Abstract
Cationic amphipathic peptides have been extensively investigated as a potential source of new antimicrobials that can complement current antibiotic regimens in the face of emerging drug-resistant bacteria. However, the suppression of antimicrobial activity under certain biologically relevant conditions (e.g., serum and physiological salt concentrations) has hampered efforts to develop safe and effective antimicrobial peptides for clinical use. We have analyzed the activity and selectivity of the human peptide LL37 and the de novo engineered antimicrobial peptide WLBU2 in several biologically relevant conditions. The host-derived synthetic peptide LL37 displayed high activity against Pseudomonas aeruginosa but demonstrated staphylococcus-specific sensitivity to NaCl concentrations varying from 50 to 300 mM. Moreover, LL37 potency was variably suppressed in the presence of 1 to 6 mM Mg2+ and Ca2+ ions. In contrast, WLBU2 maintained its activity in NaCl and physiologic serum concentrations of Mg2+ and Ca2+. WLBU2 is able to kill P. aeruginosa (106 CFU/ml) in human serum, with a minimum bactericidal concentration of <9 μM. Conversely, LL37 is inactive in the presence of human serum. Bacterial killing kinetic assays in serum revealed that WLBU2 achieved complete bacterial killing in 20 min. Consistent with these results was the ability of WLBU2 (15 to 20 μM) to eradicate bacteria from ex vivo samples of whole blood. The selectivity of WLBU2 was further demonstrated by its ability to specifically eliminate P. aeruginosa in coculture with human monocytes or skin fibroblasts without detectable adverse effects to the host cells. Finally, WLBU2 displayed potent efficacy against P. aeruginosa in an intraperitoneal infection model using female Swiss Webster mice. These results establish a potential application of WLBU2 in the treatment of bacterial sepsis.
The emergence of multiple drug resistance among bacterial pathogens has stimulated the search for new anti-infective agents that can complement current antibiotic regimens (17, 27, 41, 44, 50, 53). Cationic amphipathic peptides (CAPs) have been extensively investigated as a potential source of these agents (22, 26, 37, 44) and are a major class of antimicrobial peptides with different secondary structures (α-helix, β-sheets, loops, etc.) that provide neutrophils and epithelial surfaces of multicellular organisms with a rapid and efficient means of inactivating invading pathogens (3, 28, 29, 39, 49, 58, 59, 62). These ubiquitous peptides typically consist of 20 to 40 amino acid residues and are highly cationic. Most host-derived CAPs display broad activity against both gram-positive and gram-negative bacteria (3, 4, 6, 8, 13).
The mechanisms of action of antimicrobial peptides, although not completely elucidated, have been widely studied. The interactions of CAPs with their microbial targets are thought to occur electrostatically, mediated by lipid molecules on bacterial surfaces (3, 23, 34, 35). Numerous studies of CAP interactions with variably charged liposomes suggest that antimicrobial peptides selectively bind bacterial over eukaryotic cell membranes because of the negatively charged lipids found on the outer leaflets of the former (10, 20). The mechanisms of the antitumor and antiviral properties of CAPs are thought to be related to the presence of negatively charged phosphatidylserine on the surfaces of tumor cells (15, 60) and sialic acid and heparan sulfate associated with viruses (2, 15, 18, 38). CAP antifungal (7, 16) and immunomodulatory (1, 5, 24, 40, 52) properties have also been reported.
These peptides offer an appealing alternative as antimicrobial agents that can be used to combat multidrug resistance. Some bacteria display decreased susceptibility to several antimicrobial peptides by modifying their lipopolysaccharide or lipoteichoic acid molecules, leading to a decrease in net negative charge on the bacterial surface (12, 14). However, unlike the selection of resistance observed with current antibiotic treatments (48), the emergence of CAP-resistant phenotypes via multiple passages of bacterial strains is uncommon. In comparison to current antibacterial drugs, CAPs have the ability to kill bacteria very quickly (within 30 to 180 s) (57). Rapid killing allows little time for a particular genetic mutation in a bacterial cell to result in successful changes in the lipid bilayer (even in the presence of prolonged CAP exposure) because this process requires gross modification of enzymatic pathways.
Nevertheless, there are multiple obstacles to developing peptides that are of therapeutic consideration. Despite the broad activity of host-derived peptides, their potency is often optimal only under specific conditions. For instance, several CAPs, although highly active in phosphate buffer, display a significant decrease in antibacterial potency in the presence of physiological concentrations of NaCl and divalent cations (11, 42, 51, 56, 58, 62). In some cases (e.g., cystic fibrosis airway fluids), hypo-osmolar NaCl may be sufficient to significantly suppress antimicrobial function (25). Thus, the development of CAPs has been directed toward overcoming some of these challenges by either modifying host-derived peptides (23) or selecting specific amino acids for de novo design (38, 46, 47). Although several newly engineered CAPs demonstrate a significant degree of salt resistance (22, 47), current studies have not indicated that these peptides can overcome the obstacles encountered in physiological fluids and more complex cellular systems. Such limitations have shifted the focus to developing CAPs as topical agents (19).
We previously reported the de novo design of modular CAPs comprised of multimers of 12-residue lytic base unit (LBU) sequences of Val and Arg and sequences with Trp substitutions (the LBU and WLBU series, respectively). In these peptides, the cationic (Arg) and hydrophobic (Val and Trp) domains were maximally segregated when optimized as idealized amphipathic helical structures. The characterization of the activities of the LBU and WLBU peptide series against Pseudomonas aeruginosa and Staphylococcus aureus led to the selection of WLBU2 (a 24-mer) as the shortest peptide with the highest antimicrobial potential, maximal helical propensity, and minimal toxicity to mammalian cells (21).
The engineered peptide derivative WLBU2 (RRWVRRVRRWVRRVVRVVRRWVRR) is composed predominantly of Arg (13 residues) and Val (8 residues), with 3 Trp residues in the hydrophobic face separated from each other by at least 7 amino acids. The residues in WLBU2 were arranged to form an idealized helical and amphipathic structure, with optimal charge and hydrophobic densities (21). By comparison, LL37 (LLGDFFRKSKEKIGKEFKRIVQRIKDFLRNLVPRTES) is an α-helical peptide with mainly Lys and Arg in the hydrophilic face and several different hydrophobic amino acid residues (Phe, Val, Ile, etc.) in the hydrophobic domain. LL37 has distinct cationic and hydrophobic domains, with an amphipathic structure that is not as ideally optimized as that of WLBU2.
Based on these subtle but important structural differences, we compared the potency of WLBU2 to that of LL37 under rigorous test conditions to demonstrate how certain limitations of host antimicrobial agents can be overcome using newly designed peptides with optimized amphipathic structures. To conclude these studies, the in vivo efficacy of WLBU2 was examined in a mouse model of P. aeruginosa bacteremia. The results of our investigations suggest that WLBU2 is an antimicrobial agent that may have applications to systemic infections.
MATERIALS AND METHODS
Peptide synthesis.
The peptides WLBU2 and LL37 were synthesized using standard FMOC synthesis protocols as previously described (57). Synthetic peptides were characterized and purified by HPLC on Vydac C18 or C4 columns (The Separations Group, Hesperia, CA), and the identity of each was established by mass spectrometry (Electrospray Quatro II triple quadruple mass spectrometer; Micromass Inc., Manchester, United Kingdom). Peptide concentrations were determined using a quantitative ninhydrin assay as previously described (57). A peptide sample of known concentration was used to evaluate WLBU2 by spectrophotometric analysis, based on Trp absorbance at 280 nm. By plotting absorbances at 280 nm against peptide concentrations, a standard curve was generated from which concentrations of WLBU2 were deduced.
Bacterial killing assays.
