Abstract
Perturbation of the Staphylococcus aureus cytoplasmic membrane (CM) is felt to play a key role in the microbicidal mechanism of many antimicrobial peptides (APs). However, it is not established whether membrane permeabilization (MP) alone is sufficient to kill susceptible staphylococci or if the cell wall (CW) and/or intracellular targets contribute to AP-induced lethality. We hypothesized that the relationships between MP and killing may differ for distinct APs. In this study, we investigated the association between AP-induced MP and lethality in S. aureus whole cells versus CW-free protoplasts, and in comparison to the MP of liposomes modeled after whole CMs in terms of phospholipid composition, fluidity and charge. Four APs with different structure-activity relationships were examined: thrombin-induced platelet microbicidal protein 1 (tPMP-1), human neutrophil protein 1 (hNP-1), gramicidin D, and polymyxin B. MP was quantified fluorometrically by calcein release. All APs tested, except polymyxin B, caused concentration-dependent MP and killing of whole cells, but not of protoplasts. The reduced AP susceptibility of protoplasts was associated with increased cardiolipin and lysyl-phosphatidylglycerol content and reduced fluidity of their CMs. However, liposomal MP induced by tPMP-1, hNP-1, and gramicidin D paralleled that of whole cells. Collectively, these results indicate that (i) structurally distinct APs likely exert their staphylocidal effects by differing mechanisms, (ii) MP is not the sole event leading to AP-induced staphylocidal activity, (iii) a complex interrelationship exists between the CM and CW in AP-induced killing, and (iv) liposomes modeled upon whole cell or protoplast CMs can recapitulate the respective susceptibilities to killing by distinct APs.
Endogenous antimicrobial peptides (APs) are believed to play a major role in innate host defense against infection in species ranging from invertebrates to humans. Despite considerable variation in composition, structure, charge, and putative mechanism(s) of action, many APs appear to initially interact with and perturb microbial cytoplasmic membranes (CMs) (50). Recent data have suggested that the microbial cell wall (CW) may also be involved in AP-induced microbicidal pathways (39). However, the relative contributions of CM, CW, and other targets beyond these sites (e.g., intracellular targets [36, 45]) in the lethal mechanisms of APs have not been clearly established.
Over the last decade, a number of target CM characteristics have been shown to impact the in vitro activity of APs against microbial cells, including fatty acid and phospholipid content, CM fluidity, and transmembrane potential (Δψ) (1, 22, 24, 47-50). In addition, such parameters appear to influence AP activity in artificial model membrane systems (i.e., membrane vesicles and planar lipid bilayers) (5, 12, 16, 19, 24, 32). Likewise, CW characteristics have been shown to affect AP susceptibility in selected organisms (e.g., Staphylococcus aureus (37-39). However, few studies have investigated the relationship among the CM, CW, and microbicidal mechanisms of APs differing in structure and activity.
In the present study, we investigated AP-induced membrane permeabilization (MP) and killing in S. aureus whole cells versus CW-free protoplasts. In addition, liposomes were strategically designed to simulate the S. aureus CM. For these analyses, four different CM-targeting APs were selected on the basis of their diverse sources (mammalian, bacterial), structures, and putative mechanisms of action: thrombin-induced platelet microbicidal protein 1 (tPMP-1), human neutrophil peptide 1 (hNP-1), gramicidin D, and polymyxin B. The goals of this investigation were threefold: (i) to delineate the relationships between MP and killing among different APs, (ii) to characterize the CW contributions to AP-induced S. aureus killing, and (iii) to assess whether AP-induced MP in whole cells can be modeled in strategic liposomes. Our results indicated that distinct interrelationships exist between CM, CW, and killing of S. aureus by the individual APs studied. Moreover, use of whole cells, protoplasts, and liposomes provided complementary insights into AP-S. aureus interactions.
(This work was presented in part at the 104th General Meeting of the American Society for Microbiology in New Orleans, La., 2004 [abstr. A-111].)
MATERIALS AND METHODS
Bacterial strain.
