Abstract
TAK-220 is a member of a novel class of chemokine receptor antagonists and is highly specific to CCR5, as determined by receptor binding and calcium mobilization assays. The compound selectively inhibited coreceptor-mediated entry of human immunodeficiency virus type 1 (HIV-1) into host cells and HIV-1 infection mediated by CCR5. TAK-220 inhibited the replication of six CCR5-using (R5) HIV-1 clinical isolates in peripheral blood mononuclear cells (PBMCs) with a mean 90% effective concentration of 13 nM. The anti-HIV-1 activity of TAK-220 was not affected by addition of high concentrations of human serum. It equally inhibited R5 HIV-1 replication in PBMCs obtained from eight different donors, irrespective of the levels of viral production. Furthermore, the anti-HIV-1 activity of TAK-220 was found to be subtype independent. TAK-220 did not induce CCR5 internalization but blocked the binding of two monoclonal antibodies that recognize the second extracellular loop of CCR5 in CCR5-expressing cells. These results suggest that TAK-220 selectively inhibits R5 HIV-1 replication by interfering with coreceptor-mediated entry of the virus into host cells. At a dose of 5 mg/kg of body weight, TAK-220 showed oral bioavailabilities of 9.5 and 28.9% in rats and monkeys, respectively. Thus, TAK-220 is a promising candidate for the treatment of HIV-1 infection.
After the introduction of highly active antiretroviral therapy (HAART), the number of AIDS cases decreased dramatically (12, 13, 25). However, HAART is not able to eradicate human immunodeficiency virus type 1 (HIV-1) from patients completely (34). HIV-1 reemerges quickly after HAART discontinuation, even if the number of HIV-1 RNA copies is below a detectable level during HAART (7). Accordingly, HAART can decrease the incidence of AIDS but not the number of HIV-1-infected individuals, suggesting an increase in the number of people who need anti-HIV-1 therapy. According to treatment guidelines, symptomatic HIV-1-infected patients and individuals who are asymptomatic but who have CD4+ T-cell numbers of less than 300 cells/mm3 or plasma HIV-1 RNA levels of more than 55,000 copies/ml require antiretroviral therapy (42). Once antiretroviral therapy starts, these infected patients will require antiretroviral therapy throughout their lives. On the other hand, the complexity of dosing and toxicity make it difficult to maintain patients' adherence to antiretrovirals (9). Although combination chemotherapy can suppress the emergence of drug-resistant mutants by simultaneously attacking different targets, such as reverse transcriptase and protease, multidrug-resistant mutants have also emerged and are increasing in number (11). The rates of success of HAART for the treatment of HIV-1 infection were reported to be ranging from 60% to 90% in antiretroviral treatment-naïve patients and from 45% to 85% in zidovudine-experienced subjects (6). These success rates are predicted to decrease gradually with the increase in the emergence of drug-resistant strains. Thus, there is an urgent need for the development of anti-HIV-1 agents with novel modes of action.
In March 2003, the U.S Food and Drug Administration approved a new class of anti-HIV-1 agents, the entry inhibitors, of which enfuvirtide is a member (40). Among the entry inhibitors, enfuvirtide is further classified as a fusion inhibitor, and it inhibits HIV-1 replication by blocking the fusion between the viral envelope and the host cell membrane. Clinical trials of enfuvirtide have been proved to have sufficient efficacy against HIV-1, including multidrug-resistant strains (16, 17). However, enfuvirtide is a peptide compound and must be administered parenterally.
Chemokines and chemokine receptors form a complex network system essential to inflammation. Some chemokine receptors also act as coreceptors of HIV-1 in consort with the primary receptor CD4. As a result, certain chemokines can block HIV-1 entry by occupying their receptors. Although about 12 chemokine receptors can serve as coreceptors of HIV-1, only 2 receptors appear to play a major role in HIV-1 infection in vivo (14). It has been reported that HIV-1 associated with the initial infection predominantly uses CCR5 as a coreceptor and that CCR5-using (R5) HIV-1 is isolated exclusively during the asymptomatic stage (8). The turnover of R5 HIV-1 is very rapid not only in primary infection but also in chronic asymptomatic infection (26). Therefore, an attempt to suppress R5 HIV-1 replication may delay progression from the asymptomatic to the symptomatic (late) stage. This hypothesis has been supported by the finding that the individuals with CCR5-Δ32, a truncated and nonfunctional form of CCR5, display strong resistance to HIV-1 infection without obvious health problems (19, 32). These results suggest that CCR5 antagonists are effective as anti-HIV-1 agents without serious side effects.
In 1999, we reported that TAK-779 is a novel CCR5 antagonist that inhibits R5 HIV-1 replication in cell cultures in a highly potent and selective manner (4). However, TAK-779 has poor oral bioavailability, and its development was discontinued because of unfavorable effects at the injection sites. Our continuous efforts to find effective and orally bioavailable CCR5 antagonists have recently identified TAK-220 through chemical modification of a lead compound discovered by high-throughput screening. TAK-220 is a novel series of compounds whose chemical structures totally differ from that of TAK-779. In this study, we report the results of preclinical evaluation of TAK-220 for its anti-HIV-1 activity in cell cultures and its pharmacokinetics in animals.
MATERIALS AND METHODS
Cells.
CCR1-, CCR2b-, CCR3-, CCR4-, CCR5-, and CCR7-expressing Chinese hamster ovary (CHO) cells and CCR5-expressing HeLa cells were maintained in Ham's F-12 medium supplemented with 10% fetal bovine serum (FBS) and 50 μg/ml gentamicin. COS-7 cells were obtained through the Health Science Research Resources Bank (Osaka, Japan) and were maintained in Dulbecco's modified Eagle medium (DMEM) supplemented with 10% FBS and antibiotics (100 U/ml penicillin G and 100 μg/ml streptomycin). U87 astroglioma cells expressing human CD4 and either CCR5 or CXCR4 (U87.CD4.CCR5 cells or U87.CD4.CXCR4 cells) were obtained from D. Littman (New York University School of Medicine, New York, NY) and maintained in DMEM supplemented with 10% FBS, 300 μg/ml geneticin, 1 μg/ml puromycin, and antibiotics. The above medium without geneticin and puromycin was used in viral replication assays. MOLT-4/CCR5 cells (3), which comprise the T-lymphoblastoid cell line MOLT-4 expressing human CCR5, were maintained in RPMI 1640 medium supplemented with 10% FBS, 1 mg/ml geneticin, and antibiotics. MOLT-4/CCR5/Luc+ cells, which comprise MOLT-4 cells expressing human CCR5 and carrying an integrated copy of the HIV-1 long terminal repeat-driven luciferase reporter gene, were maintained in RPMI 1640 medium supplemented with 10%FBS, 500 μg/ml geneticin, and antibiotics. Peripheral blood mononuclear cells (PBMCs) obtained from healthy volunteers were isolated by Ficoll-Hypaque gradient density centrifugation and stimulated with 5 μg/ml phytohemagglutinin (PHA) in RPMI 1640 medium supplemented with 20% FBS, 100 U/ml recombinant human interleukin 2 (Takeda Pharmaceutical Company Ltd., Osaka, Japan), and antibiotics for 3 days. The above medium without PHA was used in viral replication assays.
Compounds.
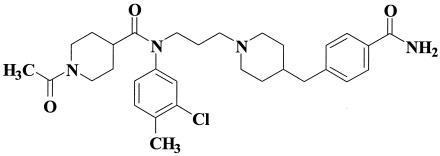
TAK-220, 1-acetyl-N-{3-[4-(4-carbamoylbenzyl)-1-piperidyl]-propyl}-N-(3-chloro-4-methylphenyl)piperidine-4-carboxamide, and AMD-3100 were synthesized by Takeda Pharmaceutical Company Ltd. The chemical structure of TAK-220 is shown in Fig. 1.
FIG. 1.
Chemical structure of TAK-220.
Viruses.
Seven R5 HIV-1 strains (JR-FL, KK, CTV, HKW, HNK, HTN, and HHA), two CXCR4-using (X4) HIV-1 strains (IIIB and SW), and one CCR5- and CXCR4-using (R5X4) HIV-1 strain (HE) were used for viral replication assays in U87 astroglioma cells and PBMCs. KK, CTV, HKW, HNK, HTN, HHA, and SW were clinical isolates from HIV-1-infected patients in Japan. For viral entry assays with recombinant HIV-1, a customized panel of 30 recombinant viruses was prepared from ViroLogic's specimen library. These viruses express genetically distinct envelope glycoproteins classified as subtypes A, B, C, D, E (now CRE01_AE), and F.
