Abstract
Due to increasing mupirocin resistance, alternatives for Staphylococcus aureus nasal decolonization are needed. Lauric acid monoesters combined with lactic, mandelic, malic, or benzoic acid are being evaluated as possible alternatives. We determined the in vitro activity of 13 lauric acid monoester (LAM) formulations and mupirocin against 30 methicillin-susceptible S. aureus (MSSA) isolates and 30 methicillin-resistant S. aureus (MRSA) isolates. We then used a murine model of MRSA nasopharyngeal colonization to compare the in vivo activity of mupirocin with three LAM formulations. MSSA and MRSA MIC90 values were 0.25 μg/ml for mupirocin and ≤4 μl/ml for all LAM formulations tested. Hsd:ICR mice were challenged with 108 CFU/naris MRSA. Five days later, S. aureus colonization was documented by culture. Treatment with bland, mupirocin, or one of three LAM ointments was then administered unblinded thrice daily for 2 days. Three days after treatment, both anterior nares were cultured for S. aureus. Administration of 128774-49E or 128774-53A was associated with greater eradication of MRSA carriage (24/34 [71%] or 33/40 [83%]) of animals, respectively) than bland ointment (12/38 [32%]) (P < 0.005). 128774-53A administration resulted in greater MRSA carriage eradication than mupirocin (19/38 [50%]) (P < 0.005) in this model. LAM formulations warrant evaluation for S. aureus nasal decolonization in humans.
Nasal carriage of methicillin-resistant Staphylococcus aureus (MRSA) by hospitalized patients has been associated with nosocomial transmission of MRSA (9). Nasal carriage is also recognized as a risk factor for S. aureus infection in patients with concomitant human immunodeficiency virus infection, with intravascular devices, undergoing surgical procedures, on hemodialysis or continuous ambulatory peritoneal dialysis, or who have undergone liver transplantation (10, 24, 41, 44). Mupirocin eradicates nasal S. aureus carriage in the short term (29). Decolonization of nasal carriers with mupirocin may reduce the incidence of S. aureus infections in surgical patients and in those on hemodialysis and continuous ambulatory peritoneal dialysis (35, 45), although this issue is controversial (26).
Mupirocin resistance in S. aureus was first reported in 1987, 2 years after mupirocin was introduced into clinical practice (3). Mupirocin resistance in staphylococci has been classified as low-level (MIC, 8 to 256 μg/ml) and high-level (MIC, >256 μg/ml) (13). Low-level resistance results from mutations in endogenous isoleucyl-tRNA synthetase (IleRS) (2, 46), whereas high-level resistance is a result of acquisition and expression of mupA, a gene encoding an exogenous IleRS which is not inhibited by mupirocin (19). Widespread use of mupirocin has been accompanied by the emergence of both types of mupirocin resistance in S. aureus (27, 31, 38). High-level-resistant strains are not eradicated from the human nasopharynx with mupirocin (1, 3). Harbarth et al. reported a statistically significant association of low-level mupirocin resistance in MRSA with failure of nasal decolonization (18). Others have corroborated this finding (42). Further, the emergence of low-level mupirocin resistance by a glycopeptide-intermediate S. aureus strain, as a result of an IleRS mutation (21), in a patient receiving nasal mupirocin (and associated with failed decolonization) has been reported (14). Because of emerging mupirocin resistance in S. aureus, alternatives to mupirocin are needed for S. aureus nasal decolonization.
The antimicrobial properties of fatty acids have been recognized for many years (12, 22). They have broad-spectrum activity against gram-positive bacteria, including S. aureus (12, 22), gram-negative bacteria, such as Neisseria gonorrhoeae and Helicobacter pylori (7, 8), Chlamydia trachomatis (4), enveloped viruses (37), and Candida albicans (5). Fatty acids and their monoglycerides have been shown, in an animal model, to be effective vaginal microbicides (30, 36). Fatty acids and their monoglycerides, commonly found in natural products, are considered nontoxic. They contribute to the antimicrobial properties of human milk (37) and skin (28). The Food and Drug Administration has listed fatty acid monoesters as generally recognized as safe (16). The antistaphylococcal activity and minimal toxicity of fatty acids make these formulations potential alternatives to mupirocin for S. aureus nasal decolonization.
