Abstract
Micafungin (FK463) is a new parenteral echinocandin. A multicenter, phase I, open-label, sequential-group dose escalation study was conducted to assess the safety, tolerability, and pharmacokinetics of micafungin in neutropenic pediatric patients. A total of 77 patients stratified by age (2 to 12 and 13 to 17 years) received micafungin. Therapy was initiated at 0.5 mg/kg per day and escalated to higher dose levels of 1.0, 1.5, 2.0, 3.0, and 4.0 mg/kg per day. Micafungin was administered within 24 h of initiating broad-spectrum antibacterial antibiotics for the new onset of fever and neutropenia. The most common overall adverse events in the study population were diarrhea (19.5%), epistaxis (18.2%), abdominal pain (16.9%), and headache (16.9%). Nine patients (12%) experienced adverse events considered by the investigator to be possibly related to the study drug. The most common related events were diarrhea, vomiting, and headache, all occurring in two patients each. There was no evidence of a dose-limiting toxicity as defined within the prespecified criteria of this clinical protocol. There was one death during the study due to septic shock. The pharmacokinetic profiles for micafungin over the 0.5- to 4.0-mg/kg dose range demonstrated dose linearity. Clearance, volume of distribution, and half-life remained relatively constant over the dose range and did not change with repeated administration. The overall plasma pharmacokinetic profile was similar to that observed in adults. However, there was an inverse relation between age and clearance. For patients 2 to 8 years old, clearance was approximately 1.35 times that of patients ≥9 years of age. In summary, micafungin over a dosage range between 0.5 and 4.0 mg/kg/day in 77 febrile neutropenic pediatric patients displayed linear pharmacokinetics and increased clearance as a function of decreasing age.
Advances in cytotoxic chemotherapy, transplantation procedures, and supportive care have increased survival in children with leukemia and other neoplasms (4, 11, 28). However, invasive fungal infections remain an important cause of infection-related mortality and morbidity in pediatric patients (1, 6, 9, 14). Recent advances in the early diagnosis, prevention, and treatment of fungal infections offer hope to these patients (2, 10, 13). Among the newer classes of antifungal compounds are the echinocandins, which have offered broad-spectrum activity and a favorable safety profile in adult patients (3).
Currently, the two most common invasive fungal infections in neutropenic adult and pediatric patients are caused by Candida spp. and Aspergillus spp. Micafungin (FK463; Fujisawa Healthcare, Inc., Deerfield, IL) is an intravenous antifungal compound of the echinocandin class. Micafungin acts by inhibiting the production of 1,3-β-d-glucan, a key component in fungal cell wall synthesis (12). A semisynthetic lipopeptide, micafungin possesses in vitro and in vivo activity against a broad spectrum of Candida and Aspergillus species, including activity against azole-resistant Candida (8, 15, 17-19, 21). Although the safety and plasma pharmacokinetics of micafungin have been studied in animal models (7, 19) and in adult patients (20), little is known about the properties of this echinocandin in pediatric patients.
We therefore conducted a multicenter study to determine the safety, tolerability, and plasma pharmacokinetics of micafungin in neutropenic pediatric patients at doses of 0.5 mg/kg per day and higher. This study provides a rationale for dosing in pediatric patients and further establishes the foundation for randomized trials designed to investigate the efficacy of micafungin in prevention and treatment of invasive fungal infections in high-risk pediatric oncology patients.
MATERIALS AND METHODS
Study design.
This phase I, open-label, sequential-group dose escalation study was conducted with pediatric patients aged 2 to 17 years at seven institutions in the United States. All relevant federal guidelines and institutional policies for conduct of clinical trials were followed. The protocol was approved by each participating institution's review board. Approximately eight patients in each age group (2 to 12 and 13 to 17 years) were to be studied per dosage level with a planned enrollment of 96 patients in all, and the dosage levels were as follows (16 patients at each of six dosage levels): 0.5, 1.0, 1.5, 2.0, 3.0, and 4.0 mg/kg per day. Micafungin was initiated as early empirical antifungal therapy within 24 h of the administration of broad-spectrum antibacterial agents for the new onset of fever in neutropenic pediatric patients considered to be at high risk for invasive fungal infections as defined by enrollment criteria. The strategy of initiation of antifungal therapy in high-risk neutropenic patients at the onset of new fever has been demonstrated to significantly reduce the development of invasive fungal infections (26, 27).
The first eight patients in each age group received micafungin at a dose of 0.5 mg/kg per day. Escalation to higher dose levels of 1.0, 1.5, 2.0, 3.0, and 4.0 mg/kg per day continued until a dose-limiting toxicity was observed. A minimum of seven patients who received at least three dosages at a dosage level and age cohort was required for assessment of toxicity before escalating to the next dosage level. A dose-limiting toxicity was defined as any grade 3 or higher toxicity (National Cancer Institute Common Toxicity Criteria, version 1 [http://www.fda.gov/cder/cancer/toxicityframe.htm]) considered at least probably related to the study drug occurred in at least two patients at that dosage level. Dose escalation within each age group was conducted independently.
Patient selection.
Neutropenic patients (absolute neutrophil count, <500 cells/mm3) 2 to 17 years of age met the inclusion criteria for treatment if one of the following conditions was present: leukemia or lymphoma, except patients on maintenance chemotherapy; bone marrow or peripheral stem cell transplant; chemotherapy anticipated to induce >10 days of neutropenia; aplastic anemia; or myelodysplastic syndrome. Patients must have been febrile and have received broad-spectrum empirical antibacterial therapy for the current episode of fever and neutropenia. Exclusion criteria included elevated serum transaminases (aspartate aminotransferase [AST] or alanine aminotransferase [ALT] level of >2.5 times the upper limit of normal) or elevated serum bilirubin (>2.5 times the upper limit of normal), evidence of a deep or disseminated fungal infection prior to enrollment, receipt of intravenous amphotericin B within 72 h of study entry, the need for treatment with a systemic antifungal agent other than micafungin, a history of anaphylaxis attributable to the echinocandin class of antifungal agents, a concomitant condition that would create a risk for the patient, or administration of an investigational drug other than those for the treatment of cancer or supportive care. Finally, patients were not permitted to be pregnant or nursing.