Chemokine-binding assay.
The CCR5-expressing CHO cells were incubated with various concentrations of TAK-220 in binding buffer (Ham's F-12 medium containing 20 mM HEPES and 0.5% bovine serum albumin [BSA], pH 7.2) containing either 200 pM 125I-labeled regulated on activation, normal T-cell expressed and secreted protein (RANTES) (Amersham Pharmacia, Piscataway, NJ), 125I-labeled macrophage inflammatory protein-1α (MIP-1α), or 125I-labeled MIP-1β (Perkin-Elmer, Inc., Wellesley, MA). Binding reactions were performed at room temperature for 40 min. The binding reaction was terminated by washing out the cell-free ligand twice with cold phosphate-buffered saline (PBS). The cell-associated radioactivity was recorded with a scintillation counter (Top-count; Packard, Tokyo, Japan). Assays for the inhibitory effect of TAK-220 on the binding of 125I-labeled RANTES to CCR1, 125I-labeled monocyte chemotactic protein-1 (MCP-1) to CCR2b, 125I-labeled eotaxin to CCR3, 125I-labeled thymus and activation-regulated chemokine (TARC) to CCR4, and125I-labeled MIP-3β to CCR7 were carried out in a similar manner, as described above.
Ca2+ mobilization assay.
CCR5- or CCR1-expressing HeLa cells were suspended in ECB buffer (Hank's balanced salt solution, 20 mM HEPES, and 0.1 mg/ml BSA, pH 7.4), loaded with 10 μM Fura-PE3AM (Teflabs, Austin, TX) at 37°C for 60 min, washed twice, and resuspended at 5 × 106 cells/ml in the same buffer. At 90 s after exposure to various concentrations of TAK-220, 20 nM RANTES was added, and the relative increase in cytoplasmic Ca2+ levels was monitored with a fluorescence spectrometer (FDSS-2000; Hamamatsu Photonics, Shizuoka, Japan).
Envelope-mediated membrane fusion assay.
COS-7 cells were seeded in a six-well plate at 5 × 105 cells per well. The culture supernatants were removed on the next day, and the cells were transfected with 0.6 μg of pSG322-env, which encodes the R5 HIV-1 (JR-FL) envelope; pHXB2-env, which encodes the X4 HIV-1 (HXB2) envelope; or pBluescript (Stratagene, La Jolla, CA) as a negative control, as well as with 0.2 μg of pSG5-rev and 1.0 μg of pSG5-tat with Lipofectamine 2000 (Invitrogen, Gaithersburg, MD). After a 6-h incubation at 37°C, the supernatants were removed and the cells were incubated with fresh culture medium for 2 days at 37°C. The transfected COS-7 cells and MOLT-4/CCR5/Luc+ cells were seeded in a 96-well plate at 1 × 104 cells each per well, and various concentrations of test compounds were added to the wells. The cell suspension was incubated at 37°C. A mixture of DMEM and RPMI 1640 medium supplemented with 10% FBS and antibiotics was used for membrane fusion. After an overnight incubation, Luc-Screen (Tropix, Foster City, CA) was added to each well and the mixtures were incubated at room temperature for 10 min. The luciferase activity was measured with a luminometer (Wallac 1420 ARVO SX; Wallac Berthold Japan, Tokyo, Japan).
Antiviral assay with U87 astroglioma cells.
U87.CD4.CCR5 or U87.CD4.CXCR4 cells were seeded into a 48-well plate (1 × 104 cells/well) and incubated overnight at 37°C. The culture supernatants were removed, and the cells were inoculated with 200 50% cell culture infectious doses (CCID50s) of R5X4 HIV-1 (HE) per well in the presence of test compounds (1,000 nM) in a total volume of 400 μl. After an overnight incubation, the cells were washed to remove unadsorbed viral particles and were further incubated in the presence of the same concentration of the test compounds for 2 days. On day 3 after infection, the culture supernatants were collected and their p24 antigen levels were determined with a sandwich enzyme-linked immunosorbent assay kit (ZeptoMetrix Corp., Buffalo, NY).
Antiviral assay with PBMCs.
PHA-stimulated PBMCs were inoculated with 1,000 to 1,400 CCID50s of R5 HIV-1 (JR-FL) or X4 HIV-1 (IIIB) or with 13 to 55 ng of p24 of HIV-1 clinical isolates per 4 × 106 cells and incubated for 4 h. The cells were washed to remove unadsorbed viral particles and seeded into a 96-well plate (2 × 105 cells/well) with culture medium containing various concentrations of the test compounds. The effects of high concentrations of human serum (HS) on the anti-HIV-1 activity of TAK-220 were examined with RPMI 1640 medium supplemented with either 20% FBS alone or 40% human type AB serum (Nabi, Boca Raton, FL) plus 10% FBS, 100 U/ml recombinant human interleukin 2, and antibiotics. On day 4 after infection, the cells were subcultured at 1:2 with culture medium containing the same concentrations of the test compounds. On day 7 after infection, the culture supernatants were collected and their p24 antigen levels were determined with a p24 antigen enzyme-linked immunosorbent assay kit.
Viral entry assay with recombinant HIV-1.
An HIV-1 entry assay has been developed by modifying the PhenoSense HIV assay, which is a novel phenotypic drug susceptibility assay for HIV-1 (28). In brief, nucleic acid amplification (reverse transcriptase PCR) was carried out to obtain HIV-1 gp160 sequences derived from HIV-1-positive plasma samples. The amplified envelope sequences were incorporated into an expression vector (pCXAS) by conventional cloning methods. Envelope expression vectors (pHIVenv) were prepared as large pools of sequences that accurately represent the viral quasispecies in patients at the time of sample collection. Recombinant HIV-1 stocks containing patient viral envelope glycoproteins were prepared by cotransfecting human embryonic kidney 293 cells with an HIV-1 genomic viral vector and an appropriate envelope expression vector. The genomic vector (pHIVlucΔU3) was replication defective and contained a luciferase expression cassette within a deleted region of the HIV-1 envelope gene. Recombinant virus particles were harvested at 48 h after transfection and were used to inoculate U87.CD4.CCR5 or U87.CD4.CXCR4 cells. The infected cells were cultured in the presence of various concentrations of TAK-220 for 48 h. Viral entry followed by a single round of replication was detected by determination of the luciferase activity in the infected cells.
Binding inhibition assay with anti-CCR5 MAbs.
Fluorescein-isothiocyanate (FITC)-conjugated anti-CCR5 monoclonal antibody (MAb) 45531.111 was purchased from R&D Systems (Minneapolis, MN). FITC-conjugated anti-CCR5 MAb 2D7 and phycoerythrin (PE)-conjugated anti-CCR5 MAb 3A9 were purchased from PharMingen (San Diego, CA). FITC- or PE-conjugated isotype-matched MAbs were purchased from PharMingen. MOLT-4/CCR5 cells (3 × 105 cells) were incubated in the presence of TAK-220 (100 nM) for 30 min at 4°C and were further incubated with a MAb or its isotype control for 45 min at 4°C. After two washes with PBS containing 0.1% BSA and 0.01% sodium azide, the cells were analyzed for their mean fluorescence intensity (MFI) with a flow cytometer (CytoACE-300; JASCO Corporation, Tokyo, Japan).
CCR5 internalization assay.
MOLT-4/CCR5 cells (3 × 105 cells) were incubated in the presence of various concentrations of the test compounds for 3 h at 37°C. After centrifugation, the cells were incubated with MAb 3A9 or an isotype control for 45 min at 4°C. After two washes with PBS containing 0.1% BSA and 0.01% sodium azide, the cells were analyzed for their MFI with a flow cytometer.
Cytotoxicity assay.
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) was purchased from Sigma Chemical Co. (St. Louis, MO) and was used to determine the cytotoxicities of the test compounds in mock-infected cells (27). MTT was added to each well, the plate was incubated at 37°C for 2 h, and then acidified isopropyl alcohol was added to dissolve the formazan crystals. The optical density was determined with a microplate reader (model 550; Bio-Rad Laboratories, Hercules, CA).
Pharmacokinetic analysis with animals.