We determined the in vitro activity against S. aureus of 13 lauric acid monoester (LAM) (Table 1) formulations or mupirocin and compared the emergence of resistance to three LAM formulations and mupirocin in S. aureus. We also compared the in vivo activity of three LAM formulations with mupirocin for experimental nasal S. aureus decolonization in mice using a modification of a previously described nasal S. aureus carriage model (23).
TABLE 1.
Lauric acid monoester formulations studied in vitroa
| Formulation | 128774-23
|
128776-53
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mandelic acid
|
Malic acid
|
Lactic acid
|
Benzoic acid | ||||||||||
| A | B | C | D | E | F | G | H | I | J | K | L | ||
| Lauric acid monoester (%) | 1 | 3 | 1 | 3 | 1 | 3 | 1 | 3 | 1 | 3 | 1 | 3 | 3 |
| Organic acid (%) | 1 | 1 | 0.5 | 0.5 | 1 | 1 | 0.5 | 0.5 | 1 | 1 | 0.5 | 0.5 | 0.5 |
Solutions of the lauric acid monoester and organic acid were dissolved in isopropanol. The lauric acid monoester CH3(CH2)10COOCH2CH(OH)CH2OH has the structure shown below: 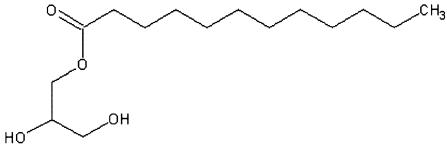
(Presented in part at the 43rd Interscience Conference on Antimicrobial Agents and Chemotherapy, Chicago, Illinois, September 2003.)
MATERIALS AND METHODS
Collection of clinical isolates.
Thirty clinical isolates each of methicillin-susceptible S. aureus (MSSA) and MRSA from the Mayo Clinic (Rochester, MN) and the Cleveland Clinic (Cleveland, OH) collected between January 1985 and December 2002 and stored at −70°C were studied. Five isolates (8%) were from patients with endocarditis, and 16 isolates (27%) were from patients with prosthetic joint infection. The source was not documented for the remaining 39 isolates. One isolate per patient was studied. The isolates were typed using SmaI pulsed-field gel electrophoresis. An isolate of MRSA which we have previously used in other animal models of infection (15) (IDRL-4293, mupirocin MIC, ≤0.125 μg/ml), was studied in vivo.
Mupirocin and LAM formulations.
Mupirocin lithium salt powder was purchased from U.S. Pharmacopeia (Rockville, MD). The MICs of 13 LAM formulations (3M Inc., St. Paul, MN) were determined. The concentration of LAM was 1 or 3% wt/wt and each organic acid (lactic, mandelic, benzoid, or malic acid) was 0.5 or 1.0% wt/wt (Table 1). For in vitro studies, solutions of the LAM and organic acid were dissolved in isopropanol (whereas for in vivo studies, formulations were in a petrolatum base). Studies selecting less susceptible isolates in vitro were performed with 128774-23A, 128774-23B, and 128776-53A.
Mupirocin calcium ointment 2% (Bactroban Nasal, SmithKlineGlaxo, Research Triangle Park, NC), and 128774-49D ointment (3% LAM and 1% lactic acid in a petrolatum base), 128774-49E ointment (3% LAM and 1% mandelic acid in a petrolatum base), 128774-53A ointment (3% LAM and 0.5% benzoic acid in a petrolatum base), and bland ointment (petrolatum base) (3M Inc., St. Paul, MN) were studied in vivo.
Determination of MICs.
MICs were determined by broth microdilution according to the Clinical and Laboratory Standards Institute guidelines with cation-adjusted Mueller-Hinton broth (MHB) and an inoculum of 105 CFU per ml (11). S. aureus ATCC 29213 was used as a control strain. For the LAM formulations, six colonies were inoculated into tryptic soy broth; the broth was incubated at 37°C until turbid, diluted to a McFarland standard of 0.5, and then further diluted 1:100 in MHB. MIC testing was performed in microtiter well plates using a total volume of 100 μl containing doubling dilutions ranging from 0.125 to 32 μl/ml of the LAM formulations tested. A well containing no LAM formulation was used as a growth control. Plates were incubated 18 to 24 h in room air at 37C; the MIC was read as the lowest antimicrobial concentration exhibiting no growth.
Selection of less susceptible isolates in vitro.
