Abstract
A simple, specific method is presented for simultaneous determination of voriconazole and itraconazole and its metabolite, hydroxyitraconazole, in human serum using one-step liquid-liquid extraction and high-performance liquid chromatography. Linearity tests ranged from 0.1 to 8.0 μg/ml; the minimum detectable concentration was 0.03 μg/ml.
Itraconazole (ITZ) and voriconazole (VRC) are two broad-spectrum antifungal triazole derivates (1, 6, 16) used for a wide range of invasive and opportunistic fungal infections. These drugs are often given to seriously ill patients receiving other drugs (e.g., immunosuppressants), with the consequent danger of serious drug interactions (3). As the metabolite hydroxyitraconazole (H-ITZ) is biologically active and can appear in concentrations nearly twice those of the unaltered drug in the steady state (8, 9), it is imperative to measure both ITZ and H-ITZ to fully evaluate patient samples.
Early clinical studies suggested that treatment with VRC carries some risk of toxicity, and plasma VRC concentrations of >6 μg/ml were associated with occasional liver function abnormalities (5). For a clinically useful plasma concentration, levels below 0.5 μg/ml may not be therapeutic while those above 8.0 μg/ml may be unnecessarily high (7, 11, 14).
The quantification of plasma concentrations of VRC and ITZ is warranted in patients with severe fungal functions and is very important for determining an optimal toxicological profile and drug tolerance in humans.
Several high-performance liquid chromatography (HPLC) assays using UV detection (4, 13, 17, 18) or mass spectrometry (2, 19, 20) have been reported for determining serum levels of voriconazole and itraconazole. The liquid chromatography (LC)-mass spectrometry methods are superior to LC-UV methods but are expensive and not generally available. Itraconazole and its active metabolite can also be measured by biological methods (12, 15), but the chromatographic methods are more specific and sensitive than bioassays (10). No HPLC-UV method has yet been published that allows simultaneous determination of ITZ, H-ITZ, and VRC in the therapeutically relevant range. The goal of the present study was to develop a fast and economical isocratic HPLC-UV method for the simultaneous determination of ITZ, H-ITZ, and VRC in serum with simple sample preparation.
All analytical reagents were purchased from Merck (Stuttgart, Germany). Voriconazole was kindly supplied by Pfizer (Vienna, Austria), and pure itraconazole, hydroxyconazole, and ketoconazole were supplied by Janssen (Beers, Belgium). Standard stock solutions were prepared in methanol and consisted of 1.0 mg/ml of ITZ, H-ITZ, and VRC, respectively. The concentration of the internal standard ketoconazole (methanolic solution) was 30 μg/ml. All standard solutions were stored at −20°C.
One milliliter of calibrator, quality control (QC), or patient sample was pipetted into a 10-ml glass tube with screw cap, followed by addition of 100 μl internal standard. The mixture was then vortexed for 10 s. For extraction, 5 ml of extraction solvent heptane-isoamyl alcohol (90:10 [vol/vol]) was added; the tubes were briefly vortexed for 5 min and then centrifuged at 5,000 × g for 5 min. The organic layer was transferred into a conical glass tube and evaporated to dryness at 50°C under a gentle stream of nitrogen gas. The residue was reconstituted with 150 μl of mobile phase.
The HPLC system (Merck-Hitachi, Stuttgart, Germany) consisted of an isocratic pump with a wavelength detector; the detector signals were recorded with an HP Chemstation and integrator, using an automatic sampling system. The separation was carried out on a 250-mm × 4.6-mm inside-diameter reverse-phase column (Zorbax SB-C18, 5 μm) maintained at 40°C. The mobile phase consisted of 50 mM phosphate buffer, pH 6.0 (adjusted with 1 M KOH), acetonitrile, and methanol (35:45:20 [vol/vol/vol]); the flow rate was 1.7 ml/min. Detection was at 255 nm, and the injected volume was 30 μl.