The animals used in this study were male albino rats [Crj:CD(SD)IGS rats; Charles River Japan Inc., Yokohama, Japan] and male cynomolgus monkeys (Keari Co. Ltd., Osaka, Japan). The body weights ranged from 250 to 349 g for the rats and 2.40 to 3.68 kg for the monkeys. TAK-220 was suspended in 0.5% (wt/vol) methylcellulose solution, and the solution was administered orally at doses of 5 mg/10 ml/kg of body weight for the rats and 5 mg/2 ml/kg for the monkeys. TAK-220 was dissolved in a mixture of dimethyl acetamide and polyethylene glycol 400 (1:1, by volume) for intravenous injection at doses of 1 mg/ml/kg for the rats and 1 mg/0.2 ml/kg for the monkeys. The compound was administered to fasted animals. Plasma samples were collected periodically up to 24 h after administration. The plasma concentration of TAK-220 was quantified by liquid chromatography-tandem mass spectrometry. Data were expressed as the mean values with standard deviations (SDs) of the results for three to four animals. The maximum concentration in plasma (Cmax) and the time to reach Cmax (Tmax) were noted directly from the concentration-time curve. Areas under the plasma concentration-time curve (AUC) were calculated by linear regression analysis and the trapezoidal rule.
Data analysis.
The 50 inhibitory concentrations (IC50s) and the IC90s were calculated by use of the SAS system procedure NLIN, which produces least-squares estimates of the parameters of a nonlinear model (logistic model).
RESULTS
Receptor-binding properties of TAK-220.
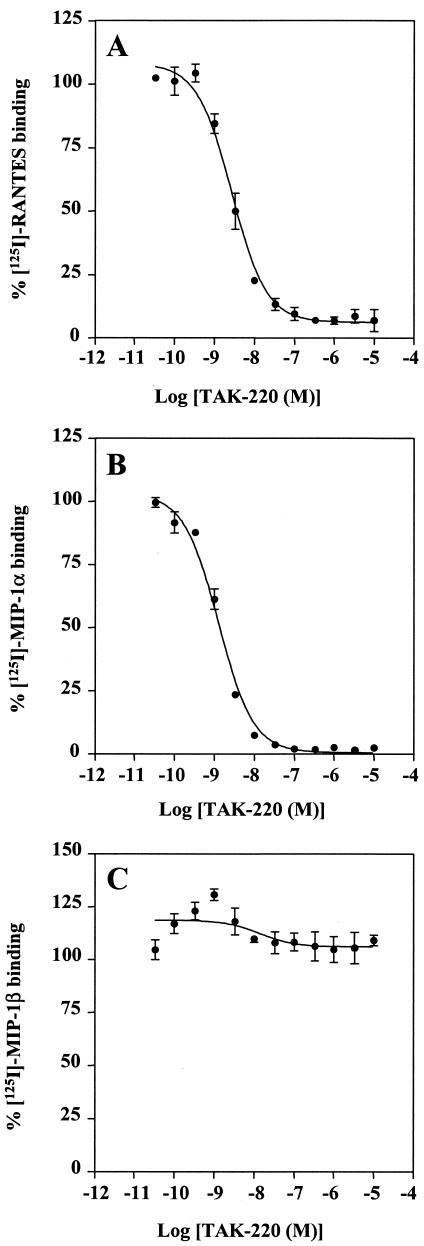
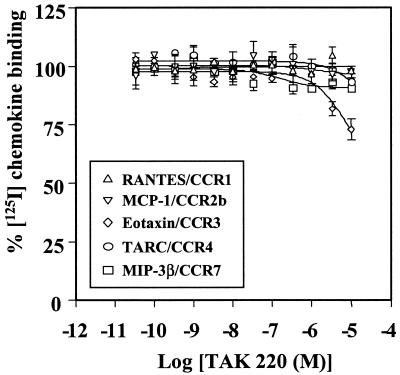
TAK-220 was examined for its inhibitory effect on the binding of RANTES, MIP-1α, and MIP-1β to CCR5-expressing CHO cells. TAK-220 inhibited the binding of RANTES to CCR5 in a dose-dependent manner, and its IC50 for RANTES binding was 3.5 nM (Fig. 2A). The compound also blocked the binding of MIP-1α to CCR5, with an IC50 of 1.4 nM (Fig. 2B). However, TAK-220 did not affect the binding of MIP-1β (Fig. 2C). To determine whether the inhibition of the chemokine binding by TAK-220 is specific to CCR5, the effect of TAK-220 on the ligand binding to CCR1-, CCR2b-, CCR3-, CCR4-, and CCR7-expressing CHO cells was examined. TAK-220 did not affect the binding of RANTES, MCP-1, eotaxin, TARC, or MIP-3β to CCR1, CCR2b, CCR3, CCR4, or CCR7, respectively (Fig. 3), indicating that the inhibition is CCR5 specific.
FIG. 2.
Effect of TAK-220 on the binding of (A) RANTES, (B) MIP-1α, and (C) MIP-1β to CCR5. The CCR5-expressing CHO cells were incubated with various concentrations of TAK-220 in the binding buffer containing 125I-labeled RANTES, MIP-1α, or MIP-1β. Binding reactions were performed at room temperature and were terminated by washing out the cell-free ligand with PBS. The cell-associated radioactivity was measured with a scintillation counter. The data represent the means ± SDs for triplicate wells.
FIG. 3.
Effect of TAK-220 on ligand binding to various chemokine receptors. The assay procedure for the inhibition of ligand binding to each chemokine receptor by TAK-220 was described in the legend to Fig. 2. The data represent the means ± SDs for triplicate wells.
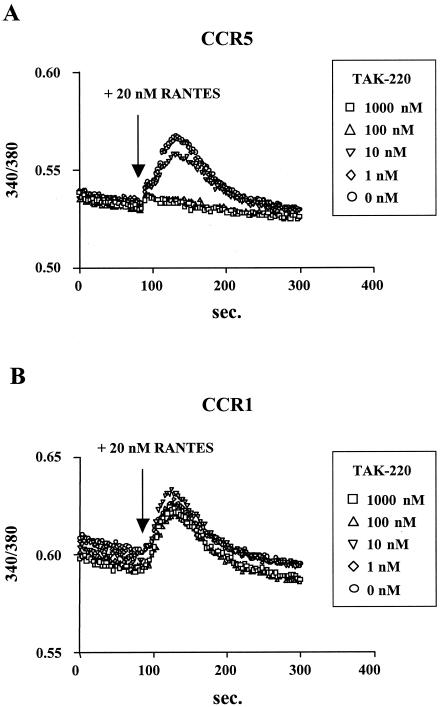
To prove that TAK-220 antagonized CCR5 but not its ligand, the inhibitory effects of TAK-220 on RANTES-induced Ca2+ mobilization in CCR5- or CCR1-expressing HeLa cells were examined. RANTES at a concentration of 20 nM clearly induced an increased intracellular Ca2+ level in CCR5-expressing cells, whereas the addition of TAK-220 abrogated the RANTES-induced increase in the intracellular Ca2+ level in a dose-dependent manner (Fig. 4A). In contrast, TAK-220 did not affect the RANTES-induced Ca2+ mobilization in CCR1-expresing HeLa cells, even when TAK-220 was used at a concentration of 1,000 nM (Fig. 4B). These results indicate that TAK-220 interacts with CCR5 but not with RANTES and inhibits the CCR5-mediated Ca2+ signaling.
FIG. 4.
Effect of TAK-220 on RANTES-induced Ca2+ mobilization in (A) CCR5- and (B) CCR1-expressing cells. CCR5- or CCR1-expressing HeLa cells were suspended in ECB buffer, loaded with 10 μM Fura-PE3AM for 60 min, washed twice, and resuspended in the same buffer. At 90 s after exposure to various concentrations of TAK-220, 20 nM RANTES was added, and the relative increase in cytoplasmic Ca2+ levels was monitored with a fluorescence spectrometer.
Selective inhibition of R5 envelope-mediated membrane fusion by TAK-220.
In the next experiment, TAK-220 was examined for its inhibitory effect on the fusion between the HIV-1 envelope and the cell membrane by using envelope-expressing cells and CD4-, CCR5-, and CXCR4-expressing cells. TAK-220 inhibited R5 HIV-1 (JR-FL) envelope-mediated membrane fusion, with an IC50 value of 0.42 nM, but did not affect X4 HIV-1 (HXB2) envelope-mediated membrane fusion, even when it was used at a concentration of 1,000 nM (Table 1). In contrast, the CXCR4 antagonist AMD-3100 inhibited X4 HIV-1 envelope-mediated membrane fusion, with an IC50 value of 34 nM, yet it had no effect on R5 HIV-1 envelope-mediated membrane fusion even when it was used at a concentration of 1,000 nM (Table 1).
TABLE 1.
Inhibitory effect of TAK-220 on HIV-1 envelope-mediated membrane fusion
Concentrations of the test compounds required to inhibit membrane fusion by 50%.