S. aureus 502A (methicillin susceptible) was obtained from the American Type Culture Collection (ATCC 27217). This strain is a well-characterized, tPMP-1-susceptible, and hNP-1-susceptible laboratory strain regularly used in the study of AP activity (22, 49). This strain was cultured from −70°C storage onto 6.6% sheep blood agar plates (Clinical Standards Laboratory, Inc., Rancho Dominguez, CA). Cells were grown to mid-logarithmic phase in brain heart infusion (BHI) broth at 37°C with shaking (Difco Laboratories, Detroit, MI). Cells were then harvested by centrifugation, washed twice with phosphate-buffered saline (pH 7.2), sonicated briefly to ensure single cells, and then adjusted spectrophotometrically (optical density at 600 nm [OD600]) to the final desired inoculum. All spectrophotometric approximations were verified by quantitative culturing.
Protoplast preparation.
As previously described (1), bacterial cells were grown to mid-log phase, harvested by centrifugation, washed twice with phosphate-buffered saline buffer, and resuspended to a bacterial density of 109 CFU/ml. This suspension was pelleted by centrifugation (5,000 × g for 15 min) and then resuspended in digestion buffer (20% [wt/vol] sucrose, 0.05 M Tris-HCl, 0.145 M NaCl, pH 7.6). The bacterial cell wall was then digested with lysostaphin (34 μg/ml; Applied Microbiology, Tarrytown, NY) in the presence of DNase I (16 μg/ml; Boehringer Mannheim, San Diego, CA) for 1 h at 37°C (1, 23). Protoplasts were collected by centrifugation at 10,000 rpm for 15 min and resuspended in fresh sucrose-containing digestion buffer. The efficiency of cell wall digestion was confirmed by Gram staining. Protoplast preparations were then adjusted spectrophotometrically (OD600) to the final desired inoculum and were stabilized in medium containing 20% (wt/vol) sucrose (23) and used within 24 h of preparation. Quantitative cultures of protoplasts were done on BHI plates containing 20% sucrose.
APs.
The charge characteristics (at pH 7.0) and the comparative mode(s) of action of all APs are summarized in Tables 1 and 2.
TABLE 1.
Comparisons of net charges of major S. aureus phospholipids and APs
| Phospholipid or AP | Chargea |
|---|---|
| Phospholipids | |
| LPG | +1 |
| PG | −1 |
| CL | −2 |
| APs | |
| tPMP-1 | +5 |
| hNP-1 | +4 |
| Gramicidin D | Neutral |
| Polymyxin B | +5 |
Net charge was at pH 7.0.
TABLE 2.
Mode of action of APs
| AP | Size (kDa) | Mode of action |
|---|---|---|
| tPMP-1 | 7.95 | MP in a voltage-dependent manner; intracellular targets; minimal depolarization |
| hNP-1 | 3.8 | Forms voltage-gated pores in the bacterial CM; depolarization |
| Gramicidin D | 1.9 | MP via the formation of monovalent, cation-specific channels |
| Polymyxin B | 1.2 | High binding affinity with lipopolysaccharides, followed by phospholipid transfer and osmotic dysregulation |
tPMP-1 is a 7.95-kDa cationic AP from rabbit platelets that is a structural and functional orthologue of human platelet factor 4 (47, 52). tPMP-1 exhibits potent microbicidal activity against S. aureus (47), with little mammalian host cell cytotoxicity in vitro (48). The mechanism of tPMP-1 activity appears to involve the initial MP of the microbial CM in a voltage-dependent manner (22-24, 49), with subsequent intracellular targeting (45). The purification quantification of tPMP-1 bioactivity is described in detail elsewhere (46, 47).
hNP-1 is a 3.8-kDa cationic α-defensin AP from human neutrophil azurophilic granules that exhibits antimicrobial activity in vitro against S. aureus and many other bacteria (11, 41, 43). Its broad in vitro antimicrobial activity (as well as its considerable host cell toxicity) has been attributed to its ability to nonselectively permeabilize diverse target CMs (30, 31). hNP-1 causes the permeabilization and depolarization of target CMs after oligomeric assembly and pore formation (11, 30, 31, 49). Purified hNP-1 was purchased from Peptide International (Louisville, KY).