Bacterial killing assays were conducted as previously described (21, 57) using the P. aeruginosa strain PAO1 (Ampr) provided by Barbara Iglewski (University of Rochester, Rochester, NY). In selected studies, a methicillin-resistant strain of S. aureus (MRSA) (obtained from Children's Hospital of Pittsburgh Microbiology Laboratory) was used as a target bacterial strain. The susceptibility of these index bacteria to the peptides described was performed using the standard NCCLS broth microdilution assay modified to compare the influences of NaCl, Mg2+, Ca2+, serum, and whole blood on the activity of WLBU2 and LL37. The medium used was 10 mM phosphate buffer (PB), pH 7.2, to which the aforementioned substances were added as described for each experiment. In all except the kinetic experiment, exposure time was held at 30 min. Bacterial suspensions (ca. 106 CFU/ml) in PB (10 mM, pH 7.0) containing NaCl, Mg2+, Ca2+, serum, or whole blood as described below were incubated with twofold dilutions of peptides for 30 min at 37°C. Following treatment, the bacterial samples were plated on tryptic soy agar (TSA) (Difco, Detroit, MI) to assay viable bacteria. Surviving colonies were counted the following day to determine the minimum bactericidal concentration (MBC), defined as the molar concentration of peptide reducing the viable bacteria within a suspension by three orders of magnitude. Results were expressed on a molar basis as an average of MBCs obtained from two to five independent experiments. This assay was also performed in the presence of heat-inactivated human serum (44) or plasma, ACES [N-(2-acetamido)-2-aminoethanesulfonic acid] buffer (0.05 mM ACES, 0.12 mM NaCl, pH 7.34), or ACES-based CaCl2 or MgCl2 (Sigma, St. Louis, MO), as indicated. As a substitute for PB, ACES buffer was used to avoid the precipitation of calcium or magnesium phosphates.
Kinetics of bacterial killing.
The procedure of bacterial killing assays was modified to determine the rate of bacterial killing by WLBU2 and LL37. P. aeruginosa strain PAO1 (ca. 106 CFU/ml) was treated with 15 μM peptide in human serum (isolated from blood samples taken from healthy donors) and 1 μM peptide in phosphate-buffered saline (PBS). Aliquots of 20 μl of the peptide-treated suspension were withdrawn at different times from 0 to 30 min at room temperature, serially diluted, and plated on TSA to determine bacterial counts. Average values of triplicates were expressed as log CFU/ml plotted against time (min).
Selective toxicity in coculture systems. (i) Selective toxicity in human whole blood.
Heparinized human blood from healthy donors (with the approval of the University of Pittsburgh Institutional Review Board) was inoculated with mid-log-phase P. aeruginosa PAO1 cells (ca. 106 CFU/ml) and then treated with variable concentrations of peptide ranging from 0 to 50 μM to a total volume of 550 μl. The reaction mixture was incubated at 37°C for 60 min with gentle shaking. To determine viable bacterial count, each sample was serially diluted using 20 μl as described above. For parallel analysis of red blood cell (RBC) lysis, the remaining suspension was spun at 14,000 rpm (20,000 × g) in an Eppendorf centrifuge model 5417C (Brinkmann Instruments, Westbury, NY) for 4 min, and 50 μl of the supernatant was diluted in 450 μl of distilled water. Similarly, 0 to 50 μl untreated blood was diluted with water to a final volume of 500 μl, and the supernatant (hemoglobin suspension) was used to produce a standard curve of RBC lysis. The average absorbance values of the supernatants of all samples (200 μl) were measured in duplicate in a plate reader at 570 nm as a measure of hemoglobin released from lysed cells. Red blood cell lysis buffer (8.3 g/liter ammonium chloride in 0.01 M Tris-HCl, pH 7.5 [Sigma]) was also used as a control (50 μl whole blood and 450 μl buffer). The percent RBC lysis and bacterial survival were compared to determine the selective potential of the peptides. These experiments were verified by four independent trials.
(ii) Coculture of human cells with P. aeruginosa.
To determine the selectivity of WLBU2 between bacteria and human cells, the activities of WLBU2 and LL37 were assayed in coculture of human monocytes or primary human skin fibroblasts (HSF) with P. aeruginosa. Peripheral blood monocytes were isolated from buffy coats obtained from sterile and heparinized human blood samples. Using histopaque gradient centrifugation (Sigma), peripheral blood mononuclear cells (PBMC) were isolated and monocytes subsequently removed by adherence on 2% gelatin (Sigma) plates. After isolation, the monocytes in RPMI were transferred to a 96-well plate (105 cells/well in RPMI or Iscove's modified Dulbecco's medium [IMDM]) and incubated at 37°C and 6% CO2 until a monolayer was formed. The medium was aspirated, and a 100-μl suspension of P. aeruginosa PAO1 (2 × 106 cells/ml in 99% human serum) was added to each well. The bacterial suspensions over the eukaryotic cell monolayer were further diluted to 106 cells/ml with equal volumes of peptide at various concentrations (0 to 100 μM) in 99% human serum. The coculture was then incubated at 37°C for 30 min. To determine bacterial survival, the coculture medium was serially diluted (after gentle pipetting) up to 1:1,000 by transferring 20-μl aliquots to another 96-well plate containing 180 μl PBS per well. Each dilution was plated (100 μl) on TSA and incubated overnight at 37°C. Bacterial counts were then determined and expressed as logarithm of CFU per ml (log CFU/ml).
To further characterize the selectivity of WLBU2 between P. aeruginosa and human cells, this coculture assay was also performed using HSF (passage 20) in IMDM (Invitrogen, Grand Island, NY).
Measurement of host cell viability after peptide treatment was accomplished using a tetrazolium-based colorimetric assay (34, 63). After two consecutive gentle washes with PBS, the cells were incubated in 100 μl IMDM (HSF) or RPMI (monocytes) containing 10% fetal bovine serum (vol/vol) and 0.5 mg/ml MTT Formazan (MTT) (Sigma). The reaction mixtures were incubated at 37°C in 5% CO2 for 4 to 6 h, after which equal volumes of 0.1 N HCl-isopropanol were added to dissolve the resulting blue crystals. The percent viability was assessed by taking absorbance measurements at 570 nm using the Dynatech MR5000 96-well plate reader (Germantown, MD). As controls, cells were first treated with 100% serum (the test medium) in the presence or absence of bacteria or with 100% lysis buffer (8.76 g NaCl, 10 g DOC, 1 M Tris-HCl [pH 8.0], and 10 g Triton X-100 per liter of distilled H2O) in the absence of bacteria. The experiments were performed in triplicate, and viability data were averaged. The final toxicity values were expressed as the mean percent toxicity for each test condition minus any observed toxicity to the human cells by bacterial treatment alone.
(iii) Host toxicity and proliferation assays in the absence of bacteria.
To investigate the influence of WLBU2 on human cells in the absence of bacteria, we sought to determine whether long-term peptide treatment of human cells would adversely affect cell viability or functionality. Thus, freshly isolated human blood monocytes were treated with 15 μM WLBU2 or LL37 for 48 h at 37°C and 6% CO2 in human serum. Cell viability in the presence or absence of peptide was evaluated by MTT staining as described above. To characterize the influence of the peptides on lymphocytic proliferation, PBMC were isolated and treated with 15 μM peptide in RPMI-based 70% fetal bovine serum for 30 min at 37°C and 6% CO2. Subsequently, equal volumes of the mitogens, phorbol myristate acetate (50 ng/ml), and ionomycin (250 ng/ml) in RPMI were added to each well. After 3 days, [3H]thymidine (1 μCi/well in 50 μl RPMI) was added to the test and control (no peptide and/or unstimulated) wells. Following incubation for 16 to 18 h, the cells were harvested onto a filtermat using a Tomtec cell harvester (Hamden, CT), and the incorporation of [3H]thymidine was detected in a Wallac Microbeta liquid scintillation counter (Turku, Finland). To calculate stimulation indices, radioactive counts per minute for mitogen-treated (stimulated) samples were divided by counts per minute for untreated samples (unstimulated).
