Abstract
Colistin, an antibiotic almost abandoned for intravenous administration for many years due to its reported toxicity, has been recently reintroduced in clinical practice due to the emergence of multidrug-resistant gram-negative bacteria and the lack of development of new antibiotics to combat them. To assess the safety and effectiveness of intravenous colistin, in combination with other antimicrobial agents, in the treatment of serious infections in patients without cystic fibrosis, a retrospective cohort study in a 450-bed tertiary-care hospital in Athens, Greece, was performed. Patients who were hospitalized from 1 October 2000 to 31 January 2004 and received intravenous colistin for more than 72 h were further analyzed. The primary outcome measure was the in-hospital mortality; secondary end points were the clinical outcome of the infections and the occurrence of colistin toxicity. Fifty patients received intravenous colistin with a median (mean) daily dose of 3 (4.5) million IU for 16.5 (21.3) days for the management of 54 episodes of infections due to multidrug-resistant gram-negative bacteria. The predominant infections were pneumonia (33.3%), bacteremia (27.8%), urinary tract infection (11.1%), and intra-abdominal infection (11.1%). The responsible pathogens were Acinetobacter baumannii (51.9%), Pseudomonas aeruginosa (42.6%), and Klebsiella pneumoniae (3.7%) strains (no pathogen was isolated from one case). In-hospital mortality was 24% (12/50 patients). Clinical response (cure or improvement) of the infection was observed in 66.7% of episodes (36/54). In the studied group, serum creatinine levels were decreased, at the end of colistin treatment, by an average of 0.2 ± 1.3 mg/dl compared to baseline levels. Deterioration of renal function during colistin therapy was observed in 4/50 patients (8%). Coadministration of other antimicrobial agents with spectrum against gram-negative microorganisms and the absence of a control group constitute the major limitations of this study. The use of intravenous colistin for the treatment of infections due to multidrug-resistant gram-negative bacteria appears to be safe and effective.
Infections due to multidrug-resistant gram-negative microorganisms pose an important clinical problem, resulting in significant morbidity and mortality worldwide (3, 17, 25). The emergence of gram-negative bacteria that develop resistance to most available antibiotics and the lack of development of new antimicrobial agents have prompted the medical community to reevaluate the use of colistin (polymyxin E).
Colistin belongs to polymyxins, a group of polypeptide antibiotics which includes five different chemical compounds (polymyxins A, B, C, D, and E). Only two of them, polymyxins B and E, have been used in clinical practice. Colistin binds to the gram-negative bacterial cell membrane phospholipids, producing a disruptive physiochemical effect, which leads to cell membrane permeability changes and ultimately cell death (7). Most gram-negative microorganisms are susceptible to colistin, including multidrug-resistant Acinetobacter baumannii and Pseudomonas aeruginosa strains. Proteus species, Neisseria species, Serratia species, and Providencia species, as well as anaerobic bacteria, are resistant to colistin (8).
The isolation of colistin from Bacillus colistinus is dated back in the year 1949 (7, 21). During the ensuing decades, colistin was used in the treatment of several types of infections, including infectious diarrhea and urinary tract infections, as well as in bowel decontamination. Early clinical experience with colistin showed a high incidence of toxicity, namely, nephrotoxicity, sometimes with fatal consequences (2, 20, 34). During the last 2 decades, the use of the antibiotic was mainly restricted to topical ophthalmic and otic use as well as to the treatment of acute exacerbations of lung infections due to multiresistant Pseudomonas aeruginosa strains in patients with cystic fibrosis (9, 28, 36, 38). Subsequently, this led to significant reduction of its administration. We reviewed our recent experience with this drug, and we present data regarding the effectiveness and particularly the safety of colistin in the treatment of infections caused by multidrug-resistant gram-negative bacilli.
MATERIALS AND METHODS
Design of the study.
Α retrospective, observational cohort study was conducted at Henry Dunant, a 450-bed tertiary-care hospital in Athens, Greece. The study was approved by the Institutional Review Board of the hospita1. All patients' records were reviewed by a physician.
Patient selection.
Patients with infections caused by multidrug-resistant gram-negative bacilli who were hospitalized during the period from 1 October 2000 (inauguration of the hospital) to 31 January 2004 and were managed with combination therapy that included intravenous colistin were identified by the pharmacy electronic databases and included in the study. Patients were excluded if they had received less than 72 h of intravenous colistin therapy. Patients who had infections due to multidrug-resistant gram-negative bacteria that were characterized as cured and who then developed a subsequent infection after a 15-day period due to a different gram-negative microorganism also requiring treatment with intravenous colistin were analyzed as two different cases.
Microbiological testing.
Identification of all causative microorganisms was performed using routine microbiological methods. Susceptibility testing was performed by both the disk diffusion method and an automated broth microdilution method (Vitek II; bioMerieux, Hazelwood, ΜΟ). The breakpoints were those defined by the Clinical and Laboratory Standards Institute (30, 31). Susceptibility to colistin was also tested by means of the disk diffusion method with the use of l0-μg colistin disks (Oxoid, Basingstoke, Hants, England); isolates were considered sensitive if the inhibition zone was more than or equal to 11 mm and resistant if the inhibition zone was less than or equal to 8 mm (29). Disk diffusion represents a common and widely used method for colistin susceptibility testing (23). Since the Clinical and Laboratory Standards Institute guidelines about the in vitro determination of the MICs for different microorganisms to colistin were established in 1970 and were not updated after 1981 (by the time of this writing), the results obtained by the broth microdilution method were verified by the disk diffusion method. Intermediate sensitivity of the isolated gram-negative pathogens to other antimicrobial agents was considered resistance.
Colistin administration.
All patients enrolled in the study had received intravenous colistin (colistin, Norma, Athens, Greece, or colistimethate sodium,Forest Laboratories, Kent, United Kingdom) as a therapeutic intervention for infections due to multidrug-resistant gram-negative bacteria at a dosage according to the discretion of attending physicians. One milligram of the colistin formulations used is approximately equal to 12,500 IU (Forest Laboratories) or 13,333 IU (Norma). Both colistin preparations (Forest Laboratories and Norma) contain sodium colistimethate, which is the active ingredient, as an amount of dry powder equivalent to 1 million IU (or equal to approximately 80 mg of sodium colistimethate). For patients with impaired renal function, dosage adjustments were done after consulting the intensive care unit (ICU) director or the infectious disease specialists of the hospital, based on the following protocol: if the serum creatinine level was 1.3 to 1.5 mg/dl, 1.6 to 2.5 mg/dl, or ≥2.6 mg/dl, the dosage of colistin administered was 2,000,000 IU every 12 h, 24 h, or 36 h, respectively. Patients who were on dialysis treatment received 1,000,000 IU of colistin after dialysis.
Definitions of infections.
Diagnosis of pneumonia required two or more serial chest radiographs with at least one of the following: new or progressive and persistent infiltrate, consolidation, cavitation, or pleural effusion. In addition, patients must have had fever of >38°C with no other recognized cause or an abnormal white blood cell (WBC) count (leukopenia [<4,000 WBC/mm3] or leukocytosis [≥12,000 WBC/mm3]) and at least two of the following: new onset of purulent sputum or change in character of sputum, increased respiratory secretions or increased suctioning requirements, new onset or worsening of cough or dyspnea or tachypnea, rales or bronchial breath sounds, or worsening gas exchange (11).
Bacteremia required either growth of a recognized pathogen from one or more blood specimen cultures or at least one of the following signs or symptoms: fever (>38°C), chills, or hypotension and (i) a common skin contaminant (e.g., diphtheroids, Bacillus sp., Propionibacterium sp., coagulase-negative staphylococci, or micrococci) grown from two or more blood cultures drawn on separate occasions and/or (ii) a common skin contaminant (e.g., diphtheroids, Bacillus sp., Propionibacterium sp., coagulase-negative staphylococci, or micrococci) grown from at least one blood culture from a patient with an intravascular line and physician-instituted antimicrobial therapy (11).
