Abstract
Nystatin is an antifungal compound with potent proinflammatory properties. Herein, we demonstrate that nystatin induces interleukin (IL)-1β, IL-8, and tumor necrosis factor alpha secretion through its activation of toll-like receptor 1 (TLR1) and TLR2. Hence, a TLR-dependent mechanism could serve as the molecular basis for the proinflammatory properties of nystatin.
The human toll-like receptors (TLR) are a family of pattern recognition receptors that detect specific molecular patterns associated with microbial pathogens (known as pathogen-associated molecular patterns [PAMP]) (1). Recently, TLR2 was observed to recognize Streptomyces nodosus-derived antifungal polyene drug amphotericin B and account for its proinflammatory characteristics (15, 16). This observation heralded a paradigm in pharmacologic toxicology whereby drugs could serve as PAMP that induce cell activation through TLR, which in turn activate signaling pathways that result in secretion of cytokines that mediate biologic effects.
Here, we report that a related polyene antifungal drug—nystatin—induces a proinflammatory response through a similar TLR-dependent mechanism. In the 1950s, nystatin was discovered as the first in-class tetraene diene macrolide (8, 9). Since then, nystatin has been used widely as a topical therapy for mucocutaneous mycoses (2). As such, topical and oral nystatin is not associated with systemic inflammation, most likely since the drug is not absorbed through skin and gastrointestinal tract. Historical and experimental data, however, suggest its potent proinflammatory properties (2). When nystatin was undergoing development for treatment of systemic mycoses, its intravenous infusion resulted in chills, fever, and thrombophlebitis (2). Hence, its clinical development as a parenteral drug was not actively pursued (until recently). Its proinflammatory nature, however, is being used in generating animal models of inflammation (11).
The molecular basis for the proinflammatory properties of nystatin is undefined. The discovery that amphotericin B induces cytokine secretion through a TLR2-dependent process (15, 16) prompted us to hypothesize that a similar mechanism could account for nystatin-associated inflammation. Several lines of evidence support our hypothesis: (i) nystatin is chemically related to amphotericin B (14), (ii) nystatin has infusion-related toxicity similar to amphotericin B (2), and (iii) nystatin is a fermentation product of Streptomyces noursei (3). Hence, a series of experiments were performed to determine the TLR2-dependent nature of nystatin-induced inflammation.
Nystatin induces the secretion of IL-1β, IL-8, and TNF-α in TLR2-expressing but not in TLR2-deficient cells.
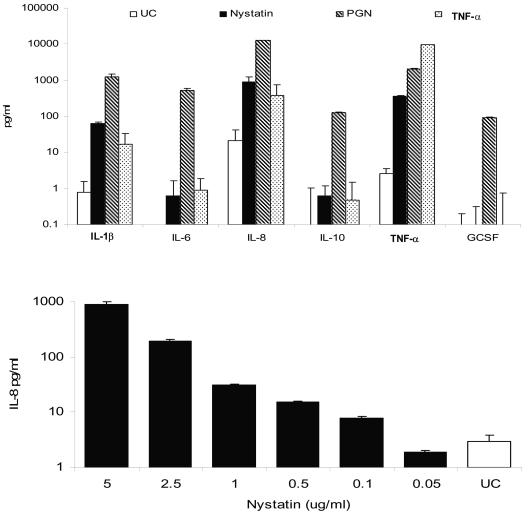
We observed that nystatin induces cytokine secretion in TLR2-expressing but not TLR2-deficient cells. When TLR2-expressing THP1 cells (ATCC number TIB-202) were stimulated with up to 5 μg/ml of nystatin (Nystatin; Sigma, St Louis, MO) for 24 h, there was a dose-dependent secretion of interleukin-1β (IL-1β), IL-8, and tumor necrosis factor alpha (TNF-α) (Fluorokine MAP multianalyte profiling; R&D Systems Inc., Minneapolis, MN) (Fig. 1). At the highest concentration tested (5 μg/ml) (demonstrated to be nontoxic by a Vi-CELL cell viability analyzer; Beckman Coulter), IL-1β, IL-8, and TNF-α levels were >70-, >40-, and >135-fold higher, respectively, than those seen with unstimulated cells. The concentration-dependent secretion of IL-1β, IL-8, and TNF-α was observed in parallel experiments using two other formulations of nystatin (Nystatin Oral Suspension [Morton Grove Pharmaceuticals, IL], for patient use, and Prestwick Chemical Library) (data not shown).