In vivo toxicity.
Before evaluating the antibacterial efficacy of WLBU2 in vivo, it was essential to examine its toxic potential, which was performed via intravenous (i.v.) administration. Female Swiss Webster mice (Taconic, Germantown, NY) were injected i.v. with 0.1 ml PBS and 3, 6, 12, or 16 mg WLBU2 per kg of body weight. Each dose was administered twice within 24 h, and all mice (10/group) were monitored for signs of toxicity, such as weight loss, piloerection, motility, histopathology, and survival.
Intraperitoneal bacterial inoculation followed by intravenous antibacterial therapy.
Mid-log-phase bacteria (P. aeruginosa PAO1, Ampr) were prepared in sterile PBS to achieve the desired bacterial suspensions for intraperitoneal (i.p.) injections. Prior to examining the efficacy of WLBU2 in vivo, the minimum Pseudomonas lethal dose (PLD), the minimum dose causing 100% mortality, was determined (107 CFU) and used in a 0.5-ml volume to inject bacteria i.p. The animals were then randomized to receive i.v. isotonic sodium chloride solution (control group) or 3 mg/kg WLBU2 30 to 45 min after bacterial challenge. The animals in each group, which included 14 mice (7/group for each of two independent trials), were returned to individual cages and subsequently monitored for 7 to 10 days. The end points of the study were indicated either by 7 to 10 days of survival or by complete absence of motility and hypothermia (Thermistor thermometer; Kent Scientific, Torrington, Connecticut) as signs of terminal illness or lethality.
Evaluation of treatment.
Quantitative blood cultures on carbenicillin TSA plates (200 μg/ml) were performed to determine bacterial loads over the course of the infection. Blood samples were obtained from the tail vein by aseptic percutaneous puncture 0.5 h to 36 h after bacterial challenge and serially diluted. Then, a 0.1-ml volume of each dilution was spread on carbenicillin TSA plates and incubated at 37°C overnight for enumeration of developed colonies. At the disease end point, animals were euthanized, and tissues were weighed and homogenized using 70-μm cell strainers (Becton Dickinson, Franklin Lakes, NJ) to determine the bacterial CFU/g of tissue. To compare fatality rates between different groups (treated and mock treated), the log rank test was performed using GraphPad Prism version 3.00 for Windows (GraphPad Software, San Diego, California). Significance was accepted when P values were less than 0.05.
RESULTS
Influence of physiological salt concentrations on antipseudomonal activity.
As previously mentioned, the activity of host-derived antimicrobial peptides can be inhibited in the presence of physiological serum concentrations of sodium chloride and divalent cations (43, 58). For instance, we initially demonstrated that LL37 was inactive against S. aureus in PBS (21). However, the activity of the de novo engineered peptide WLBU2 was not altered under such conditions. To further examine the influence of salt on antibacterial activity, we treated P. aeruginosa with different peptide concentrations in various NaCl conditions up to 300 mM. As indicated in Table 1, the peptides were highly toxic to P. aeruginosa in NaCl (at all tested concentrations), with MBCs ranging from 0.5 to 1 μM (2.3 to 4.5 μg/ml) for LL37 and ≤0.5 μM (1.7 μg/ml) for WLBU2. However, LL37 displayed a significant salt-dependent decrease in activity against MRSA, with an MBC greater than 2 μM in as little as 50 mM NaCl (data not shown). In contrast, the activity of WLBU2 against MRSA remained unchanged under various NaCl conditions, with MBCs of <0.5 μM (data not shown). These results provide evidence that, unlike LL37, WLBU2 has the ability to resist broad changes in NaCl concentrations regardless of the test organisms (gram-negative P. aeruginosa or gram-positive MRSA strains).
TABLE 1.
Influence of sodium, magnesium, and calcium chloride on antibacterial activitya
| Salt and concn (mM) | MBC (μM)
|
|
|---|---|---|
| LL37 | WLBU2 | |
| NaCl | ||
| 0 | 0.5 | 0.5 |
| 50 | 0.5 | 0.5 |
| 150 | 0.5 | 0.25 |
| 300 | 1.2 | 0.5 |
| MgCl2 | ||
| 0 | 0.5 | <0.5 |
| 1 | <2.5 | <0.5 |
| 3 | >5 | <0.5 |
| 6 | >5 | <1 |
| CaCl2 | ||
| 0 | 0.5 | 0.5 |
| 1 | >5 | 0.5 |
| 3 | >5 | <1 |
| 6 | >5 | <2 |
P. aeruginosa PAO1 was incubated with twofold serially diluted peptides at 37°C for 30 min. Bacterial survival at corresponding peptide concentrations was evaluated by broth dilution assays as described in Materials and Methods. Sodium chloride, even above physiological concentration, had negligible effects on the activities of LL37 and WLBU2 against P. aeruginosa. However, LL37 showed an Mg2+- and Ca2+-dependent decrease in potency, while the activity of WLBU2 was slightly inhibited at 6 mM divalent cations. The MBCs were derived from representative values of two to three independent experimental trials.
Divalent cations bridge lipopolysaccharides on bacterial surfaces, suggesting that they may serve as competitive inhibitors to CAPs. To investigate whether divalent cations can inhibit peptide activity, we determined the dose-dependent effects of Mg2+ and Ca2+ on peptide activity using 1, 3, and 6 mM MgCl2 and CaCl2 in ACES buffer (normal physiological serum concentrations of Mg2+ and Ca2+ are in the range of 2 ± 0.5 mM). The activity of LL37 was significantly inhibited in a dose-dependent manner, as MBCs were greater than 5 μM at 3 mM Mg2+ (Table 1). In contrast, WLBU2 activity was unaffected at concentrations as high as 3 mM Mg2+ (MBC < 0.5). However, there was a small increase in MBC (<1 μM) at 6 mM. The effects of Ca2+ on the activities of both peptides were slightly more apparent, with LL37 not reaching an MBC within 0 to 5 μM even in as low as 1 mM CaCl2. In addition, WLBU2 was fourfold less active in calcium concentrations from 1 mM (MBC = 0.5 μM) to 6 mM (MBC < 2 μM) (Table 1). Notably, calcium ions had only a minor inhibitory effect on WLBU2 within its physiological concentration range (MBC < 1 μM). These results suggest that WLBU2 has been successfully engineered to relatively resist broad variations in NaCl and divalent cation concentrations that may be challenging to host-derived peptides.
Antibacterial activity and selectivity in human serum.
The inactivation of CAPs in human serum has hindered efforts to develop antimicrobial peptides for systemic use (19, 37). As a consequence, less challenging conditions (e.g., phosphate buffer) are commonly used to examine antibacterial activity and host toxicity. Moreover, the characterization of antimicrobial selectivity has been based on the exposure of mammalian cells to peptides in the absence of microbial pathogens (e.g., red blood cell lysis assay in PBS) (57). To evaluate antibacterial selectivity more appropriately, we have developed a comprehensive series of experiments in which both bacteria and host cells are mixed prior to the addition of peptide. This assay was previously described by a study in which HSF and P. aeruginosa PA1244 were combined and then treated with peptide in PBS-based IMDM (21). Although this assay provides important information about the selective target of a peptide, it remains unclear whether similar results would be expected in biological conditions.