We exposed 5 × 109 CFU of each S. aureus isolate to increasing concentrations of mupirocin in 10 ml of MHB containing the following concentrations: 0.125 μg/ml (day 1), 0.25 μg/ml (day 2), 0.5 μg/ml (day 3), 1 μg/ml (day 4), 2 μg/ml (day 5), 4 μg/ml (day 6), 8 μg/ml (day 7), and 16 μg/ml (day 8). Following overnight incubation, the broth culture was centrifuged for 10 min at 900 × g and the pellet was divided into two aliquots. One aliquot was suspended in 10 ml of fresh MHB containing twice the mupirocin concentration of the previous day; the other aliquot was spread on the surface of a Mueller-Hinton agar (MHA) plate containing 4 μg/ml of mupirocin to screen for resistant S. aureus. The plate was incubated in room air at 37°C for 48 h. The MIC of 5 to 10 colonies recovered from screening agar was determined at each day of serial passage. This procedure was repeated for 8 consecutive days.
The same procedure (with minor modifications) was used for three LAM formulations. Concentrations of LAM formulations in MHB were doubled each day as follows: 0.25 μl/ml (day 1), 0.5 μl/ml (day 2), 1 μl/ml (day 3), 2 μl/ml (day 4), 4 μl/ml (day 5), 8 μl/ml (day 6), 16 μl/ml (day 7), and 32 μl/ml (day 8). MHB with 20 μl/ml of 128774-23A, 128774-23B, or 128776-53A was substituted for MHA plates due to the insolubility of the LAM formulations in agar (precluding identification of individual colonies). Following overnight incubation, the MHB was subcultured to a sheep blood agar plate and LAM formulation MIC testing was performed on six colonies.
Experimental model of nasal MRSA decolonization.
A previously described nasal S. aureus carriage model (23) was modified as described below. Healthy 4-week-old 25- to 30-g Hsd:ICR mice housed in “shoebox” cages of five animals each with mouse chow and water available ad libitum were used. The anterior nasopharynx of 10 mice sedated with 60 mg/kg ketamine plus 6 mg/kg xylazine was cultured to assess S. aureus carriage. Anterior nasopharyneal specimens were collected by aseptically inserting 7 mm of sterile 6.0 vicryl suture material (hereafter termed swab) into each naris. The swabs were inserted and withdrawn 10 times and then left inserted for 10 min. Both swabs were then placed in a single microcentrifuge tube containing 150 μl of tryptic soy broth and vortexed; 75 μl of tryptic soy broth and both swabs were removed and spread on the surface of a sheep blood agar plate. The plates were incubated at 35°C in 5% CO2 for 48 h, following which they were inspected for growth. Colonies morphologically resembling staphylococci were tested to determine whether they were S. aureus or not using a latex agglutination test (Staphaurex, Lenexa, KS).
We challenged 279 mice with a 10-μl suspension containing 108 CFU of MRSA IDRL-4293 pipetted into each naris. This inoculum was shown to result in colonization of 7 of 10 (70%) mice. Five days after challenge, anterior nasopharyngeal cultures were collected. Then the animals were arbitrarily assigned to one of five unblinded treatment regimens consisting of 10 μl of bland, mupirocin, 128774-49D, 128774-49E, or 128774-53A ointment per naris, administered three times daily for 2 days. All mice in a single cage were assigned to the same treatment regimen. The numbers of animals in each treatment group are shown in Table 2. Ointments were administered with a 100-μl glass syringe through a 22-gauge feeding needle (1.25 ball diameters) pushed snuggly against the naris. The ointment-loaded syringe was warmed with a lamp and maintained at 42°C during use (maximum, 10 min) to reduce viscosity and facilitate instillation into the nares. (Maintenance at 42°C for 10 min demonstrated no effect on mupirocin activity in vitro.)
TABLE 2.
Treatment regimen assignment, number of animals successfully colonized, and results of treatment
| Treatment | No. of animals challenged | No. of animals colonized before treatment | No. (%) of animals decolonized |
|---|---|---|---|
| Bland ointment | 53 | 38 | 12 (32) |
| Mupirocin | 56 | 38 | 19 (50) |
| 128774-49D | 60 | 39 | 18 (46) |
| 128774-49E | 50 | 34 | 24 (71) |
| 128774-53A | 60 | 40 | 33 (83) |
Animals not colonized with S. aureus were initially treated (because results of nasopharyngeal cultures performed to detect colonization were not complete until after treatment), but were excluded from the final analysis. Three days after discontinuing treatment, nasopharyngeal cultures for S. aureus were performed. Results of treatment were analyzed using Fisher's exact test. We used Bonferoni's correction after comparing the results of five treatment regimens to each other (i.e., 10 tests total); a P value of <0.005 represented a statistically significant difference.