Patients were considered to have an intra-abdominal infection if they had at least two of the following signs or symptoms: fever (>38°C), nausea, vomiting, abdominal pain, or jaundice with no other recognized cause and (i) organisms cultured from drainage from a surgically placed drain (e.g., closed suction drainage system, open drain, T-tube drain), (ii) organisms seen on a Gram stain of drainage or tissue obtained during surgical operation or needle aspiration, and/or (iii) organisms cultured from blood and radiographic evidence of infection, e.g., abnormal findings on ultrasound, CT scan, magnetic resonance imaging, radiolabel scans (gallium, technetium, etc.), or abdominal X ray (11).
Infections at other body sites or fluids, such as urinary tract infections and central venous catheter-related infections, were defined based on guidelines from the Centers for Disease Control and Prevention (11).
Definition of outcomes.
The primary outcome measure was in-hospital mortality. Secondary end points included clinical outcome of the infection and occurrence of renal dysfunction and were defined as follows: cure was defined as resolution of presenting symptoms and signs of the infection by the end of colistin treatment and discharge from the hospital; improvement was defined as partial resolution of presenting symptoms and signs of the infection; unresponsiveness was defined as persistence or worsening of presenting symptoms and/or signs of the infection during colistin administration.
Normal renal function was defined as a serum creatinine level of 1.3 mg/dl or lower. Deterioration of renal function during colistin treatment was defined as an increase of more than 50% of the baseline creatinine level to a value higher than 1.3 mg/dl or as a decline in renal function requiring renal replacement therapy. Baseline creatinine was defined as the creatinine level on the initial day of intravenous colistin administration.
Data collection.
Using a detailed case report form designed specifically for this study, we collected data from all available medical records, including age, sex, Acute Physiological and Chronic Health Evaluation II score (APACHE II score) on the day of the patient's admission to the ICU and on the first day of colistin administration (if the patient was admitted to the ICU) (6, 19), site(s) of infection, duration of colistin treatment, concomitant antibiotic treatment, prior antibiotic or antifungal use, mechanical ventilatory support, renal support, and duration of hospitalization. Microbiological data included the causative organism(s) isolated from the site(s) of infection, the date of isolation, and the in vitro susceptibilities to several antibiotics, including colistin. In addition, data from laboratory tests, such as renal and liver function, serum electrolytes, erythrocyte sedimentation rate, C-reactive protein, and complete blood count, on admission day as well as on the first and last day of colistin treatment were also collected. The information collected in the case report forms was entered into a computer database. Using a random number selection web page, 20% of the registered data were double-checked by an independent reviewer. In addition, the type of the infection, the causative pathogen(s), and the clinical outcome were determined by two blinded reviewers.
Data analysis.
Categorical variables were compared by the Fisher exact test. For continuous variables, we used Student's t test or the Mann-Whitney test for normally and nonnormally distributed variables, respectively. Variables associated with mortality in the univariable analysis (P < 0.05) were included in a backward stepwise multiple logistic regression model. All statistical analyses were performed using SPSS 11.0 and S-PLUS 6.1 Professional.
RESULTS
Study population.
From 1 October 2000 through 31 January 2004, 152 patients received treatment with intravenous colistin for infections due to multidrug-resistant gram-negative bacteria. Fifty-five patients were excluded from the study because they had received less than 72 h of intravenous colistin therapy. The mortality rate among these 55 patients was 34.5% (19/55 patients). Results from 43 patients who were admitted to the ICU during the first part of the study period and for whom data were collected and maintained by the ICU medical staff had been previously reported (27). Thus, 54 patients were included in this study. Medical records were not available for three patients (one of them died); in addition, one patient was in the hospital during data collection. Consequently, data from 50 patients were analyzed. Α total of 54 courses of intravenous colistin were given, because four patients developed two episodes of infection that were included as two different cases.
Patient characteristics.
Table 1 describes the demographic and clinical features, including comorbidity, of the study cohort (50 patients). None of the patients had received organ transplantation, radiotherapy, or interferon treatment. All patients had received other antimicrobial agents prior to colistin treatment.
TABLE 1.
Demographic and clinical features of patients managed with intravenous colistin for infections caused by multidrug-resistant gram-negative bacteria (n = 50)
| Characteristic of patients | Mean ± SD (range) or n (%) |
|---|---|
| Demographic | |
| Age [mean ± SD (range)] | 59.2 ± 17.7 (24-90) |
| Sex (male) | 29 (58%) |
| APACHE II score on admission to ICUa | 16.1 ± 6.1 (2-32) |
| APACHE II score on first day of colistin treatmenta | 17.1 ± 5.4 (7-28) |
| Comorbidity | |
| Malignancy | 14 (28) |
| Heart dysfunction | 27 (54) |
| Lung dysfunction | 15 (30) |
| Diabetes mellitusb | 15 (30) |
| Urogenital disorders | 17 (34) |
| Chronic renal failure | 5 (10) |
| Acute renal failure on admission | 3 (6) |
| Hemodialysis | 6 (12) |
| Liver failure | 2 (4) |
| Hematological disorders | 8 (16) |
| Neurological disorders | 27 (54) |
| Prior hospitalization | 32 (64) |
| Prior surgeryc | 36 (72) |
| Central nervous system | 15 (30) |
| Intestinal | 13 (26) |
| Cardiac/vascular | 3 (6) |
| Orthopedic | 3 (6) |
| Urinary | 1 (2) |
| Plastic | 1 (2) |
| Admission to the ICU | 40 (80) |
| Catheters | |
| Urinary catheters | 48 (96) |
| Cerebrospinal fluid drainage | 10 (20) |
| Abdominal drainage | 12 (24) |
| Mechanical ventilation support | 35 (70) |
| Tracheostomy | 26 (52) |
| Gastrostomy | 3 (6) |
| Colostomy | 2 (4) |
| Prior antibiotic use | 50 (100) |
| Prior antifungal use | 18 (36) |
| Antitumor treatment | 6 (12) |
| Steroid treatment | 20 (40) |
| Blood transfusion | 38 (76) |
| Trauma | 7 (14) |
| Prosthetic materiald | 24 (48) |
| Time to develop infection for which colistin was given (days) [mean ± SD (range)] | 18.3 ± 28.1 (0-140) |
| Duration of ICU stay (days) [mean ± SD (range)] | 32.2 ± 30.4 (1-131) |
| Duration of hospitalization (days) [mean ± SD (range)] | 74.7 ± 62.1 (11-267) |
APACHE II score refers only to the patients who were admitted to the ICU, i.e., 40 patients.
Two patients with diabetes mellitus were on oral medication treatment, and 13 patients were receiving insulin treatment.
Three patients underwent two different surgical operations during their hospitalization; one patient had intestinal and urinary tract surgeries, one patient had central nervous system and orthopedic surgeries, and one patient had central nervous system and cardiac/vascular surgeries.
Prosthetic material included ventriculo-peritoneal shunts, stents, heart valves, pacemakers, vascular grafts, orthopedic pins, wires, vena cava filters, and radiation clips.
Types of infection.