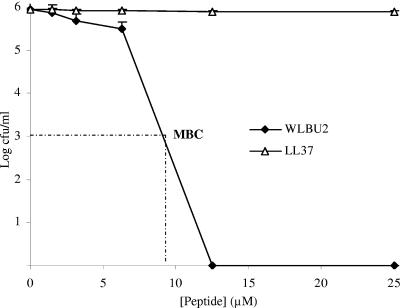
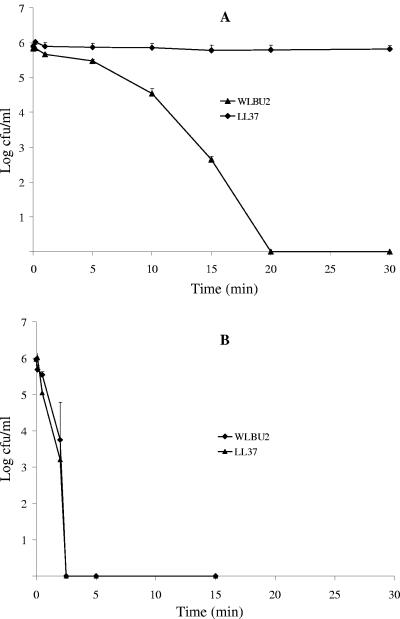
To investigate the potential of WLBU2 for systemic applications, we initially examined the influence of human serum on its antibacterial activity and compared it with that of LL37. Thus, P. aeruginosa suspensions were treated with increasing peptide concentrations in the presence of human serum (98%). As indicated in Fig. 1, LL37 displayed no significant activity, even at concentrations up to 100 μM (data not shown). By contrast, WLBU2 was highly active against P. aeruginosa, with no bacterial survival observed at 12.5 μM (MBC < 9 μM). Because peptide clearance may have a profound impact on activity in vivo, we compared the rates of bacterial killing in human serum and PBS (Fig. 2). The results of this experiment indicate that the peptide WLBU2 requires at most 20 min to kill about 106 CFU/ml of P. aeruginosa at 15 μM in approximately 98% human serum. As expected, LL37 did not show significant activity over time in human serum. Not surprisingly, both peptides sterilized a similar bacterial suspension of P. aeruginosa PAO1 (106 CFU/ml) in PBS at a concentration of 1 μM in less than 5 min. These data provide evidence that WLBU2 should be further investigated for systemic use.
FIG. 1.
Activity of WLBU2 in human plasma. To determine activity in human plasma, bacterial isolates of P. aeruginosa PAO1 (∼106 cells/ml) were incubated with twofold serially diluted peptides at 37°C for 30 min either in heat-inactivated human plasma or in serum. Bacterial survival at corresponding peptide concentrations was evaluated by broth dilution assays as previously described. WLBU2 was able to sterilize the bacterial suspension at 12.5 μM, while LL37 demonstrated no significant activity even at 100 μM (not shown). Results of activity in serum and plasma were identical. The graphs were derived from average values of four independently generated and almost identical triplicate sets of data.
FIG. 2.
Kinetics of bacterial killing. P. aeruginosa PAO1 was treated with 15 μM peptide in human serum (A) and 1 μM peptide in PBS (B). Bacterial survival was monitored over time (up to 30 min) and determined as described in Materials and Methods. The results reveal that a minimum period of 20 min is required for complete bacterial killing in about 98% human serum. The peptide LL37 was inactive under this condition, as expected. However, both LL37 and WLBU2 achieved complete killing within the first 5 min of treatment in PBS. Data plotted are representative average values of one triplicate set of three independent experimental trials.
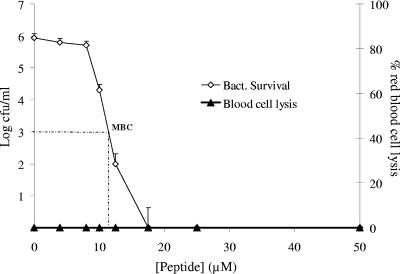
The higher MBC of WLBU2 in human serum raised a concern for potential toxic effects on mammalian cells. Moreover, it remained unclear whether the complete cellular composition of whole blood would affect antibacterial potency. To address these two issues, we examined the antibacterial selectivity of WLBU2 in human whole blood. In this system, a bacteremic condition was established ex vivo with P. aeruginosa PAO1 (106 CFU/ml) prior to addition of peptide. Bacterial survival was determined by serial dilution assay, and red blood cell lysis was deduced from a standard curve generated by spectrophotometric analysis of water-treated blood. As predicted, LL37 did not show any antibacterial activity against P. aeruginosa (data not shown). Conversely, WLBU2 demonstrated the ability to “sterilize” the Pseudomonas-inoculated blood at 15 to 20 μM peptide (Fig. 3), consistent with its activity in human serum. In addition, there was no detectable toxicity to the erythrocytes at all test concentrations (up to 50 μM). These results further support the superior potency of WLBU2 over the host peptide LL37 and may serve as a preliminary indicator of the antibacterial potential of WLBU2 in vivo.
FIG. 3.
Peptide efficacy in an ex vivo bacteremic model. Human whole blood was inoculated with P. aeruginosa PAO1 (∼106 cells/ml) and treated with peptide at various concentrations for 45 min at 37°C. Bacterial count was determined by standard bacterial dilution assay and blood cell lysis by spectrophotometric analysis of hemoglobin release. The erythrolytic effect of red blood cell lysis buffer was comparable to that of water, which was used to generate the standard curve (not shown) in this assay, as described in Materials and Methods. The peptide WLBU2 was remarkably selective against the bacterial cells, with no detectable lytic effects on the blood cells. The graph was derived from average values of four independently generated and almost identical triplicate sets of data.
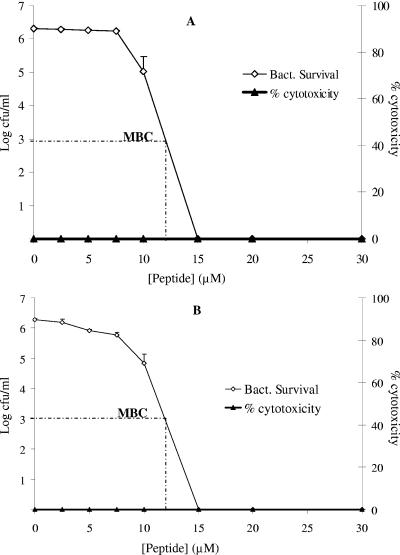
Because the blood cell lysis assay did not reveal any specific information about the fate of the leukocytes in the ex vivo bacteremic model, the cytotoxic effect of the peptide on human blood monocytes was investigated. To accomplish this, cultures of freshly isolated peripheral blood monocytes were inoculated with P. aeruginosa in heat-inactivated human plasma or serum (44). Subsequently, the coculture was treated with peptides as described in the legend to Fig. 4. Using a tetrazolium-based staining method (MTT), the level of toxicity to the monocytes was determined. WLBU2 did not affect the viability of the monocytes (0% cytotoxicity). As expected, bacterial killing was optimal at 15 μM peptide for both peripheral blood monocytes (Fig. 4A) and primary HSF (Fig. 4B). Again, LL37 was inactive under these conditions (data not shown). Taken together, these results support the high antibacterial selectivity of WLBU2 as a potentially effective and safe antimicrobial agent.
FIG. 4.
WLBU2 selectively targeted P. aeruginosa in a coculture model in human plasma. Approximately 105 human monocytes (A) or primary human skin fibroblasts (HSFCCD-986sk) at passage 20 (B) were inoculated with P. aeruginosa in 98% heat-inactivated human serum. The coculture was then incubated for 60 min with twofold dilutions of peptides as described in Materials and Methods. Bacterial survival was determined as in the standard broth dilution assay, and the percent viability of the human PBMC or HSF was evaluated by MTT staining. The peptide WLBU2 displayed high antibacterial selectivity in comparison to host-derived peptide LL37 (not shown). Data plotted are representative averages of triplicate sets of three independent experimental trials.
Effects of WLBU2 on host cells in the absence of bacteria.