In vivo emergence of resistance.
S. aureus recovered from mice treated with mupirocin, 128774-49D, 128774-49E, or 128774-53A were tested for susceptibility to the topical agent used for treatment (11).
RESULTS
In vitro studies.
There were 18 unique MSSA and 17 unique MRSA pulsed-field gel electrophoresis patterns. MSSA/MRSA MIC90 (range) were 0.25 (≤0.125 to 0.5)/0.25 (0.125 to 0.5) μg/ml for mupirocin, 2 (1 to 2)/2 (1 to 2) μl/ml for 128776-53A, 1 (0.25 to 1)/2 (0.25 to 2) μl/ml for 128774-23B, and 2 (0.25 to 2)/4 (1 to 4) μl/ml for 128774-23J. Similar MICs were found for the other LAM formulations and MICs were similar for MSSA and MRSA (data not shown).
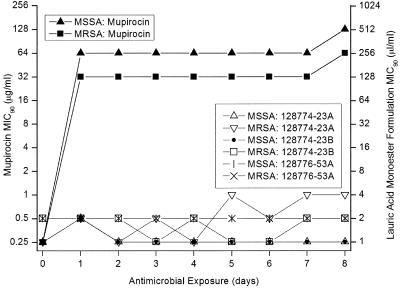
After 1 day of in vitro exposure, the mupirocin MIC90 values had increased by eight and seven twofold dilutions for MSSA and MRSA, respectively. On subsequent days no further significant changes in the mupirocin MIC90 values were detected. After 5 days of LAM formulation exposure, MIC90 values had increased a maximum of two twofold dilutions (Fig. 1).
FIG. 1.
MIC90 values (μg/ml for mupirocin, μl/ml for LAM formulations) of methicillin-susceptible S. aureus and methicillin-resistant S. aureus over the days of exposure.
Experimental model of nasal MRSA decolonization.
No S. aureus was detected from cultures of the 10 healthy, unchallenged animals tested. S. aureus was detected in 189 colonization cultures from 279 mice challenged with MRSA (Table 2). The results of treatment are shown as the number of animals decolonized following treatment and the percentage of colonized animals decolonized following treatment. Treatment with 128774-53A or 128774-49E resulted in greater eradication of MRSA carriage than bland ointment (P < 0.005). Mupirocin or 128774-49D treatment results were not significantly different than bland ointment treatment results (P = 0.08 and 0.14, respectively). 128774-53A treatment resulted in greater MRSA carriage eradication than mupirocin (P < 0.005). 128774-49D and 128774-49E treatment results were not significantly different than mupirocin treatment results (P = 0.46 and 0.06, respectively).
Emergence of resistance.
S. aureus isolates with mupirocin MICs of ≥4 mg/ml were detected in 4 of 19 (21%) animals failing mupirocin decolonization (Table 3). Animals found to be colonized with mupirocin-resistant MRSA after treatment had not been housed in the same cages. A threefold doubling dilution increase in MIC to 128774-49D was the maximum observed in the animals treated with 128774-49D. The maximum increase in MIC to 128774-53A in the animals treated with this formulation was two doubling dilutions. The maximum increase in MIC to 128774-49E in the animals treated with this formulation was a single doubling dilution.
TABLE 3.
Pre- and posttreatment susceptibility results
| Treatment | No. of animals | MIC before treatment | MIC range after treatment | No. of mice with resistantaS. aureus |
|---|---|---|---|---|
| Mupirocin | 19 | ≤0.125 μg/ml | ≤0.125-8 μg/ml | 4 |
| 128774-49D | 21 | 1 μl/ml | 1-8 μl/ml | —b |
| 128774-49E | 10 | 2 μl/ml | 0.5-4 μl/ml | — |
| 128774-53A | 7 | 1 μl/ml | 2-4 μl/ml | — |
Defined as mupirocin MIC of ≥4 μg/ml.
—, susceptibility breakpoints not established.
