ultrastructure of mitochondria, where the loss of cristae and condensation (Fig. 3D) became apparent upon cocultivation with this drug. However, morphological alterations of mitochondria have also been observed upon incubation with other drugs. Albendazole, a benzimidazole component with activity on tubulin formation, resulted in rounded mitochondria, which were abnormally increased in size and which contained altered cristae (16). The presence of aberrant mitochondria was recently reported as an effect of nitazoxanide against E. multilocularis metacestode tissue in vitro, but no detailed information was provided (32). Hence, the mitochondrial alterations that we observed do not prove that clarithromycin directly affects the mitochondrial ribosome. However, in a comparable study with the amoebal species Acanthamoeba castellanii, where genetic analyses predicted the resistance of the cytoplasmic ribosome and the susceptibility of the mitochondrial ribosome to macrolides, the fortuitous isolation of a macrolide-resistant mutant and its genetic characterization showed that the expected point mutation 2058A→G of the mitochondrial lsrRNA was associated with drug resistance (no encystment of the amoebae) and, hence, with a lack of ultrastructural changes to the mitochondria upon cocultivation with clarithromycin (21).
The other structural changes observed in the E. multilocularis vesicles upon incubation with clarithromycin (e.g., loss of microtriches and separation of the germinal and the laminated layers) seem to be rather nonspecific effects, as they have also been observed in experiments with benzimidazoles or nitazoxanide (16, 32).
Rather surprising findings were the rapid (within 2 days) and distinct effects of clarithromycin (100 μg/ml) on adult worms (highly reduced motility, swelling, vacuolization, constriction of proglottids) which were comparable to the effects of antibiotic or anthelmintic compounds with unrelated modes of action (hygromycin B, praziquantel). The disruption of energy production in the mitochondria by clarithromycin appears to be a fast event, and the alternative, low-efficiency cytosolic respiratory metabolism in tapeworms (23) does not seem to have the capacity to compensate. Again, the similar changes induced by the different drugs may simply reflect a state of stress in the parasite that stems from the disruption of different cell organelles.
Clarithromycin showed potent anthelmintic activity in vitro, but whether such an effect will extend to the in vivo situation remains to be investigated. Of crucial importance is whether the drug would penetrate into larger metacestode tissues. In our in vitro experiments, by using metacestode material cut into small blocks, this possible barrier was not of concern. Furthermore, we would expect that macrolides act only in a parasitostatic manner and not in a parasitocidal manner, according to the results of experiments done with A. castellanii (21), which revealed that the drug-induced effects were nearly perfectly reversible. Hence, macrolides might be promising agents for the treatment of alveolar echinococcosis, particularly because of their synergistic actions with other existing drugs (benzimidazoles, nitazoxanide).
The experiments described in this study have considerable relevance for the search for drugs endowed with anthelmintic activities. Our observations endorse the use of sequence-based in silico approaches for the possible exploitation of the available ribosomal drugs as anthelmintic agents. Ultimately, elucidation of the mechanisms governing the selectivities and the specificities of ribosomal drugs should provide ample opportunities for the development of anthelmintic agents by rational drug design.
Acknowledgments
We thank Elisabeth M. Schraner for technical assistance with electron microscopy.
This work was supported in part by the University of Zürich.