FIG. 1.
(Upper panel) Cytokine secretion in human monocytic-derived THP1 cells during exposure to nystatin, unpurified peptidoglycan (PGN), and tumor necrosis factor alpha (TNF-α). (Lower panel) Concentration-dependent secretion of IL-8 by THP1 cells during nystatin stimulation. In triplicate experiments conducted on two separate occasions, THP1 human monocytic cells were seeded into 96-well plates at a density of 106/ml and incubated with various stimuli—nystatin (5 μg/ml), unpurified PGN (which contains the TLR2 ligand lipoteichoic acid; TLR2-positive control) (10 μg/ml), and TNF-α (TLR-independent positive control) (10 ng/ml)—for 24 h. The cytokine profile was measured in cell-free culture supernatant by use of Fluorokine MAP multianalyte profiling (R&D Systems Inc., Minneapolis, MN). IL, interleukin; GSCF, granulocyte colony-stimulating factor; UC, unstimulated cells.
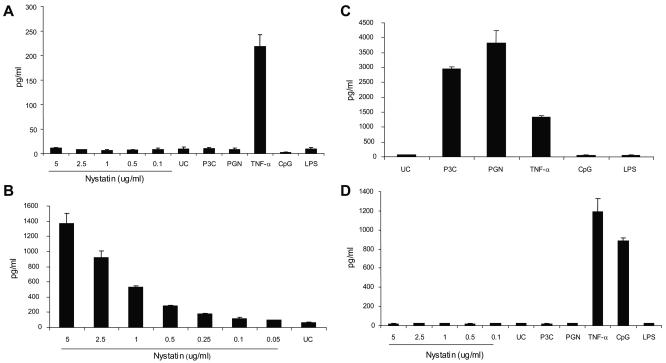
In contrast, TLR2-deficient HEK293 (ATCC CRL-1573) did not respond to nystatin. When stimulated with up to 5 μg/ml of nystatin, HEK293 did not significantly secrete IL-8 (Fig. 2A), IL-1β, and TNF-α. Consistent with its lack of TLR2 and TLR4, HEK293 did not secrete IL-8 in response to unpurified Staphylococcus aureus-derived peptidoglycan (PGN), which contains the TLR2 ligand lipoteichoic acid (Sigma) (10 μg/ml) (19), tripalmitoyl cysteinyl lipopeptide (P3C) (Calbiochem) (10 ng/ml), and TLR4 ligand lipopolysaccharide (purified lipopolysaccharide [LPS] from Escherichia coli) (Sigma) (10 g/ml).
FIG. 2.
Cellular activation, as measured by IL-8 secretion, during nystatin exposure requires TLR2. (A) TLR2-deficient HEK293 cells do not respond to nystatin, unpurified peptidoglycan (PGN), tripalmitoyl cysteinyl lipopeptide (P3C), unmethylated CpG, or purified lipopolysaccharide (LPS) but secrete IL-8 in response to TNF-α. (B) Transfection of HEK293 with TLR2 (HEK293-TLR2) rendered responsiveness to nystatin. (C) HEK293-TLR2 cells also acquired responsiveness to the TLR2 ligands P3C and unpurified PGN, but not to LPS and CpG. (D) Transfection of HEK293 with TLR9 (HEK293-TLR9) rendered responsiveness to unmethylated CpG but not to nystatin, P3C, PGN, or LPS. Human embryonic kidney 293 (HEK293), HEK293-TLR2, and HEK293-TLR9 cells were seeded in 96-well plates at a density of 106/ml and stimulated with 0.05 to 5 μg/ml of nystatin, LPS (10 μg/ml), P3C (10 ng/ml), PGN (10 μg/ml), unmethylated CpG (2 uM), and tumor necrosis factor alpha (TNF-α) for 18 h. HEK293 and HEK293-TLR9 do not respond to nystatin exposure. Stable transfection of TLR2 in HEK293 (HEK293-TLR2) rendered a dose-dependent responsiveness, as indicated by IL-8 secretion, to nystatin and to the TLR2 ligands P3C and unpurified PGN but not to unmethylated CpG and LPS. The experiments were conducted in triplicate on at least three occasions.