The results of the previous experiments demonstrate the effects of WLBU2 on human cells only after a short-term treatment. Furthermore, the influence of WLBU2 on the functionality of these cells remained unclear. To address these issues, the influence of WLBU2 on the viability of human PBMC after treatment for 48 h in human serum using MTT staining was determined. PBMC remained 100% viable in the MTT assay (data not shown). Despite the evidence shown here in support of the safety of WLBU2 in a host environment, it could still be argued that, although nontoxic to mammalian cells in serum, WLBU2 could affect their functionality. To address this issue, we examined the influence of WLBU2 on lymphocytic proliferation. Thus, human blood lymphocytes were treated with 15 μM peptide for 30 min prior to the addition of phorbol myristate acetate and ionomycin to determine the proliferation level by [H3]thymidine incorporation within 4 days of stimulation (data not shown). The results show that lymphocyte proliferation either remained unchanged or increased by up to 15% for both LL37- and WLBU2-treated samples compared with untreated samples. This study demonstrates that it is possible to design antimicrobial peptides with optimized amphipathic helical structures to overcome the challenges of physiological salt concentrations and biological fluids.
In vivo toxicity and antipseudomonal efficacy in an i.p. infection model.
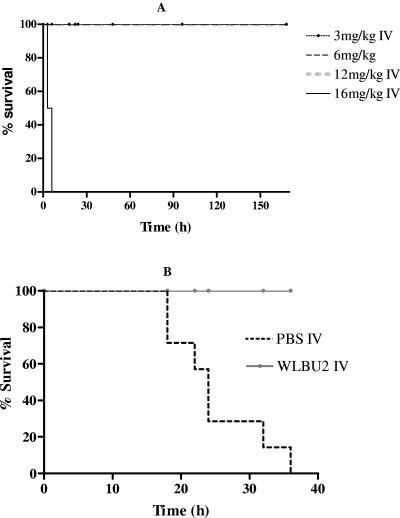
The in vitro and ex vivo observations suggest the possible application of WLBU2 in the treatment of bacteremia. Thus, a logical extension was to examine the potential therapeutic application of WLBU2 in a septicemic animal model of P. aeruginosa infection. Initially, we characterized the lethal potential of the peptide within a range of concentrations to determine the maximum tolerated dose (MTD). For these experiments, groups of 10 mice (25 g each) were given i.v. two equal doses of WLBU2 varying from 3 to 16 mg/kg body weight within 24 h (Fig. 5A). WLBU2 induced no lethality and no apparent toxic effect at up to 12 mg per kg of body weight in comparison with PBS-injected mice (not shown), but the mice receiving the highest peptide dose (16 mg/kg) died within 30 min postinjection. Thus, the MTD i.v. was 12 mg/kg, the highest i.v. dose of WLBU2 that led to no obvious toxicity to the mice. We are currently in the process of identifying the physiological basis for this toxicity.
FIG. 5.
WLBU2 in vivo efficacy against P. aeruginosa. To characterize the in vivo efficacy of WLBU2, we first determined the maximum tolerated dose (MTD) i.v. by injecting female Swiss Webster mice with PBS (10 mice) or 3 to 16 mg WLBU2 (10 mice) per kg of body weight i.v. The animals were then followed up for a minimum of 7 days for survival. Mice treated i.v. with 16 mg WLBU2/kg body weight died within 30 min posttreatment. As shown in the Kaplan-Meier survival curve, the i.v. MTD is 12 mg WLBU2/kg body weight (A). Therefore, the 50% lethal dose is between 12 and 16 mg/kg. (B) The therapeutic potential of WLBU2 was determined by injecting mice i.p. with PAO1 (1.0 × 107 to 1.5 × 107 CFU). The animals were treated 30 to 45 min postinjection with PBS, and bacterial load was determined over time by blood culture on carbenicillin (200 μg/ml) tryptic soy agar plates. The establishment of bacteremia began in about 2 to 3 h (data not shown) and progressed to septicemia and fatality within 36 h (seven mice). In contrast, when Pseudomonas-infected mice were treated i.v. with 3 mg of WLBU2 per kg of body weight 30 to 45 min after the administration of the minimum PLD, not a single case of bacteremia and fatality was observed (seven mice). A log rank test reveals a P value of less than 0.0001.
Based on the toxicity profile of WLBU2, a bacteremic mouse model was developed to test the therapeutic potential of the peptide using female Swiss Webster mice. Initially, the minimum PLD was determined by i.p. injections of various bacterial doses. It was determined that mice (∼25 g each) subjected to i.p. administration of 107 CFU became terminally ill and required euthanasia within 36 h (data not shown). Mice injected i.p. with P. aeruginosa (107 CFU) and treated i.v. with isotonic NaCl (PBS) became bacteremic after 2 h postinjection (approximately 1,000 CFU/ml of blood). Bacterial loads increased to 1 to 10 billion CFU/ml of blood at the disease end point (data not shown). Consistent with this observation was the dissemination of bacteria to the internal organs (liver, lungs, kidney, and spleen), with bacterial loads varying from 108 to 109 CFU per gram of tissue during terminal illness (absence of motility and hypothermia). Identically inoculated mice injected i.v. with 3 mg WLBU2 per kg 30 to 45 min after bacterial injections showed no signs of disease (normal motility, no piloerection, etc.). Moreover, no bacteria were recovered from the blood or the internal organs (liver, kidney, lungs, and spleen) after 7 to 10 days posttreatment (data not shown). Figure 5B illustrates a Kaplan-Meier survival analysis of the difference between mice treated with i.v. PBS and mice treated i.v. with WLBU2 30 to 45 min after i.p. injection of bacteria. No lethality was observed in WLBU2-treated mice. Not determined in this study was the minimum effective dose required to prevent infection. These data are currently under investigation. It is concluded from this model that WLBU2 demonstrated high efficacy against P. aeruginosa PAO1 in vivo when systemically administered.
DISCUSSION
In this study, the activity of a reference natural antimicrobial peptide (LL37) was compared with that of a de novo engineered peptide (WLBU2) under biologically relevant conditions proven to be challenging to many of the most commonly studied antimicrobial peptides (19, 26). As shown in this study, LL37 displayed no saline sensitivity against P. aeruginosa, but its activity was variably suppressed in the presence of Mg2+, Ca2+, and human serum. In contrast, WLBU2 was resistant to physiological concentrations of NaCl, MgCl2, and CaCl2. An important observation was the complete killing of P. aeruginosa by WLBU2 in human serum and whole blood, without detectable adverse effects to human leukocytes, erythrocytes, and primary skin fibroblasts at concentrations fivefold above the MBC. However, the most critical finding was the antipseudomonal efficacy of WLBU2 in i.p. infected mice.
Studies of natural peptides indicate that NaCl and divalent cations (even at subphysiologic levels) may affect CAP activity (25). In this study, LL37 sensitivity to NaCl for MRSA indicates that salt resistance is sometimes dependent on the test organism. Conversely, the highly cationic peptide WLBU2, a derivative of the de novo engineered 24-mer sequence (LBU2), was shown to be highly potent against both P. aeruginosa and MRSA in similar NaCl conditions. Based on the fact that this staphylococcus-specific salt sensitivity (displayed by LBU2 or LL37) was previously observed for parent peptide LBU2 (21), it is evident that the strategic substitution of three Trp residues in the peptide hydrophobic face is responsible for the observed salt resistance against S. aureus. In support of this observation, several studies indicate that Trp may be an important determinant of antimicrobial activity (30, 32). For instance, a single substitution of Phe for Trp in lactoferricin considerably decreases its antimicrobial potency (61). To examine the underlying mechanism of salt resistance, Park et al. demonstrated that salt resistance can be enhanced by increasing helical stability (45). As helical conformation is normally induced by peptide interaction with the bacterial surface, increasing the affinity of a peptide for the lipid bilayer should enhance its propensity to form an α-helix. In a salt environment, the hydrophobic and bulky rings of the Trp residues (by virtue of their membrane-seeking property) are likely to enhance the affinity of WLBU2 for the bacterial membrane, thereby leading to a greater stability of the helical structure. However, raising the concentrations of Mg2+ and Ca2+ beyond serum physiologic levels had a minor inhibitory effect on the activity of WLBU2. This finding suggests that there is an optimal salt concentration (in this case, twice the physiologic concentrations) beyond which the helical stability may be affected. One explanation could be that the divalent cations (at high concentrations) may more effectively compete with the peptide for the negatively charged bacterial surface. Nevertheless, an important lesson is that CAPs can be designed to overcome salt sensitivity using Trp substitutions. Further, the property of salt resistance displayed by WLBU2 may be particularly critical to treatment of infections in diseases that may disturb the normal salt homeostasis in certain human tissues (e.g., cystic fibrosis airway) (25).