REFERENCES
- 1.Ammann, R. W., and J. Eckert. 1996. Cestodes. Echinococcus. Gastroenterol. Clin. N. Am. 25:655-689. [DOI] [PubMed] [Google Scholar]
- 2.Araujo, F. G., D. R. Guptill, and J. S. Remington. 1988. Azithromycin, a macrolide antibiotic with potent activity against Toxoplasma gondii. Antimicrob. Agents Chemother. 32:755-757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beckers, C. J., D. S. Roos, R. G. Donald, B. J. Luft, J. C. Schwab, Y. Cao, and K. A. Joiner. 1995. Inhibition of cytoplasmic and organellar protein synthesis in Toxoplasma gondii. Implications for the target of macrolide antibiotics. J. Clin. Investig. 95:367-376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boettger, E. C., B. Springer, T. Prammananan, Y. Kidan, and P. Sander. 2001. Structural basis for selectivity and toxicity of ribosomal antibiotics. EMBO Rep. 2:318-323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Botero, D. 1970. Paromomycin as effective treatment of Taenia infections. Am. J. Trop. Med. Hyg. 19:234-237. [DOI] [PubMed] [Google Scholar]
- 6.Camps, M., G. Arrizabalaga, and J. Boothroyd. 2002. An rRNA mutation identifies the apicoplast as the target for clindamycin in Toxoplasma gondii. Mol. Microbiol. 43:1309-1318. [DOI] [PubMed] [Google Scholar]
- 7.Clough, B., M. Strath, P. Preiser, P. Denny, and I. R. Wilson. 1997. Thiostrepton binds to malarial plastid rRNA. FEBS Lett. 406:123-125. [DOI] [PubMed] [Google Scholar]
- 8.Derouin, F. 2001. Anti-toxoplasmosis drugs. Curr. Opin. Investig. Drugs 2:1368-1374. [PubMed] [Google Scholar]
- 9.Eckert, J., and P. Deplazes. 2004. Biological, epidemiological, and clinical aspects of echinococcosis, a zoonosis of increasing concern. Clin. Microbiol. Rev. 17:107-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eckert, J., M. A. Gemmell, F.-X. Meslin, and Z. S. Pawlowski (ed.). 2001. WHO/OIE manual on echinococcosis in humans and animals: a public health problem of global concern. Office International des Epizooties, Paris, France.
- 11.Eckert, J., R. C. Thompson, S. A. Michael, L. M. Kumaratilake, and H. M. el-Sawah. 1989. Echinococcus granulosus of camel origin: development in dogs and parasite morphology. Parasitol. Res. 75:536-544. [DOI] [PubMed] [Google Scholar]
- 12.Fichera, M. E., and D. S. Roos. 1997. A plastid organelle as a drug target in apicomplexan parasites. Nature 390:407-409. [DOI] [PubMed] [Google Scholar]
- 13.Ginsburg, H., A. A. Divo, T. G. Geary, M. T. Boland, and J. B. Jensen. 1986. Effects of mitochondrial inhibitors on intraerythrocytic Plasmodium falciparum in in vitro cultures. J. Protozool. 33:121-125. [DOI] [PubMed] [Google Scholar]
- 14.Hemphill, A., M. Stettler, M. Walker, M. Siles-Lucas, R. Fink, and B. Gottstein. 2002. Culture of Echinococcus multilocularis metacestodes: an alternative to animal use. Trends Parasitol. 18:445-451. [DOI] [PubMed] [Google Scholar]
- 15.Hoerauf, A., O. Adjei, and D. W. Buttner. 2002. Antibiotics for the treatment of onchocerciasis and other filarial infections. Curr. Opin. Investig. Drugs 3:533-537. [PubMed] [Google Scholar]
- 16.Ingold, K., P. Bigler, W. Thormann, T. Cavaliero, B. Gottstein, and A. Hemphill. 1999. Efficacies of albendazole sulfoxide and albendazole sulfone against in vitro-cultivated Echinococcus multilocularis metacestodes. Antimicrob. Agents Chemother. 43:1052-1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kapel, C. M. O., P. R. Torgerson, R. C. A. Thompson, and P. Deplazes. Int. J. Parasitol., in press. [DOI] [PubMed]
- 18.Kern, P. 2003. Echinococcus granulosus infection: clinical presentation, medical treatment and outcome. Langenbeck's Arch. Surg. 388:413-420. [DOI] [PubMed] [Google Scholar]
- 19.Kiatfuengfoo, R., T. Suthiphongchai, P. Prapunwattana, and Y. Yuthavong. 1989. Mitochondria as the site of action of tetracycline on Plasmodium falciparum. Mol. Biochem. Parasitol. 34:109-115. [DOI] [PubMed] [Google Scholar]
- 20.Krolewiecki, A., S. Leon, P. Scott, and D. Abraham. 2002. Activity of azithromycin against Leishmania major in vitro and in vivo. Am. J. Trop. Med. Hyg. 67:273-277. [DOI] [PubMed] [Google Scholar]
- 21.Mathis, A., P. Wild, P. Deplazes, and E. C. Boettger. 2004. The mitochondrial ribosome of the protozoan Acanthamoeba castellanii is the target for macrolide antibiotics. Mol. Biochem. Parasitol. 135:223-227. [DOI] [PubMed] [Google Scholar]
- 22.McFadden, G. I., and D. S. Roos. 1999. Apicomplexan plastids as drug targets. Trends Microbiol. 7:328-333. [DOI] [PubMed] [Google Scholar]
- 23.McManus, M. C., and C. Bryant. 1995. Biochemistry, physiology and molecular biology of Echinococcus, p. 135-181. In R. C. A. Thompson and A. J. Lymbery (ed.), Echinococcosis and hydatid disease. CAB International, Wallingford, United Kingdom.
