Abstract
Haemophilus influenzae isolates vary widely in their susceptibilities to the peptide deformylase inhibitor LBM415 (MIC range, 0.06 to 32 μg/ml); however, on average, they are less susceptible than gram-positive organisms, such as Staphylococcus aureus and Streptococcus pneumoniae. Insertional inactivation of the H. influenzae acrB or tolC gene in strain NB65044 (Rd strain KW20) increased susceptibility to LBM415, confirming a role for the AcrAB-TolC pump in determining resistance. Consistent with this, sequencing of a PCR fragment generated with primers flanking the acrRA region from an LBM415-hypersusceptible H. influenzae clinical isolate revealed a genetic deletion of acrA. Inactivation of acrB or tolC in several clinical isolates with atypically reduced susceptibility to LBM415 (MIC of 16 μg/ml or greater) significantly increased susceptibility, confirming that the pump is also a determinant of decreased susceptibility in these clinical isolates. Examination of acrR, encoding the putative repressor of pump gene expression, from several of these strains revealed mutations introducing frameshifts, stop codons, and amino acid changes relative to the published sequence, suggesting that loss of pump repression leads to decreased susceptibility. Supporting this, NB65044 acrR mutants selected by exposure to LBM415 at 8 μg/ml had susceptibilities to LBM415 and other pump substrates comparable to the least sensitive clinical isolates and showed increased expression of pump genes.
The continuing emergence and spread of cellular target-based antibiotic resistance mechanisms is a serious and increasing threat to the effective treatment of microbial infections. There is an obvious need for the development of new compounds, directed at novel cellular functions, which will be active against current resistant strains. In general, target-based antibiotic development strategies are very effective for identifying potent and specific inhibitors of target proteins; however, historically there have been significant hurdles to the effective use of these inhibitors as broad-spectrum antibiotics. This has much to do with intrinsic resistance imparted by bacterial membrane impermeability and efflux. These issues are especially problematic in the case of gram-negative bacteria, where the outer membrane and efflux pumps have been shown to act synergistically to minimize intracellular accumulation of a variety of structurally unrelated compounds (reviewed in references 17, 18, and 19).
There are five general groups of bacterial efflux pumps currently described: the major facilitator superfamily, small multidrug resistance family, the multidrug and toxic compound extrusion family, the ATP-binding cassette family, and the resistance-nodulation-division (RND) family (25). The RND family appears to have the broadest substrate range, and these pumps are therefore most generally relevant vis-a-vis drug resistance in gram-negative bacteria. Architecturally, they consist of an inner membrane proton-drug antiporter, an outer membrane channel, and a so-called membrane fusion protein that is thought to function in facilitating the interaction between the inner and outer membrane components in the periplasm. Substrate extrusion is driven by the proton motive force, and recent data indicate that many substrates may be pumped from the periplasm or the cytoplasmic membrane (9, 14, 33).
Along with intrinsic resistance conferred by efflux, regulatory mutations turning on or increasing efflux pump expression (presumably selected for by exposure to antimicrobial agents or biocides) can confer increased resistance to several or all of the substrates for a given pump (5). Efflux pump overexpressors have been isolated clinically (3, 15, 34); therefore, while cross-resistance to novel agents may not preexist in the form of target-based mutations selected by commonly used antibiotics, these exposures may select pump mutants with decreased susceptibility to novel antibiotics.
Pseudomonas aeruginosa, an important emerging opportunistic pathogen, represents one end of the spectrum of efflux-based resistance, having multiple RND family pumps of overlapping substrate range and a notably impermeable outer membrane which has been shown to significantly increase the efficiency of the pumps by limiting influx (24). Perhaps representing the other end is Haemophilus influenzae, an important respiratory pathogen (7, 11, 22, 29) that has only one known RND family (AcrAB-TolC homolog) pump (10, 27) and is characterized by a relatively permeable outer membrane. The permeability of the outer membrane has been implicated in limiting the efficiency of the efflux pump even for relatively large substrates such as erythromycin (27). Therefore, H. influenzae may represent an example of a gram-negative pathogen where efflux-based intrinsic and acquired resistance may be expected to pose less of a problem. Despite this and consistent with erythromycin being a substrate of the AcrAB-TolC pump of H. influenzae (27), moderate levels of intrinsic resistance to macrolides in H. influenzae clinical isolates has been associated with efflux (21). Recently, high-level resistance to macrolides related to mutations in the L22 ribosomal protein has also been shown to require a contribution from efflux (20).
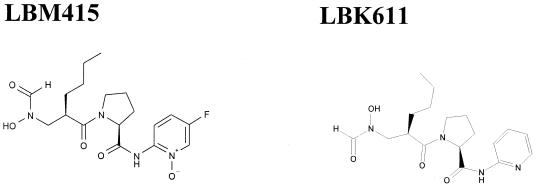
A new class of antimicrobial compounds, typified by the reverse hydroxamates LBM415 and LBK611 (Fig. 1), are potent inhibitors of bacterial peptide deformylase (PDF), an antibacterial target that has been the focus of much recent interest (2, 6, 32). LBM415 shows significant promise, particularly towards gram-positive bacteria, including well-characterized resistant strains (10a). We have noticed that H. influenzae isolates exhibit, overall, somewhat reduced susceptibilities to LBM415 (MIC90 of 4 μg/ml against a panel of 33 isolates) and related compounds, although they also have a very wide range of susceptibilities (LBM415 MIC range, 0.06 to 32 μg/ml). Therefore, we have investigated the contribution of AcrAB-TolC-mediated efflux in determining the susceptibility of several H. influenzae strains to LBM415 and the structurally related compound LBK611.