The recognition that CAP activity is usually suppressed in serum has restricted the development of antimicrobial peptides mainly to topical or local applications (e.g., skin and respiratory tract infections) (36, 37, 46, 54). As described in this report, standard bacterial killing assays revealed that LL37 was totally inactivated in human serum, suggesting that, like most widely studied host-derived peptides, LL37 may not have evolved to fight systemic infections. In contrast, WLBU2 was not only highly potent against P. aeruginosa in human serum but also achieved complete killing within 20 min, a potential advantage against rapid peptide clearance in a complete host environment. This phenomenon could be explained by the existence of an equilibrium between free and protein-bound peptide molecules. As more free molecules become associated with their bacterial target, the equilibrium would shift toward the progressive release of more peptide molecules, thus leading to effective but slower bacterial killing in human serum.
Once WLBU2 was proven effective in serum, a logical extension was to investigate whether it would be as potent in human blood (in the context of biological plus host cell environments). The efficacy of WLBU2 in the bacteremic model implies that it overcame the potential inhibitory effects of physiological serum salt concentrations, including divalent cations. In fact, it is more likely that serum proteins, such as albumin or apolipoproteins (54), are responsible for the lower activity (in comparison with PBS) in human serum and whole blood. Notably, the antibacterial activity of WLBU2 was unaltered when blood samples derived from donors with hyperlipidemic disorders (milky blood or serum) were used (data not shown). Another important finding was the slightly lower (20 to 30%) antibacterial activity in coculture assays in comparison to bacterial killing alone in human serum. This minor difference in activity might be due to interactions of the host cells (erythrocytes and leukocytes) with the peptide. Similar to the immunomodulatory properties of some host-derived CAPs (52), WLBU2 may have other effects on host cells, particularly on leukocytes, which are worthy of further investigation. These results provide new information that is critical to the characterization of the therapeutic potential of WLBU2 for systemic applications.
In light of all of this evidence for the antibacterial efficacy of WLBU2 in vitro, it was important to know whether this peptide could display any toxicity to mammalian cells after a short- or long-term exposure. Consistent with the bacteremic model, the MTT staining assays in human serum demonstrated that, given both bacterial and host cell targets, the peptide could discriminate against the bacterial cells while sparing the leukocytes and HSF cells. Likewise, a long-term treatment of these cells in the absence of bacteria had no effects on their viability. Moreover, the lymphocyte proliferation assay ruled out the concern that the peptide might specifically and adversely affect host cell functionality.
One of the greatest obstacles in the field of antimicrobial peptides is the transition from test tube to animal models because of the sensitivity of CAPs to biological environments. Due to these limitations, antimicrobial properties of CAPs fall short of supporting their clinical use, with a few exceptions (e.g., the polymyxins) (9, 49).
The in vivo toxicity and in vivo efficacy of WLBU2 were also characterized using an i.p. model of P. aeruginosa bacteremia. The results indicated that WLBU2 was nontoxic at up to fourfold the effective therapeutic dose when administered systemically. Initially, WLBU2 was able to rescue a group of 14 mice from the progression of the P. aeruginosa infection. The reproducibility of these results provides convincing evidence for the systemic efficacy of WLBU2 against P. aeruginosa. Of note, an interesting feature of this i.p. infection model is the successful induction of Pseudomonas bacteremia without the need for an immunosuppressive drug (33, 45). This important aspect of the mouse model will render possible the characterization of the immune modulatory properties of WLBU2 in vivo alone or in the context of a P. aeruginosa infection associated with a fully immunocompetent host.
In conclusion, we have demonstrated that a de novo engineered antimicrobial peptide was able to overcome the challenges of physiological serum concentrations of NaCl, Mg2+, and Ca2+, while the synthetic form of the human peptide LL37 displayed a high sensitivity to NaCl and divalent cations. In addition, LL37 activity was completely suppressed in human serum. In contrast, WLBU2 displayed high efficacy in human serum and the ability to eradicate a bacteremic condition ex vivo. Furthermore, there were no observed adverse effects on mammalian cells in terms of both cytotoxicity and functionality of blood lymphocytes. Finally, the in vivo efficacy of WLBU2 was demonstrated in an i.p. model of P. aeruginosa infection. These results, while promising, underscore the need for comparative studies of WLBU2 and standard antipseudomonal therapeutics (e.g., colistin and tobramycin) prior to further consideration for clinical trials. Such studies should include, but must not be limited to, dose-dependent response to bacteremia treatments in an i.v. infection model, peptide pharmacokinetics, immunogenicity, and influence on cytokine levels with or without the setting of an infection.
Acknowledgments
Support for this project was supplied in part by grants to the University of Pittsburgh Cystic Fibrosis Program Project Grant FRIZZE97R0 (Ray Frizzell), National Institutes of Health Minority Supplement grant 1 U19 AI51661-01 (Sharon L. Hillier and Michael Parniak), NIH grants AR-99-005 1P30 AR47372-01 and P01 AI039061-09 (T.A.M.), the Cystic Fibrosis Foundation Fellowship (S.M.P.), and developmental funds from Children's Hospital of Pittsburgh (S.M.P.).
We thank Barbara Iglewski (University of Rochester) for providing the P. aeruginosa strain PAO1 and Phalguni Gupta (Graduate School of Public Health of the University of Pittsburgh, PA) for the blood samples used in this study. We greatly appreciate helpful discussions with Michael Parniak, Bruce McClane, Michael Cascio, and Sharon L. Hillier about this study. Finally, we thank Denise Capozzi and JoAnn Flynn for their suggestions and comments on the animal protocol, which was approved by the Institutional Animal Care and Use Committee (IACUC animal protocol number 0402610A-1) of the University of Pittsburgh.