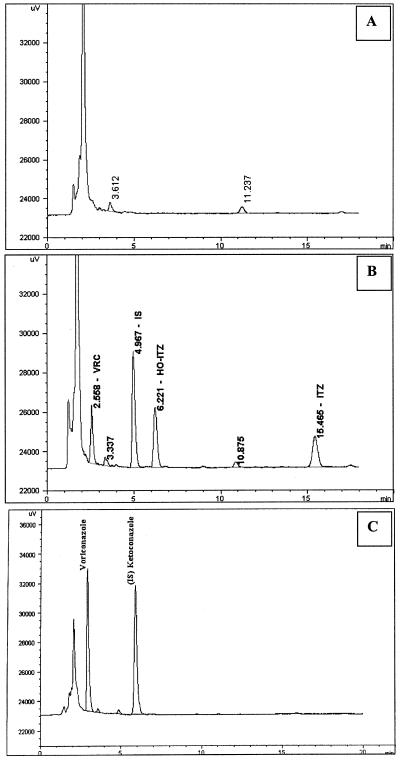
Figure 1 illustrates the influence of the biological matrix with typical chromatograms of blank serum (Fig. 1A) and pooled human serum spiked with 1.0 μg/ml of each drug (Fig. 1B), as well as a serum sample obtained from a patient undergoing VRC treatment with a concentration of 2.14 μg/ml (Fig. 1C). No peaks were seen to interfere with the peaks of either the drugs or the internal standard. The retention times were 2.56 min for voriconazole, 4.97 min for ketoconazole (internal standard), 6.22 min for hydroxyitraconazole, and 15.46 min for itraconazole.
FIG. 1.
Representative chromatograms of VRC, ITZ, H-ITZ and the internal standard (IS [ketoconazole]) in human serum. (A) blank human serum; (B) pooled human serum spiked with 1.0 μg/ml of each compound; (C) serum samples obtained from patient with VRC concentration of 2.14 μg/ml.
This method was evaluated for accuracy, linearity, specificity, and precision (expressed as the percent coefficient of variation [%CV]). We constructed calibration curves for each component with six concentrations ranging from 0.1 to 8.0 μg/ml (0.1, 0.5, 1.0, 2.0, 4.0, and 8.0 μg/ml).
The calibration curves showed an excellent linear relationship between the peak height ratio for drug and internal standard. The correlation coefficient (r) was greater than 0.996 in all compounds (n = 5). The limit of quantitation was determined as the lowest concentration of standard (0.1 μg/ml), and the limit of detection, defined as the concentration of drug giving a signal-to-noise ratio of >3:1, was 0.03 μg/ml for all drugs. Intra- and interday precision and accuracy were assessed by analyzing three quality control samples (nominal values of 0.75, 3.0, and 6.0 μg/ml, stored at −70°C) at each concentration on the same day and mean values of three QCs for 6 days. Intraday %CVs for itraconazole, hydroxconazole, and voriconazole were 2.2%, 1.6% and 2.6%, respectively. Interday %CVs for the corresponding compounds were 2.5%, 1.6% and 2.1% (Table 1), respectively.
TABLE 1.