FIG. 1.
Structures of the novel peptide deformylase inhibitors LBM415 and LBK611.
MATERIALS AND METHODS
Bacterial strains, plasmids, and growth media.
Bacterial strains and plasmids used in this study are listed in Table 1. Luria-Bertani (LB) broth or LB agar (Difco) was used for routine growth of Escherichia coli. Chocolate agar plates (Remel) were used for routine growth of H. influenzae. Brain heart infusion broth (Remel) supplemented with 10 μg/ml of β-NAD (Fluka) and 10 μg/ml hemin, provided from a hemin-vitamin K solution (sBHI) (Remel), was used for liquid broth cultivation of H. influenzae. For induction of natural competence in H. influenzae, nutritional downshift was induced using M-IV medium as described previously (23). Kanamycin was added to growth media at 50 μg/ml (E. coli) or 5 μg/ml (H. influenzae) as required.
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Genotype or relevant characteristic(s) | Reference or source |
|---|---|---|
| H. influenzae | ||
| NB65044 | Rd KW20 | 10 |
| NB65001 | ATCC 49247 | |
| NB65062 | Clinical isolate, LBM415 hypersusceptible | R. N. Jones |
| NB65016 | Clinical isolate, decreased LBM415 susceptibility | I. A. Chopra |
| NB65027 | Clinical isolate, decreased LBM415 susceptibility | B. Willinger |
| NB65051 | Clinical isolate, decreased LBM415 susceptibility | R. N. Jones |
| NB65063 | Clinical isolate, decreased LBM415 susceptibility | R. N. Jones |
| NB65069 | Clinical isolate, decreased LBM415 susceptibility | R. N. Jones |
| NB65076 | Clinical isolate, decreased LBM415 susceptibility | R. N. Jones |
| NB65062-CDS0038 | NB65062 derivative, complemented for acrA deficiency | This study |
| NB65062-CDS0039 | NB65062 derivative, complemented for acrA deficiency | This study |
| NB65044-CDS0001 | NB65044 acrB::Km | This study |
| NB65044-CDS0020 | NB65044 tolC::Km | This study |
| NB65044-CDS0011 | NB65044 acrR mutant selected on 8 μg/ml LBM415 | This study |
| NB65044-CDS0014 | NB65044 acrR mutant selected on 8 μg/ml LBM415 | This study |
| NB65016-CDS0004 | NB65016 acrB::Km | This study |
| NB65027-CDS0021 | NB65027 acrB::Km | This study |
| NB65027-CDS0003 | NB65027 tolC::Km | This study |
| NB65051-CDS0002 | NB65051 acrB::Km | This study |
| NB65051-CDS0022 | NB65051 tolC::Km | This study |
| NB65063-CDS0005 | NB65063 acrB::Km | This study |
| NB65069-CDS0007 | NB65069 acrB::Km | This study |
| NB65076-CDS0006 | NB65076 acrB::Km | This study |
| E. coli | ||
| Top 10 | Invitrogen, Inc. | |
| Plasmids | ||
| pCR 2.1 topo | Cloning vector | Invitrogen, Inc. |
| pEX18Tc | Cloning vector | 13 |
| pCDBKmUS | pEX18Tc derivative containing the H. influenzae uptake sequence and acrB interrupted by a Tn903-derived Kmr cassette | This study |
| pCD14Km | pBluescript containing ORF tolC interrupted by a Tn903-derived Kmr cassette | This study |
| pACYC177 | Cloning vector, source of Tn903 Kmr cassette | NEBa |
| pBAD18Kan | Cloning/expression vector, source of Tn903 Kmr marker | 12 |
| pBluescript SK | Cloning vector | Stratagene, Inc. |
NEB, New England BioLabs.
Antimicrobial susceptibility testing.
Antibiotic MICs were determined by broth microdilution using twofold dilution in Haemophilus test medium (HTM; Remel) in accordance with the procedures established by the CLSI (formerly NCCLS) (16). Peptide deformylase inhibitors were synthesized at the Novartis Institutes for Biomedical Research, Cambridge, MA. All remaining antibiotics were obtained from Sigma (St. Louis, MO).
DNA manipulations.
H. influenzae genomic DNA was isolated using the Puregene tissue kit (Gentra Systems, Inc., Minneapolis, MN) according to the manufacturer's instructions. Oligonucleotides for PCR and sequencing were obtained from Genelink (Hawthorne, NY) and are listed in Table 2. PCRs were carried out using the Easystart mix-in-a-tube system (Molecular Bio-Products, Inc., San Diego, CA) according to the supplied instructions, with prepared genomic DNA or cells from isolated colonies used as the template. Restriction endonucleases and modifying enzymes were used according to the instructions supplied with the enzymes. DNA fragments were purified or isolated following agarose gel electrophoresis, using the QIAquick PCR cleanup or gel extraction kit (QIAGEN, Inc., Valencia, CA) as specified in the instructions. Nucleotide sequencing was performed by Agencourt, Inc. (Beverly, MA).
TABLE 2.
Oligonucleotides used for PCRs in this study
| Primer pair | Sequence | Product size and target gene |
|---|---|---|
| AcrBHIF | 5′-CCACTTTAATGTATGAGGAAATCCG-3′ | 3.1 kb, acrB |
| AcrBHIR3 | 5′-CGAATTACGTAAGATAACCTAAGTGCG-3′ | |
| HI1462IF | 5′-CACGCTTGCTTTGTTGATGTCTGGTGC-3′ | 1.3 kb, tolC |
| HI1462IR | 5′-TCCCGCCATTGAGCTATATACCGCA-3′ | |
| AcrRHIF2 | 5′-TAATGATGAAAAGTGCGGTTAATT-3′ | 0.752 kb, acrR |
| AcrRHIR | 5′-TTTCTGAATCGCACGCCAAGAGCGT-3′ | |
| AcrRHIF1 | 5′-GTGCGGTGCCACCGCAAGGACATA-3′ | 2 kb, acrR-acrA |
| AcrAHIR | 5′-TGCAGGCTCTATTGCACCCACAATG-3′ |
In vitro insertion mutagenesis.