HEK293 cells acquire responsiveness to nystatin upon transfection with human TLR2.
The activation of TLR2-expressing THP1 but not TLR2-deficient HEK293 suggests that TLR2 ligation may be mediating cytokine secretion in response to nystatin. To prove this, we demonstrate that we can render nystatin responsiveness to HEK293 by transfection with human TLR2 (Fig. 2B). Concentration-dependent IL-8, IL-1β, and TNF-α secretion was observed in HEK293-TLR2 cells during nystatin stimulation. At 5-μg/ml of nystatin, the level of IL-8 was 23-fold higher than that seen with unstimulated cells (1,372.67 ± 127.12 [mean ± standard deviation] versus 59.08 ± 9.29 pg/ml; P = 0.003) (Fig. 2B). HEK293-TLR2 also acquired responsiveness to P3C and unpurified PGN, but it remained unresponsive to LPS (Fig. 2C). The acquired responsiveness of HEK293-TLR2 to nystatin was specific to TLR2, since a similar clone of HEK293, which was transfected with a non-TLR2 construct (a human TLR9 gene construct), failed to respond to nystatin (while it gained responsiveness to CpG2006S [Integrated DNA Technologies, Coralville, IA]) (2 μM) (Fig. 2D).
Anti-TLR2 MAb inhibits nystatin-induced cytokine secretion.
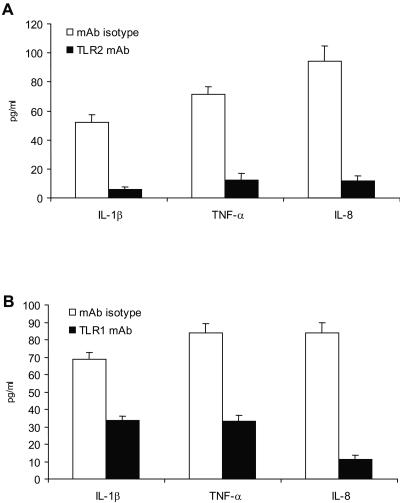
We subsequently investigated whether we can abrogate cytokine secretion during TLR2 neutralization. Preincubation of HEK293-TLR2 and THP1 cells with anti-human TLR2 monoclonal antibody (MAb) (functional-grade purified anti-human TLR2, clone TLR2.1; eBioscience) reduced nystatin-induced IL-1β, IL-8, and TNF-α secretion (P ≤ 0.001) (Fig. 3A). Anti-human TLR2 monoclonal antibody (MAb) also reduced cytokine secretion in response to unpurified PGN (P = 0.007) and P3C (P = 0.006).
FIG. 3.
Anti-TLR2 and -TLR1 monoclonal antibody attenuates interleukin-8 (IL-8) and IL-1β and tumor necrosis factor alpha (TNF-α) secretion in response to nystatin. (A) THP1 human monocytic cells that were preincubated with murine anti-human TLR2 monoclonal antibody (MAb) secreted significantly less IL-1β and TNF-α than the isotype control. Likewise, human embryonic kidney (HEK) 293-TLR2 cells that were preincubated with murine anti-human TLR2 MAb had significantly lower levels of IL-8 secretion in response to nystatin compared to cells that were preincubated with the isotype control. (B) THP1 human monocytic cells that were preincubated with murine anti-human TLR1 MAb secreted significantly less IL-1β and TNF-α than the isotype control. Likewise, HEK293-TLR2 cells that were preincubated with murine anti-human TLR1 MAb had significantly lower levels of IL-8 secretion in response to nystatin than cells preincubated with the isotype control. The experiments were performed in triplicate and were repeated on at least three occasions.
Taken together, these results implicate TLR2 in the proinflammatory response to nystatin. We therefore propose a model wherein TLR2 recognizes nystatin as a PAMP and the ensuing TLR2-nystatin interaction activates intracellular signaling pathways that result in IL-1β, IL-8, and TNF-α secretion. In turn, IL-1β and TNF-α mediate the manifestations of fever and chills (4), while IL-8 augments inflammation by recruiting inflammatory cells (13). Hence, a TLR2-mediated activation serves as the molecular basis for nystatin-induced local inflammation (in animal models of inflammation) (11) and systemic inflammation that has precluded its use as parenteral therapy (2).