REFERENCES
- 1.Aarbiou, J., K. F. Rabe, and P. S. Hiemstra. 2002. Role of defensins in inflammatory lung disease. Ann. Med. 34:96-101. [DOI] [PubMed] [Google Scholar]
- 2.Andersen, J. H., S. A. Osbakk, L. H. Vorland, T. Traavik, and T. J. Gutteberg. 2001. Lactoferrin and cyclic lactoferricin inhibit the entry of human cytomegalovirus into human fibroblasts. Antivir. Res. 51:141-149. [DOI] [PubMed] [Google Scholar]
- 3.Anderson, R. C., R. E. Hancock, and P. L. Yu. 2004. Antimicrobial activity and bacterial-membrane interaction of ovine-derived cathelicidins. Antimicrob. Agents Chemother. 48:673-676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bals, R., X. Wang, M. Zasloff, and J. M. Wilson. 1998. The peptide antibiotic LL-37/hCAP-18 is expressed in epithelia of the human lung where it has broad antimicrobial activity at the airway surface. Proc. Natl. Acad. Sci. USA 95:9541-9546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bals, R., and J. M. Wilson. 2003. Cathelicidins—a family of multifunctional antimicrobial peptides. Cell Mol. Life Sci. 60:711-720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baltch, A. L., R. P. Smith, M. A. Franke, W. J. Ritz, P. Michelsen, L. H. Bopp, and J. K. Singh. 2000. Microbicidal activity of MDI-P against Candida albicans, Staphylococcus aureus, Pseudomonas aeruginosa, and Legionella pneumophila. Am. J. Infect. Control 28:251-257. [DOI] [PubMed] [Google Scholar]
- 7.Bellamy, W., H. Wakabayashi, M. Takase, K. Kawase, S. Shimamura, and M. Tomita. 1993. Killing of Candida albicans by lactoferricin B, a potent antimicrobial peptide derived from the N-terminal region of bovine lactoferrin. Med. Microbiol. Immunol. (Berlin) 182:97-105. [DOI] [PubMed] [Google Scholar]
- 8.Bellm, L., R. I. Lehrer, and T. Ganz. 2000. Protegrins: new antibiotics of mammalian origin. Expert Opin. Investig. Drugs 9:1731-1742. [DOI] [PubMed] [Google Scholar]
- 9.Beringer, P. 2001. The clinical use of colistin in patients with cystic fibrosis. Curr. Opin. Pulm. Med. 7:434-440. [DOI] [PubMed] [Google Scholar]
- 10.Blazyk, J., R. Wiegand, J. Klein, J. Hammer, R. M. Epand, R. F. Epand, W. L. Maloy, and U. P. Kari. 2001. A novel linear amphipathic beta-sheet cationic antimicrobial peptide with enhanced selectivity for bacterial lipids. J. Biol. Chem. 276:27899-27906. [DOI] [PubMed] [Google Scholar]
- 11.Brewer, D., and G. Lajoie. 2000. Evaluation of the metal binding properties of the histidine-rich antimicrobial peptides histatin 3 and 5 by electrospray ionization mass spectrometry. Rapid Commun. Mass Spectrom. 14:1736-1745. [DOI] [PubMed] [Google Scholar]
- 12.Brodsky, I. E., R. K. Ernst, S. I. Miller, and S. Falkow. 2002. mig-14 is a Salmonella gene that plays a role in bacterial resistance to antimicrobial peptides. J. Bacteriol. 184:3203-3213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Callaway, J. E., J. Lai, B. Haselbeck, M. Baltaian, S. P. Bonnesen, J. Weickmann, G. Wilcox, and S. P. Lei. 1993. Modification of the C terminus of cecropin is essential for broad-spectrum antimicrobial activity. Antimicrob. Agents Chemother. 37:1614-1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cao, M., and J. D. Helmann. 2004. The Bacillus subtilis extracytoplasmic-function σX factor regulates modification of the cell envelope and resistance to cationic antimicrobial peptides. J. Bacteriol. 186:1136-1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chernysh, S., S. I. Kim, G. Bekker, V. A. Pleskach, N. A. Filatova, V. B. Anikin, V. G. Platonov, and P. Bulet. 2002. Antiviral and antitumor peptides from insects. Proc. Natl. Acad. Sci. USA 99:12628-12632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cho, Y., J. S. Turner, N. N. Dinh, and R. I. Lehrer. 1998. Activity of protegrins against yeast-phase Candida albicans. Infect. Immun. 66:2486-2493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cohen, S. P., L. M. McMurry, D. C. Hooper, J. S. Wolfson, and S. B. Levy. 1989. Cross-resistance to fluoroquinolones in multiple-antibiotic-resistant Escherichia coli selected by tetracycline or chloramphenicol: decreased drug accumulation associated with membrane changes in addition to OmpF reduction. Antimicrob. Agents Chemother. 33:1318-1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Daher, K. A., M. E. Selsted, and R. I. Lehrer. 1986. Direct inactivation of viruses by human granulocyte defensins. J. Virol. 60:1068-1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Darveau, R. P., M. D. Cunningham, C. L. Seachord, L. Cassiano-Clough, W. L. Cosand, J. Blake, and C. S. Watkins. 1991. β-Lactam antibiotics potentiate magainin 2 antimicrobial activity in vitro and in vivo. Antimicrob. Agents Chemother. 35:1153-1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dathe, M., J. Meyer, M. Beyermann, B. Maul, C. Hoischen, and M. Bienert. 2002. General aspects of peptide selectivity towards lipid bilayers and cell membranes studied by variation of the structural parameters of amphipathic helical model peptides. Biochim. Biophys. Acta 1558:171-186. [DOI] [PubMed] [Google Scholar]
- 21.Deslouches, B., S. M. Phadke, V. Lazarevic, M. Cascio, K. Islam, R. C. Montelaro, and T. A. Mietzner. 2005. De novo generation of cationic antimicrobial peptides: influence of length and tryptophan substitution on antimicrobial activity. Antimicrob. Agents Chemother. 49:316-322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Friedrich, C., M. G. Scott, N. Karunaratne, H. Yan, and R. E. Hancock. 1999. Salt-resistant alpha-helical cationic antimicrobial peptides. Antimicrob. Agents Chemother. 43:1542-1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fujii, G., M. E. Selsted, and D. Eisenberg. 1993. Defensins promote fusion and lysis of negatively charged membranes. Protein Sci. 2:1301-1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ganz, T. 2002. Immunology. Versatile defensins. Science 298:977-979. [DOI] [PubMed] [Google Scholar]
- 25.Goldman, M. J., G. M. Anderson, E. D. Stolzenberg, U. P. Kari, M. Zasloff, and J. M. Wilson. 1997. Human beta-defensin-1 is a salt-sensitive antibiotic in lung that is inactivated in cystic fibrosis. Cell 88:553-560. [DOI] [PubMed] [Google Scholar]
- 26.Gough, M., R. E. Hancock, and N. M. Kelly. 1996. Antiendotoxin activity of cationic peptide antimicrobial agents. Infect. Immun. 64:4922-4927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Grisaru-Soen, G., L. Lerner-Geva, N. Keller, H. Berger, J. H. Passwell, and A. Barzilai. 2000. Pseudomonas aeruginosa bacteremia in children: analysis of trends in prevalence, antibiotic resistance and prognostic factors. Pediatr. Infect. Dis. J. 19:959-963. [DOI] [PubMed] [Google Scholar]
- 28.Hancock, R. E., and M. G. Scott. 2000. The role of antimicrobial peptides in animal defenses. Proc. Natl. Acad. Sci. USA 97:8856-8861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Harder, J., J. Bartels, E. Christophers, and J. M. Schroder. 2001. Isolation and characterization of human beta-defensin-3, a novel human inducible peptide antibiotic. J. Biol. Chem. 276:5707-5713. [DOI] [PubMed] [Google Scholar]
- 30.Haug, B. E., M. L. Skar, and J. S. Svendsen. 2001. Bulky aromatic amino acids increase the antibacterial activity of 15-residue bovine lactoferricin derivatives. J. Pept. Sci. 7:425-432. [DOI] [PubMed] [Google Scholar]