Assays were performed in triplicate, and data are expressed as the means ± SDs of three separate experiments.
Selective inhibition of CCR5-mediated HIV-1 infection by TAK-220.
The anti-HIV-1 activity of TAK-220 was examined in two different assays. In the replication assay with U87 astroglioma cells, TAK-220 completely inhibited the replication of R5X4 HIV-1 (HE) in U87.CD4.CCR5 cells at a concentration of 1,000 nM but was totally inactive against the same strain in U87.CD4.CXCR4 cells (data not shown). On the contrary, AMD-3100 displayed apparent inhibition of R5X4 HIV-1 replication only in U87.CD4.CXCR4 cells (data not shown). In the replication assay in PBMCs obtained from two different donors, TAK-220 inhibited the replication of R5 HIV-1 (JR-FL) in a dose-dependent manner (data not shown), and its 50% effective concentrations (EC50s) with PBMCs from donors 1 and 2 were 0.60 and 0.68 nM, respectively (Table 2). However, TAK-220 did not inhibit the replication of X4 HIV-1 (IIIB) even at a concentration of 10,000 nM (Table 2). A clear difference was observed with AMD-3100, where it could inhibit X4 HIV-1 replication in PBMCs. The EC50s of AMD-3100 with PBMCs from donors 1 and 2 were 60 and 77 nM, respectively. The compound was totally inactive against R5 HIV-1 replication even at 10,000 nM (Table 2).
TABLE 2.
Anti-HIV-1 activity of TAK-220 against laboratory-adapted strains of R5 and X4 HIV-1 in PBMCs
| Compound | EC50 (nM)a
|
|||
|---|---|---|---|---|
| Expt 1b
|
Expt 2
|
|||
| JR-FL (R5) | IIIB (X4) | JR-FL (R5) | IIIB (X4) | |
| TAK-220 | 0.60c | >10,000 | 0.68 | >10,000 |
| AMD-3100 | >10,000 | 60 | >10,000 | 77 |
Concentrations of the test compounds required to inhibit the replication of HIV-1 by 50%.
PBMCs from two different donors (donors 1 and 2) were used in experiments 1 and 2, respectively.
Assays were performed in triplicate.
Anti-HIV-1 activity of TAK-220 against clinical isolates in PBMCs.
To estimate the efficacy of TAK-220 in HIV-1-infected patients, the activity of TAK-220 was examined against six R5 HIV-1 clinical isolates and one X4 HIV-1 clinical isolate in PBMCs from three different donors. Also, its activity was tested against one R5X4 HIV-1 laboratory-adapted strain. TAK-220 could inhibit the replication of all R5 isolates, with EC50s and EC90s ranging from 0.55 to 1.7 nM and from 4.0 to 28 nM, respectively (Table 3). There was a little difference in the anti-HIV-1 activity of TAK-220 among the test strains. The mean EC50 and EC90 were 1.1 and 13 nM, respectively. On the other hand, the compound was not inhibitory to the replication of R5X4 HIV-1 (HE) and X4 HIV-1 (SW), even at a concentration of 10,000 nM, in PBMCs (Table 3). TAK-220 did not reduce the viability or proliferation of mock-infected PBMCs at concentrations up to 10,000 nM (data not shown). Thus, TAK-220 was found to be a highly potent and selective inhibitor of R5 HIV-1 clinical isolates in PBMCs.
TABLE 3.
Anti-HIV-1 activity of TAK-220 against HIV-1 clinical isolates in PBMCsa
| Strain | Tropismb | No. of assays | EC50 (nM)
|
EC90 (nM)
|
||
|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | |||
| KK | R5 | 3 | 1.2 | 0.92 | 12 | 15 |
| CTV | R5 | 3 | 0.72 | 0.10 | 5.0 | 0.50 |
| HKW | R5 | 3 | 1.7 | 1.9 | 12 | 11 |
| HNK | R5 | 3 | 1.7 | 1.2 | 28 | 18 |
| HTN | R5 | 3 | 0.93 | 0.32 | 15 | 11 |
| HHA | R5 | 3 | 0.55 | 0.25 | 4.0 | 1.8 |
| Overall mean | 1.1 | 13 | ||||
| HE | R5X4 | 1 | >10,000 | >10,000 | ||
| SW | X4 | 2 | >10,000 | >10,000 | ||
Cells from different donors were used in each assay. Cells were infected with HIV-1 and incubated in the presence of various concentrations of TAK-220. The anti-HIV-1 activity was determined by measurement of p24 antigen levels on day 7 after virus infection. Assays were performed in triplicate wells.
The tropisms of clinical isolates were determined by their infectivity to U87.CD4.CCR5 and U87.CD4.CXCR4 cells.
Anti-HIV-1 activity of TAK-220 in the presence of HS.
To further estimate the efficacy of TAK-220 in vivo, the influence of HS on its anti-HIV-1 activity was examined. No substantial difference in the replication of R5 HIV-1 (JR-FL) was observed between the condition of 20% FBS alone and that of 40% HS plus 10% FBS. Similar p24 antigen levels in the control cultures (no compound) were obtained, irrespective of the presence of HS (Table 4). The EC50s of TAK-220 in the presence of 40% HS plus 10% FBS in experiments 1 and 2 were 0.41 and 1.1 nM, respectively, which were almost same as those obtained in the presence of 20% FBS (Table 4). Furthermore, the levels of protein binding of TAK-220 in human plasma were as low as 53.0 to 56.7% (data not shown). These results suggest that the activity of TAK-220 is not strongly affected by HS in vivo.
TABLE 4.
Anti-HIV-1 activity of TAK-220 in PBMCs in the presence of high concentrations of human seruma
PBMCs obtained from two different donors (donors 1 and 2) were used in experiments 1 and 2, respectively. Cells were infected with R5 HIV-1 (JR-FL) and incubated in the presence of various concentrations of TAK-220 and either 20% FBS alone or 40% HS plus 10% FBS.
The p24 levels of the control cultures (no compound) in the absence (20% FBS alone) or in the presence of HS (40% HS plus 10% FBS) were 47 and 44 ng/ml, respectively, in experiment 1 and 8.1 and 12 ng/ml, respectively, in experiment 2.
Ratio of the EC50 in the presence of 20% FBS to the EC50 in the presence of 40% HS plus 10% FBS.
Anti-HIV-1 activity of TAK-220 in PBMCs from different donors.
It appears that the anti-HIV-1 activities of compounds are often affected by the host cells obtained from different donors. Therefore, the activities of TAK-220 were examined in PBMCs from eight different donors. The p24 antigen levels in culture supernatants ranged from 0.47 to 69 ng/ml on day 7 after virus infection (Table 5), indicating that the replication efficiency of R5 HIV-1 (JR-FL) differed considerably from one donor to another. However, this difference in HIV-1 replication did not influence the anti-HIV-1 activity of TAK-220. TAK-220 inhibited R5 HIV-1 replication, with EC50s ranging from 0.17 to 2.5 nM and EC90s ranging from 5.1 to 13 nM (Table 5). In particular, the EC90s varied less than threefold among the PBMCs from the eight donors.
TABLE 5.
Anti-HIV-1 activity of TAK-220 in PBMCs from eight different donorsa
| Donor no. | p24 level (ng/ml) | EC50 (nM) | EC90 (nM) |
|---|---|---|---|
| 1 | 21 | 0.36 | 5.1 |
| 2 | 8.1 | 0.46 | 5.8 |
| 3 | 8.2 | 1.3 | 5.3 |
| 4 | 3.9 | 0.24 | 6.6 |
| 5 | 0.47 | 1.6 | 12 |
| 6 | 68 | 2.5 | 13 |
| 7 | 20 | 0.38 | 6.6 |
| 8 | 69 | 0.17 | 7.3 |
Cells were infected with R5 HIV-1 (JR-FL) and then incubated in the presence of various concentrations of TAK-220. The anti-HIV-1 activity was determined by measurement of p24 antigen levels on day 7 after virus infection. Assays were performed in triplicate wells.
Activity of TAK-220 against recombinant HIV-1 expressing different subtypes of envelope proteins.
When TAK-220 was examined for its inhibitory effect on recombinant viruses containing R5 (n = 23), X4 (n = 4), and R5X4 (n = 3) HIV-1 envelope glycoproteins in U87.CD4.CCR5 or U87.CD4.CXCR4 cells, it blocked the infection of all R5 and R5X4 HIV-1 isolates in U87.CD4.CCR5 cells, with EC50s ranging from 1.1 to 34 nM (mean EC50 = 8.9 nM) (Table 6). All subtypes evaluated in this study (subtypes A, B, C, D, E, and F) were found to be almost equally susceptible to TAK-220. The variation of their susceptibility to TAK-220 was approximately 30-fold and was independent of the envelope subtype. TAK-220 could not inhibit the infection of X4 or R5X4 HIV-1 in U87.CD4.CXCR4 cells even at a concentration of 19,000 nM (Table 6). These results suggest that the anti-HIV-1 activity of TAK-220 is coreceptor dependent and subtype independent.