DISCUSSION
This study shows that mupirocin, 128774-23A, 128774-23B, and 128776-53A and 10 other LAM formulations demonstrate in vitro activity against S. aureus. MICs for mupirocin before antimicrobial exposure were similar to those reported previously (17). The MICs of lauric acid monoesters were similar to those reported by Holland et al. (i.e., MIC90 of 15 μg/ml and range of 10 to 20 μg/ml in 29 isolates of S. aureus) (20), considering that the formulations tested herein contained 1 to 3% lauric acid monoester. In general, the MIC50 values of the 3% LAM formulations were one twofold dilution below the MIC50 values of the 1% LAM formulations (data not shown). Assuming that LAM is the active component of the formulations, the MIC50 of the 3% LAM formulations should be three times lower than that of the 1% LAM formulation; however, we only tested twofold dilutions, so the results were as expected. For this reason, 3% LAM formulations were selected for in vivo testing. Additionally, 128774-53A was chosen for in vivo study because it was expected to be more chemically stable on account of containing benzoic acid, which does not have hydroxyl groups, which might undergo transesterification with the lauric acid ester.
We modified a previously described murine nasal S. aureus model (23) to evaluate topical agents being developed for nasal S. aureus decolonization. Overall, we achieved staphylococcal colonization in 68% of mice. A cotton rat model of S. aureus colonization has recently been used to evaluate lysostaphin cream, mupirocin ointment, and nisin cream for experimental S. aureus nasal decolonization (25). Although the cotton rat model results in a higher nasal colonization rate than does the mouse model used herein, we found no S. aureus in unchallenged mice, in contrast to the situation in cotton rat nares (25), making assessment of colonization easier in the mice studied herein. Furthermore, we used a nasal swab in our study, permitting multiple colonization evaluations of the same animal; we analyzed treatment results only from animals with pretreatment cultures positive for MRSA. The cotton rat model and the original mouse model we modified both used nasal excision, which precluded pretreatment microbiological confirmation of MRSA colonization (23, 25).
The LAM formulations studied herein are lipophilic surfactant/emulsifiers. Their exact mechanism of action is unknown but likely involves effects on the bacterial cell envelope and/or induction of autolysin activity and inhibition of protein synthesis. For example, Bergsson et al. demonstrated that S. aureus is killed by fatty acids, and especially by monocaprin, through disintegration of the cell membrane, leaving the cell wall intact (6). Ved et al. showed that dodecyl glycerol inhibits peptidoglycan synthesis and stimulates a proteinase which activates peptidoglycan-degrading enzyme autolysin (39, 40). Several investigators have reported effects on toxin synthesis. For example, Schlievert et al. demonstrated that S. aureus elaboration of hemolysin, toxic shock syndrome toxin 1, and exfoliative toxin A was inhibited at glycerol monolaurate concentrations below those necessary to inhibit growth (34). Mechanistic studies performed by Projan et al. showed that glycerol monolaurate inhibits synthesis of staphylococcal toxins (and other exoproteins) at the level of transcription by interfering with signal transduction (32). Interference with signal transduction has also been shown in other genera; Rusin and Novick demonstrated that glycerol monolaurate suppresses growth of vancomycin-resistant Enterococcus faecalis in the presence of vancomycin and blocks the induction of vancomycin resistance, which involves a membrane-associated signal transduction mechanism, either at or before initiation of transcription (33).
Mupirocin had low in vivo activity in our model. Our study was not, however, designed to demonstrate the efficacy of intranasal mupirocin (as used in humans). There are differences between our model and the administration of intranasal mupirocin to humans. For example, in this study, animals were only treated for 2 days, whereas humans are typically treated for 5 days. There are differences between the anatomy of the nasal passages and types of cells present between humans and mice that may impact on the efficacy of mupirocin in the model described.
We were able to select mupirocin-resistant mutants in vivo and in vitro. It is likely that a mupirocin concentration gradient existed in the nasopharynx of the mice following intranasal mupirocin application, similar to the situation in humans, facilitating selection for mupirocin resistance (43).
In conclusion, our in vitro studies show that mupirocin and 13 LAM formulations are active against S. aureus. Two of the three LAM formulations selected for testing in vivo showed activity in a murine nasal S. aureus decolonization model. The LAM formulations studied herein, or modifications thereof, may be an alternative to mupirocin ointment for nasal S. aureus decolonization in humans.
Acknowledgments
This study was supported by 3M Inc. (St. Paul, MN), the Mayo Foundation, and the Spanish Society of Infectious Diseases and Clinical Microbiology (Madrid, Spain).
We thank Rajesh M. Prabhu, Paloma Anguita-Alonso, Andrej Trampuz, and Melanie M. Hein for useful suggestions and Jennifer Milverstedt for technical help.