Procedures.
Parents or guardians provided written informed consent. Patients underwent physical examination, clinical assessments for possible invasive fungal infection, and blood collection for determination of baseline clinical laboratory profiles. Vital signs were recorded within 1 h prior to the first dose, within 60 min after administration of micafungin for the first 3 days, and at the first-week posttreatment assessment. Blood for hematology and serum chemistry profiles was collected within 24 h before the first dose of micafungin, on days 3, 5, and 7, and then twice weekly during dosing, at the end of study drug therapy, and at the 1-week posttreatment assessment. Absolute neutrophil count was measured daily during therapy.
An adverse event (AE) was defined as any reaction, side effect, or other untoward event, regardless of relationship to study drug, that was not present at baseline and that occurred during the conduct of the clinical trial. Treatment-emergent adverse events with onset after the first administration of micafungin through 72 h after the last dose were recorded. Ongoing adverse events were followed for as long as necessary until the event resolved or stabilized. Patient survival was followed for 1 month after the last dose. Deaths occurring within 1 month after discontinuation of study drug were reported as a serious adverse event.
Safety monitoring.
The medical monitor (D.H.B.) initially reviewed the safety data (adverse events and laboratory parameters) in order to ensure that the maximum tolerated dose criteria had not been fulfilled before escalating to the next dosage cohort. These safety findings were then thoroughly reviewed and discussed with the protocol chair and investigators before escalation to the next cohort.
Pharmacokinetic sampling.
Blood samples (2 ml) for plasma separation were collected for determination of pharmacokinetic parameters on days 1 and 4 of study drug dosing at 0 h (predose), 60 min (end of infusion), 90 min, and 2, 4, 6, 8, 10, and 24 h (before the next dose). Blood samples were obtained from a port site separate from that used to administer study drug.
Assay methodology.
Plasma concentrations of micafungin and two metabolites (M1 and M2) were assayed using a validated high-performance liquid chromatography method with a fluorescence detection system. Acidified plasma samples were precipitated with acetonitrile and centrifuged prior to dilution with buffer and injection into a high-performance liquid chromatography system. Separation of micafungin, M1, and M2 was achieved with a reverse-phase TSK-GEL ODS80 column (TosoHaas, Montgomeryville, PA). The analytes were quantified by fluorescence. The lower limit of quantitation for micafungin was 0.05 μg/ml in plasma. Quantitation was based on the ratio of the peak height response of the compound to the peak height of the internal standard, FR195743, an analogue of FK463. Eight-point standard curves (range of concentrations, 0.05 μg/ml to 25 μg/ml for FK463 and 0.05 μg/ml to 25 μg/ml for M1 and M2) were linear, with r values greater than 0.996; the linear least-squares regression was weighted 1/x. Accuracies were within ±12%. Quality control samples analyzed with each analytical run had coefficients of variation less than or equal to 11.54% for FK463, 14.87% for M1, and 6.34% for M2. All individual sample concentration data reported for FK463 and metabolites M1 and M2 were within the statistical performance of the assay.
Treatment.
Micafungin, administered intravenously once daily as a 1-hour infusion, was initiated within 24 h of the start of broad-spectrum antibacterial therapy for the new onset of fever and neutropenia. Patients received 0.5 mg/kg per day (not to exceed 25 mg per day), 1 mg/kg per day (not to exceed 50 mg per day), 1.5 mg/kg per day (not to exceed 75 mg per day), 2.0 mg/kg per day (not to exceed 100 mg per day), 3.0 mg/kg per day (not to exceed 150 mg per day), or 4.0 mg/kg per day (not to exceed 200 mg per day). Micafungin was to be administered until recovery from neutropenia (absolute neutrophil count, ≥250 cells/mm3). The maximum length of time a patient could receive micafungin was 4 weeks. If a patient developed a proven or probable systemic fungal infection or if a patient required another systemic antifungal agent for empirical therapy, micafungin was discontinued. The requirement for empirical antifungal therapy was based on the participating institution's protocol. The duration of follow-up for monitoring for emergence of posttreatment invasive fungal infections was 1 week.
Criteria for evaluation.
The data set for the primary analysis included all patients who received at least one dose of the study drug. Safety parameters included treatment-emergent adverse events, laboratory measurements, and changes in vital signs. Dose-limiting toxicity was reached if two separate patients at the same dosage level and age group developed the same National Cancer Institute grade 3 or greater toxicity and if toxicity was considered at least probably related to the study drug. Because the trial was primarily designed to be a study of safety and pharmacokinetics, no primary efficacy endpoint was established. Efficacy parameters included frequency of emergent fungal infections during treatment and posttreatment periods and a requirement for empirical antifungal therapy.
Statistical methods.
At each dosage level within each age group, and for both age groups combined, the rates of treatment-emergent adverse events, serious adverse events, and those events related to micafungin were summarized. The rate of invasive fungal infections at the end of therapy, the proportion of patients with invasive fungal infections during the posttreatment period, and the proportion of patients requiring additional systemic antifungal therapy during the posttreatment period were calculated.