For use in generating chromosomal insertion knockouts of acrB in H. influenzae, the acrB gene was inactivated in vitro as follows: primers AcrBHIF and AcrBHIR3 (Table 2) were used to generate a PCR fragment containing acrB from H. influenzae NB65001 genomic template DNA. This was directly cloned into pCR 2.1-Topo (Invitrogen, Carlsbad, CA) according to the instructions provided with the kit, then excised using EcoRI, and ligated into pEX18Tc. The resulting construct was linearized at the unique MfeI site within acrB, blunt ended with T4 DNA polymerase, and ligated to a blunt PCR fragment encompassing the kanamycin resistance marker from pACYC177 to give plasmid pCDBKm. This construct has the resistance determinant in the orientation opposite that of acrB. The presence of an H. influenzae DNA uptake sequence has been shown to facilitate much greater levels of natural transformation, which help to introduce DNA into various isolates which may not efficiently take up DNA by natural transformation (1). To introduce the uptake sequence into pCDBKm, a 177-bp DNA fragment containing the uptake sequence was amplified from NB65044 genomic DNA using previously described primers (1). The product was cloned into pCR2.1-Topo (Invitrogen, Carlsbad, CA), excised with KpnI, and cloned into the KpnI site within the multicloning site of pCDBKm to give pCDBKmUS.
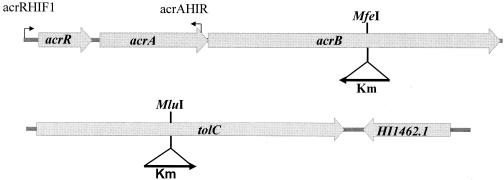
To obtain an insertion in the open reading frame (ORF) encoding TolC (30), primers HI1462IF and HI1462IR (Table 2) were used to generate a PCR fragment encompassing most of tolC from H. influenzae NB65001 genomic DNA template. This was ligated directly into pCR 2.1-Topo (Invitrogen, Carlsbad, CA), recovered as an EcoRI fragment, and ligated into the EcoRI site of pBluescript SK. A kanamycin resistance gene, isolated from pBAD18Kan as a 1.8-kb HaeII fragment and blunt ended with T4 DNA polymerase, was then ligated into the unique MluI site within tolC, which had been rendered blunt, to generate pCD14Km. This construct has the kanamycin resistance determinant in the same orientation as HI1462 (Fig. 2).
FIG. 2.
Genetic arrangement of the acrAB and tolC efflux pump genes in H. influenzae showing the positions and orientations of kanamycin resistance markers (Km) used for insertional inactivation. The positions of PCR primers AcrRHIF1 and AcrAHIR (arrows) are shown.
Introduction of insertions onto the chromosome by gene replacement.
For introduction of the acrB::Km insertion into the genome, H. influenzae strains were grown to early log phase (optical density at 600 nm of approximately 0.2) in sBHI, and natural competence was induced by nutritional downshift into M-IV medium by the method of Poje and Redfield (23). Competent cells were transformed with pCDBKmUS (linearized with XbaI) or pCDRKm (linearized with ScaI) as previously described (23) and plated on chocolate agar containing 5 μg/ml kanamycin. For introduction of the tolC::Km insertion into the genome of H. influenzae strain NB65044, competent cells were transformed with pCD14Km (linearized with ScaI) and selected as described above. For introduction of this insertion into strains NB65027 and NB65051, the recipient cells were made competent as described above, transformed with genomic DNA isolated from strain NB65044-CDS0001 (which has the insertion) and selected on chocolate agar plates containing 5 μg/ml kanamycin. All insertions were confirmed by PCR, and sequencing of fragments was generated using primers flanking the insertion sites.
PCR analysis of hypersusceptible strain NB65062 and complementation of the acrA deletion.
For analysis of the region encompassing the acrA gene in LBM415-hypersusceptible strain NB65062, primers AcrRHIF1 and AcrAHIR (Table 2) were used to generate an approximately 1.1-kb PCR fragment from H. influenzae NB65062 genomic DNA. The fragment was gel purified, and the nucleotide sequence was determined. For complementation of the acrA defect of strain NB65062, genomic DNA from NB65044, which contains a functional AcrAB-TolC pump, was used to transform NB65062 using the nutritional downshift method described above. Transformed cells were plated on chocolate agar containing either 4 μg/ml LBM415 or 2 μg/ml erythromycin, both substrates of the AcrAB-TolC efflux pump, and representative isolated colonies arising after 24 h were picked for further examination.
RNA isolation.
H. influenzae cultures were grown in triplicate in HTM (Remel) liquid medium with shaking at 37°C to late log phase (optical density at 600 nm of 0.8 to 1.0) and collected by centrifugation, and the cell pellets were frozen at −80°C. Total RNA was isolated from cell pellets using the Purescript RNA isolation kit (Gentra Systems, Minneapolis, MN) according to the supplied instructions. Approximately 100 μg of total RNA was then treated with 10 units RNase-free DNase I (RQ1; Promega, Madison, WI) for 1 h at 37°C to remove contaminating genomic DNA. Treated samples were then purified by processing over RNeasy minicolumns (QIAGEN, Inc., Valencia, CA) with an additional on-column DNase I treatment, according to the supplied protocol. RNA integrity was confirmed by formaldehyde agarose gel electrophoresis. Standard PCR using 20 ng RNA as template and primers specific for acrA was carried out (45 cycles) to confirm that there was no measurable contaminating genomic DNA.