REFERENCES
- 1.Annigeri, R., J. Conly, S. Vas, H. Dedier, K. P. Prakashan, J. M. Bargman, V. Jassal, and D. Oreopoulos. 2001. Emergence of mupirocin-resistant Staphylococcus aureus in chronic peritoneal dialysis patients using mupirocin prophylaxis to prevent exit-site infection. Perit. Dial. Int. 21:554-559. [PubMed] [Google Scholar]
- 2.Antonio, M., N. McFerran, and M. J. Pallen. 2002. Mutations affecting the Rossman fold of isoleucyl-tRNA synthetase are correlated with low-level mupirocin resistance in Staphylococcus aureus. Antimicrob. Agents Chemother. 46:438-442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baird, D., and J. Coia. 1987. Mupirocin-resistant Staphylococcus aureus. Lancet ii:387-388. [PubMed] [Google Scholar]
- 4.Bergsson, G., J. Arnfinnsson, S. M. Karlsson, O. Steingrimsson, and H. Thormar. 1998. In vitro inactivation of Chlamydia trachomatis by fatty acids and monoglycerides. Antimicrob. Agents Chemother. 42:2290-2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bergsson, G., J. Arnfinnsson, O. Steingrimsson, and H. Thormar. 2001. In vitro killing of Candida albicans by fatty acids and monoglycerides. Antimicrob. Agents Chemother. 45:3209-3212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bergsson, G., J. Arnfinnsson, O. Steingrimsson, and H. Thormar. 2001. Killing of gram-positive cocci by fatty acids and monoglycerides. APMIS 109:670-678. [DOI] [PubMed] [Google Scholar]
- 7.Bergsson, G., O. Steingrimsson, and H. Thormar. 2002. Bactericidal effects of fatty acids and monoglycerides on Helicobacter pylori. Int. J. Antimicrob. Agents 20:258-262. [DOI] [PubMed] [Google Scholar]
- 8.Bergsson, G., O. Steingrimsson, and H. Thormar. 1999. In vitro susceptibilities of Neisseria gonorrhoeae to fatty acids and monoglycerides. Antimicrob. Agents Chemother. 43:2790-2792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boyce, J. M. 2001. MRSA patients: proven methods to treat colonization and infection. J. Hosp. Infect. 48(Suppl. A):S9-S14. [DOI] [PubMed] [Google Scholar]
- 10.Chang, F. Y., N. Singh, T. Gayowski, S. D. Drenning, M. M. Wagener, and I. R. Marino. 1998. Staphylococcus aureus nasal colonization and association with infections in liver transplant recipients. Transplantation 65:1169-1172. [DOI] [PubMed] [Google Scholar]
- 11.Clinical and Laboratory Standards Institute. 2003. Methods for dilution. antimicrobial susceptibility tests for bacteria that grow aerobically, 6th ed. Approved standard M7-A6. Clinical and Laboratory Standards Institute, Wayne, Pa.
- 12.Conley, A. J., and J. J. Kabara. 1973. Antimicrobial action of esters of polyhydric alcohols. Antimicrob. Agents Chemother. 4:501-506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cookson, B. D. 1998. The emergence of mupirocin resistance: a challenge to infection control and antibiotic prescribing practice. J. Antimicrob. Chemother. 41:11-18. [DOI] [PubMed] [Google Scholar]
- 14.Decousser, J. W., P. Pina, J. C. Ghnassia, J. P. Bedos, and P. Y. Allouch. 2003. First report of clinical and microbiological failure in the eradication of glycopeptide-intermediate methicillin-resistant Staphylococcus aureus carriage by mupirocin. Eur. J. Clin. Microbiol. Infect. Dis. 22:318-319. [DOI] [PubMed] [Google Scholar]
- 15.Fernandez-Guerrero, M., M. Rouse, N. Henry, and W. Wilson. 1988. Ciprofloxacin therapy of experimental endocarditis caused by methicillin-susceptible or methicillin-resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 32:747-751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Food and Drug Administration. 1997. Substances generally recognized as safe; proposed rule (21 CFR 172.860). Food and Drug Administration, Washington, D.C.