The pharmacokinetic profiles for micafungin were computed from the drug concentration-time data using noncompartmental methods (5) using a reduced data set where outlier concentrations were removed. The peak drug concentration (Cmax) and time of peak drug concentration were obtained directly from the observed data. The terminal elimination rate constant (kel) was obtained from a log-linear regression of the plasma concentration compared to time data in the terminal postdistribution phase. The elimination half-life (t1/2) was calculated from 0.693/kel. The area under the plasma drug concentration-versus-time curve (AUC0-24) and the area under the moment curve from 0 to 24 h (AUMC0-24) were calculated by the log-linear trapezoidal rule. The area under the plasma drug concentration-versus-time curve from time zero to time infinity (AUC0-∞) was obtained from the following equation: AUC0-24 + Ct/kel, where Ct was the last measurable concentration and kel was the terminal elimination rate constant. The total body clearance (CL) for micafungin on day 1 and day 4 was obtained from the equations dose/AUC0-∞ and dose/AUC0-24 (presumed steady state, where 24 h was the dosing interval), respectively. The volume of distribution (V) for micafungin was calculated as follows: V = CL/kel. The steady-state volume of distribution (Vss) was calculated from the product of CL and the mean residence time. The mean residence time was obtained from the following equation: (AUMC0-∞/AUC0-∞) T/2, where AUMC0-∞ was extrapolated from [(Ct × t)/kel + Ct/kel2] and where T was the infusion time, all other terms having been previously defined.
Prior to the analysis, drug concentration data and case report forms were reviewed for outliers or documentation about errors in the timing of blood samples or drug infusion or contamination of blood samples. Based on this review, mistiming was found and selected drug concentration values were excluded for five patients. The second step was to apply a method described previously by Tukey (22). Tukey defined “outer fences” that are three times the interquartile range above the upper (75th percentile) quartile and three times the interquartile range below the lower (25th percentile) quartile. This procedure was applied to the set of data for each time point for the patients in each dosage group.
RESULTS
Patients.
Between November 1998 and August 2000, 78 patients were enrolled in the study (Table 1). Due to slower-than-expected enrollment in the 13- to 17-year age group, entry of patients in this age group was terminated after full patient accrual in the 4.0-mg/kg/day dosage level of the 2- to 12-year age group. Of the 78 patients participating in the study, 58 were in the 2- to 12-year group and 20 were in the 13- to 17-year group. Patients in the 2- to 12-year age group received the full range of dosages from 0.5 to 4.0 mg/kg per day, while patients in the 13- to 17-year age cohort received 0.5 to 1.5 mg/kg per day. The primary analysis set consisted of the 77 patients who received at least one dose of the study drug. These patients (44 males and 33 females) had a mean (±standard deviation) age of 9.0 ± 4.49 years. The mean age of the patients in the 2- to 12-year age cohort was 6.9 ± 3.05 years; the mean age for patients in the 13- to 17-year age group was 15.0 ± 1.34.
TABLE 1.
Patient characteristics
| Characteristic | Dose (mg/kg) cohort
|
Total | |||||
|---|---|---|---|---|---|---|---|
| 0.5 | 1.0 | 1.5 | 2.0 | 3.0 | 4.0 | ||
| Groupa | |||||||
| 2 to 12 years | 8 | 10 | 9 | 12 | 10 | 8 | 57 |
| 13 to 17 years | 8 | 8 | 4 | * | * | * | 20 |
| All enrolled patients | 16 | 18 | 13 | 12 | 10 | 8 | 77 |
| Gender, age, and wt | |||||||
| No. male (%) | 10 (63) | 12 (67) | 7 (54) | 6 (50) | 5 (50) | 4 (50) | 44 (57) |
| No. white (%) | 12 (75) | 11 (61) | 9 (69) | 10 (83) | 10 (100) | 6 (75) | 58 (75) |
| Mean age (yr) ±SD | 9.9 ± 5.54 | 10.5 ± 4.97 | 9.2 ± 4.80 | 7.9 ± 2.71 | 7.8 ± 3.36 | 6.4 ± 2.77 | 9.0 ± 4.49 |
| Mean wt (kg) ±SD | 38.6 ± 19.59 | 45.9 ± 32.91 | 36.7 ± 18.33 | 29.5 ± 11.98 | 30.9 ± 13.18 | 28.0 ± 11.61 | 36.5 ± 21.59 |
| No. of patients (%) with underlying neoplastic diseasesb | |||||||
| ALL | 4 (25) | 6 (33) | 5 (39) | 4 (33) | 4 (40) | 2 (25) | 25 (33) |
| Solid tumor | 5 (31) | 6 (33) | 2 (15) | 5 (42) | 2 (20) | 2 (25) | 22 (29) |
| AML | 3 (19) | 4 (22) | 0 (0) | 1 (8) | 3 (30) | 0 (0) | 11 (14) |
| NHL | 2 (13) | 0 (0) | 0 (0) | 2 (17) | 1 (10) | 3 (38) | 8 (10) |
| Other | 2 (13) | 2 (11) | 6 (46) | 0 (0) | 0 (0) | 1 (13) | 11 (14) |
Due to slower-than-expected enrollment in the 13- to 17-year age group, entry of patients in this age group was terminated after full patient accrual in the 4.0-mg/kg per day dosage level of the 2- to 12-year age group (indicated by asterisks).
ALL, acute lymphocytic leukemia; AML, acute myelogenous leukemia; NHL, non-Hodgkin's lymphoma.
A total of 27 (35%) patients were recipients of an allogeneic stem cell transplant (n = 19) or an autologous stem cell transplant (n = 8). Approximately one-half of the patients (39/77 [51%]) had initial active disease at the time of entry into the study, 33% (n = 25) were in remission, and 17% (n = 13) were in relapse.