GeneChip analysis.
Efflux pump gene expression was examined using custom Affymetrix GeneChips designed to interrogate all predicted ORFs for H. influenzae strain RdKW20 (10). RNA was reverse transcribed, and 1 μg of cDNA was fragmented, labeled, and hybridized to GeneChips using the standard prokaryotic GeneChip protocol supplied by Affymetrix. GeneChip data were obtained by scanning with an Affymetrix autoloading scanner, and data were normalized and compared using Genespring (Silicon Genetics) analysis software.
Real-time RT-PCR.
Primers and probes for real-time reverse transcription-PCR (RT-PCR) (Table 3) were designed using Primer Express v. 2.0 software (Applied Biosystems, Foster City, CA) and were synthesized by Applied Biosystems Assays by Design service. The levels of acrB transcripts were monitored by real-time RT-PCR analysis using Applied Biosystems' EZ RT-PCR Core Reagents kit based on a one-step RT-PCR for RNA quantitation on an Applied Biosystems PRISM model 7500 Sequence Detection system. Relative quantitation was done by the comparative cycle threshold method using the endogenous internal control rpsL (ribosomal protein S12) for sample normalization which had been shown to be invariant in this study (data not shown). Cycle threshold values were calculated using Applied Biosystems Sequence Detection software v.1.2.2. For each one-step RT-PCR run, 10 μl (10 ng) of total RNA was added to a reaction mixture prepared on ice containing 1× EZ RT-PCR TaqMan buffer, 3 mM manganese acetate, 300 μM dATP, dCTP, and dGTP, 600 μM dUTP, 0.9 μM of forward and reverse primers, 0.25 μM fluorogenic TaqMan-labeled probe, and 5 U of rTth DNA polymerase in a final volume of 50 μl. The thermocycling conditions were as follows: 60°C for 30 min, 95°C for 5 min, followed by 45 cycles of 95°C for 15 s and 60°C for 1 min. A preliminary experiment was performed to show that both the target and endogenous control transcripts (acrB and rpsL, respectively) were amplified with approximately equal efficiencies.
TABLE 3.
Primers used for real-time RT-PCR
| Primer | Sequence | Target |
|---|---|---|
| HIAcrBST1F | 5′-AAAATGATAATTCGCGTGCTACGG-3′ | acrB, unlabeled |
| HIAcrBST1R | 5′-GAGGTTGGATTGATGGCTAACAC-3′ | acrB, unlabeled |
| HIRpsLSt1F | 5′-AACCTTCAAGAGCACAGTGTTGTAT-3′ | rpsL, unlabeled |
| HIRpsLST1R | 5′-GCACACCCGGTAAGTCTTTAA-3′ | rpsL, unlabeled |
| HIAcrBST1M2 | 5′-AACGGACTCTGCAACATT-3′ | acrB, TaqMan MGB fluorogenic (FAM labeled)a |
| HIRpsLST1M2 | 5′-ACGACCACCACGGATTA-3′ | rpsL, TaqMan MGB fluorogenic (FAM labeled) |
MGB, minor groove binder; FAM, 6-carboxyfluorescein.
RESULTS AND DISCUSSION
Role of the AcrAB-TolC pump in determining susceptibility to LBM415.
Insertional inactivation of acrB or tolC in the H. influenzae laboratory strain NB65044 (Fig. 2) increased susceptibility to LBM415 16- to 32-fold (NB65044-CDS0001 and NB65044-CDS0020 [Table 4 ]). The insertion also increased susceptibility to the structurally related PDF inhibitor LBK611 and the known pump substrate erythromycin (27), and more dramatically to clindamycin, while not significantly affecting susceptibility to the non-pump substrate tetracycline (27) (Table 4). Another known non-pump substrate, chloramphenicol, was also unaffected by pump deletion (data not shown). This confirms that the AcrAB-TolC pump of H. influenzae plays a significant role in reducing intrinsic susceptibility to LBM415 and LBK611.
TABLE 4.