Pneumonia was the predominant infection (33.3%), followed by bacteremia (27.8%), urinary tract infection (UTI) (11.1%), intra-abdominal infection (11.1%), meningitis (5.6%), central venous catheter-related infection (3.7%), surgical site infection (3.7%), and skin and subcutaneous tissue infection (1.9%). In one of the cases, the site of infection was not discovered. This patient received empirical treatment with intravenous colistin in the ICU due to persistent fever unresponsive to broad-spectrum antibiotics, since no microorganism was isolated from repeated specimen cultures. Eight out of the 15 cases of bacteremia were not associated with any other identifiable infected site, while the remaining 7 were associated with other site infections, namely, pneumonia (2 cases), abdominal infections (2 cases), meningitis, UTI, and surgical site infection (1 case each). Eight cases out of the 54 (14.8%) were infections at two different sites at the same time, specifically, 3 cases of pneumonia and UTI, 3 cases of central venous catheter-related infection and pneumonia, 1 case of surgical site infection and pneumonia, and 1 case of abdominal infection and pneumonia.
Responsible pathogens.
Acinetobacter baumannii was the causative pathogen in 28/54 episodes of infection (51.9%), Pseudomonas aeruginosa in 23/54 (42.6%), and Klebsiella pneumoniae in 2/54 (3.7%) (no pathogen was isolated from one patient). In 21 out of 54 episodes of infection (38.9%), a second strain was isolated from the same culture specimen(s). Specifically, 38.1% (8/21) of the isolated organisms in these cases were gram-negative bacilli (Pseudomonas aeruginosa [2 strains], Klebsiella pneumoniae [2], Acinetobacter baumannii [1], Escherichia coli [1], Enterobacter aerogenes [1], and Proteus mirabilis [1]); 42.9% (9/21) were gram-positive cocci (coagulase-negative staphylococci [4], Enterococcus faecium [3], Enterococcus faecalis [1], and Streptococcus sp. [1]); and 19% (4/21) were fungi (Candida albicans [3] and Aspergillus niger [1]).
Table 2 presents the in vitro susceptibilities of the main isolated pathogens for several antimicrobial agents from 52 episodes of infection (one patient received empirical treatment and for one patient the susceptibility test was not available). In 24 of 52 cases (44.5%), the isolated gram-negative microorganisms were sensitive only to colistin (colistin-only sensitive). Only 1 out of the 28 (3.6%) strains of Acinetobacter baumannii was found to be resistant to colistin; it was isolated from a patient who was referred to our hospital after a long-standing hospitalization in another institution for ventilator-associated pneumonia. No strain of Pseudomonas aeruginosa was found to be resistant to colistin.
TABLE 2.
Description of in vitro antimicrobial susceptibility of isolated pathogens (28 strains of Acinetobacter baumannii, 22 strains of Pseudomonas aeruginosa, and 2 strains of Klebsiella pneumoniae)a
| Antimicrobial agent |
Acinetobacter baumanniib
|
Pseudomonas aeruginosa
|
Klebsiella pneumoniae
|
|||
|---|---|---|---|---|---|---|
| No. R/total (%) | No. R + I/total (%) | No. R/total (%) | No. R + I/total (%) | No. R/total (%) | No. R + I/total (%) | |
| Piperacillin | 25/27 (92.6) | 25/27 (92.6) | 11/21 (52.4) | 14/21 (66.7) | 2/2 (100) | 2/2 (100) |
| Ticarcillin | 26/27 (96.3) | 26/27 (96.3) | 19/20 (95.0) | 19/20 (95.0) | 1/1 (100) | 1/1 (100) |
| Piperacillin-tazobactam | 23/27 (85.2) | 24/27 (88.9) | 8/22 (36.4) | 11/22 (50.0) | 0/2 (0) | 2/2 (100) |
| Ticarcillin-clavulanic acid | 27/28 (96.4) | 27/28 (96.4) | 19/21 (90.5) | 20/21 (95.2) | 1/1 (100) | 1/1 (100) |
| Ampicillin-sulbactam | 1/11 (9.1) | 5/11 (45.5) | Not done | Not done | Not done | Not done |
| Ceftazidime | 27/28 (96.4) | 27/28 (96.4) | 15/22 (68.2) | 21/22 (95.5) | 2/2 (100) | 2/2 (100) |
| Cefepime | 25/28 (89.3) | 27/28 (96.4) | 16/22 (72.7) | 21/22 (95.5) | 0/2 (0) | 2/2 (100) |
| Aztreonam | 27/28 (96.4) | 27/28 (96.4) | 14/21 (66.7) | 20/21 (95.2) | 2/2 (100) | 2/2 (100) |
| Imipenemc | 17/28 (60.7) | 21/28 (75.0) | 9/21 (42.9) | 20/21 (95.2) | 0/1 (0) | 0/1 (0) |
| Meropenem | 1/28 (3.6) | 20/28 (71.4) | 11/22 (50.0) | 20/22 (90.9) | 0/2 (0) | 0/2 (0) |
| Ciprofloxacin | 27/28 (96.4) | 27/28 (96.4) | 21/22 (95.5) | 21/22 (95.5) | 1/2 (50) | 1/2 (50) |
| Amikacin | 25/28 (89.3) | 25/28 (89.3) | 18/22 (81.8) | 20/22 (90.9) | 0/2 (0) | 1/2 (50) |
| Gentamicin | 8/28 (28.6) | 24/28 (85.7) | 16/22 (72.7) | 21/22 (95.5) | 1/2 (50) | 1/2 (50) |
| Tobramycin | 10/28 (35.7) | 26/28 (92.9) | 19/22 (86.4) | 20/22 (90.9) | 1/2 (50) | 1/2 (50) |
| Netilmicin | 26/27 (96.3) | 26/27 (96.3) | 17/20 (85.0) | 19/20 (95.0) | 1/1 (100) | 1/1 (100) |
| Colistin | 1/28 (3.6) | 1/28 (3.6) | 0/21 (0.0) | 0/21 (0/0) | 0/2 (0) | 0/2 (0) |
The total number of isolates was 52 (one patient was treated empirically and one patient was referred from another hospital with diagnosed pneumonia due to a Pseudomonas aeruginosa strain, but the susceptibility test was not available). R, resistant; I, intermediate sensitive.
Not all of the Acinetobacter baumannii strains were tested for susceptibility to ampicillin-sulbactam.
Intermediate susceptibility of the microorganisms to imipenem was defined by a MIC of 8 mg/liter.
Colistin administration.
Patients received treatment with intravenous colistin at a median/mean (± standard deviation [SD]) daily dose of 3/4.5 (±2.3) million IU (range, 1.5 to 9.0 million IU) for a median/mean (± SD) duration of 16.5/21.3 (±16.0) days (range, 4 to 72 days). The mean (± SD) total cumulative dose was 95.3 (±54.5) million IU (range, 15 to 308 million IU). Patients received the first dose of intravenous colistin after a mean (± SD) duration of hospitalization of 23.4 (±27.7) days (range, 0 to 143 days; 25th/50th/75th percentiles, 9/15.5/31). In 3 out of 50 patients, colistin was administered by continuous intravenous infusion at daily doses from 2 to 6 million IU over 24 h.
In 6 of 50 patients, colistin was administered by an alternative way in addition to the intravenous infusion. Specifically, two patients also received intraventricular colistin (for both of them, the infection [meningitis] improved, although one subsequently died) and three patients received it in a nebulized form (for all of them, pneumonia was cured), and for one patient with surgical site infection, colistin was used as an irrigation solution instilled directly to the wound to control sternotomy wound infection (this patient died).
Supplementary treatment.
Nineteen of 50 patients were initially treated with intravenous colistin monotherapy. For 15 out of these 19 patients, additional antibiotics against gram-negative microorganisms were subsequently given during the course of colistin treatment (11 patients received one additional agent, while 4 patients received two additional agents). In 31 of 50 patients, one or two additional antimicrobial agents with spectrum against gram-negative bacilli were concurrently administered during the whole course of colistin administration. Specifically, 60% of patients received meropenem intravenously, 34% of patients received ampicillin-sulbactam, 22% received ciprofloxacin, 20% received piperacillin-clavulanic acid, 16% received imipenem, and 14% received amikacin and gentamicin. (The total number is more than 100%, since most of the patients received more than one antimicrobial agent.)