Study drug administration.
The mean duration of study drug administration was 6.6 ± 4.81 days (range, 1 to 27 days). There was no difference in the mean duration of study drug exposure in each individual age group compared to the overall exposure.
Safety.
Overall, mean serum AST, ALT, total bilirubin, and alkaline phosphatase levels remained unchanged from baseline (prior to the first dose of micafungin) to the end of study drug therapy. Two patients, both of whom were 13 to 17 years of age and receiving micafungin at 0.5 mg/kg per day, experienced modest increases in liver-related laboratory parameters that were assessed by the investigator to be possibly related to study drug. The first patient experienced a transient increase in ALT from 61 U/liter at baseline to 104 U/liter on day 3 that resolved on day 3. The second patient experienced an increase in total bilirubin from 2.7 mg/dl on day 8 to 3.2 mg/dl on day 17. There was no evidence of nephrotoxicity noted in any study patients. Mean serum creatinine levels did not change from baseline (0.39 ± 0.11 mg/dl) to the end of therapy (0.38 ± 0.13 mg/dl), nor were there any clinically significant changes in mean values from baseline to the end of therapy observed for any other laboratory parameters.
Tolerability.
A total of 68 (88%) patients experienced at least one adverse event during study drug therapy. The most frequently occurring adverse events regardless of causality were diarrhea (19%), epistaxis (18%), abdominal pain (17%), headache (17%), vomiting (17%), chills (16%), mucositis (16%), and rash (14%). There was no apparent association with age or study drug dosage and the frequency or type of adverse event. Nine patients (12%) experienced adverse events considered by the investigator to be possibly related to micafungin. The most frequent related events were diarrhea, vomiting, and headache, occurring in two patients each; all other related events occurred in one patient each. Based on the National Cancer Institute Common Toxicity Criteria, all but three of the attributable events were grade 1 or 2 in severity. The three grade 3-related events were vomiting, headache, and hyperbilirubinemia. The patient who experienced hyperbilirubinemia was a 15-year-old female who received an allogeneic stem cell transplant for relapsed acute myelogenous leukemia. Her total bilirubin was 2.0 mg/dl on day 1 (first day of dosing), 1.2 mg/dl on day 4, 2.1 mg/dl on day 6, and 2.7 mg/dl on day 8 (1 day after the last dose of 0.5 mg/kg micafungin). Eleven patients (14%) experienced mild to moderate rash, and seven patients (9%) experienced mild to moderate pruritus; none of these conditions was considered to be related to micafungin.
Serious adverse events.
There was one death during the study. A 9-year-old female patient receiving treatment for Ewing's sarcoma and primitive neuroectodermal tumors died on day 8 after receiving 4 days of micafungin at 2.0 mg/kg. The patient died due to septic shock that was considered unrelated to study drug or a fungal infection.
Maximum tolerated dose assessment.
There was no dose-limiting toxicity observed. The maximum tolerated dose was not obtained as defined by the prespecified parameters of this study's protocol.
Pharmacokinetics.
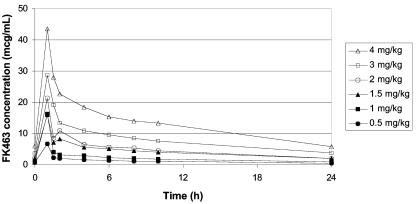
A total of 73 patients, who had data for plasma concentrations of micafungin, were included in the analysis. The mean plasma micafungin concentration-versus-time profiles obtained from all patients on days 1 and 4 at doses of 0.5, 1.0, 1.5, 2.0, 3.0, and 4.0 mg/kg/day declined in a biexponential manner postinfusion (Fig. 1).
FIG. 1.
Mean plasma concentration-time profiles of micafungin in neutropenic pediatric patients between 2 and 17 years of age (day 4).
Table 2 demonstrates that there did not appear to be any significant difference in plasma half-life or other pharmacokinetic parameters over time or across dosage cohort. For example, the range across the dosage cohorts of the mean terminal t1/2 values measured from the data from day 1 was 11.6 to 13.2 h, and the range from the data from day 4 was 12.2 to 17.3 h. Mean values of CL and Vss also remained constant across dosage groups, indicating no change in the systemic disposition of micafungin across the dose range of 0.5 to 4.0 mg/kg. None of these values changed appreciably with repeated administration.
TABLE 2.