TABLE 6.
Anti-HIV-1 activity of TAK-220 against recombinant HIV-1 expressing different subtype envelope glycoproteins
| Tropism | Subtype and sample no. | EC50 (nM)
|
|
|---|---|---|---|
| U87.CD4.CCR5 | U87.CD4.CXCR4 | ||
| R5 | A_R5_1 | 2.9a | NRb |
| A_R5_2 | 14 | NR | |
| A_R5_3 | 5.7 | NR | |
| B_R5_1 | 10 | NR | |
| B_R5_2 | 7.1 | NR | |
| B_R5_3 | 1.3 | NR | |
| B_R5_4 | 7.2 | NR | |
| B_R5_5 | 12 | NR | |
| B_R5_6 | 7.0 | NR | |
| B_R5_7 | 10 | NR | |
| B_R5_8 | 16 | NR | |
| B_R5_9 | 13 | NR | |
| B_R5_10 | 34 | NR | |
| C_R5_1 | 14 | NR | |
| C_R5_2 | 7.4 | NR | |
| C_R5_3 | 4.5 | NR | |
| D_R5_1 | 1.1 | NR | |
| E_R5_1 | 1.5 | NR | |
| E_R5_2 | 23 | NR | |
| E_R5_3 | 6.4 | NR | |
| F_R5_1 | 8.0 | NR | |
| F_R5_2 | 5.5 | NR | |
| F_R5_3 | 6.0 | NR | |
| R5X4 | B_Dual_1 | 7.0 | >19,000 |
| B_Dual_2 | 6.3 | >19,000 | |
| B_Dual_3 | 1.1 | >19,000 | |
| Overall mean | 8.9 | ||
| X4 | B_X4_1 | NR | >19,000 |
| B_X4_2 | NR | >19,000 | |
| B_X4_3 | NR | >19,000 | |
| D_X4_2 | NR | >19,000 | |
Data are expressed as the means of two separate experiments.
NR, not replicable.
Effect of TAK-220 on anti-CCR5 antibody binding to CCR5-expressing cells.
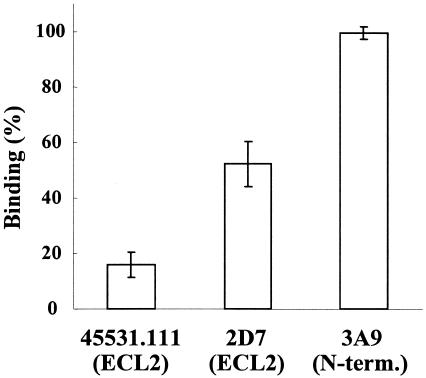
It has been reported that R5 HIV-1 gp120 interacts with the N terminus and the second extracellular loop (ECL2) of CCR5 (2, 5, 18, 31). Therefore, it is assumed that TAK-220 inhibits HIV-1 entry by interacting with these domains of CCR5. To clarify the binding site of TAK-220, the effect of TAK-220 on the binding of three anti-CCR5 MAbs was investigated. These MAbs are known to recognize different CCR5 domains. At a concentration of 100 nM, TAK-220 achieved more than 80% inhibition of the binding of 45531.111, which specifically recognizes the region including Tyr184 to Phe189 of the ECL2 of CCR5 (18) (Fig. 5). TAK-220 also inhibited by about 50% the binding of 2D7, which recognizes the region that includes Lys171 and Asp172 of the ECL2 (18) (Fig. 5). However, TAK-220 did not inhibit the binding of MAb 3A9, which is specific to the N terminus of CCR5 (41) (Fig. 5).
FIG. 5.
Effect of TAK-220 on anti-CCR5 MAb binding to CCR5-expressing cells. MOLT-4/CCR5 cells were incubated in the presence of TAK-220 (100 nM) and examined for the binding of anti-CCR5 MAbs. MAbs 45531.111 and 2D7 recognize ECL2 of CCR5, while MAb 3A9 recognizes the N terminus (N-term.) of CCR5. The MFI obtained in the absence of compound was regarded as 100% binding of each MAb. The data are expressed as the means ± SDs of three separate experiments.
Effect of TAK-220 on CCR5 internalization.
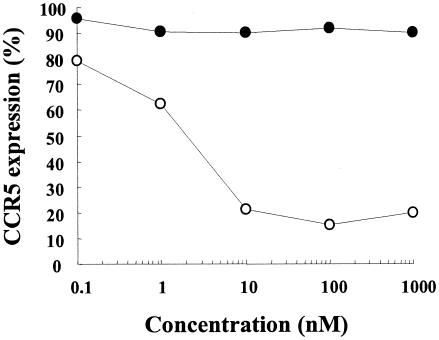
To determine whether TAK-220 inhibits HIV-1 entry through the induction of CCR5 internalization, its effect on surface CCR5 expression was examined in CCR5-expressing cells. It is known that RANTES does not inhibit the binding of the anti-CCR5 MAb 3A9 to CCR5-expressing cells (41). Since TAK-220 did not inhibit the binding of this MAb to the cells (Fig. 5), the effects of TAK-220 and RANTES on CCR5 expression in MOLT-4/CCR5 cells were determined with MAb 3A9 by flow cytometry. After incubation of MOLT-4/CCR5 cells with 10 nM RANTES for 3 h, the binding of MAb 3A9 to the cells was reduced by approximately 80% due to CCR5 internalization by RANTES (Fig. 6), as reported previously (20). In contrast, TAK-220 did not affect the binding of MAb 3A9 to CCR5 even at a concentration of 1,000 nM, indicating that TAK-220 does not induce CCR5 internalization in CCR5-expressing cells (Fig. 6).
FIG. 6.
Effect of TAK-220 on cell surface CCR5 expression. MOLT-4/CCR5 cells were incubated in the presence of various concentrations of either TAK-220 (•) or RANTES (○) for 3 h at 37°C. The expression of CCR5 on the cell surface was detected with the anti-CCR5 MAb 3A9. The MFI obtained in the absence of compound was regarded as 100% expression. The data are expressed as the means for triplicate tubes.
Pharmacokinetics of TAK-220 in animals.
After oral administration of TAK-220 at a dose of 5 mg/kg, the bioavailabilities of TAK-220 in rats and monkeys were estimated to be 9.5% and 28.9%, respectively, based on the AUC (Table 7). Thus, TAK-220 was orally bioavailable in both rats and monkeys.
TABLE 7.
Pharmacokinetics of TAK-220 after single oral administration in rats and monkeysa
| Parameter | Rat (n = 3) | Monkey (n = 4) |
|---|---|---|
| i.v./oral doses (mg/kg) | 1/5 | 1/5 |
| Bioavailability (%)b | 9.5 ± 3.8 | 28.9 ± 8.3 |
| i.v. AUC0-24c (ng · h/ml) | 141.0 ± 27.8 | 367.2 ± 48.2 |
| Oral Cmax (ng/ml) | 31.2 ± 8.1 | 298.0 ± 169.0 |
| Tmax (h) | 0.083 ± 0.0 | 0.7 ± 0.4 |
| Oral AUC0-24 (ng · h/ml) | 67.1 ± 23.7 | 538.3 ± 204.1 |
TAK-220 was administered orally or intravenously (i.v.) to fasted animals. Data are expressed as the means ± SDs.
Bioavailability = (AUCoral/AUCi.v.) × (dosei.v./doseoral) × 100. SD of bioavailability = bioavailability × [(SD of AUCoral/AUCoral)2 + (SD of AUCi.v./AUCi.v.)2]1/2.
AUC0-24, AUC from time zero to 24 h.