- 31.Haug, B. E., and J. S. Svendsen. 2001. The role of tryptophan in the antibacterial activity of a 15-residue bovine lactoferricin peptide. J. Pept. Sci. 7:190-196. [DOI] [PubMed] [Google Scholar]
- 32.Hirakata, Y., M. Kaku, K. Tomono, K. Tateda, N. Furuya, T. Matsumoto, R. Araki, and K. Yamaguchi. 1992. Efficacy of erythromycin lactobionate for treating Pseudomonas aeruginosa bacteremia in mice. Antimicrob. Agents Chemother. 36:1198-1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hongo, T., and Y. Fujii. 1991. In vitro chemosensitivity of lymphoblasts at relapse in childhood leukemia using the MTT assay. Int. J. Hematol. 54:219-230. [PubMed] [Google Scholar]
- 34.Hristova, K., M. E. Selsted, and S. H. White. 1997. Critical role of lipid composition in membrane permeabilization by rabbit neutrophil defensins. J. Biol. Chem. 272:24224-24233. [DOI] [PubMed] [Google Scholar]
- 35.Hwang, P. M., and H. J. Vogel. 1998. Structure-function relationships of antimicrobial peptides. Biochem. Cell Biol. 76:235-246. [DOI] [PubMed] [Google Scholar]
- 36.Jacob, L., and M. Zasloff. 1994. Potential therapeutic applications of magainins and other antimicrobial agents of animal origin. Ciba Found. Symp. 186:197-216. [DOI] [PubMed] [Google Scholar]
- 37.Javadpour, M. M., M. M. Juban, W. C. Lo, S. M. Bishop, J. B. Alberty, S. M. Cowell, C. L. Becker, and M. L. McLaughlin. 1996. De novo antimicrobial peptides with low mammalian cell toxicity. J. Med. Chem. 39:3107-3113. [DOI] [PubMed] [Google Scholar]
- 38.Jenssen, H., J. H. Andersen, L. Uhlin-Hansen, T. J. Gutteberg, and O. Rekdal. 2004. Anti-HSV activity of lactoferricin analogues is only partly related to their affinity for heparan sulfate. Antivir. Res. 61:101-109. [DOI] [PubMed] [Google Scholar]
- 39.Joerger, R. D. 2003. Alternatives to antibiotics: bacteriocins, antimicrobial peptides and bacteriophages. Poult. Sci. 82:640-647. [DOI] [PubMed] [Google Scholar]
- 40.Lillard, J. W., Jr., P. N. Boyaka, O. Chertov, J. J. Oppenheim, and J. R. McGhee. 1999. Mechanisms for induction of acquired host immunity by neutrophil peptide defensins. Proc. Natl. Acad. Sci. USA 96:651-656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Matuszczyk, S. A. 1983. Antibiotic therapy in the light of the multiple drug resistance of bacteria. Czas. Stomatol. 36:595-600. (In Polish.) [PubMed] [Google Scholar]
- 42.Minahk, C. J., and R. D. Morero. 2003. Inhibition of enterocin CRL35 antibiotic activity by mono- and divalent ions. Lett. Appl. Microbiol. 37:374-379. [DOI] [PubMed] [Google Scholar]
- 43.Moore, M. A., Z. W. Hakki, R. L. Gregory, L. E. Gfell, W. K. Kim-Park, and M. J. Kowolik. 1997. Influence of heat inactivation of human serum on the opsonization of Streptococcus mutans. Ann. N. Y. Acad. Sci. 832:383-393. [DOI] [PubMed] [Google Scholar]
- 44.Navon-Venezia, S., R. Feder, L. Gaidukov, Y. Carmeli, and A. Mor. 2002. Antibacterial properties of dermaseptin S4 derivatives with in vivo activity. Antimicrob. Agents Chemother. 46:689-694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Park, I. Y., J. H. Cho, K. S. Kim, Y. B. Kim, and S. C. Kim. 2004. Helix stability confers salt resistance upon helical antimicrobial peptides. J. Biol. Chem. 279:13896-13901. [DOI] [PubMed] [Google Scholar]
- 46.Phadke, S. M., K. Islam, B. Deslouches, S. A. Kapoor, D. Beer Stolz, S. C. Watkins, R. C. Montelaro, J. M. Pilewski, and T. A. Mietzner. 2003. Selective toxicity of engineered lentivirus lytic peptides in a CF airway cell model. Peptides 24:1099-1107. [DOI] [PubMed] [Google Scholar]
- 47.Pitt, T. L., M. Sparrow, M. Warner, and M. Stefanidou. 2003. Survey of resistance of Pseudomonas aeruginosa from UK patients with cystic fibrosis to six commonly prescribed antimicrobial agents. Thorax 58:794-796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Reddy, K. V., R. D. Yedery, and C. Aranha. 2004. Antimicrobial peptides: premises and promises. Int. J. Antimicrob. Agents 24:536-547. [DOI] [PubMed] [Google Scholar]
- 49.Rinaldi, A. C. 2002. Antimicrobial peptides from amphibian skin: an expanding scenario. Curr. Opin. Chem. Biol. 6:799-804. [DOI] [PubMed] [Google Scholar]
- 50.Sanders, C. C., W. E. Sanders, Jr., R. V. Goering, and V. Werner. 1984. Selection of multiple antibiotic resistance by quinolones, β-lactams, and aminoglycosides with special reference to cross-resistance between unrelated drug classes. Antimicrob. Agents Chemother. 26:797-801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Scane, T. M., and D. F. Hawkins. 1986. Antibacterial activity in human amniotic fluid: dependence on divalent cations. Br. J. Obstet. Gynaecol. 93:577-581. [DOI] [PubMed] [Google Scholar]
- 52.Scott, M. G., D. J. Davidson, M. R. Gold, D. Bowdish, and R. E. Hancock. 2002. The human antimicrobial peptide LL-37 is a multifunctional modulator of innate immune responses. J. Immunol. 169:3883-3891. [DOI] [PubMed] [Google Scholar]
- 53.Shepard, B. D., and M. S. Gilmore. 2002. Antibiotic-resistant enterococci: the mechanisms and dynamics of drug introduction and resistance. Microbes Infect. 4:215-224. [DOI] [PubMed] [Google Scholar]
- 54.Sorensen, O., T. Bratt, A. H. Johnsen, M. T. Madsen, and N. Borregaard. 1999. The human antibacterial cathelicidin, hCAP-18, is bound to lipoproteins in plasma. J. Biol. Chem. 274:22445-22451. [DOI] [PubMed] [Google Scholar]
- 55.Tam, J. P., Y. A. Lu, and J. L. Yang. 2002. Correlations of cationic charges with salt sensitivity and microbial specificity of cystine-stabilized beta-strand antimicrobial peptides. J. Biol. Chem. 277:50450-50456. [DOI] [PubMed] [Google Scholar]
- 56.Tencza, S. B., J. P. Douglass, D. J. Creighton, Jr., R. C. Montelaro, and T. A. Mietzner. 1997. Novel antimicrobial peptides derived from human immunodeficiency virus type 1 and other lentivirus transmembrane proteins. Antimicrob. Agents Chemother. 41:2394-2398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tomita, T., S. Hitomi, T. Nagase, H. Matsui, T. Matsuse, S. Kimura, and Y. Ouchi. 2000. Effect of ions on antibacterial activity of human beta defensin 2. Microbiol. Immunol. 44:749-754. [DOI] [PubMed] [Google Scholar]
- 58.Tossi, A., L. Sandri, and A. Giangaspero. 2000. Amphipathic, alpha-helical antimicrobial peptides. Biopolymers 55:4-30. [DOI] [PubMed] [Google Scholar]
- 59.Uthaisangsook, S., N. K. Day, S. L. Bahna, R. A. Good, and S. Haraguchi. 2002. Innate immunity and its role against infections. Ann. Allergy Asthma Immunol. 88:253-264. [DOI] [PubMed] [Google Scholar]
- 60.Vogel, H. J., D. J. Schibli, W. Jing, E. M. Lohmeier-Vogel, R. F. Epand, and R. M. Epand. 2002. Towards a structure-function analysis of bovine lactoferricin and related tryptophan- and arginine-containing peptides. Biochem. Cell Biol. 80:49-63. [DOI] [PubMed] [Google Scholar]
- 61.Yu, Q., R. I. Lehrer, and J. P. Tam. 2000. Engineered salt-insensitive alpha-defensins with end-to-end circularized structures. J. Biol. Chem. 275:3943-3949. [DOI] [PubMed] [Google Scholar]
- 62.Zasloff, M. 2002. Antimicrobial peptides of multicellular organisms. Nature 415:389-395. [DOI] [PubMed] [Google Scholar]
- 63.Zhang, W., M. Torabinejad, and Y. Li. 2003. Evaluation of cytotoxicity of MTAD using the MTT-tetrazolium method. J. Endod. 29:654-657. [DOI] [PubMed] [Google Scholar]