- 17.Fuchs, P. C., R. N. Jones, and A. L. Barry. 1990. Interpretive criteria for disk diffusion susceptibility testing of mupirocin, a topical antibiotic. J. Clin. Microbiol. 28:608-609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Harbarth, S., S. Dharan, N. Liassine, P. Herrault, R. Auckenthaler, and D. Pittet. 1999. Randomized, placebo-controlled, double-blind trial to evaluate the efficacy of mupirocin for eradicating carriage of methicillin-resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 43:1412-1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hodgson, J. E., S. P. Curnock, K. G. Dyke, R. Morris, D. R. Sylvester, and M. S. Gross. 1994. Molecular characterization of the gene encoding high-level mupirocin resistance in Staphylococcus aureus J2870. Antimicrob. Agents Chemother. 38:1205-1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Holland, K. T., D. Taylor, and A. M. Farrell. 1994. The effect of glycerol monolaurate on growth of, and production of toxic shock syndrome toxin-1 and lipase by, Staphylococcus aureus. J. Antimicrob. Chemother. 33:41-55. [DOI] [PubMed] [Google Scholar]
- 21.Hurdle, J. G., A. J. O'Neill, and I. Chopra. 2004. The isoleucyl-tRNA synthetase mutation V588F conferring mupirocin resistance in glycopeptide-intermediate Staphylococcus aureus is not associated with a significant fitness burden. J. Antimicrob. Chemother. 53:102-104. [DOI] [PubMed] [Google Scholar]
- 22.Kabara, J. J., D. M. Swieczkowski, A. J. Conley, and J. P. Truant. 1972. Fatty acids and derivatives as antimicrobial agents. Antimicrob. Agents Chemother. 2:23-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kiser, K. B., J. M. Cantey-Kiser, and J. C. Lee. 1999. Development and characterization of a Staphylococcus aureus nasal colonization model in mice. Infect. Immun. 6:5001-5006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kluytmans, J., A. van Belkum, and H. Verbrugh. 1997. Nasal carriage of Staphylococcus aureus: epidemiology, underlying mechanisms, and associated risks. Clin. Microbiol. Rev. 10:505-520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kokai-Kun, J. F., S. M. Walsh, T. Chanturiya, and J. J. Mond. 2003. Lysostaphin cream eradicates Staphylococcus aureus nasal colonization in a cotton rat model. Antimicrob. Agents Chemother. 47:1589-1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Laupland, K. B., and J. M. Conly. 2003. Treatment of Staphylococcus aureus colonization and prophylaxis for infection with topical intranasal mupirocin: an evidence-based review. Clin. Infect. Dis. 37:933-938. [DOI] [PubMed] [Google Scholar]
- 27.Miller, M. A., A. Dascal, J. Portnoy, and J. Mendelson. 1996. Development of mupirocin resistance among methicillin-resistant Staphylococcus aureus after widespread use of nasal mupirocin ointment. Infect. Control Hosp. Epidemiol. 17:811-813. [DOI] [PubMed] [Google Scholar]
- 28.Miller, S. J., R. Aly, H. R. Shinefeld, and P. M. Elias. 1988. In vitro and in vivo antistaphylococcal activity of human stratum corneum lipids. Arch. Dermatol. 124:209-215. [PubMed] [Google Scholar]
- 29.Mody, L., C. A. Kauffman, S. A. McNeil, A. T. Galecki, and S. F. Bradley. 2003. Mupirocin-based decolonization of Staphylococcus aureus carriers in residents of 2 long-term care facilities: a randomized, double-blind, placebo-controlled trial. Clin. Infect. Dis. 37:1467-1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Neyts, J., T. Kristmundsdottir, E. De Clercq, and H. Thormar. 2000. Hydrogels containing monocaprin prevent intravaginal and intracutaneous infections with HSV-2 in mice: impact on the search for vaginal microbicides. J. Med. Virol. 61:107-110. [DOI] [PubMed] [Google Scholar]
- 31.Petinaki, E., I. Spiliopoulou, F. Kontos, M. Maniati, Z. Bersos, N. Stakias, H. Malamou-Lada, C. Koutsia-Carouzou, and A. N. Maniatis. 2004. Clonal dissemination of mupirocin-resistant staphylococci in Greek hospitals. J. Antimicrob. Chemother. 53:105-108. [DOI] [PubMed] [Google Scholar]