Impact of AcrAB-TolC efflux pump status on susceptibility to LBM415 and LBK611 in H. influenzae clinical isolates and role of AcrR mutation in decreasing susceptibility
| Strain or isolate | Pump status | MIC (μg/ml)a
|
||||
|---|---|---|---|---|---|---|
| LBM415 | LBK611 | ERY | CLI | TET | ||
| Strains | ||||||
| NB65044 | 4 | 4 | 4 | 8 | 0.5 | |
| NB65044-CDS0001 | Derivative of NB65044 lacking acrB | 0.25 | 0.25 | 0.25 | 0.5 | 0.5 |
| NB65044-CDS0020 | Derivative of NB65044 lacking tolC | 0.125 | 0.125 | 0.25 | 0.5 | 1 |
| NB65044-CDS0011b | 32 | 32 | 8 | 32 | 1 | |
| NB65044-CDS0014b | 32 | 32 | 8 | 32 | 1 | |
| NB65062 | LBM415-hypersusceptible clinical isolate, lacks acrA | ≤0.25 | ND | NDc | ≤0.25 | 0.5 |
| NB65062-CDS0038d | NB65062 complemented for acrA | 8 | ND | NDc | 16 | 1 |
| NB65062-CDS0039e | NB65062 complemented for acrA | 8 | ND | NDc | 16 | 1 |
| Clinical isolates with decreased susceptibility | ||||||
| NB65016 | 16 | 16 | 16 | 16 | 1 | |
| NB65016-CDS0004 | Derivative of NB65016 lacking acrB | 0.25 | 0.25 | 0.25 | 0.5 | 1 |
| NB65027 | 32 | 32 | 16 | 32 | 1 | |
| NB65027-CDS0021 | Derivative of NB65027 lacking acrB | 0.5 | 0.5 | 0.25 | 2 | 0.5 |
| NB65027-CDS0003 | Derivative of NB65016 lacking tolC | 0.5 | 0.25 | 0.125 | 2 | 1 |
| NB65051 | 32 | 32 | 16 | 32 | 1 | |
| NB65051-CDS0002 | Derivative of NB65051 lacking acrB | 0.25 | 0.25 | 0.25 | 0.5 | 1 |
| NB65051-CDS0022 | Derivative of NB65051 lacking tolC | 0.25 | 0.25 | 0.125 | 0.5 | 1 |
| NB65063 | 32 | 64 | 8 | 16 | 1 | |
| NB65063-CDS0005 | Derivative of NB65063 lacking acrB | 1 | 1 | 0.25 | 0.5 | 1 |
| NB65069 | 16 | 32 | 16 | 16 | 1 | |
| NB65069-CDS0007 | Derivative of NB65069 lacking acrB | 0.25 | 0.5 | 0.125 | 0.5 | 1 |
| NB65076 | 16 | 32 | 16 | 32 | 2 | |
| NB65076-CDS0006 | Derivative of NB65076 lacking acrB | 0.25 | 0.25 | 0.125 | 0.5 | 1 |
Abbreviations: ERY, erythromycin; CLI, clindamycin; TET, tetracycline; ND, not determined.
Strains NB65044-CDS0011 and NB65044-CDS0014 are acrR mutants selected on chocolate agar plates containing 8 μg/ml LBM415. NB65044-CDS0011 acrR has a C-to-T nucleotide change at position 253, introducing a stop codon. NB65044-CDS0014 acrR has a T-to-C nucleotide change at position 164, resulting in an L-to-P amino acid substitution.
MICs determined by Etest (AB Biodisk, Solna, Sweden); NB65062, 0.19 μg/ml; NB65062-CDS0038 and NB65062-CDS0039, 12 μg/ml.
Transformant selected on ERY.
Transformant selected on LBM415.
Decreased susceptibility to antibiotics in clinical isolates of a number of bacteria is frequently associated with overexpression of efflux pumps. Identification of pump repressor mutations and/or pump gene overexpression in resistant clinical isolates is a strong indicator of efflux-based resistance; however, there is not always a clear association between pump status and resistance to specific antibiotics (28). Therefore, to directly address whether the AcrAB-TolC pump plays a significant role in decreasing the susceptibility of H. influenzae clinical isolates with the lowest susceptibilities to the PDF inhibitors, acrB was inactivated in strains NB65016, NB65027, NB65051, NB65063, NB65069, and NB65076, all of which exhibit decreased susceptibilities to LBM415, LBK611, and clindamycin. In all cases, pump loss substantially increased susceptibility to these compounds, while having no impact on non-pump substrates (Table 4). This finding provides direct confirmation that the AcrAB-TolC pump is widely distributed and is a major contributor to the decreased susceptibility exhibited by these strains. Moreover, inactivation of tolC in strains NB65027 and NB65051 also specifically increased susceptibility to pump substrates (Table 4), further substantiating the role of this outer membrane channel in AcrAB-TolC pump function in clinical isolates.
Efflux has previously been implicated in mediating moderate levels of macrolide resistance in H. influenzae clinical isolates, although a small percentage of isolates were macrolide susceptible and lacked the efflux mechanism (21). We also noticed a small percentage of clinical isolates were hypersusceptible to erythromycin, as well as clindamycin and PDF inhibitors. Using PCR diagnostics, acrR-, acrA-, or acrB-derived products were obtained for several of these strains (data not shown), suggesting that the pump genes are widely distributed, even in hypersusceptible strains. However, for hypersusceptible strain NB65062 (LBM415 MIC of ≤0.25 μg/ml), the acrA gene was not generated. The use of primers AcrRHIF1 and AcrAHIR (Table 2 and Fig. 2) encompassing most of acrR-acrA did, however, generate a product of smaller size than the predicted 2 kb. Nucleotide sequencing of the fragment revealed an 873-bp deletion resulting in the loss of most of acrA. Transformation of H. influenzae NB65062 with genomic DNA from NB65044, which possesses an intact acrAB locus, and selection on chocolate agar containing LBM415 (4 μg/ml) or erythromycin (2 μg/ml), resulted in isolates with decreased susceptibilities to both classes of antibiotics and no change in susceptibility to non-pump substrates (strains NB65062-CDS0038 and NB65044-CDS0039 [Table 4]). Pulsed-field gel electrophoretic analysis of genomic DNA from both transformants gave identical restriction patterns to that of NB65062, while PCR and sequencing revealed the restoration of full-length acrA, confirming that hypersusceptibility is due to a lack of the AcrAB-TolC pump in strain NB65062. This observation is consistent with the role of the AcrAB-TolC pump in providing intrinsic resistance to PDF inhibitors and other pump substrates and indicates that hypersusceptibility can result from mutational loss of efflux pump components. The remaining hypersusceptible strains that gave predicted PCR products for pump genes may have other small deletions or point mutations compromising pump function, but this remains to be confirmed.