Mortality.
The in-hospital mortality in this study of 50 patients was 24% (12/50). Five of the patients who died had bacteremia, three had pneumonia, one had a surgical site infection, one had meningitis, and one had an abdominal infection. In one additional patient who died, colistin was administered empirically. Only one of the 12 patients who died had responded to colistin treatment, but this patient died of another cause (the patient with meningitis reported above). Eight out of the 12 patients died while receiving intravenous colistin (on treatment days 4, 5, 10, 10, 11, 21, 25, and 72). There was no statistical difference in mortality rates between the patients who had infections due to colistin-only-sensitive microorganisms and those who had multidrug-resistant gram-negative isolates (P > 0.05). The mean (± SD) length of hospital stay was 74.7 (±62.1) days (range, 11 to 267 days), and the mean (± SD) duration of ICU stay was 32.2 (±30.4) days (range, 1 to 131 days).
Predictors of death.
Table 3 shows the results of univariable analysis of factors possibly associated with mortality. Age, history of diabetes mellitus, the time until the infection for which colistin was given occurred, and temperature on admission to the hospital were significantly associated with death (P < 0.05). Variables that were significantly associated with mortality in the univariable analysis were included in a backward stepwise multiple logistic regression model. Multivariable analysis showed that age (odds ratio = 1.059; 95% confidence interval [CI] = 1.004 to 1.118) and temperature on admission to the hospital (odds ratio = 0.383; 95% CI = 0.148 to 0.991) were independent predictors of in-hospital mortality.
TABLE 3.
Univariable analysis of factors associated with death
| Variable | Patients who died (n = 12)a | Patients who survived (n = 38)a | P value |
|---|---|---|---|
| Age (years) | 68.9 ± 12.8 | 56.1 ± 18.1 | 0.03 |
| Sex (male) | 6 (50.0) | 23 (60.5) | 0.74 |
| APACHE II score on admission | 18.6 ± 3.9 | 15.0 ± 6.7 | 0.09 |
| APACHE II score on first day of colistin treatment | 19.2 ± 5.6 | 16.2 ± 5.3 | 0.17 |
| Malignancy | 6 (50.0) | 8 (21.1) | 0.07 |
| Heart dysfunction | 9 (75.0) | 18 (47.4) | 0.11 |
| Lung dysfunction | 6 (50.0) | 9 (23.7) | 0.15 |
| Diabetes mellitus | 7 (58.3) | 8 (21.1) | 0.03 |
| Chronic renal failure | 2 (16.7) | 3 (7.9) | 0.58 |
| Acute renal failure on admission | 0 (0) | 3 (7.9) | 1.00 |
| Hemodialysis | 2 (16.7) | 4 (10.5) | 0.62 |
| Liver failure | 1 (8.3) | 1 (2.6) | 0.43 |
| Hematological disorders | 3 (25.0) | 5 (13.2) | 0.38 |
| Neurological disorders | 7 (58.3) | 20 (52.6) | 1.00 |
| Prior hospitalization | 5 (41.7) | 27 (71.1) | 0.09 |
| Prior surgery | 0.88 | ||
| Intestinal | 2 (16.7) | 11 (28.9) | |
| Central nervous system | 4 (33.3) | 11 (28.9) | |
| Other surgery | 4 (33.3) | 10 (26.3) | |
| No surgery | 6 (15.8) | 2 (16.7) | |
| Admission to the ICU | 12 (100) | 28 (73.7) | 0.09 |
| Mechanical ventilation support | 10 (83.3) | 25 (65.8) | 0.30 |
| Tracheostomy | 6 (50.0) | 20 (52.6) | 1.00 |
| Cerebrospinal fluid drainage | 4 (33.3) | 6 (15.8) | 0.23 |
| Abdominal drainage | 3 (25.0) | 9 (23.7) | 1.00 |
| Prior antibiotic use | 12 (100) | 37 (97.4) | 1.00 |
| Prior antifungal use | 6 (50.0) | 12 (31.6) | 0.31 |
| Antitumor treatment | 2 (16.7) | 4 (10.5) | 0.62 |
| Steroid treatment | 6 (50.0) | 14 (36.8) | 0.51 |
| Blood transfusion | 10 (83.3) | 28 (73.7) | 0.71 |
| Trauma | 1 (8.3) | 6 (15.8) | 1.00 |
| Prosthetic material | 7 (58.3) | 17 (44.7) | 0.51 |
| Time to develop infection for which colistin was given (days) | 28.9 ± 37.9 | 15.2 ± 24.3 | 0.04 |
| Site of infection | 0.65 | ||
| Bacteremia | 5 (41.7) | 10 (26.3) | |
| Pneumonia | 3 (25.0) | 14 (36.8) | |
| Other | 4 (33.3) | 14 (36.8) | |
| Pathogens | 0.73 | ||
| Acinetobacter | 5/10 (50.0) | 21/37 (56.8) | |
| Pseudomonas | 5/10 (50.0) | 16/37 (43.2) | |
| Number of isolates | 0.27 | ||
| One | 8/10 (80.0) | 20/36 (55.6) | |
| Two | 2/10 (20.0) | 16/36 (44.4) | |
| Admission temperature (°C) | 36.8 ± 0.7 | 37.4 ± 0.9 | 0.05 |
| White blood cells (cells/μl)b | 11,469.2 ± 3,988.4 | 11,842.9 ± 6,148.8 | 0.90 |
| Neutrophils (%)b | 81.3 ± 8.3 | 77.1 ± 11.2 | 0.23 |
| C-reactive protein (mg/dl)b | 12.0 ± 9.6 | 9.9 ± 8.3 | 0.58 |
| ESR (mm/first h)b | 76.0 ± 51.6 | 69.6 ± 29.5 | 0.77 |
| SGPT (U/liter)b | 69.3 ± 45.5 | 89.5 ± 120.4 | 0.93 |
| ALP (U/liter)b | 119.9 ± 91.0 | 155.5 ± 214.0 | 0.42 |
| Creatinine (mg/dl)b | 1.2 ± 0.8 | 1.5 ± 1.9 | 0.71 |
| Urea (mg/dl)b | 75.5 ± 48.4 | 63.3 ± 63.0 | 0.22 |
| Total bilirubin (mg/dl)b | 2.5 ± 4.4 | 1.7 ± 2.4 | 0.99 |
The given values are either mean ± SD or n (percentage).
Values refer to the first day of intravenous colistin administration. SGPT, serum glutamic pyruvic transaminase; ESR, erythrocyte sedimentation rate; ALP, alkaline phosphatase.
Clinical response of the infection.
Clinical response of the infection (cure or improvement) was observed in 36 out of 54 episodes (66.7%) (cure in 53.7% [29/54 episodes] and improvement in 13% [7/54 episodes]). Four patients experienced a second episode of infection, which was fully treated with an additional course of intravenous colistin in three of them. Unresponsiveness was observed in 18 out of 54 episodes (33.3%) in 50 patients. Eleven out of 17 patients whose infections were unresponsive to colistin therapy died. Table 4 presents the clinical response associated with the site of the infection and the responsible pathogen.
TABLE 4.