- 32.Projan, S. J., S. Brown-Skrobot, P. M. Schlievert, F. Vandenesch, and R. P. Novick. 1994. Glycerol monolaurate inhibits the production of beta-lactamase, toxic shock toxin-1, and other staphylococcal exoproteins by interfering with signal transduction. J. Bacteriol. 176:4204-4209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ruzin, A., and R. P. Novick. 1998. Glycerol monolaurate inhibits induction of vancomycin resistance in Enterococcus faecalis. J. Bacteriol. 180:182-185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schlievert, P. M., J. R. Deringer, M. H. Kim, S. J. Projan, and R. P. Novick. 1992. Effect of glycerol monolaurate on bacterial growth and toxin production. Antimicrob. Agents Chemother. 36:626-631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tacconelli, E., Y. Carmeli, A. Aizer, G. Ferreira, M. G. Foreman, and E. M. C. D'Agata. 2003. Mupirocin prophylaxis to prevent Staphylococcus aureus infection in patients undergoing dialysis: a meta-analysis. Clin. Infect. Dis. 37:1629-1638. [DOI] [PubMed] [Google Scholar]
- 36.Thormar, H., G. Bergsson, E. Gunnarsson, G. Georgsson, M. Witvrouw, O. Steingrimsson, E. De Clercq, and T. Kristmundsdottir. 1999. Hydrogels containing monocaprin have potent microbicidal activities against sexually transmitted viruses and bacteria in vitro. Sex. Transm. Infect. 75:181-185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Thormar, H., C. E. Isaacs, H. R. Brown, M. R. Barshatzky, and T. Pessolano. 1987. Inactivation of enveloped viruses and killing of cells by fatty acids and monoglycerides. Antimicrob. Agents Chemother. 31:27-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Upton, A., S. Lang, and H. Heffernan. 2003. Mupirocin and Staphylococcus aureus: a recent paradigm of emerging antibiotic resistance. J. Antimicrob. Chemother. 51:613-617. [DOI] [PubMed] [Google Scholar]
- 39.Ved, H. S., E. Gustow, V. Mahadevan, and R. A. Pieringer. 1984. Dodecylglycerol. A new type of antibacterial agent which stimulates autolysin activity in Streptococcus faecium ATCC 9790. J. Biol. Chem. 259:8115-8121. [PubMed] [Google Scholar]
- 40.Ved, H. S., E. Gustow, and R. A. Pieringer. 1984. Inhibition of peptidoglycan synthesis of Streptococcus faecium ATCC 9790 and Streptococcus mutans BHT by the antibacterial agent dodecyl glycerol. Biosci. Rep. 4:659-664. [DOI] [PubMed] [Google Scholar]
- 41.von Eiff, C., K. Becker, K. Machka, H. Stammer, and G. Peters. 2001. Nasal carriage as a source of Staphylococcus aureus bacteremia. N. Engl. J. Med. 344:11-16. [DOI] [PubMed] [Google Scholar]
- 42.Walker, E. S., J. E. Vasquez, H. Bullock, and F. A. Sarubbi. 2003. Mupirocin-resistant, methicillin-resistant Staphylococcus aureus: does mupirocin remain effective? Infect. Control Hosp. Epidemiol. 24:342-346. [DOI] [PubMed] [Google Scholar]
- 43.Watanabe, H., H. Masaki, N. Asoh, K. Watanabe, K. Oishi, S. Kobayashi, A. Sato, R. Sugita, and T. Nagatake. 2001. Low concentrations of mupirocin in the pharynx following intranasal application may contribute to mupirocin resistance in methicillin-resistant Staphylococcus aureus. J. Clin. Microbiol. 39:3775-3777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Weinke, T., R. Schiller, F. J. Fehrenbach, and H. D. Pohle. 1992. Association between Staphylococcus aureus nasopharyngeal colonization and septicemia in patients infected with the human immunodeficiency virus. Eur. J. Clin. Microbiol. Infect. Dis. 11:985-989. [DOI] [PubMed] [Google Scholar]
- 45.Wilcox, M. H., J. Hall, H. Pike, P. A. Templeton, W. N. Fawley, P. Parnell, and P. Verity. 2003. Use of perioperative mupirocin to prevent methicillin-resistant Staphylococcus aureus (MRSA) orthopaedic surgical site infections. J. Hosp. Infect. 54:196-201. [DOI] [PubMed] [Google Scholar]
- 46.Yun, H. J., S. W. Lee, G. M. Yoon, S. Y. Kim, S. Choi, Y. S. Lee, E. C. Choi, and S. Kim. 2003. Prevalence and mechanisms of low- and high-level mupirocin resistance in staphylococci isolated from a Korean hospital. J. Antimicrob. Chemother. 51:619-623. [DOI] [PubMed] [Google Scholar]