Gramicidin D is a neutral, linear pentadecapeptide antibiotic produced by Bacillus brevis, which possesses mainly anti-gram-positive activity and considerable eukaryotic cytotoxicity (3). Gramicidin D is a combination of several gramicidin species (A, B, and C) but is predominantly composed of gramicidin A (80 to 85%) (3) (Tables 1 and 2). The lethal mechanism of gramicidin D is believed to involve CM permeabilization via the formation of monovalent, cation-specific channels (2, 3). Gramicidin D was purchased from Sigma Chemicals (St. Louis, MO). Stock solutions were prepared in dimethyl sulfoxide, and final assay solutions were made in HEPES buffer as per the manufacturer's recommendations.
Polymyxin B is an amphiphilic antibiotic complex produced by Bacillus polymyxa, with activity against many gram-negative (but not gram-positive) bacteria (4, 40). This AP binds to lipopolysaccharide and causes microbial killing by inducing rapid and selective phospholipid exchanges between the outer and inner CMs of gram-negative bacteria, which leads to osmotic instability (6, 7, 40). Polymyxin B is generally inactive against gram-positive bacteria, as their cell walls have been postulated to prevent the access of drug to a putative CM target(s) (29, 40). Polymyxin B (sulfate) was purchased from Sigma Chemicals (St. Louis, MO) and stock solutions prepared in HEPES buffer as per the manufacturers' recommendations.
In vitro AP susceptibility testing.
The MICs of gramicidin D and polymyxin B against S. aureus strain 502A were determined in cation-supplemented Mueller-Hinton broth (Difco Laboratories, Detroit, MI) by a microdilution technique according to National Committee for Clinical Laboratory Standards guidelines, with a final S. aureus inoculum of 105 CFU/ml (34). The MIC was defined as the lowest drug concentration preventing visible turbidity after 18 h of incubation at 37°C. For MP and killing assays with these two APs, fixed multiples of the MICs (1- to 10-fold) were used to encompass a likely bactericidal concentration (see below).
Standard MICs of tPMP-1 and hNP-1 are not routinely performed, since conventional nutrient media tends to mitigate the activity of these peptides. Thus, bactericidal assays were carried out only with these latter APs (see below). The concentrations of tPMP-1 (0.5 to 2 μg/ml) and hNP-1 (5 to 20 μg/ml) used in this study were selected to encompass a sublethal to lethal range as established in pilot studies.
Bactericidal activity of APs.
S. aureus cells and protoplasts were diluted into the test AP solutions (HEPES buffer for gramicidin D and polymyxin B; Eagle's minimal essential medium, pH 7.4, for tPMP-1; and 10 mM potassium phosphate, pH 7.4, containing 1% BHI broth for hNP-1) to achieve a final inoculum of 106 CFU/ml and then incubated at 37°C for 60 min. At the indicated time point, samples were removed and processed for quantitative culturing to assess the extent of killing by each AP (as previously described in detail [22]). The standard 24-h quantification assay was modified for protoplasts which were plated onto BHI plates containing 20% sucrose (48 h, 37°C). Controls for staphylocidal activity consisted of cells in appropriate buffer lacking AP but containing the appropriate amount of the respective AP diluent. The AP assay solution did not contain 20% sucrose. This lack of sucrose in the reaction mixture (1-h assay period) did not impact the viability of protoplasts. There were no decreases in protoplast counts in the absence of APs over the assay time period. Experiments were repeated independently at least three times on separate days. The mean (± standard deviation [SD]) surviving log10 CFU/ml was plotted against different concentrations of AP.
MP of S. aureus whole cells and protoplasts by APs: calcein loading.
MP of whole cells and protoplasts was detected via the release of a preloaded fluorescent probe, calcein. Calcein acetoxymethylester (calcein AM) is a nonfluorescent derivative of calcein that is lipid soluble and therefore can readily diffuse across CMs to load the bacterial cell (9, 15). Once within the cytoplasm of bacterial cells, calcein AM is hydrolyzed by cytoplasmic esterases, yielding the fluorescent derivative calcein. Calcein (C30H26N2O13, molecular weight of 623) has excitation and emission wavelengths of 494 and 517 nm, respectively.