Accuracy and intra- and interday precision data for measurement of itraconazole, hydroxyitraconazole, and voriconazole in human serum
| Sample/analyte | Amt of analyte added (μg/ml) | Intraday run (n = 6)
|
Interday run (n = 6)
|
||||
|---|---|---|---|---|---|---|---|
| Amt of analyte detected (mean μg/ml ± SD) | CV (%) | Bias (%) | Amt of analyte detected (mean μg/ml ± SD) | CV (%) | Bias (%) | ||
| Itraconazole | |||||||
| 0.75 | 0.735 ± 0.068 | 9.2 | −2.0 | 0.721 ± 0.071 | 9.9 | −4.0 | |
| 3.00 | 3.066 ± 0.115 | 3.8 | 2.2 | 2.912 ± 0.071 | 2.4 | −3.0 | |
| 6.00 | 5.888 ± 0.187 | 3.2 | −1.9 | 5.808 ± 0.143 | 2.5 | −3.3 | |
| Hydroxyitraconazole | |||||||
| 0.75 | 0.732 ± 0.026 | 3.4 | 1.8 | 0.763 ± 0.033 | 4.3 | 1.7 | |
| 3.00 | 3.020 ± 0.062 | 2.1 | 0.7 | 2.918 ± 0.115 | 3.9 | −2.8 | |
| 6.00 | 6.122 ± 0.097 | 1.6 | 2.0 | 5.990 ± 0.092 | 1.6 | −0.2 | |
| Voriconazole | |||||||
| 0.75 | 0.758 ± 0.070 | 9.3 | 1.1 | 0.784 ± 0.041 | 5.2 | 4.3 | |
| 3.00 | 2.978 ± 0.105 | 3.5 | −0.8 | 3.083 ± 0.203 | 6.6 | 2.7 | |
| 6.00 | 6.110 ± 0.158 | 2.6 | 1.8 | 5.795 ± 0.122 | 2.1 | −3.6 | |
The effect of repeated freezing (−70°C) and thawing at ambient temperature (25°C) was tested using the QC samples (five replicates/concentration). The experiments showed that serum and precipitates containing VRC, ITZ, and H-ITZ can be stored for 1 week without any change in the material. The sample extract was stable after extraction over 24 h in the autosampler tray.
To determine recovery, known concentrations of VRC, ITZ, and H-ITZ were added to the pooled patient serum samples and the concentrations were determined in five replicates. The mean rates of recovery at a range of concentrations of 0.5, 1.0, 2.5, and 5.0 μg/ml for the three compounds were 95.7% for ITZ, 97.7% for H-ITZ, and 96.5% for VRC.
Our HPLC-UV method enables reliable quantification of voriconazole and itraconazole and its metabolite in serum. The short and relatively simple sample preparation and ease of use make it suitable for routine determinations. The advantage of this cost- and time-effective method is that two different drug samples can be analyzed with the same sample preparation and the same chromatographic conditions, which is particularly helpful when patients are receiving both drugs.
REFERENCES
- 1.Barchiesi, F., D. Arzeni, F. Caselli, and G. Scalise. 2000. Primary resistance to flucytosine among clinical isolates of Candida spp. J. Antimicrob. Chemother. 45:408-409. [DOI] [PubMed] [Google Scholar]
- 2.Carrier, A., and J. Parent. 2000. Liquid chromatographic-mass spectrometric determination of itraconazole and its major metabolite, hydroxyitraconazole, in dog plasma. J. Chromatogr. B 745:413-420. [DOI] [PubMed] [Google Scholar]
- 3.Crane, J. K., and H. T. Shih. 1993. Syncope and cardiac arrhythmia due to an interaction between itraconazole and terfenadine. Am. J. Med. 95:445-446. [DOI] [PubMed] [Google Scholar]
- 4.Darouiche, R. O., A. Setoodeh, and E. J. Anaissie. 1995. Potential use of a simplified method for determination of itraconazole levels in plasma and esophageal tissue by using high-performance liquid chromatography. Antimicrob. Agents Chemother. 39:757-759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Denning, D. W., P. Ribaud, N. Milpied, D. Caillot, R. Herbrecht, E. Thiel, A. Haas, M. Ruhnke, and H. Lode. 2002. Efficacy and safety of voriconazole in the treatment of acute invasive aspergillosis. Clin. Infect. Dis. 34:563-571. [DOI] [PubMed] [Google Scholar]
- 6.Denning, D. W., R. M. Tucker, L. H. Hanson, and D. A. Stevens. 1989. Treatment of invasive aspergillosis with itraconazole. Am. J. Med. 86:791-800. [DOI] [PubMed] [Google Scholar]