- 24.Pfister, P., M. Risch, D. E. Brodersen, and E. C. Bottger. 2003. Role of 16S rRNA helix 44 in ribosomal resistance to hygromycin B. Antimicrob. Agents Chemother. 47:1496-1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pukrittayakamee, S., R. Clemens, A. Chantra, A. Nontprasert, T. Luknam, S. Looareesuwan, and N. J. White. 2001. Therapeutic responses to antibacterial drugs in vivax malaria. Trans. R. Soc. Trop. Med. Hyg. 95:524-528. [DOI] [PubMed] [Google Scholar]
- 26.Reuter, S., M. Merkle, K. Brehm, P. Kern, and B. Manfras. 2003. Effect of amphotericin B on larval growth of Echinococcus multilocularis. Antimicrob. Agents Chemother. 47:620-625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rodriguez-Fonseca, C., R. Amils, and R. A. Garrett. 1995. Fine structure of the peptidyl transfease centre on 23S-like rRNAs deduced from chemical probing of antibiotic-ribosome complexes. J. Mol. Biol. 247:224-235. [DOI] [PubMed] [Google Scholar]
- 28.Roos, D. S. 1999. The apicoplast as a potential therapeutic target in Toxoplasma and other apicomplexan parasites: some additional thoughts. Parasitol. Today 15:41. [DOI] [PubMed] [Google Scholar]
- 29.Sadiq, S. T., K. W. Glasgow, C. J. Drakeley, O. Muller, B. M. Greenwood, D. C. Mabey, and R. L. Bailey. 1995. Effects of azithromycin on malariometric indices in The Gambia. Lancet 346:881-882. [DOI] [PubMed] [Google Scholar]
- 30.Salem, H. H., and G. al-Allaf. 1967. Paromomycin and Taenia saginata. Lancet ii:1360. [DOI] [PubMed] [Google Scholar]
- 31.Sander, P., T. Prammananan, A. Meier, K. Frischkorn, and E. C. Bottger. 1997. The role of ribosomal RNAs in macrolide resistance. Mol. Microbiol. 26:469-480. [DOI] [PubMed] [Google Scholar]
- 32.Stettler, M., R. Fink, M. Walker, B. Gottstein, T. G. Geary, J. F. Rossignol, and A. Hemphill. 2003. In vitro parasiticidal effect of nitazoxanide against Echinococcus multilocularis metacestodes. Antimicrob. Agents Chemother. 47:467-474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stettler, M., J. F. Rossignol, R. Fink, M. Walker, B. Gottstein, M. Merli, R. Theurillat, W. Thormann, E. Dricot, R. Segers, and A. Hemphill. 2004. Secondary and primary murine alveolar echinococcosis: combined albendazole/nitazoxanide chemotherapy exhibits profound anti-parasitic activity. Int. J. Parasitol. 34:615-624. [DOI] [PubMed] [Google Scholar]
- 34.Taylor, W. R., T. L. Richie, D. J. Fryauff, H. Picarima, C. Ohrt, D. Tang, D. Braitman, G. S. Murphy, H. Widjaja, E. Tjitra, A. Ganjar, T. R. Jones, H. Basri, and J. Berman. 1999. Malaria prophylaxis using azithromycin: a double-blind, placebo-controlled trial in Irian Jaya, Indonesia. Clin. Infect. Dis. 28:74-81. [DOI] [PubMed] [Google Scholar]
- 35.Uip, D. E., A. L. Lima, V. S. Amato, M. Boulos, V. A. Neto, and D. Bem David. 1998. Roxithromycin treatment for diarrhoea caused by Cryptosporidium spp. in patients with AIDS. J. Antimicrob. Chemother. 41:93-97. [DOI] [PubMed] [Google Scholar]
- 36.Van der Auwera, G., S. Chappelle, and R. de Wachter. 1994. Structure of the large ribosomal subunit RNA of Phytophthora megasperma, and phylogeny of oomycetes. FEBS Lett. 338:133-136. [DOI] [PubMed] [Google Scholar]