Pharmacokinetic parameters for plasma micafungin concentrationsa
| Daily dose (mg/kg) | No. of patients | Study day | Cmax (μg/ml) | AUC0-24 (h · μg/ml) | AUC0-∞ (h · μg/ml) | t1/2 (h) | CL (ml/h/kg) | Vss (liter/kg) |
|---|---|---|---|---|---|---|---|---|
| 0.5 | 15 | 1 | 3.7 ± 1.0 | 19.0 ± 1.9 | 25.9 ± 2.8 | 12.6 ± 0.7 | 22.7 ± 2.6 | 0.39 ± 0.051 |
| 16 | 4 | 6.4 ± 1.4 | 27.9 ± 2.3 | 38.3 ± 3.6 | 12.3 ± 1.0 | 19.4 ± 2.0 | 0.32 ± 0.031 | |
| 1.0 | 16 | 1 | 10.8 ± 1.9 | 40.3 ± 3.5 | 52.4 ± 4.7 | 12.5 ± 1.1 | 21.8 ± 2.4 | 0.33 ± 0.039 |
| 11 | 4 | 16.2 ± 3.8 | 52.4 ± 4.6 | 78.2 ± 10.0 | 17.3 ± 3.1 | 20.6 ± 2.0 | 0.39 ± 0.068 | |
| 1.5 | 13 | 1 | 13.2 ± 3.1 | 79.4 ± 12.5 | 106.8 ± 16.7 | 12.8 ± 1.1 | 17.4 ± 3.2 | 0.27 ± 0.037 |
| 10 | 4 | 16.3 ± 2.5 | 100.6 ± 10.1 | 143.8 ± 20.7 | 12.9 ± 1.7 | 16.5 ± 2.5 | 0.28 ± 0.045 | |
| 2.0 | 11 | 1 | 15.3 ± 3.8 | 83.0 ± 7.4 | 113.8 ± 16.0 | 13.2 ± 2.0 | 20.8 ± 2.9 | 0.31 ± 0.023 |
| 8 | 4 | 21.4 ± 9.7 | 94.3 ± 16.3 | 132.3 ± 27.1 | 12.2 ± 0.7 | 24.3 ± 4.7 | 0.42 ± 0.077 | |
| 3.0 | 9 | 1 | 35.8 ± 7.1 | 162.9 ± 19.8 | 206.6 ± 23.0 | 11.7 ± 0.6 | 15.9 ± 1.6 | 0.24 ± 0.030 |
| 5 | 4 | 30.4 ± 8.9 | 190.5 ± 24.6 | 264.8 ± 40.0 | 13.2 ± 1.2 | 17.0 ± 2.5 | 0.29 ± 0.034 | |
| 4.0 | 7 | 1 | 30.3 ± 6.9 | 191.4 ± 21.2 | 247.1 ± 23.8 | 11.6 ± 1.0 | 17.4 ± 2.5 | 0.28 ± 0.053 |
| 7 | 4 | 43.5 ± 9.2 | 301.9 ± 42.7 | 415.9 ± 56.0 | 13.5 ± 1.5 | 14.2 ± 2.3 | 0.26 ± 0.043 |
Values are expressed as mean ± standard error of the mean.
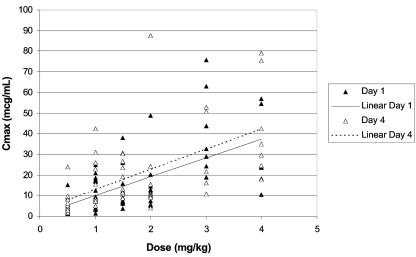
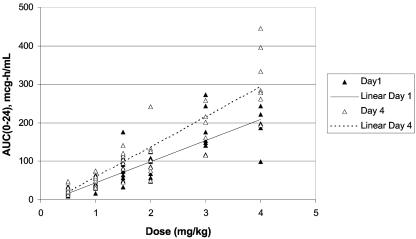
On day 1, the Cmax and AUC0-24 values, although variable, increased in an approximately dose-proportional linear fashion. On day 4 (presumed steady state), both mean Cmax and mean AUC0-24 values correlated more closely with dose in a linear fashion and were higher relative to those on day 1 (Fig. 2 and 3). The variability found between the day 1 and day 4 mean CL and Vss values was minimal. Furthermore, the mean CL and Vss values remained constant across the dosage range.
FIG. 2.
Individual maximum plasma concentration (Cmax) values of micafungin in neutropenic pediatric patients between 2 and 17 years of age as a function of micafungin dose on days 1 and 4.
FIG. 3.
Individual plasma AUC0-24 values of micafungin in neutropenic pediatric patients between 2 and 17 years of age as a function of micafungin dose on days 1 and 4.
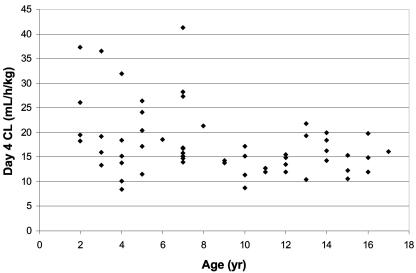
Pharmacokinetic profiles obtained on days 1 and 4 for the two predefined age cohorts (2 to 12 and 13 to 17 years) were relatively consistent with mean data derived from both groups combined. However, mean estimates of CL and Vss were numerically greater and Cmax and AUC were numerically smaller in the 2- to 12-year-old group, although the values spanned similar ranges as those in the 13- to 17-year-old group. Individual CL values evaluated as a function of age revealed that patients <8 years old had CL rates for micafungin that were on the average 1.5 to 2.0 times greater than rates in pediatric patients 8 years of age and older (Fig. 4).
FIG. 4.
Individual plasma clearance values of micafungin in neutropenic pediatric patients as a function of age on day 4 over the micafungin dose range (0.5 to 4.0 mg/kg/day).
To better understand the effect of age on the pharmacokinetic disposition of micafungin, pairwise comparison of the pharmacokinetic parameters was performed for the age groups 2 to 8 years old and 9 to 17 years old. CL (0.385 ± 0.15 versus 0.285 ± 0.12 ml/min/kg for 2 to 8 years and 9 to 17 years, respectively) and Vss (0.35 ± 0.18 versus 0.28 ± 0.09 liters/kg) were significantly greater and t1/2 (11.6 ± 2.8 versus 13.3 ± 4.3 h) was significantly shorter in patients 2 to 8 years of age compared to those 9 to 17 years of age.
Efficacy.
One patient enrolled into this study developed a documented invasive fungal infection. This patient was a 15-year-old female who received 0.5 mg/kg per day who died after completing the study. This patient received her last dose of study drug on day 14 and completed the study on day 22 with no evidence of a fungal infection; no additional antifungals were administered. On day 33, the patient died; autopsy revealed pulmonary aspergillosis. Micafungin was discontinued in one patient in the 13- to 17-year age group 0.5-mg/kg/day cohort on day 7 due to persistent fever and neutropenia and was initiated on amphotericin B on day 9. A computed tomography scan of the chest on day 14 revealed nodular densities in the left lower lobe of the lung, consistent with either fungal pneumonia or leukemic infiltrate. No further procedures were performed to characterize these infiltrates. The patient was alive at the end of the study.