This study has contemporary clinical relevance, since nystatin has, in recent years, undergone reformulation into lipid carriers (6, 7, 10, 12, 20)—in similarity to the lipid formulations of amphotericin B. Indeed, intravenous liposomal nystatin has undergone early-phase clinical development (2). While data from clinical trials are yet to be published, we anticipate that the incorporation of nystatin into lipid carriers could result in reduction of proinflammatory toxicities—an effect analogous to the reduced toxicity associated with lipid-based amphotericin B. The incorporation of nystatin (and amphotericin B) into lipid carriers could create hindrance that prevents interaction between the polyene and TLR2 (15, 16). In the only clinical data reported, only one of five patients receiving liposomal nystatin developed fever, chills, and dyspnea (10).
TLR1 cooperates with TLR2 in mediating the cellular response to nystatin.
Mechanistically, TLR2 activation results in the cooperative activation of TLR1 or TLR6 (17, 18). Here, we demonstrate that TLR1 is required in nystatin-induced TLR2 activation (Fig. 3B). Preincubation of HEK293-TLR2 and THP1 with anti-human TLR1 MAb (functional-grade purified anti-human TLR1, clone GD2.F4; eBioscience) significantly reduced IL-8 and IL-1β and TNF-α secretion. Anti-TLR1 MAb also reduced cytokine secretion in response to P3C (a TLR2-TLR1 ligand) (17, 18) but not to TNF-α. Our multiple attempts to assess TLR6 were limited by a lack of reliable neutralizing antibodies.
In conclusion, this study demonstrated that a TLR2- and TLR1-dependent process serves as the molecular basis for the proinflammatory properties of nystatin. This study expands the concept that certain “nonimmunologic” drugs serve as agonists for TLR, whose consequent activation mediates additional and often unintended beneficial and detrimental drug effects. On the one hand, it could lead to more-effective antimicrobial properties—as exemplified by the antiviral efficacies of the imidazoquinolines (5). On the other hand, an uncontrolled proinflammatory response could manifest as adverse toxicities—as was previously described for two clinically useful polyenes, nystatin and amphotericin B (15, 16). An appreciation of the role of TLR in pharmacologic immunotoxicology should highlight their potential as novel therapeutic targets whose inhibition could alleviate the morbid outcomes of otherwise effective drug treatments.
Acknowledgments
Conflict of interest statement: R.R.R. was a Mayo Foundation Scholar and Visiting Scientist at the Lilly Research Laboratories (Indianapolis, IN) during the conduct of this study. H.L.W. is currently with the National Institutes of Health (Bethesda, MD).
REFERENCES
- 1.Akira, S., and S. Sato. 2003. Toll-like receptors and their signaling mechanisms. Scand. J. Infect. Dis. 35:555-562. [DOI] [PubMed] [Google Scholar]
- 2.Arikan, S., and J. H. Rex. 2001. Nystatin LF (Aronex/Abbott). Curr. Opin. Investig. Drugs 2:488-495. [PubMed] [Google Scholar]
- 3.Bruheim, P., S. E. Borgos, P. Tsan, H. Sletta, T. E. Ellingsen, J. M. Lancelin, and S. B. Zotchev. 2004. Chemical diversity of polyene macrolides produced by Streptomyces noursei ATCC 11455 and recombinant strain ERD44 with genetically altered polyketide synthase NysC. Antimicrob. Agents Chemother. 48:4120-4129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dinarello, C. A. 2000. Proinflammatory cytokines. Chest 118:503-508. [DOI] [PubMed] [Google Scholar]
- 5.Dockrell, D. H., and G. R. Kinghorn. 2001. Imiquimod and resiquimod as novel immunomodulators. J. Antimicrob. Chemother. 48:751-755. [DOI] [PubMed] [Google Scholar]