It should be noted that among clinical strains examined here, not all LBM415-hypersusceptible isolates were also hypersusceptible to macrolides. Indeed, some strains were LBM415 hypersusceptible while exhibiting high levels of macrolide resistance, suggesting a possible disconnect between the two resistances in some instances. This suggests the presence of target-based macrolide resistance in some strains that might lack the efflux pump. A recent report indicates that certain target mutations in L22 ribosomal proteins confer resistance to macrolides only in the presence of efflux (20). Further examination of macrolide resistance in certain LBM415-hypersusceptible strains will shed more light on the relationship between efflux and target-based macrolide resistance.
AcrR is a repressor of AcrAB expression, and mutations in acrR are related to susceptibility to LBM415.
The demonstration that the AcrAB-TolC efflux pump is a major contributor to decreased susceptibility to LBM415 and other antimicrobials in H. influenzae clinical isolates suggests that increased pump expression may lead to decreases in susceptibility. Although the emerging picture of efflux pump regulation is becoming increasingly complex, there are many cases where pump overexpression is related to simple mutations in regulatory genes. For example, P. aeruginosa nalB strains overexpress MexAB-OprM due to mutations in the mexR gene encoding a repressor, located immediately upstream of the mexAB-oprM genes (26). In H. influenzae, a putative acrR gene (HI0983), located immediately upstream of acrAB (Fig. 2), encodes an AcrR/TetR family repressor which may be involved in controlling the expression of acrAB. Nucleotide sequencing of acrR from H. influenzae strains NB65016, NB65027, NB65051, and NB65063 revealed the presence of insertion/deletions or point mutations generating either frameshifts or stop codons, as follows: NB65016, 1-base (C) insertion after nucleotide 442 (frameshift); NB65027, 8-bp deletion and GTT insertion after nucleotide 366 (frameshift) and an additional 1-base insertion downstream; NB65051, 4-bp deletion after nucleotide 322 (frameshift); and NB65063, C252T substitution (stop). The acrR genes from NB65069 and NB65076 revealed point mutations leading to amino acid changes relative to the published sequence for the acrR gene. The preponderance of acrR mutations, combined with the clear role of the AcrAB-TolC efflux pump in decreasing susceptibility to LBM415, erythromycin, and clindamycin in these strains strongly suggests that the AcrAB-TolC efflux pump is being overexpressed due to loss of AcrR repressor function.
To further examine the relationship between AcrR and decreased susceptibility to LBM415, we tested whether exposure of H. influenzae NB65044 to LBM415 at 8 μg/ml would select mutants with altered acrR genes. Mutants of strain NB65044 were selected on chocolate agar containing 8 μg/ml of LBM415 (typical frequency of 10−7), and examination of the acrR genes from 10 isolated mutants revealed mutations in all 10 isolates (data not shown). Susceptibility testing of two of these mutants (NB65044-CDS0011 and NB65044-CDS0014, possessing an introduced stop codon and an amino acid change, respectively) (see footnote b of Table 4) revealed an eightfold decrease in susceptibility to LBM415 and LBK611 and a fourfold decrease in susceptibility to clindamycin with no change in susceptibility to tetracycline (Table 4). To relate the increase in resistance to increased pump expression, transcriptional profiling revealed that there was a modest increase in expression of acrR (approximately 3.44-fold), acrA (approximately 2.65-fold), and acrB (approximately 1.88-fold) in strain NB65044-CDS0011 compared to the parent strain NB65044. Real-time RT-PCR analysis for acrB (2.17-fold ± 0.03-fold upregulated) confirmed the increase measured by GeneChips. Similar results were obtained for a strain insertionally inactivated for acrR using a kanamycin cassette (data not shown).
Taken together, these data show that decreased susceptibility to LBM415 can be acquired mutationally in the form of acrR mutations, presumably by affecting expression of AcrAB. It also strongly supports the notion that the mutations in acrR found in the less susceptible clinical strains examined here result in pump overexpression. It was previously reported that inactivation of acrR in H. influenzae did not alter susceptibility to a wide range of compounds, including erythromycin (30). In our investigation the change observed in erythromycin susceptibility upon acrR mutation was small, but susceptibility to clindamycin (and LBM415) was apparently much more responsive to AcrAB pump level, potentially reflecting differences between the compounds as regards recognition by the pump and/or influx across the outer membrane. The GeneChip data also indicate that acrR is autoregulated, which is typical for many efflux pump repressors.
Conclusions.
In summary, we have shown that the AcrAB-TolC efflux pump of H. influenzae is responsible for decreasing susceptibility to the PDF inhibitors LBM415 and the structurally related compound LBK611. Susceptibility is further decreased upon mutational loss of AcrR. Significantly, acrR mutants apparently exist within the clinical population, suggesting that previous exposure to antimicrobials may have selected for decreased susceptibility to these novel PDF inhibitors. Furthermore, exposure of H. influenzae to LBM415 in vitro selected for acrR mutations and corresponding decreases in susceptibility to both LBM415 and other pump substrates. This highlights the potential impact of efflux with respect to novel antimicrobial compound development, even in bacteria, such as H. influenzae, with relatively less efficient efflux.
In light of the comparative inefficiency of the H. influenzae AcrAB-TolC pump and the relative permeability of the outer membrane, it may be possible to modify novel compounds to influx rapidly and/or escape recognition by the pump. Indeed, glycyl modifications of minocycline were previously shown to alter the relative contributions of pumps mediating efflux in Pseudomonas aeruginosa from MexAB-OprM to MexXY-OprM (8), suggesting that a specific pump can be evaded through chemical modification. Since many H. influenzae strains likely have only a single RND-type pump, this strategy may be more successful in this organism. Alternatively, efflux may be overcome through the development of specific pump inhibitors. At least one compound, MC 04,124 has been reported to inhibit macrolide efflux in H. influenzae (4, 31). The increasing prevalence and importance of gram-negative infections demand a more concerted effort directed at overcoming efflux in order to increase the odds of successful development of novel antimicrobials targeted at these bacteria.