The methods for preparation of calcein AM and whole-cell loading have been described previously (25). The only modification in the present study was utilization of a 20% sucrose-HEPES buffer solution for maintaining protoplast stability during the calcein loading. Then calcein-loaded S. aureus whole cells or protoplasts were diluted into the test AP solutions described above (final inoculum, 106 CFU/ml) and incubated at 37°C for 60 min. As pointed out above, the test AP solution did not contain 20% sucrose. At the same sampling times used to assess the bactericidal effects of each AP, calcein retention was quantified as previously detailed using a Turner digital fluorometer (model 450; Barnstead/Thermolyne Corp., Dubuque, IA) equipped with a 490-nm excitation filter and a 517-nm emission filter. Cobalt (Co2+, chloride salt, 2 μM; Sigma) was used to quench the fluorescence of calcein released into the extracellular supernatant (18). Controls for MP consisted of cells and protoplasts in appropriate buffer lacking AP but containing the relevant AP diluent. For whole cells and protoplasts, MP (%) was defined as the absolute percent calcein leakage by APs with respect to calcein-loaded, AP-untreated cells (25). Each experiment included a concomitant quantitative culture to ensure that calcein loading did not alter S. aureus susceptibility to the APs. Experiments were repeated independently at least three times on separate days.
Assessment of liposomal permeabilization. (i) Liposome preparation.
The S. aureus CM contains three major phospholipids species: negatively charged phosphatidylglycerol (PG) and cardiolipin (CL) and positively charged lysyl-PG (LPG) (37). For formulating model liposomes, synthetic 1,2-dipalmitoyl-sn-glycero-3-phosphoglycerol (sodium salt; DPPG) and CL were purchased from Avanti Polar Lipids, Inc. (Alabaster, AL). Since LPG is not commercially available due to its instability, we used synthetic octadecylamine (stearylamine [SA]), a positively charged lipid (Sigma Chemicals, St. Louis, Mo.). Stable liposomes containing the following phospholipids (in molar ratio) were formulated for use in this study: DPPG:CL (20:1), SA:DPPG:CL (1:9:2), and SA:DPPG:CL (1:4:1).
(ii) Calcein loading.
Liposomes loaded with calcein (50 mM) were prepared via a modification of the whole-cell protocol given above. Phospholipids (in chloroform; total, 20 mg) were dried under a stream of N2 (∼15 min), followed by desiccation overnight. The lipid film was hydrated with 2 ml HEPES buffer containing 9% (wt/vol) sucrose and 50 mM calcein (10 min, 65°C; final phospholipid concentration, 10 mg/ml), and then sonicated (5 min, model 450; Branson Ultrasonics Corporation, Danbury, CT). Calcein-encapsulated liposomes were then separated from free calcein using a 10 DG chromatography column (30 × 10 ml; Bio-Rad Laboratories, Hercules, CA). Liposomes were sized using a Microtrac ultrafine particle analyzer (model 150; Leeds and Northrup, North Wales, PA), and mean diameters were found to be consistently less than 0.2 μm.
(iii) Permeabilization assay.
At concentrations of >20 mM, calcein fluorescence is self-quenching, i.e., the fluorescence emitted by calcein is reabsorbed by the fluorophore, and thus, the net fluorescence detected is minimal. Therefore, intact liposomes prepared in the present study (encapsulating 50 mM calcein) emit relatively low levels of fluorescence. However, permeabilization of liposomes by APs causes the leakage and dilution of calcein into the buffer supernatant, resulting in an increase in total supernatant fluorescence. Thus, in contrast to the assay for whole S. aureus cells and protoplasts, liposome permeabilization was detected by an increase in fluorescence over time. Liposomes were diluted 20-fold into HEPES buffer, and stock AP solutions were added to the liposomal suspension at the concentrations described above for the whole cell or protoplasts studies. Fluorescence of the liposomal reaction mixture was monitored via fluorometry immediately prior to AP addition and 60 min after AP addition. At the end of each experiment, Triton X-100 (0.5% [vol/vol]) was added to completely release any residually entrapped calcein from the liposomes. The percentage of calcein leakage was then calculated using the following formula: calcein leakage (%) = [(F -F0)/(Ft -F0)] × 100, where F is the fluorescence intensity of AP-treated liposomes, F0 is the fluorescence intensity of control liposomes, and Ft is the fluorescence intensity after the addition of Triton X-100 (42).