Clinical response associated with the type of the infection and the responsible pathogen (n = 54 episodes)
| Type of infection or pathogen responsible | Outcome of the infection
|
|
|---|---|---|
| No. with clinical response/ total (%)a | No. unresponsive/ total (%) | |
| Type of infection | ||
| Pneumonia | 10/18 (55.6) | 8/18 (44.4) |
| Bacteremia | 9/15 (60) | 6/15 (40) |
| Urinary tract infection | 6/6 (100) | 0/6 (0) |
| Abdominal infection | 5/6 (83.3) | 1/6 (16.7) |
| Meningitis | 2/3 (66.7) | 1/3 (33.3) |
| Surgical site infection | 1/2 (50) | 1/2 (50) |
| Catheter-related infection | 2/2 (100) | 0/2 (0) |
| Skin (subcutaneous tissue infection) | 1/1 (100) | 0/1 (0) |
| Not found | 0/1 (0) | 1/1 (100) |
| Total | 36/54 (66.7) | 18/54 (33.3) |
| Responsible pathogen | ||
| Acinetobacter baumannii | 19/28 (67.9) | 9/28 (32.1) |
| Pseudomonas aeruginosa | 16/23 (69.6) | 7/23 (30.4) |
| Klebsiella pneumoniae | 1/2 (50) | 1/2 (50) |
| Not found | 0/1 (0) | 1/1 (100) |
| Total | 36/54 (66.7) | 18/54 (33.3) |
Clinical response includes episodes defined as clinical cured or improved.
Renal function during colistin treatment.
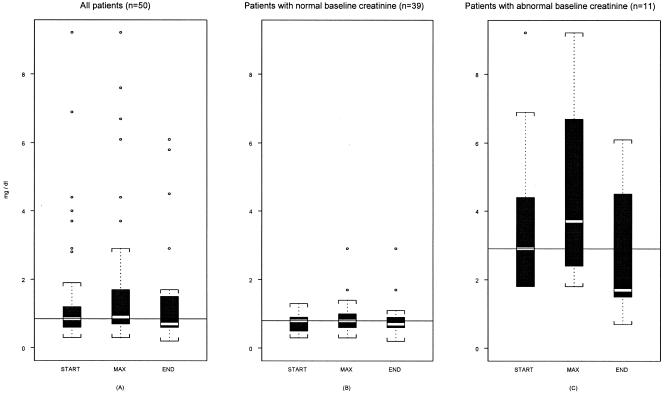
Figure 1 summarizes the distribution of serum creatinine values at the initiation and the end of intravenous colistin treatment, as well as the distribution of peak values in this group of patients. No deterioration of renal function was observed at the end of colistin treatment compared to the baseline value in 46/50 patients (92%). Although baseline serum creatinine levels were increased by a mean of 0.3 (±0.8) mg/dl during treatment with colistin in the study group, at the end of treatment serum creatinine levels were decreased by 0.2 (±1.3) mg/dl on average compared to baseline values. The maximum value of creatinine was observed at a mean of 6.7 (±10.7) days following colistin therapy (range, 0 to 53 days; 25th/50th/75th percentiles, 0/3.5/9.25 days).
FIG. 1.
The distribution of serum creatinine levels on the first day of colistin treatment (START), at the peak value (MAX), and at the end of colistin treatment (END) in all studied patients (A), in the group of patients with normal baseline creatinine values (B), and in the group of patients with abnormal baseline creatinine values (C). The horizontal lines within the boxes represent the median creatinine baseline value at the first day of colistin treatment.
Eleven out of 50 patients (22%) had abnormal baseline creatinine values at the onset of colistin treatment (>1.3 mg/dl), and 3 of them had acute renal failure requiring renal replacement therapy. Deterioration of renal function was observed in 4 out of 50 patients (8%), and only 2 of them required renal replacement therapy. Two out of the four patients who developed renal dysfunction during colistin treatment had normal baseline renal function, and two had abnormal baseline renal function. Death occurred in two out of the four patients with deterioration of renal function; one had a normal baseline creatinine value and the other had an abnormal value. Both of them had metastatic cancer of terminal stage and died from septic shock and multiple organ failure. Urea values showed a mean (± SD) decrease of 12.5 (±51.2) mg/dl (10th/25th/50th/75th/90th percentiles, −66/−20/−7/10/26 mg/dl).
Laboratory data.
An improvement of the C-reactive protein values was observed during colistin treatment. At the initiation of colistin therapy, the mean (± SD) C-reactive protein value was 11.3 (±8.9) mg/dl (25th/50th/75th percentiles, 2.9/10.1/16.1 mg/dl); this value decreased at the end of therapy to 6.4 (±5.4) mg/dl (25th/50th/75th percentiles, 1.5/5.8/9.8 mg/dl). Normal values of C-reactive protein in our hospital are 0 to 0.5 mg/dl.
No significant elevation of liver function tests was noted during the administration of colistin. The difference between the values at the end of colistin treatment and baseline values of liver enzymes, cholestatic enzymes, and total bilirubin expressed as the mean (± SD) and 25th/50th/75th percentiles were as follows: for serum glutamic oxalacetic transaminase (aspartate aminotransferase), 2.2 (±50.4) U/liter and −19/−5/11 U/liter; for serum glutamic pyruvic transaminase (alanine aminotransferase), −3 (±59) U/liter and −27/−5/11 U/liter; for alkaline phosphatase, 34.4 (±194.9) U/liter and −31.8/4.5/32.5 U/liter; for gamma-glutamyl transpeptidase, −62.6 (±190.7) U/liter and −158.3/−25/51.3 U/liter; and for total bilirubin, 0.2 (±2.7) mg/dl and −1.1/−0.1/0.2 mg/dl.
Neurotoxicity.
During treatment, all patients were closely monitored for possible neurological adverse episodes, including dizziness, weakness, paresthesia, and ataxia, as well as neuromuscular blockade and apnea. Only one patient developed polyneuropathy; the symptoms appeared while she was on her 25th day of treatment with colistin. From then on, and although colistin was continued for 11 more days, the symptoms gradually subsided. No confirmatory electromyography testing was performed.
DISCUSSION
Our results show a satisfactory profile of effectiveness and safety of intravenous colistin in combination with other antibiotics for the treatment of infections due to multidrug-resistant gram-negative bacilli in patients without cystic fibrosis. Colistin was given as a salvage therapeutic regimen when treatment with other antimicrobial agents had failed or there was no alternative therapeutic option. In this population of 50 patients, 80% of whom were admitted to the ICU and who had a mean APACHE II score on admission to the ICU of 16.1, a positive clinical response was observed in 66.7% of cases. In addition, the observed mortality was 24%, which compares favorably to mortality reported in most other studies of patients with infections caused by multidrug-resistant gram-negative bacteria, such as Pseudomonas aeruginosa and Acinetobacter baumannii (18, 33).
An interesting finding of the multivariable analysis of predictors of death in our study was that lower temperature on admission to the hospital was associated with death. It is known that in patients with sepsis, hypothermia is associated with worse outcomes than high fever. Our analysis suggests that the temperature during admission to the hospital may have a prognostic value, a finding that may deserve further exploration.
In Table 5, we summarize the characteristics (number of patients, demographics, site[s] of infection, and pathogen[s]) and the treatment outcomes (mortality, outcome of infection, and nephrotoxicity) of all recently published studies of patients who received colistin in four different countries (10, 22, 24, 26, 32). It is interesting that the percentages of clinical cure of infection are relatively similar between the studies (57% to 73%). However, mortality and nephrotoxicity vary considerably. Specifically, mortality ranged from 20% in a study of 60 cases in Manhattan, New York, to 61.9% in another study from Seville, Spain. Comparison of the data presented in Table 5 shows that there are several explanations for the observed variability of mortality. For example, high mortality was observed in a study of solid organ transplant recipients, a population that frequently has associated comorbidities. An important additional observation is that the dosage and duration of colistin administration also vary in the presented studies (12.6 to 17 days).