Acknowledgments
We are grateful to Stacey Esterow for pulsed-field gel analysis. We also thank Herbert Schweizer and Jon Beckwith for kindly providing pEX18Tc and pBAD18Kan, respectively, and Ian Chopra, Ronald Jones, and Birgit Willinger for providing H. influenzae clinical isolates. We are also grateful to Genedata, Inc., for assistance in developing the custom-designed GeneChip.
REFERENCES
- 1.Akerley, B. J., E. J. Rubin, V. L. Novick, K. Amaya, N. Judson, and J. J. Mekalanos. 2002. A genome-scale analysis for identification of genes required for growth or survival of Haemophilus influenzae. Proc. Natl. Acad. Sci. USA 99:966-971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Apfel, C. M., H. Locher, S. Evers, B. Takacs, C. Hubschwerlen, W. Pirson, M. G. Page, and W. Keck. 2001. Peptide deformylase as an antibacterial drug target: target validation and resistance development. Antimicrob. Agents Chemother. 45:1058-1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beinlich, K. L., R. Chuanchuen, and H. P. Schweizer. 2001. Contribution of multidrug efflux pumps to multiple antibiotic resistance in veterinary clinical isolates of Pseudomonas aeruginosa. FEMS Microbiol. Lett. 198:129-134. [DOI] [PubMed] [Google Scholar]
- 4.Cho, D., D. Lofland, J. Blais, K. Tangen, D. Cotter, O. Lomovskaya, S. Chamberland, and M. N. Dudley. 2000. An efflux pump inhibitor (EPI), MC-04,124, enhances the activity of macrolides against gram-negative bacteria, abstr. 1497, p. 208. Program Abstr. 40th Intersci. Conf. Antimicrob. Agents Chemother. 2000. American Society for Microbiology, Washington, D.C.
- 5.Chuanchuen, R., K. Beinlich, T. T. Hoang, A. Becher, R. R. Karkhoff-Schweizer, and H. P. Schweizer. 2001. Cross-resistance between triclosan and antibiotics in Pseudomonas aeruginosa is mediated by multidrug efflux pumps: exposure of a susceptible mutant strain to triclosan selects nfxB mutants overexpressing MexCD-OprJ. Antimicrob. Agents Chemother. 45:428-432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clements, J. M., R. P. Beckett, A. Brown, G. Catlin, M. Lobell, S. Palan, W. Thomas, M. Whittaker, S. Wood, S. Salama, P. J. Baker, H. F. Rodgers, V. Barynin, D. W. Rice, and M. G. Hunter. 2001. Antibiotic activity and characterization of BB-3497, a novel peptide deformylase inhibitor. Antimicrob. Agents Chemother. 45:563-570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dagan, R., C. E. Johnson, S. McLinn, N. Abughali, J. Feris, E. Leibovitz, D. J. Burch, and M. R. Jacobs. 2000. Bacteriologic and clinical efficacy of amoxicillin/clavulanate vs. azithromycin in acute otitis media. Pediatr. Infect. Dis. J. 19:95-104. [DOI] [PubMed] [Google Scholar]
- 8.Dean, C. R., M. A. Visalli, S. J. Projan, P. E. Sum, and P. A. Bradford. 2003. Efflux-mediated resistance to tigecycline (GAR-936) in Pseudomonas aeruginosa PAO1. Antimicrob. Agents Chemother. 47:972-978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Elkins, C. A., and H. Nikaido. 2002. Substrate specificity of the RND-type multidrug efflux pumps AcrB and AcrD of Escherichia coli is determined predominantly by two large periplasmic loops. J. Bacteriol. 184:6490-6498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fleischmann, R. D., M. D. Adams, O. White, R. A. Clayton, E. F. Kirkness, A. R. Kerlavage, C. J. Bult, J. F. Tomb, B. A. Dougherty, J. M. Merrick, et al. 1995. Whole-genome random sequencing and assembly of Haemophilus influenzae Rd. Science 269:496-512. [DOI] [PubMed] [Google Scholar]
- 10a.Fritsche, T. R., H. S. Sader, R. Cleeland, and R. N. Jones. 2005. Comparative antimicrobial characterization of LBM415 (NVP PDF-713), a new peptide deformylase inhibitor of clinical importance. Antimicrob. Agents Chemother. 49:1468-1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gotfried, M. H. 2001. Epidemiology of clinically diagnosed community-acquired pneumonia in the primary care setting: results from the 1999-2000 respiratory surveillance program. Am. J. Med. 111(Suppl. 9A):25S-29S. [DOI] [PubMed] [Google Scholar]
- 12.Guzman, L. M., D. Belin, M. J. Carson, and J. Beckwith. 1995. Tight regulation, modulation, and high-level expression by vectors containing the arabinose PBAD promoter. J. Bacteriol. 177:4121-4130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hoang, T. T., R. R. Karkhoff-Schweizer, A. J. Kutchma, and H. P. Schweizer. 1998. A broad-host-range Flp-FRT recombination system for site-specific excision of chromosomally-located DNA sequences: application for isolation of unmarked Pseudomonas aeruginosa mutants. Gene 212:77-86. [DOI] [PubMed] [Google Scholar]