- 7.Espinel-Ingroff, A. 2001. In vitro fungicidal activities of voriconazole, itraconazole, and amphotericin B against opportunistic moniliaceous and dematiaceous fungi. J. Clin. Microbiol. 39:954-958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Haria, M., H. M. Bryson, and K. L. Goa. 1996. Itraconazole. A reappraisal of its pharmacological properties and therapeutic use in the management of superficial fungal infections. Drugs 51:585-620. [DOI] [PubMed] [Google Scholar]
- 9.Heykants, J., A. Van Peer, V. Van de Velde, P. Van Rooy, W. Meuldermans, K. Lavrijsen, R. Woestenborghs, J. Van Cutsem, and G. Cauwenbergh. 1989. The clinical pharmacokinetics of itraconazole: an overview. Mycoses 32(Suppl. 1):67-87. [DOI] [PubMed] [Google Scholar]
- 10.Hostetler, J. S., J. Heykants, K. V. Clemons, R. Woestenborghs, L. H. Hanson, and D. A. Stevens. 1993. Discrepancies in bioassay and chromatography determinations explained by metabolism of itraconazole to hydroxyitraconazole: studies of interpatient variations in concentrations. Antimicrob. Agents Chemother. 37:2224-2227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jeu, L., F. J. Piacenti, A. G. Lyakhovetskiy, and H. B. Fung. 2003. Voriconazole. Clin. Ther. 25:1321-1381. [DOI] [PubMed] [Google Scholar]
- 12.Law, D., C. B. Moore, and D. W. Denning. 1994. Bioassay for serum itraconazole concentrations using hydroxyitraconazole standards. Antimicrob. Agents Chemother. 38:1561-1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pennick, G. J., M. Clark, D. A. Sutton, and M. G. Rinaldi. 2003. Development and validation of a high-performance liquid chromatography assay for voriconazole. Antimicrob. Agents Chemother. 47:2348-2350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Perea, S., G. J. Pennick, A. Modak, A. W. Fothergill, D. A. Sutton, D. J. Sheehan, and M. G. Rinaldi. 2000. Comparison of high-performance chromatographic and microbiological methods for determination of voriconazole levels in plasma. Antimicrob. Agents Chemother. 44:1209-1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Perfect, J. R., D. V. Savani, and D. T. Durack. 1986. Comparison of itraconazol and fluconazole in treatment of cryptococcal meningitis and candida pyelonephritis in rabbits. Antimicrob. Agents Chemother. 29:579-583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sharkey, P. K., M. G. Rinaldi, J. F. Dunn, T. C. Hardin, R. J. Fetchick, and J. R. Graybill. 1991. High-dose itraconazole in the treatment of severe mycoses. Antimicrob. Agents Chemother. 35:707-713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Srivatsan, V., A. K. Dasgupta, P. Kale, R. R. Datla, D. Soni, M. Patel, R. Patel, and C. Mavadhiya. 2004. Simultaneous determination of itraconazole and hydroxyitraconazole in human plasma by high-performance liquid chromatography. J. Chromatogr. A 1031:307-313. [DOI] [PubMed] [Google Scholar]
- 18.Wong, J. W., U. R. Nisar, and K. H. Yuen. 2003. Liquid chromatographic method for the determination of plasma itraconazole and its hydroxy metabolite in pharmacokinetic/bioavailability studies. J. Chromatogr. B 798:355-360. [DOI] [PubMed] [Google Scholar]
- 19.Yao, M., L. Chen, and N. R. Srinivas. 2001. Quantitation of itraconazole in rat heparinized plasma by liquid chromatography-mass spectrometry. J. Chromatogr. B 752:9-16. [DOI] [PubMed] [Google Scholar]
- 20.Zhou, L., R. D. Glickman, N. Chen, W. E. Sponsel, J. R. Graybill, and K. W. Lam. 2002. Determination of voriconazole in aqueous humor by liquid chromatography-electrospray ionization-mass spectrometry. J. Chromatogr. B 776:213-220. [DOI] [PubMed] [Google Scholar]