TABLE 5.
Characteristics and treatment outcomes of all recently reported studies of patients who received intravenous colistin for infections due to multiresistant gram-negative bacteriaa
| Reference or source | City, state, country | Setting | Number of patients | Demographics/ APACHE II score | Duration/ dosage of colistin | Site(s) of infection | Pathogen(s) | Mortality rate(s) | Clinical cure by infection typeb | Definition of nephrotoxicity | Nephrotoxicityb |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 24c | Pittsburgh, Pennsylvania | Abdominal organ transplantation (ICU) | 23 pts (20 had received organ transplantation, 3 abdominal surgery) | Mean age, 52 years | Mean duration, 17 days (range, 7-36) | Pneumonia, 18 pts (43.9%) | P. aeruginosa | In-hospital mortality, 14 pts (61%) | Pneumonia, 10/15 pts Intra-abdominal, 5/6 pts Wound, 1/3 pts | RF was defined by a requirement either for intermittent hemodialysis or for continuous venous hemofiltration | 1/2 pts developed RF requiring artificial kidney support (the other 21 pts were already receiving artificial kidney support) |
| Bacteremia, 8 pts (19.5%) | |||||||||||
| Wound, 3 pts (7.3%) | |||||||||||
| Intra-abdominal, 6 pts (14.6%) | |||||||||||
| Endocarditis, 1 patient (2.4%) | |||||||||||
| Multiple types, 5 pts (12.2%) | |||||||||||
| 26 | Athens, Greece | ICU | 24 critically ill patients with sepsis, 26 episodes of infection | Mean age, 44.3 years; Mean APACHE II, 20.6 | Mean duration, 13.5 d (4-24d) Dosage, 3 MIU × 3 | VAP, 15 cases (57.7%) Catheter-related sepsis, 3 cases (11.5%) | 20 cases P. aeruginosa (76.9%) | 30-day mortality, 42.3% | VAP, 11/15 cases Catheter-related sepsis, 2/3 cases | RF was defined as an increase in serum creatinine > 1 mg/dl during treatment | 3 pts (14.3%); only 1 pt required continuous venovenous hemodiafiltration |
| Empyema thoracic, 1 case (3.8%) | 6 cases A. baumannii (23.1%) | Empyema thoracic, 0/1 case | |||||||||
| Posttraumatic meningitis, 1 case (3.8%) | Posttraumatic meningitis, 1/1 case | ||||||||||
| Sinusitis, 1 case (3.8%) | Sepsis of unknown primary origin, 3/4 cases | ||||||||||
| UTI, 1 case (3.8%) | |||||||||||
| Sepsis of unknown primary origin, 4 cases (15.4%) | |||||||||||
| 22 | Sao Paolo, Brazil | ICU, 52%; transplant unit 13%; surgical and medical wards, 35% | 59 patients, 60 episodes of infection | Mean age, 42.1 years; Mean APACHE II, 13.1 | Mean duration, 12.6 d (2-34) Mean daily dose, 152.8 mg (60-300 mg) | Pneumonia, 20 pts (33%) UTI, 12 pts (20%) | 39 A. baumannii (65%) | 22 cases (37%) | Pneumonia, 5/20 cases | No definition provided (information about creatinine values are provided) | 22 pts (37%) [11/41 (27%) pts with normal baseline renal function had worsening during treatment (mean increase, 0.9 ± 0.6); 11/19 pts (58%) with abnormal baseline renal function had worsening during treatment (mean increase, 1.5 ± 1.4)] |
| Primary bloodstream infection, 9 pts (15%) | 21 P. aeruginosa (35%) | Primary bloodstream infection, 7/9 cases | |||||||||
| CNS infection, 5 pts (8%) | CNS infection, 4/5 cases | ||||||||||
| Surgical site, 5 pts (8%) | Surgical site, 3/5 cases | ||||||||||
| Peritonitis, 4 pts (7%) | Peritonitis, 2/4 cases | ||||||||||
| Catheter-related infection, 4 pts (7%) | Catheter-related infection, 3/4 cases | ||||||||||
| Otitis media, 1 patient (2%) | Otitis media, 1/1 case | ||||||||||
| 32 | New York, New Yorke | Tertiary-care hospital | 60 receiving polymyxin B (parenterally) | Mean age, 61 years | Mean duration, 13.5 d (1-56d) Mean daily dose, 1.1 MIU (0.12-2.25 MIU) | Lung, 39 pts (65%) | 46 A. baumannii (77%) | 12/60 pts (20%) | Not provided | RF was defined as doubling of serum creatinine value of ≥2.0 mg/dl | 7/50 evaluable pts (14%) |
| Blood, 5 pts (8%) | 2 P. aeruginosa (3%) | Mortality rate of pts who developed RF, 4/7 (57%) vs 8/53 (15%) | |||||||||
| Abdomen, 3 pts (5%) | 2 A. baumannii plus P. aeruginosa (3%) | ||||||||||
| Urine, 2 pts (3%) | 10 None identified (17%) | ||||||||||
| Bone, 2 pts (3%) | |||||||||||
| CSF, Jackson Pratt drain pleural effusion 1 patient each (2%) | |||||||||||
| Undetermined, 7 pts (12%) | |||||||||||
| 10 | Seville, Spaind | ICU | 21 in colistin (CO) group and 14 in imipenem (IM) group | Mean age: CO group, 56.9 years; IM group, 64.5 years | CO group: mean duration, 14.7d (10-21d); dose, 2.5-5 mg/kg/d | VAP | 21 A. baumannii susceptible to colistin | In-hospital mortality, 13/21 pts (CO) (61.9%); 9/14 pts (IM) (64.2%) | CO group, 12/21 pts | In patients with normal renal function (cr. <1.2), RF was defined as cr. value >2 mg/dl, as a reduction of cr. clearance of 50% relative to antibiotic initiation, or as need for RRT. In patients with abnormal renal function, RF was defined as an increase of 50% of the baseline cr. level, as a reduction of cr. clearance of 50% relative to antibiotic initiation, or as a need for RRT. | 5/21 pts (CO) (24%) |
| Mean APACHE II: CO group, 19.6; IM group, 20.5 Mean SOFA: CO group, 10; IM group, 11. | 14 A. baumannii susceptible to imipenem | VAP-related mortality, 8/21 pts (CO) (38%); 5/14 pts (IM) (35.7%) | IM group, 8/14 pts | 6/14 pts (IM) (42%) | |||||||
| This study | Athens, Greece | ICU, 80%; medical, surgical wards, 20% | 50 pts, 54 episodes | Mean age, 59.2 years | Mean duration, 21.5d (4-72 d) | Pneumonia, 18 episodes (33.3%) | 28 A. baumannii (51.9%) | In-hospital mortality, 12/50 pts (24%) | Pneumonia, 10/18 episodes | RF was defined as an increase more than 50% of the baseline creatinine level to a value higher than 1.3 mg/dl or as a decline in renal function requiring renal replacement therapy. | 4/50 pts (8%) |
| Mean APACHE II: 16.1 | Mean daily dose, 4.5 MIU (1.5-9 MIU) | Bacteremia, 15 episodes (27.8%) | 23 P. aeruginosa (42.6%) | Bacteremia, 9/15 episodes | |||||||
| Urinary tract infection, 6 episodes (11.1%) | 2 K. pneumoniae (3.7%) | Urinary tract infection, 6/6 episodes | |||||||||
| Intra-abdominal infection, 6 episodes (11.1%) | 1 Not found (1.9%) | Abdominal infection, 5/6 episodes | |||||||||
| Meningitis, 3 episodes (5.6%) | Meningitis, 2/3 episodes | ||||||||||
| Catheter-related infection, 2 episodes (3.7%) | Surgical site infection, 1/2 episodes | ||||||||||
| Surgical site infection, 2 episodes (3.7%) | Catheter-related infection, 2/2 episodes | ||||||||||
| Skin and subcutaneous tissue infection, 1 episode (1.9%) | Skin (subcutaneous tissue infection), 1/1 episode | ||||||||||
| Not found, 1 episode (1.9%) | Not found, 0/1 episode |
Abbreviations: pts, patients; pt, patient; RRT, renal replacement therapy; cr., creatinine; RF, renal failure; MIU, million international units; CNS, central nervous system.