DISCUSSION
The present study clearly demonstrated that the inhibition of chemokine receptors by TAK-220 is highly specific to CCR5 (Fig. 3) and that the inhibition is not due to the interaction of TAK-220 with RANTES (Fig. 3 and 4). These results indicate that TAK-220 is a potent and specific antagonist of CCR5. Interestingly, TAK-220 strongly inhibited the binding of RANTES and MIP-1α to CCR5 but had no effect on the binding of MIP-1β (Fig. 2). This finding seems to be very important from an immunological viewpoint. It has been reported that CCR5-knockout mice have some immunological defects and impaired defenses against some pathogens (15, 30, 43). Therefore, sustained and long-term suppression of ligand binding to CCR5 may generate unexpected adverse effects. The nature of TAK-220 that does not inhibit the binding of MIP-1β to CCR5 could be beneficial to immune responses through this signaling pathway. In fact, TAK-220 had no effect on MIP-1β-induced chemotaxis (data not shown). On the other hand, individuals with the CCR5-Δ32 mutation that resist HIV-1 infection have no obvious health problems (19, 32). In addition, patients homozygous for the CCR5-Δ32 allele show longer survivals after renal transplants and have no obvious health problems (10). These findings imply that suppression of ligand binding to CCR5 might be compensated for by certain host defenses.
TAK-220 selectively blocked R5 HIV-1 envelope-mediated membrane fusion and R5 HIV-1 replication in PBMCs at nanomolar concentrations (Tables 1 and 2). TAK-220 also inhibited the replication of R5X4 (dual-tropic) HIV-1 in CCR5-expressing cells but not in CXCR4-expressing cells, suggesting that TAK-220 inhibits HIV-1 replication through blocking of CCR5-mediated viral entry into the host cells without affecting any other molecules involved in HIV-1 replication. Thus, the mode of action of TAK-220 is unrelated to those of the currently available anti-HIV-1 drugs, such as nucleoside or nucleotide reverse transcriptase inhibitors, nonnucleoside reverse transcriptase inhibitors, protease inhibitors, and the gp41-mediated fusion inhibitor enfuvirtide. Therefore, it would be possible that the combination of TAK-220 with these anti-HIV-1 agents could lead to favorable results in terms of clinical efficacy. In fact, TAK-220 displayed synergistic anti-HIV-1 activity when it was combined with zidovudine, lamivudine, efavirenz, indinavir, and enfuvirtide in cell culture experiments (36a).
TAK-220 inhibited the replication of six R5 HIV-1 clinical isolates with a mean EC90 of 13 nM (7 ng/ml) (Table 3). It is noteworthy that most of these isolates are multidrug-resistant strains (unpublished data). In addition, no strong PBMC donor or HIV-1 subtype dependency was observed for the anti-HIV-1 activity of TAK-220 (Tables 5 and 6). These results suggest that TAK-220 may be effective in a variety of patients infected with non-subtype B viruses. Furthermore, the anti-HIV-1 activity of TAK-220 was not affected by the presence of high concentrations of HS (Table 4). Therefore, it is considered that a plasma TAK-220 concentration of more than 7 ng/ml should be the trough level for the exertion of its clinical efficacy. This concentration could be attainable by oral administration in humans, since TAK-220 was orally bioavailable in both rats and monkeys (Table 7).
We previously reported that there was close correlation among the inhibitory effects of TAK-779 derivatives on membrane fusion, viral replication, and RANTES binding (36). In this study, however, there was some discrepancy between RANTES binding and membrane fusion or viral replication. TAK-220 inhibited RANTES binding to CCR5-expressing cells, with an IC50 of 3.5 nM (Fig. 2), whereas it could inhibit membrane fusion and viral replication, with EC50s of 0.42 nM and 0.60 to 0.68 nM (Table 1 and 2), respectively. These results indicate that TAK-220 is more inhibitory of membrane fusion and viral replication than of RANTES binding. It has been reported that the binding site of β-chemokines to CCR5 does not completely overlap with that of either recombinant gp120 or the virion (41). It is possible that the binding site of TAK-220 to CCR5 is closer to the gp120-binding site than to the β-chemokine-binding site. Unlike RANTES (20), TAK-220 did not induce CCR5 internalization in CCR5-expressing cells (Fig. 6). It blocked the binding of anti-CCR5 MAbs 45531.111 and 2D7, which recognize different regions of ECL2 of CCR5 (18) (Fig. 5). TAK-220 had no effect on the binding of anti-CCR5 MAb 3A9, which is specific to the N terminus of CCR5 (41) (Fig. 5). R5 HIV-1 gp120 interacts with the N terminus and ECL2 of CCR5 (2, 5, 18, 31). TAK-220 may inhibit viral entry through the interaction with ECL2. However, it is still unclear whether TAK-220 directly binds to ECL2 or induces its conformational change, followed by binding to a different region of CCR5. It was recently demonstrated that the small-molecule CCR5 antagonists SCH-351125 and SCH-350581 bound within a putative ligand-binding cavity formed by transmembrane helices 1, 2, 3, and 7 of CCR5 and inhibited the binding of several ECL2-recognizing MAbs to CCR5 (39). Like such SCH compounds, TAK-220 may also bind within the putative ligand-binding cavity and directly or indirectly inhibit the binding of ECL2-recognizing anti-CCR5 MAbs, yet the exact binding site of TAK-220 remains to be determined.
Since HIV-1 mutates at a considerable rate (3 in every 10,000 bp of DNA transcribed) (23), a common problem for all anti-HIV-1 agents is the rapid emergence of drug-resistant viruses. Therefore, it is anticipated that mutants resistant to TAK-220 will also emerge after its clinical use. To circumvent this problem as much as possible, in vitro isolation and characterization of the resistant mutants seems to be extremely important. A phenotypic switch from R5 to X4 HIV-1 is associated with rapid disease progression and an accelerated decrease in the numbers of CD4+ T cells (8, 29, 33). It was reported that the use of a modified RANTES (NNY-RANTES) in human peripheral blood lymphocyte-SCID mice infected with R5 HIV-1 selected X4 HIV-1 variants (24). On the other hand, HIV-1 mutants less susceptible to the anti-CCR5 MAb 2D7, MIP-1α, or the small-molecule CCR5 antagonist AD101 were isolated in vitro, but they did not switch coreceptor usage from CCR5 to CXCR4 (1, 22, 38). Accordingly, the envelope amino acid mutations of TAK-220-resistant viruses and their coreceptor usage should be elucidated. To this end, a long-term culture experiment with the KK strain, an isolate from a treatment-naïve patient, has been conducted with PBMCs and increasing concentrations of TAK-220. However, viruses significantly less susceptible to TAK-220 have not been isolated yet even after a 2-year cultivation period (unpublished data), and this experiment is still continuing.
In general, HIV-1 strains resistant to an existing class of anti-HIV-1 agents often show cross-resistance to other compounds of the same class. Once such strains have emerged in patients, the choice of alternative agents is limited in current HAART. Several pharmaceutical companies are now developing CCR5 antagonists, such as UK-427,857 (P. Dorr et al., 10th Conf. Retrovir. Opportunistic Infect., abstr. 12, 2003), SCH-D (D. Schurmann et al., 11th Conf. Retrovir. Opportunistic Infect., abstr. 140LB, 2004), AK602/ONO4128/GW873140 (21), and PRO 140 (37). In the near future, it must be clarified whether HIV-1 isolates resistant to a certain CCR5 antagonist also show cross-resistance to other CCR5 antagonists. The Russian subtype G strain RU570 was reported to be not susceptible to SCH-C (35). In our hands, however, TAK-220 could inhibit the replication of RU570, with an EC50 of 4.0 nM, as well as the other strains used in this study (data not shown).
In conclusion, TAK-220, a novel small-molecule CCR5-specific antagonist, is a highly potent and selective inhibitor of R5 HIV-1 replication. Pharmacokinetic studies of TAK-220 indicate that the compound is orally available in rats and monkeys. Thus, TAK-220 has proved to be a promising therapeutic agent for HIV-1 infection and should be evaluated for its clinical efficacy in humans.
Acknowledgments
We thank K. Kuroshima, M. Inanami, and S. Shiki for excellent technical assistance. pHXB2-env was obtained through the National Institutes of Health AIDS Research and Reference Reagent Program, National Institute of Allergy and Infectious Diseases, Bethesda, MD. Clinical isolates CTV and HHA were kindly provided by S. Matsushita (Kumamoto University, Kumamoto, Japan). Clinical isolates HKW, HNK, and HTN were kindly provided by S. Oka (International Medical Center of Japan, Tokyo, Japan).
This study was supported in part by a grant-in-aid for scientific research from the Japan Society for the Promotion of Science to M. Baba.