- 6.Groll, A. H., C. E. Gonzalez, N. Giri, K. Kligys, W. Love, J. Peter, E. Feuerstein, J. Bacher, S. C. Piscitelli, and T. J. Walsh. 1999. Liposomal nystatin against experimental pulmonary aspergillosis in persistently neutropenic rabbits: efficacy, safety and non-compartmental pharmacokinetics. J. Antimicrob. Chemother. 43:95-103. [DOI] [PubMed] [Google Scholar]
- 7.Groll, A. H., V. Petraitis, R. Petraitiene, A. Field-Ridley, M. Calendario, J. Bacher, S. C. Piscitelli, and T. J. Walsh. 1999. Safety and efficacy of multilamellar liposomal nystatin against disseminated candidiasis in persistently neutropenic rabbits. Antimicrob. Agents Chemother. 43:2463-2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hazen, E. L., and R. Brown. 1950. Two antifungal agents produced by a soil actinomycete. Science 112:423. [PubMed] [Google Scholar]
- 9.Holt, R. J. 1980. Progress in antimycotic chemotherapy 1945-1980. Infection 8(Suppl. 3):S284-S287. [PubMed] [Google Scholar]
- 10.Krupova, Y., M. Mistrik, E. Bojtarova, D. Sejnova, I. Ilavska, and V. Krcmery, Jr. 2001. Liposomal nystatin (L-NYS) in therapy of pulmonary aspergillosis refractory to conventional amphotericin B in cancer patients. Support. Care Cancer 9:209-210. [DOI] [PubMed] [Google Scholar]
- 11.Macedo, S. B., L. R. Ferreira, F. F. Perazzo, and J. C. Carvalho. 2004. Anti-inflammatory activity of Arnica montana 6cH: preclinical study in animals. Homeopathy 93:84-87. [DOI] [PubMed] [Google Scholar]
- 12.Mehta, R. T., R. L. Hopfer, T. McQueen, R. L. Juliano, and G. Lopez-Berestein. 1987. Toxicity and therapeutic effects in mice of liposome-encapsulated nystatin for systemic fungal infections. Antimicrob. Agents Chemother. 31:1901-1903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mukaida, N. 2000. Interleukin-8: an expanding universe beyond neutrophil chemotaxis and activation. Int. J. Hematol. 72:391-398. [PubMed] [Google Scholar]
- 14.Ng, A. W., K. M. Wasan, and G. Lopez-Berestein. 2003. Development of liposomal polyene antibiotics: an historical perspective. J. Pharm. Pharm. Sci. 6:67-83. [PubMed] [Google Scholar]
- 15.Razonable, R. R., M. Henault, L. N. Lee, C. Laethem, P. A. Johnston, H. L. Watson, and C. V. Paya. 2005. Secretion of proinflammatory cytokines and chemokines during amphotericin B exposure is mediated by coactivation of toll-like receptors 1 and 2. Antimicrob. Agents Chemother. 49:1617-1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sau, K., S. S. Mambula, E. Latz, P. Henneke, D. T. Golenbock, and S. M. Levitz. 2003. The antifungal drug amphotericin B promotes inflammatory cytokine release by a Toll-like receptor- and CD14-dependent mechanism. J. Biol. Chem. 278:37561-37568. [DOI] [PubMed] [Google Scholar]
- 17.Takeda, K., O. Takeuchi, and S. Akira. 2002. Recognition of lipopeptides by Toll-like receptors. J. Endotoxin Res. 8:459-463. [DOI] [PubMed] [Google Scholar]
- 18.Takeuchi, O., S. Sato, T. Horiuchi, K. Hoshino, K. Takeda, Z. Dong, R. L. Modlin, and S. Akira. 2002. Cutting edge: role of Toll-like receptor 1 in mediating immune response to microbial lipoproteins. J. Immunol. 169:10-14. [DOI] [PubMed] [Google Scholar]
- 19.Travassos, L. H., S. E. Girardin, D. J. Philpott, D. Blanot, M. A. Nahori, C. Werts, and I. G. Boneca. 2004. Toll-like receptor 2-dependent bacterial sensing does not occur via peptidoglycan recognition. EMBO Rep. 5:1000-1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wallace, T. L., V. Paetznick, P. A. Cossum, G. Lopez-Berestein, J. H. Rex, and E. Anaissie. 1997. Activity of liposomal nystatin against disseminated Aspergillus fumigatus infection in neutropenic mice. Antimicrob. Agents Chemother. 41:2238-2243. [DOI] [PMC free article] [PubMed] [Google Scholar]