Nephrotoxicity and outcome of the infection are not uniformly defined in the five papers.
61% of patients had septic shock. Univariate analysis of factors related to unfavorable response showed an association with Pseudomonas bacteremia.
In the colistin group, 47.6% of patients had septic shock, while in the imipenem group, 57.1% did.
In the New York study, all patients received polymyxin B.
Toxicity, specifically nephrotoxicity, is a major concern when colistin is administered, mainly because of some previous reports (during the period of 1960 to 1970) on the safety of medication which reported significant nephrotoxicity (1, 16, 20, 34). However, recent data, including results from our study, in which nephrotoxicity was 8%, suggest that colistin toxicity is less prominent than previously reported (1, 5). A possible explanation is that fluid supplementation, as well as supportive treatment of patients, has been improved. Furthermore, physicians are focused on these aspects in their clinical practices. In addition, the previous established opinion on the nephrotoxicity of colistin led to vigilance of the medical community, so that renal function and the factors that affect it were subsequently assiduously monitored. We cannot assume that deterioration of renal function developed exclusively due to colistin treatment, because other concomitantly administered medications or other factors, such as aminoglycosides, glycopeptides, and shock, may be associated with nephrotoxicity, too. It is notable that in our study we observed, on average, a decrease of serum creatinine at the end of colistin treatment compared to baseline levels. In 1962, Fekety et al. reported the same observation about serum urea nitrogen levels for 48 patients treated with colistin (9).
In our study, the majority of patients received other antimicrobial agents with spectrum against gram-negative bacteria concomitantly with colistin, namely, β-lactams, especially meropenem and ampicillin-sulbactam, aminoglycosides (amikacin and gentamicin), and/or quinolones, especially ciprofloxacin, despite reported resistance. The effect of the combination treatment is unclear, although the possibility of a beneficial effect cannot be excluded (35). Few experimental or clinical studies are found in the literature on the synergistic activity of colistin with other antimicrobial agents, such as β-lactams, rifampin, amikacin, trimethoprim-sulfamethoxazole, and ciprofloxacin, against multidrug-resistant gram-negative bacteria (12, 13, 14, 15, 37). One clinical trial on the effectiveness of colistin in pulmonary exacerbations of infections due to Pseudomonas aeruginosa strains in patients with cystic fibrosis showed that the combination of colistin with an antipseudomonal agent was more effective than colistin alone (4). As mentioned above, colistin acts by increasing the permeability of the cell membrane and thus could act synergistically with other antimicrobial agents by facilitating their entrance into the bacterial cell.
Despite the reported findings, our study has several limitations. First, it is a retrospective study with the inherent problems related to this study design. Second, we should acknowledge that this study did not individualize exposure for the organism, that is, pharmacodynamic properties of colistin were not taken into account. Third, there is no control group for the comparison of outcomes, including mortality, cure of infection, and nephrotoxicity. Fourth, a significant proportion of our patients received other antimicrobial agents with activity against gram-negative bacilli, including Pseudomonas aeruginosa and Acinetobacter baumannii. Finally, a great proportion of the Acinetobacter baumannii isolates was not tested for susceptibility to ampicillin-sulbactam.
In conclusion, our study shows that intravenous colistin constitutes a relatively safe and effective therapeutic intervention in cases of severe nosocomial infections due to multidrug-resistant gram-negative bacteria. A restriction of its use to decrease the rate of the emergence of bacteria resistant to colistin should be implemented. In addition, randomized controlled trials on the effectiveness and safety of monotherapy with colistin or a combination of colistin with other antimicrobial agents, such as carbapenems, for the treatment of multiresistant gram-negative bacteria are urgently needed.
Acknowledgments
There was no conflict of interest in this study.
REFERENCES
- 1.Bosso, J. A., C. A. Liptak, D. K. Seilheimer, and G. M. Harrison. 1991. Toxicity of colistin in cystic fibrosis patients. DICP 25:1168-1170. [DOI] [PubMed] [Google Scholar]
- 2.Brown, J. M., D. C. Dorman, and L. P. Roy. 1970. Acute renal failure due to overdosage of colistin. Med. J. Aust. 2:923-924. [DOI] [PubMed] [Google Scholar]
- 3.Carmeli, Y., N. Troillet, A. W. Karchmer, and M. H. Samore. 1999. Health and economic outcomes of antibiotic resistance in Pseudomonas aeruginosa. Arch. Intern. Med. 159:1127-1132. [DOI] [PubMed] [Google Scholar]
- 4.Conway, S. P., M. N. Pond, A. Watson, C. Etherington, H. L. Robey, and M. H. Goldman. 1997. Intravenous colistin sulphomethate in acute respiratory exacerbations in adult patients with cystic fibrosis. Thorax 52:987-993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Conway, S. P., C. Etherington, J. Munday, M. H. Goldman, J. J. Strong, and M. Wootton. 2000. Safety and tolerability of bolus intravenous colistin in acute respiratory exacerbations in adults with cystic fibrosis. Ann. Pharmacother. 34:1238-1242. [DOI] [PubMed] [Google Scholar]
- 6.Damiano, A. M., M. Bergner, E. A. Draper, W. A. Knaus, and D. P. Wagner. 1992. Reliability of a measure of severity of illness: acute physiology of chronic health evaluation—II. J. Clin. Epidemiol. 45:93-101. [DOI] [PubMed] [Google Scholar]
- 7.Evans, M. E., D. J. Feola, and R. P. Rapp. 1999. Polymyxin B sulfate and colistin: old antibiotics for emerging multiresistant gram-negative bacteria. Ann. Pharmacother. 33:960-967. [DOI] [PubMed] [Google Scholar]
- 8.Falagas, M. E., and S. K. Kasiakou. 2005. Colistin: the revival of polymyxins for the management of multidrug-resistant gram-negative bacterial infections. Clin. Infect. Dis. 40:1333-1341. [DOI] [PubMed] [Google Scholar]
- 9.Fekety, F. R., Jr., P. S. Norman, and L. E. Cluff. 1962. The treatment of gram-negative bacillary infections with colistin. The toxicity and efficacy of large doses in forty-eight patients. Ann. Intern. Med. 57:214-229. [DOI] [PubMed] [Google Scholar]
- 10.Garnacho-Montero, J., C. Ortiz-Leyba, F. J. Jimenez-Jimenez, A. E. Barrero-Almodovar, J. L. Garcia-Garmendia, M. Bernabeu-Wittell, et al. 2003. Treatment of multidrug-resistant Acinetobacter baumannii ventilator-associated pneumonia (VAP) with intravenous colistin: a comparison with imipenem-susceptible VAP. Clin. Infect. Dis. 36:1111-1118. [DOI] [PubMed] [Google Scholar]
- 11.Gaynes, R. P., and T. C. Horan. 1996. Surveillance of nosocomial infections. Appendix A: CDC definitions of nosocomial infections, p. 1-14. In C. G. Mayhall (ed.), Hospital epidemiology and infection control. Williams & Wilkins, Baltimore, Md.