REFERENCES
- 1.Aarons, E. J., S. Beddows, T. Willinghaml Wu, and R. A. Koup. 2001. Adaptation to blockade of human immunodeficiency virus type 1 entry imposed by the anti-CCR5 monoclonal antibody 2D7. Virology 287:382-390. [DOI] [PubMed] [Google Scholar]
- 2.Alkhatib, G., S. S. Ahuja, D. Light, S. Mummidi, E. A. Berger, and S. K. Ahuja. 1997. CC chemokine receptor 5-mediated signaling and HIV-1 co-receptor activity share common structural determinants. Critical residues in the third extracellular loop support HIV-1 fusion. J. Biol. Chem. 272:19771-19776. [DOI] [PubMed] [Google Scholar]
- 3.Baba, M., H. Miyake, M. Okamoto, Y. Iizawa, and K. Okonogi. 2000. Establishment of a CCR5-expressing T-lymphoblastoid cell line highly susceptible to R5 HIV type 1. AIDS Res. Hum. Retrovir. 16:935-941. [DOI] [PubMed] [Google Scholar]
- 4.Baba, M., O. Nishimura, N. Kanzaki, M. Okamoto, H. Sawada, Y. Iizawa, M. Shiraishi, Y. Aramaki, K. Okonogi, Y. Ogawa, K. Meguro, and M. Fujino. 1999. A small-molecule, nonpeptide CCR5 antagonist with highly potent and selective anti-HIV-1 activity. Proc. Natl. Acad. Sci. USA 96:5698-5703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bieniasz, P. D., R. A. Fridell, I. Aramori, S. S. Ferguson, M. G. Caron, and B. R. Cullen. 1997. HIV-1-induced cell fusion is mediated by multiple regions within both the viral envelope and the CCR-5 co-receptor. EMBO J. 16:2599-2609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carpenter, C. C., M. A. Fischl, S. M. Hammer, M. S. Hirsch, D. M. Jacobsen, D. A. Katzenstein, J. S. Montaner, D. D. Richman, M. S. Saag, R. T. Schooley, M. A. Thompson, S. Vella, P. G. Yeni, and P. A. Volberding. 1998. Antiretroviral therapy for HIV infection in 1998: updated recommendations of the International AIDS Society-USA Panel. JAMA 280:78-86. [DOI] [PubMed] [Google Scholar]
- 7.Chun, T. W., R. T. Davey, Jr., D. Engel, H. C. Lane, and A. S. Fauci. 1999. Re-emergence of HIV after stopping therapy. Nature 401:874-875. [DOI] [PubMed] [Google Scholar]
- 8.Connor, R. I., K. E. Sheridan, D. Ceradini, S. Choe, and N. R. Landau. 1997. Change in coreceptor use correlates with disease progression in HIV-1-infected individuals. J. Exp. Med. 185:621-628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Deeks, S. G., M. Smith, M. Holodniy, and J. O. Kahn. 1997. HIV-1 protease inhibitors. A review for clinicians. JAMA 277:145-153. [PubMed] [Google Scholar]
- 10.Fischereder, M., B. Luckow, B. Hocher, R. P. Wuthrich, U. Rothenpieler, H. Schneeberger, U. Panzer, R. A. Stahl, I. A. Hauser, K. Budde, H. Neumayer, B. K. Kramer, W. Land, and D. Schlondorff. 2001. CC chemokine receptor 5 and renal-transplant survival. Lancet 357:1758-1761. [DOI] [PubMed] [Google Scholar]
- 11.Grant, R. M., F. M. Hecht, M. Warmerdaml Liu, T. Liegler, C. J. Petropoulos, N. S. Hellmann, M. Chesney, M. P. Busch, and J. O. Kahn. 2002. Time trends in primary HIV-1 drug resistance among recently infected persons. JAMA 288:181-188. [DOI] [PubMed] [Google Scholar]
- 12.Gulick, R. M., J. W. Mellors, D. Havlir, J. J. Eron, C. Gonzalez, D. McMahon, D. D. Richman, F. T. Valentine, L. Jonas, A. Meibohm, E. A. Emini, and J. A. Chodakewitz. 1997. Treatment with indinavir, zidovudine, and lamivudine in adults with human immunodeficiency virus infection and prior antiretroviral therapy. N. Engl. J. Med. 337:734-739. [DOI] [PubMed] [Google Scholar]
- 13.Hammer, S. M., K. E. Squires, M. D. Hughes, J. M. Grimes, L. M. Demeter, J. S. Currier, J. J. Eron, Jr., J. E. Feinberg, H. H. Balfour, Jr., L. R. Deyton, J. A. Chodakewitz, M. A. Fischl, et al. 1997. A controlled trial of two nucleoside analogues plus indinavir in persons with human immunodeficiency virus infection and CD4 cell counts of 200 per cubic millimeter or less. N. Engl. J. Med. 337:725-733. [DOI] [PubMed] [Google Scholar]
- 14.Huang, Z. 2002. Structure, function and modulation of chemokine receptors: members of the g-protein-coupled receptor superfamily. Mini Rev. Med. Chem. 2:373-383. [DOI] [PubMed] [Google Scholar]
- 15.Huffnagle, G. B., L. K. McNeil, R. A. McDonald, J. W. Murphy, G. B. Toews, N. Maeda, and W. A. Kuziel. 1999. Cutting edge: role of C-C chemokine receptor 5 in organ-specific and innate immunity to Cryptococcus neoformans. J. Immunol. 163:4642-4646. [PubMed] [Google Scholar]
- 16.Lalezari, J. P., K. Henry, M. O'Hearn, J. S. Montaner, P. J. Piliero, B. Trottier, S. Walmsley, C. Cohen, D. R. Kuritzkes, J. J. Eron, Jr., J. Chung, R. DeMasi, L. Donatacci, C. Drobnes, J. Delehanty, M. Salgo, et al. 2003. Enfuvirtide, an HIV-1 fusion inhibitor, for drug-resistant HIV infection in North and South America. N. Engl. J. Med. 348:2175-2185. [DOI] [PubMed] [Google Scholar]
- 17.Lazzarin, A., B. Clotet, D. Cooper, J. Reynes, K. Arasteh, M. Nelson, C. Katlama, H. J. Stellbrink, J. F. Delfraissy, J. Lange, L. Huson, R. DeMasi, C. Wat, J. Delehanty, C. Drobnes, M. Salgo, et al. 2003. Efficacy of enfuvirtide in patients infected with drug-resistant HIV-1 in Europe and Australia. N. Engl. J. Med. 348:2186-2195. [DOI] [PubMed] [Google Scholar]
- 18.Lee, B., M. Sharron, C. Blanpain, B. J. Doranz, J. Vakili, P. Setoh, E. Berg, G. Liu, H. R. Guy, S. R. Durell, M. Parmentier, C. N. Chang, K. Price, M. Tsang, and R. W. Doms. 1999. Epitope mapping of CCR5 reveals multiple conformational states and distinct but overlapping structures involved in chemokine and coreceptor function. J. Biol. Chem. 274:9617-9626. [DOI] [PubMed] [Google Scholar]
- 19.Liu, R., W. A. Paxton, S. Choe, D. Ceradini, S. R. Martin, R. Horuk, M. E. MacDonald, H. Stuhlmann, R. A. Koup, and N. R. Landau. 1996. Homozygous defect in HIV-1 coreceptor accounts for resistance of some multiply-exposed individuals to HIV-1 infection. Cell 86:367-377. [DOI] [PubMed] [Google Scholar]
- 20.Mack, M., B. Luckow, P. J. Nelson, J. Cihak, G. Simmons, P. R. Clapham, N. Signoret, M. Marsh, M. Stangassinger, F. Borlat, T. N. Wells, D. Schlondorff, and A. E. Proudfoot. 1998. Aminooxypentane-RANTES induces CCR5 internalization but inhibits recycling: a novel inhibitory mechanism of HIV infectivity. J. Exp. Med. 187:1215-1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maeda, K., H. Nakata, Y. Koh, T. Miyakawa, H. Ogata, Y. Takaoka, S. Shibayama, K. Sagawa, D. Fukushima, J. Moravek, Y. Koyanagi, and H. Mitsuya. 2004. Spirodiketopiperazine-based CCR5 inhibitor which preserves CC-chemokine/CCR5 interactions and exerts potent activity against R5 human immunodeficiency virus type 1 in vitro. J. Virol. 78:8654-8662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maeda, Y., M. Foda, S. Matsushita, and S. Harada. 2000. Involvement of both the V2 and V3 regions of the CCR5-tropic human immunodeficiency virus type 1 envelope in reduced sensitivity to macrophage inflammatory protein 1α. J. Virol. 74:1787-1793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mansky, L. M., and H. M. Temin. 1995. Lower in vivo mutation rate of human immunodeficiency virus type 1 than that predicted from the fidelity of purified reverse transcriptase. J. Virol. 69:5087-5094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mosier, D. E., G. R. Picchio, R. J. Gulizia, R. Sabbe, P. Poignard, L. Picard, R. E. Offord, D. A. Thompson, and J. Wilken. 1999. Highly potent RANTES analogues either prevent CCR5-using human immunodeficiency virus type 1 infection in vivo or rapidly select for CXCR4-using variants. J. Virol. 73:3544-3550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Palella, F. J., Jr., K. M. Delaney, A. C. Moorman, M. O. Loveless, J. Fuhrer, G. A. Satten, D. J. Aschman, S. D. Holmberg, et al. 1998. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. N. Engl. J. Med. 338:853-860. [DOI] [PubMed] [Google Scholar]