- 14.Li, X. Z., D. Ma, D. M. Livermore, and H. Nikaido. 1994. Role of efflux pump(s) in intrinsic resistance of Pseudomonas aeruginosa: active efflux as a contributing factor to beta-lactam resistance. Antimicrob. Agents Chemother. 38:1742-1752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mazzariol, A., Y. Tokue, T. M. Kanegawa, G. Cornaglia, and H. Nikaido. 2000. High-level fluoroquinolone-resistant clinical isolates of Escherichia coli overproduce multidrug efflux protein AcrA. Antimicrob. Agents Chemother. 44:3441-3443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.National Committee for Clinical Laboratory Standards. 2003. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. Approved standard M7-A5. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 17.Nikaido, H. 1996. Multidrug efflux pumps of gram-negative bacteria. J. Bacteriol. 178:5853-5859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nikaido, H. 1998. Multiple antibiotic resistance and efflux. Curr. Opin. Microbiol. 1:516-523. [DOI] [PubMed] [Google Scholar]
- 19.Nikaido, H., and H. I. Zgurskaya. 2001. AcrAB and related multidrug efflux pumps of Escherichia coli. J. Mol. Microbiol. Biotechnol. 3:215-218. [PubMed] [Google Scholar]
- 20.Peric, M., B. Bozdogan, C. Galderisi, D. Krissinger, T. Rager, and P. C. Appelbaum. 2004. Inability of L22 ribosomal protein alteration to increase macrolide MICs in the absence of efflux mechanism in Haemophilus influenzae HMC-S. J. Antimicrob. Chemother. 54:393-400. [DOI] [PubMed] [Google Scholar]
- 21.Peric, M., B. Bozdogan, M. R. Jacobs, and P. C. Appelbaum. 2003. Effects of an efflux mechanism and ribosomal mutations on macrolide susceptibility of Haemophilus influenzae clinical isolates. Antimicrob. Agents Chemother. 47:1017-1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pfaller, M. A., A. F. Ehrhardt, and R. N. Jones. 2001. Frequency of pathogen occurrence and antimicrobial susceptibility among community-acquired respiratory tract infections in the respiratory surveillance program study: microbiology from the medical office practice environment. Am. J. Med. 111(Suppl. 9A):4S-12S. [DOI] [PubMed] [Google Scholar]
- 23.Poje, G., and R. J. Redfield. 2003. Transformation of Haemophilus influenzae. Methods Mol. Med. 71:57-70. [DOI] [PubMed] [Google Scholar]
- 24.Poole, K. 2001. Multidrug efflux pumps and antimicrobial resistance in Pseudomonas aeruginosa and related organisms. J. Mol. Microbiol. Biotechnol. 3:255-264. [PubMed] [Google Scholar]
- 25.Poole, K., and R. Srikumar. 2001. Multidrug efflux in Pseudomonas aeruginosa: components, mechanisms and clinical significance. Curr. Top. Med. Chem. 1:59-71. [DOI] [PubMed] [Google Scholar]
- 26.Poole, K., K. Tetro, Q. Zhao, S. Neshat, D. E. Heinrichs, and N. Bianco. 1996. Expression of the multidrug resistance operon mexA-mexB-oprM in Pseudomonas aeruginosa: mexR encodes a regulator of operon expression. Antimicrob. Agents Chemother. 40:2021-2028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sanchez, L., W. Pan, M. Vinas, and H. Nikaido. 1997. The acrAB homolog of Haemophilus influenzae codes for a functional multidrug efflux pump. J. Bacteriol. 179:6855-6857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sobel, M. L., G. A. McKay, and K. Poole. 2003. Contribution of the MexXY multidrug transporter to aminoglycoside resistance in Pseudomonas aeruginosa clinical isolates. Antimicrob. Agents Chemother. 47:3202-3207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sokol, W. 2001. Epidemiology of sinusitis in the primary care setting: results from the 1999-2000 respiratory surveillance program. Am. J. Med. 111(Suppl. 9A):19S-24S. [DOI] [PubMed] [Google Scholar]
- 30.Trepod, C. M., and J. E. Mott. 2004. Identification of the Haemophilus influenzae tolC gene by susceptibility profiles of insertionally inactivated efflux pump mutants. Antimicrob. Agents Chemother. 48:1416-1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Watkins, W. J., Y. Landaverry, R. Leger, R. Litman, T. E. Renau, N. Williams, R. Yen, J. E. Zhang, S. Chamberland, D. Madsen, D. Griffith, V. Trembe, K. Huie, and M. N. Dudley. 2004. The relationship between physicochemical properties, in vitro activity and pharmacokinetic profiles of analogues of diamine-containing efflux pump inhibitors. Bioorg. Med. Chem. Lett. 13:4241-4244. [DOI] [PubMed] [Google Scholar]
- 32.Wise, R., J. M. Andrews, and J. Ashby. 2002. In vitro activities of peptide deformylase inhibitors against gram-positive pathogens. Antimicrob. Agents Chemother. 46:1117-1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yu, E. W., G. McDermott, H. I. Zgurskaya, H. Nikaido, and D. E. Koshland, Jr. 2003. Structural basis of multiple drug-binding capacity of the AcrB multidrug efflux pump. Science 300:976-980. [DOI] [PubMed] [Google Scholar]
- 34.Ziha-Zarifi, I., C. Llanes, T. Kohler, J. C. Pechere, and P. Plesiat. 1999. In vivo emergence of multidrug-resistant mutants of Pseudomonas aeruginosa overexpressing the active efflux system MexA-MexB-OprM. Antimicrob. Agents Chemother. 43:287-291. [DOI] [PMC free article] [PubMed] [Google Scholar]