By the end of therapy, 21 patients (27%) had a suspected deeply invasive fungal infection, defined as the initiation of empirical amphotericin B for persistent fever and neutropenia despite broad-spectrum antibiotics. In both age groups of patients, fewer than 30% of patients received empirical antifungal therapy (30% [17/57] and 20% [4/20], respectively). There was no apparent relationship with dose.
DISCUSSION
This trial was designed to evaluate the safety, tolerability, and plasma pharmacokinetics of micafungin in pediatric patients in two age cohorts with increasing doses. A maximum tolerated dose was not identified within the protocol-specified criteria in this study. There were also no dose-related adverse events in either age group, a finding consistent with two previous studies of micafungin in adult bone marrow transplant patients (J. Hiemenz, P. Cagnoni, D. Simpson, S. Devine, and N. Chao, Abstr. 39th Intersci. Conf. Antimicrob. Agents Chemother., abstr. 1648, 1999; R. Powles, B. Sirohi, R. Chopra, N. Russel, and H. G. Prentice, Abstr. 41st Intersci. Conf. Antimicrob. Agents Chemother., abstr. 676, 2001). Most adverse events were mild to moderate in intensity and were expected given the patient population. There was no attributable nephrotoxicity or infusion-related toxicity. No allergic or histamine-like reactions to micafungin were noted. Evidence of hepatotoxicity was minimal.
The absence of dose-limiting toxicity at 4 mg/kg/day in this pediatric trial is compatible with laboratory animal toxicity studies. Results in animals administered micafungin indicate that only at comparatively high dosages was toxicity observed (Investigator's Brochure, Micafungin [FK463; Fujisawa Healthcare, Inc., Deerfield IL]). For example, only at dosages ≥32 mg/kg were rats observed to have adverse reactions, which consisted of increased plasma histamine concentrations and increased heart rate. At a dosage of 100 mg/kg, adverse reactions included loss of urinary electrolytes, tremor, hypotonia, and loss of reflexes. The lethal dosage of micafungin in rats was 125 mg/kg in males and 250 mg/kg in females. A dosage of 200 mg in dogs produced palpebral swelling and flushing, flushing of the ear auricle and skin, decreased hemoglobin and hematocrit, and increased plasma AST, ALT, and total bilirubin levels. Multiple-dose studies demonstrated adverse laboratory results at 32 mg/kg consisting of elevated AST, ALT, total bilirubin, and blood urea nitrogen levels.
The safety data in this pediatric clinical trial are compatible with those of the randomized double-blind trial of micafungin versus fluconazole for prophylaxis during neutropenia in hematopoietic stem cell transport recipients (23). Among the 425 patients receiving micafungin and 457 patients receiving fluconazole (23), a total of 64 (15.1%) micafungin-treated patients and 77 (16.8%) fluconazole-treated patients experienced an adverse event that was considered by the investigator to have some relation to study drug. Study drug administration was interrupted for adverse events in 2.8% of micafungin-treated patients and 2.4% of fluconazole-treated patients. Fewer micafungin-treated patients discontinued study drug due to an adverse event (4.2% for micafungin and 7.2% for fluconazole [P = 0.058]). Moreover, there were no significant differences in any single AE, including markers of hepatic and renal injury, between micafungin and fluconazole. Furthermore, there were no differences in infusion-related AEs. There also were no significant differences in the frequency of related AEs in pediatric patients compared with those in adults in this double-blinded trial. However, as the current study enrolled 77 pediatric patients for a mean of 6 days, one should be cautious in drawing definitive conclusions on safety and tolerability in children that may become apparent only when larger numbers are studied for a longer period of time.
The overall pharmacokinetic profile of micafungin in all febrile neutropenic pediatric patients enrolled in this study was consistent with that obtained in healthy adults and adult bone marrow transplant patients.
However, patients between 2 and 8 years of age exhibited clearance values approximately 1.3 to 1.5 times greater than those for adult patients, while patients between 9 and 17 years of age had clearance rates comparable to those of adult bone marrow transplant patients. Mean CL values from the older pediatric patients were consistent with values obtained from adult bone marrow transplant patients (13.6 ml/h/kg) who received micafungin at a dosage of 12.5 to 200 mg per day (Hiemenz et al., 39th ICAAC). As this study demonstrated linear plasma pharmacokinetics over the dosage range examined, the relationship of clearance = dose/AUC pertains. Given that the clearance in the younger cohort is approximately 1.35 to 1.5 times that of adults, a dosage 1.5 times that of adults would be appropriate in order to achieve similar drug exposure. Thus, if one anticipated administering an adult-equivalent dosage to children 2 to 8 years old of 2 mg/kg or 3 mg/kg, one would use 3 mg/kg or 4.5 mg/kg, respectively, in order to achieve comparable exposure.
Such adjustments of dosage for pediatric patients with greater clearance and reduced exposure in comparison to those of adults have been recommended for fluconazole (16), voriconazole (25), and caspofungin (24). A dosage of 12 mg of fluconazole/kg is recommended in pediatric patients for comparable exposure to that obtained with the adult dosage of 400 mg/day. A dosage of 50 mg/m2 of caspofungin is recommended in pediatrics for comparability of exposure to the 50-mg dosage in adults. In addition, the dosage of voriconazole in pediatric patients displays linear plasma pharmacokinetics, while that of adults demonstrates saturation kinetics. These differences are associated with significantly greater clearance rates and reduced AUCs in pediatric patients receiving voriconazole in comparison to those of adults. Current studies are under way for voriconazole in order to further define optimal dosing to achieve drug exposure comparable to that of adults.