- 12.Giamarellos-Bourboulis, E. J., E. Xirouchaki, and H. Giamarellou. 2001. Interactions of colistin and rifampin on multidrug-resistant Acinetobacter baumannii. Diagn. Microbiol. Infect. Dis. 40:117-120. [DOI] [PubMed] [Google Scholar]
- 13.Giamarellos-Bourboulis, E. J., L. Karnesis, and H. Giamarellou. 2002. Synergy of colistin with rifampin and trimethoprim/sulfamethoxazole on multidrug-resistant Stenotrophomonas maltophilia. Diagn. Microbiol. Infect. Dis. 44:259-263. [DOI] [PubMed] [Google Scholar]
- 14.Giamarellos-Bourboulis, E. J., H. Sambatakou, I. Galani, and H. Giamarellou. 2003. In vitro interaction of colistin and rifampin on multidrug-resistant Pseudomonas aeruginosa. J. Chemother. 15:235-238. [DOI] [PubMed] [Google Scholar]
- 15.Gunderson, B. W., K. H. Ibrahim, L. B. Hovde, T. L. Fromm, M. D. Reed, and J. C. Rotschafer. 2003. Synergistic activity of colistin and ceftazidime against multiantibiotic-resistant Pseudomonas aeruginosa in an in vitro pharmacodynamic model. Antimicrob. Agents Chemother. 47:905-909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Holmes, K. K. 1964. Toxicity of colistin and polymyxin B. N. Engl. J. Med. 271:633-634. [DOI] [PubMed] [Google Scholar]
- 17.Hsueh, P. R., L. J. Teng, C. Y. Chen, W. H. Chen, C. J. Yu, S. W. Ho, et al. 2002. Pandrug-resistant Acinetobacter baumannii causing nosocomial infections in a university hospital, Taiwan. Emerg. Infect. Dis. 8:827-832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kang, C. I., S. H. Kim, H. B. Kim, S. W. Park, Y. J. Choe, M. D. Oh, et al. 2003. Pseudomonas aeruginosa bacteremia: risk factors for mortality and influence of delayed receipt of effective antimicrobial therapy on clinical outcome. Clin. Infect. Dis. 37:745-751. [DOI] [PubMed] [Google Scholar]
- 19.Knaus, W. A., E. A. Draper, D. P. Wagner, and J. E. Zimmerman. 1985. APACHE II: a severity of disease classification system. Crit. Care Med. 13:818-829. [PubMed] [Google Scholar]
- 20.Koch-Weser, J., V. W. Sidel, E. B. Federman, P. Kanarek, D. C. Finer, and A. E. Eaton. 1970. Adverse effects of sodium colistimethate. Manifestations and specific reaction rates during 317 courses of therapy. Ann. Intern. Med. 72:857-868. [DOI] [PubMed] [Google Scholar]
- 21.Koyama, Y., A. Kurosasa, A. Tsuchiya, and K. Takakuta. 1950. A new antibiotic “colistin” produced by spore-forming soil bacteria. J. Antibiot. (Tokyo) 3:457-458. [Google Scholar]
- 22.Levin, A. S., A. A. Barone, J. Penco, M. V. Santos, I. S. Marinho, E. A. Arruda, et al. 1999. Intravenous colistin as therapy for nosocomial infections caused by multidrug-resistant Pseudomonas aeruginosa and Acinetobacter baumannii. Clin. Infect. Dis. 28:1008-1011. [DOI] [PubMed] [Google Scholar]
- 23.Li, J., R. L. Nation, R. W. Milne, J. D. Turnidge, and K. Coulthard. 2005. Evaluation of colistin as an agent against multi-resistant Gram-negative bacteria. Int. J. Antimicrob. Agents 25:11-25. [DOI] [PubMed] [Google Scholar]
- 24.Linden, P. K., S. Kusne, K. Coley, P. Fontes, D. J. Kramer, and D. Paterson. 2003. Use of parenteral colistin for the treatment of serious infection due to antimicrobial-resistant Pseudomonas aeruginosa. Clin. Infect. Dis. 37:e154-e160. [DOI] [PubMed] [Google Scholar]
- 25.Livermore, D. M. 2002. Multiple mechanisms of antimicrobial resistance in Pseudomonas aeruginosa: our worst nightmare? Clin. Infect. Dis. 34:634-640. [DOI] [PubMed] [Google Scholar]
- 26.Markou, N., H. Apostolakos, C. Koumoudiou, M. Athanasiou, A. Koutsoukou, I. Alamanos, et al. 2003. Intravenous colistin in the treatment of sepsis from multiresistant Gram-negative bacilli in critically ill patients. Crit. Care 7:R78-R83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Michalopoulos, A. S., S. Tsiodras, K. Rellos, S. Mentzelopoulos, and M. E. Falagas. 2005. Colistin treatment in patients with ICU-acquired infections caused by multiresistant Gram-negative bacteria: the renaissance of an old antibiotic. Clin. Microbiol. Infect. 11:115-121. [DOI] [PubMed] [Google Scholar]
- 28.Nakajima, S. 1965. Clinical use of colimycin F otic solution. Jibiinkoka 37:693-697. (In Japanese.) [PubMed] [Google Scholar]
- 29.National Committee for Clinical Laboratory Standards. 1981. Performance standards for antimicrobial disc susceptibility tests. Approved standard M2-A2 S2. National Committee for Clinical Laboratory Standards, Villanova, Pa.
- 30.National Committee for Clinical Laboratory Standards. 2000. Methods for dilution antimicrobial susceptibility test for bacteria that grow aerobically. Approved standards document M7-A5, 5th ed. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 31.National Committee for Clinical Laboratory Standards. 2000. Performance standard for antimicrobial susceptibility testing. Document M100-S10. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 32.Ouderkirk, J. P., J. A. Nord, G. S. Turett, and J. W. Kislak. 2003. Polymyxin B nephrotoxicity and efficacy against nosocomial infections caused by multiresistant gram-negative bacteria. Antimicrob. Agents Chemother. 47:2659-2662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rello, J., E. Quintana, V. Ausina, J. Castella, M. Luquin, A. Net, et al. 1991. Incidence, etiology, and outcome of nosocomial pneumonia in mechanically ventilated patients. Chest 100:439-444. [DOI] [PubMed] [Google Scholar]
- 34.Ryan, K. J., L. I. Schainuck, R. O. Hickman, and G. E. Striker. 1969. Colistimethate toxicity. Report of a fatal case in a previously healthy child. JAMA 207:2099-2101. [DOI] [PubMed] [Google Scholar]
- 35.Rynn, C., M. Wootton, K. E. Bowker, H. H. Alan, and D. S. Reeves. 1999. In vitro assessment of colistin's antipseudomonal antimicrobial interactions with other antibiotics. Clin. Microbiol. Infect. 5:32-36. [DOI] [PubMed] [Google Scholar]
- 36.Takigami, T., S. Tani, and O. Kitamoto. 1962. Clinical trials of colistin. Effect of the oral administration of the colistin on the intestinal bacterial flora. Jpn. J. Exp. Med. 32:107-116. [PubMed] [Google Scholar]
- 37.Tascini, C., S. Ferranti, F. Messina, and F. Menichetti. 2000. In vitro and in vivo synergistic activity of colistin, rifampin, and amikacin against a multiresistant Pseudomonas aeruginosa isolate. Clin. Microbiol. Infect. 6:690-691. [DOI] [PubMed] [Google Scholar]
- 38.Yow, E. M., E. Tan, L. Shane, S. Schonfeld, and H. Abu-Nassar. 1961. Colistin (coly-mycin) in resistant bacterial infections. A clinical appraisal. Arch. Intern. Med. 108:664-670. [DOI] [PubMed] [Google Scholar]