- 26.Pantaleo, G., O. J. Cohen, T. Schacker, M. Vaccarezza, C. Graziosi, G. P. Rizzardi, J. Kahn, C. H. Fox, S. M. Schnittman, D. H. Schwartz, L. Corey, and A. S. Fauci. 1998. Evolutionary pattern of human immunodeficiency virus (HIV) replication and distribution in lymph nodes following primary infection: implications for antiviral therapy. Nat. Med. 4:341-345. [DOI] [PubMed] [Google Scholar]
- 27.Pauwels, R., J. Balzarini, M. Baba, R. Snoeck, D. Schols, P. Herdewijn, J. Desmyter, and E. De Clercq. 1988. Rapid and automated tetrazolium-based colorimetric assay for the detection of anti-HIV compounds. J. Virol. Methods 20:309-312. [DOI] [PubMed] [Google Scholar]
- 28.Petropoulos, C. J., N. T. Parkin, K. L. Limoli, Y. S. Lie, T. Wrin, W. Huang, H. Tian, D. Smith, G. A. Winslow, D. J. Capon, and J. M. Whitcomb. 2000. A novel phenotypic drug susceptibility assay for human immunodeficiency virus type 1. Antimicrob. Agents Chemother. 44:920-928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Richman, D. D., and S. A. Bozzette. 1994. The impact of the syncytium-inducing phenotype of human immunodeficiency virus on disease progression. J. Infect. Dis. 169:968-974. [DOI] [PubMed] [Google Scholar]
- 30.Salazar-Mather, T. P., J. S. Orange, and C. A. Biron. 1998. Early murine cytomegalovirus (MCMV) infection induces liver natural killer (NK) cell inflammation and protection through macrophage inflammatory protein 1α (MIP-1α)-dependent pathways. J. Exp. Med. 187:1-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Samson, M., G. LaRosa, F. Libert, P. Paindavoine, M. Detheux, G. Vassart, and M. Parmentier. 1997. The second extracellular loop of CCR5 is the major determinant of ligand specificity. J. Biol. Chem. 272:24934-24941. [DOI] [PubMed] [Google Scholar]
- 32.Samson, M., F. Libert, B. J. Doranz, J. Rucker, C. Liesnard, C. M. Farber, S. Saragosti, C. Lapoumeroulie, J. Cognaux, C. Forceille, G. Muyldermans, C. Verhofstede, G. Burtonboy, M. Georges, T. Imai, S. Rana, Y. Yi, R. J. Smyth, R. G. Collman, R. W. Doms, G. Vassart, and M. Parmentier. 1996. Resistance to HIV-1 infection in Caucasian individuals bearing mutant alleles of the CCR-5 chemokine receptor gene. Nature 382:722-725. [DOI] [PubMed] [Google Scholar]
- 33.Schuitemaker, H., N. A. Kootstra, M. H. Koppelman, S. M. Bruisten, H. G. Huisman, M. Tersmette, and F. Miedema. 1992. Proliferation-dependent HIV-1 infection of monocytes occurs during differentiation into macrophages. J. Clin. Investig. 89:1154-1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Siliciano, J. D., J. Kajdas, D. Finzi, T. C. Quinn, K. Chadwick, J. B. Margolick, C. Kovacs, S. J. Gange, and R. F. Siliciano. 2003. Long-term follow-up studies confirm the stability of the latent reservoir for HIV-1 in resting CD4+ T cells. Nat. Med. 9:727-728. [DOI] [PubMed] [Google Scholar]
- 35.Strizki, J. M., S. Xu, N. E. Wagner, L. Wojcik, J. Liu, Y. Hou, M. Endres, A. Palani, S. Shapiro, J. W. Clader, W. J. Greenlee, J. R. Tagat, S. McCombie, K. Cox, A. B. Fawzi, C. C. Chou, C. Pugliese-Sivo, L. Davies, M. E. Moreno, D. D. Ho, A. Trkola, C. A. Stoddart, J. P. Moore, G. R. Reyes, and B. M. Baroudy. 2001. SCH-C (SCH 351125), an orally bioavailable, small molecule antagonist of the chemokine receptor CCR5, is a potent inhibitor of HIV-1 infection in vitro and in vivo. Proc. Natl. Acad. Sci. USA 98:12718-12723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Takashima, K., H. Miyake, R. A. Furuta, J. Fujisawa, Y. Iizawa, N. Kanzaki, M. Shiraishi, K. Okonogi, and M. Baba. 2001. Inhibitory effects of small-molecule CCR5 antagonists on human immunodeficiency virus type 1 envelope-mediated membrane fusion and viral replication. Antimicrob. Agents Chemother. 45:3538-3543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36a.Tremblay, C. L., F. Giguel, Y. Guan, T.-C. Chou, K. Takashima, and M. S. Hirsch. 2005. TAK-220, a novel small-molecule CCR5 antagonist, has favorable anti-human immunodeficiency virus interactions with other antiretrovirals in vitro. Antimicrob. Agents Chemother. 49:3490-3492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Trkola, A., T. J. Ketas, K. A. Nagashima, L. Zhao, T. Cilliers, L. Morris, J. P. Moore, P. J. Maddon, and W. C. Olson. 2001. Potent, broad-spectrum inhibition of human immunodeficiency virus type 1 by the CCR5 monoclonal antibody PRO 140. J. Virol. 75:579-588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Trkola, A., S. E. Kuhmann, J. M. Strizki, E. Maxwell, T. Ketas, T. Morgan, P. Pugach, S. Xu, L. Wojcik, J. Tagat, A. Palani, S. Shapiro, J. W. Clader, S. McCombie, G. R. Reyes, B. M. Baroudy, and J. P. Moore. 2002. HIV-1 escape from a small molecule, CCR5-specific entry inhibitor does not involve CXCR4 use. Proc. Natl. Acad. Sci. USA 99:395-400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tsamis, F., S. Gavrilov, F. Kajumo, C. Seibert, S. Kuhmann, T. Ketas, A. Trkola, A. Palani, J. W. Clader, J. R. Tagat, S. McCombie, B. Baroudy, J. P. Moore, T. P. Sakmar, and T. Dragic. 2003. Analysis of the mechanism by which the small-molecule CCR5 antagonists SCH-351125 and SCH-350581 inhibit human immunodeficiency virus type 1 entry. J. Virol. 779:5201-5208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wild, C., T. Greenwell, and T. Matthews. 1993. A synthetic peptide from HIV-1 gp41 is a potent inhibitor of virus-mediated cell-cell fusion. AIDS Res. Hum. Retrovir. 9:1051-1053. [DOI] [PubMed] [Google Scholar]
- 41.Wu, L., G. LaRosa, N. Kassam, C. J. Gordon, H. Heath, N. Ruffing, H. Chen, J. Humblias, M. Samson, M. Parmentier, J. P. Moore, and C. R. Mackay. 1997. Interaction of chemokine receptor CCR5 with its ligands: multiple domains for HIV-1 gp120 binding and a single domain for chemokine binding. J. Exp. Med. 186:1373-1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yeni, P. G., S. M. Hammer, M. S. Hirsch, M. S. Saag, M. Schechter, C. C. Carpenter, M. A. Fischl, J. M. Gatell, B. G. Gazzard, D. M. Jacobsen, D. A. Katzenstein, J. S. Montaner, D. D. Richman, R. T. Schooley, M. A. Thompson, S. Vella, and P. A. Volberding. 2004. Treatment for adult HIV infection: 2004 recommendations of the International AIDS Society-USA Panel. JAMA 292:251-265. [DOI] [PubMed] [Google Scholar]
- 43.Zhou, Y., T. Kurihara, R. P. Ryseck, Y. Yang, C. Ryan, J. Loy, G. Warr, and R. Bravo. 1998. Impaired macrophage function and enhanced T cell-dependent immune response in mice lacking CCR5, the mouse homologue of the major HIV-1 coreceptor. J. Immunol. 160:4018-4025. [PubMed] [Google Scholar]