Assessment of the frequency of invasive fungal infections emerging on study drug and frequency of the use of empirical antifungal therapy were prespecified secondary endpoints. Only 1 patient among the 77 neutropenic patients receiving micafungin as early empirical therapy in this trial was found to have a documented invasive fungal infection. This patient notably received the lowest dosage of 0.5 mg/kg/day, which is consistent with the dose dependency of the experimental antifungal efficacy of echinocandins. However, the true efficacy of micafungin as early empirical therapy is not possible to reliably assess in this study due to the small numbers of patients, the lack of available comparative data, and the relatively short period of follow-up. Nonetheless, these data are consistent with the findings of micafungin as a prophylactic agent in adult and pediatric hematopoietic stem cell transplant recipients (23). In that large, randomized, blinded trial, micafungin was more effective than fluconazole in preventing breakthrough systemic fungal infections and reducing the need for empirical antifungal therapy. However, dosage optimization studies are still warranted in neutropenic adult and pediatric patients for treatment and prevention of invasive fungal infections.
In summary, micafungin demonstrated dose-proportional linear pharmacokinetics in these pediatric patients consistent with that observed in adults. Clearance was higher in younger patients, suggesting a need for increased dosage in the very young. Additional clinical trials are warranted for pediatric patients to assess the efficacy and optimum dosage of micafungin for treatment and prevention of invasive fungal infections.
Acknowledgments
We thank the following research coordinators for their valuable contributions to the implementation of this clinical trial: Maureen Roden (National Cancer Institute, Bethesda, MD), Micki Roy and Kim Allison (St. Jude Children's Research Hospital, Memphis, TN), Kathy Rubel and Tammy Scott (Johns Hopkins Hospital, Baltimore, MD), Anjum Zaki (Georgetown University Hospital, Washington, DC), Cindy Love (Children's National Medical Center, Washington, DC), and Ofelia Vargas-Shiraishi (Children's Hospital of Orange County, Orange, CA).
This study was supported in part by grants to the individual sites from Fujisawa Healthcare, Inc., Deerfield, Illinois.
REFERENCES
- 1.Abassi, S., J. L. Shenep, W. T. Hughes, and P. M. Flynn. 1999. Aspergillosis in children with cancer: a 34-year experience. Clin. Infect. Dis. 29:1210-1219. [DOI] [PubMed] [Google Scholar]
- 2.Chanock, S. J., and T. J. Walsh. 1996. Evolving concepts of prevention and treatment of invasive fungal infections in pediatric bone marrow transplant recipients. Bone Marrow Transpl. 18(Suppl. 3):S15-S20. [PubMed] [Google Scholar]
- 3.Denning, D. W. 2003. Echinocandin antifungal drugs. Lancet 362:1142-1151. [DOI] [PubMed] [Google Scholar]
- 4.Gatta, G., R. Capocaccia, M. P. Coleman, L. A. G. Ries, and F. Berrino. 2002. Childhood cancer survival in Europe and the United States. Cancer 95:1767-1772. [DOI] [PubMed] [Google Scholar]
- 5.Gibaldi, M., and D. Perrier. 1982. Pharmacokinetics, 2nd ed., p. 409-416. Marcel Dekker, Inc., New York, N.Y.
- 6.Groll, A. H., M. Kurz, W. Schneider, V. Witt, H. Schmidt, M. Schneider, and D. Schwabe. 1999. Five-year survey of invasive aspergillosis in a pediatric cancer centre. Epidemiology, management and long-term survival. Mycoses 42:431-442. [DOI] [PubMed] [Google Scholar]
- 7.Groll, A. H., D. Mickiene, V. Petraitis, R. Petraitiene, K. H. Ibrahim, S. C. Piscitelli, I. Bekersky, and T. J. Walsh. 2001. Compartmental pharmacokinetics and tissue distribution of the antifungal echinocandin-like lipopeptide FK463 in rabbits. Antimicrob. Agents Chemother. 45:3322-3327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Groll, A. H., and T. J. Walsh. 2000. Evaluation of the antifungal echinocandin FK463. Curr. Opin. Investig. Drugs 2:405-412. [Google Scholar]
- 9.Groll, A. H., and T. J. Walsh. 2003. Fungal infections in pediatric patients, p. 417-422. In M. McGinnis, M. Pfaller, and E. Anaissie (ed.), Clinical mycology. Churchill Livingstone, Philadelphia, Pa.
- 10.Groll, A., and T. J. Walsh. 2003. Antifungal agents, p. 3075-3108. In R. D. Feigin, J. D. Cherry, G. J. Demmler, and S. L. Kaplan (ed.), Textbook of pediatric infectious diseases.
- 11.Harris, R. E., H. N. Sather, and S. A. Feig. 1998. High-dose cytosine arabinoside and L-asparaginase in refractory acute lymphoblastic leukemia: the Children's Cancer Group experience. Med. Pediatr. Oncol. 30:233. [DOI] [PubMed] [Google Scholar]
- 12.Hatano, K., Y. Morishita, T. Nakai, and F. Ikeda. 2002. Antifungal mechanism of FK463 against Candida albicans and Aspergillus fumigatus. J. Antibiot. 55:219-222. [DOI] [PubMed] [Google Scholar]
- 13.Hayes-Lattin, B., and R. T. Maziarz. 2004. Update in the epidemiology, prophylaxis, and treatment of fungal infections in patients with hematologic disorders. Leuk. Lymphoma 45:669-680. [DOI] [PubMed] [Google Scholar]
- 14.Hovi, L., U. Saarinen-Pihkala, K. Vettenranta, and H. Saxen. 2000. Invasive fungal infections in pediatric bone marrow transplant recipients: single center experience of 10 years. Bone Marrow Transplant. 26:999-1004. [DOI] [PubMed] [Google Scholar]
- 15.Ikeda, F., Y. Wakai, S. Matsumoto, K. Maki, E. Watabe, S. Tawara, T. Goto, Y. Watanabe, F. Matsumoto, and S. Kuwahara. 2000. Efficacy of FK463, a new lipopeptide antifungal agent, in mouse models of disseminated candidiasis and aspergillosis. Antimicrob. Agents Chemother. 44:614-618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee, J. W., N. I. Seibel, M. A. Amantea, P. Whitcomb, P. A. Pizzo, and T. J. Walsh. 1992. Safety, tolerance, and pharmacokinetics of fluconazole in children with neoplastic diseases. J. Pediatr. 120:987-993. [DOI] [PubMed] [Google Scholar]
- 17.Matsumoto, S., Y. Wakai, T. Nakai, K. Hatano, T. Ushitani, F. Ikeda, S. Tawara, T. Goto, F. Matusumoto, and S. Kuwahara. 2000. Efficacy of FK463, a new lipopeptide antifungal agent, in mouse models of pulmonary aspergillosis. Antimicrob. Agents Chemother. 44:619-621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mikamo, H., Y. Sato, and T. Tamaya. 2000. In vitro antifungal activity of FK463, a new water soluble echinocandin-like lipopeptide. J. Antimicrob. Chemother. 46:485-487. [DOI] [PubMed] [Google Scholar]
- 19.Petraitis, V., R. Petraitiene, A. H. Groll, K. Roussillon, M. Hemmings, C. A. Lyman, T. Sein, J. Bacher, I. Bekersky, and T. J. Walsh. 2002. Comparative antifungal activity and plasma pharmacokinetics of micafungin (FK463) against disseminated candidiasis and invasive pulmonary aspergillosis in persistently neutropenic rabbits. Antimicrob. Agents Chemother. 46:1857-1869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pettengell, K., J. Mynhardt, T. Kluyts, W. Lau, D. Facklam, and D. Buell. 2004. Successful treatment of oesophageal candidiasis by micafungin: a novel systemic antifungal agent. Aliment. Pharmacol. Ther. 20:475-481. [DOI] [PubMed] [Google Scholar]
- 21.Tawara, S., F. Ikeda, K. Maki, Y Morishita, K. Otomo, N. Teratani, T. Goto, M. Tomishima, H. Ohki, A. Yamada, K. Kawabata, H. Takasugi, K. Sakane, H. Tanaka, F. Matsumoto, and S. Kuwahara. 2000. In vitro activities of a new lipopeptide antifungal agent, FK463, against a variety of clinically important fungi. Antimicrob. Agents Chemother. 44:57-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tukey, J. W. 1977. Exploratory data analysis, p. 43-45. Addison-Wesley, Reading, Mass.
- 23.van Burik, J.-A., V. Ratanatharathorn, D. E. Stepan, C. B. Miller, J. H. Lipton, D. H. Vesole, N. Bunin, D. A. Wall, J. W. Hiemenz, Y. Satoi, J. M. Lee, and T. J. Walsh for the National Institute of Allergy and Infectious Diseases Mycoses Study Group. 2004. Micafungin versus fluconazole for prophylaxis against invasive fungal infections during neutropenia in patients undergoing hematopoietic stem cell transplantation. Clin. Infect. Dis. 39:1407-1416. [DOI] [PubMed] [Google Scholar]
- 24.Walsh, T. J., P. C. Adamson, N. L. Seibel, P. M. Flynn, M. N. Neely, C. Schwartz, A. Shad, S. Kaplan, M. M. Roden, J. A. Stone, A. Miller, S. K. Bradshaw, S. X. Li, C. A. Sable, and N. A. Kartsonis. An open-label, sequential dose-escalation study to investigate the pharmacokinetics, safety, and tolerability of caspofungin in children and adolescents. Antimicrob. Agents Chemother., in press. [DOI] [PMC free article] [PubMed]
- 25.Walsh, T. J., M. O. Karlsson, T. Driscoll, A. G. Arguedas, P. Adamson, X. Saez-Llorens, A. J. Vora, A. C. Ariettas, J. Blumer, I. Lutsar, P. Milligan, and N. Wood. 2004. Pharmacokinetics and safety of intravenous voriconazole in children after single- or multiple-dose administration. Antimicrob. Agents Chemother. 48:2166-2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wiley, J. M., N. Smith, B. G. Leventhal, M. L. Graham, L. C. Strauss, C. A. Hurwitz, J. Modlin, D. Mellits, R. Baumgardner, and B. J. Corden. 1990. Invasive fungal disease in pediatric acute leukemia patients with fever and neutropenia during induction chemotherapy: a multivariate analysis of risk factors. J. Clin. Oncol. 8:280-286. [DOI] [PubMed] [Google Scholar]
- 27.Wingard, J. R., W. P. Vaughan, H. G. Braine, W. G. Merz, and R. Saral. 1987. Prevention of fungal sepsis in patients with prolonged neutropenia: a randomized, double-blind, placebo-controlled trial of intravenous miconazole. Am. J. Med. 83:1103-1110. [DOI] [PubMed] [Google Scholar]
- 28.Xie, Y., S. M. Davies, Y. Xiang, L. L. Robison, and J. A. Ross. 2003. Trends in leukemia incidence and survival in the United States (1973-1998). Cancer 97:2229-2235. [DOI] [PubMed] [Google Scholar]