Determination of phospholipids profiles.
The three major phospholipid species PG, CL, and LPG were quantitatively compared in S. aureus whole cells versus protoplasts. Lipids were extracted, evaporated to dryness, and stored by standard techniques, as previously detailed (10). Individual phospholipids were separated by two-dimensional thin-layer chromatography (2D-TLC) using Silica 60 F254 high-pressure TLC plates (Merck, Darmstadt, Germany) and subsequently developed as detailed elsewhere (8, 17). LPG was specifically identified by ninhydrin staining (37). Other phospholipids were visualized by exposure of the TLC plate to iodine vapor. PG and CL were purchased from Avanti Polar Lipids, Inc., and used as standards to determine the positions of their spots in 2D-TLC.
For quantitative analysis, isolated phospholipids were individually recovered from TLC plates and digested at 180°C for 3 h with 0.3 ml of 70% perchloric acid. The digested samples were incubated with colorometric reagent (10% ascorbic acid, 2.5% ammonium molybdate, 5% perchloric acid [1:1:8, vol/vol/vol]) for 2 h at 37°C and quantified spectrophotometrically at OD660. The content of each phospholipid species was expressed as a percentage of total phospholipid content.
CM fluidity measurement.
CM fluidity of whole cells, protoplasts, and liposomes was determined by fluorescence polarization using the fluoresecent probe 1,6-diphenyl-1,3,5-hexatriene (DPH) as described earlier (1). DPH is a probe that localizes in the hydrophobic core of the lipid bilayer and is highly fluorescent when bound therein. The protocol for DPH incorporation into target CMs, the measurement of fluorescence polarization, and calculation of the degree of fluorescence polarization (p) is described in detail elsewhere (Biotek model SFM 25 spectrofluorometer with excitation and emission wavelengths of 360 and 426 nm, respectively) (1). The lower the p value, the higher the degree of membrane fluidity (1).
Statistical analyses.
Linear regression analysis was performed to compare the relationship between AP-induced MP (percentage of calcein leakage) and killing of whole cells (−Δlog10 CFU/ml) using Microsoft Excel software's statistical program. Correlation coefficients (r2s) of ≥0.5 were considered significant at the P<0.05 level. Kruskal-Wallis analysis of variance was used to compare membrane fluidity among whole cells, protoplasts, and liposomes. P values of <0.05 were considered significant (Epistat).
RESULTS
In vitro susceptibility.
The MICs of gramicidin D and polymyxin B against S. aureus 502A were 4 and 32 μg/ml, respectively.
Staphylocidal activity and MP of APs against whole cells (Fig. 1A and B).
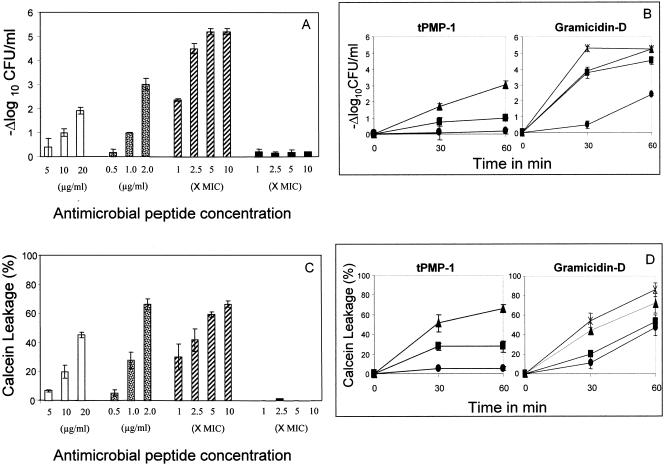
FIG. 1.
(A) Staphylocidal activity of antimicrobial peptides on S. aureus whole cells. Killing of S. aureus whole cells exposed to different concentrations (as discussed in Materials and Methods) of antimicrobial peptides for 1 h with respect to the untreated control cells is shown. hNP-1 (□) tPMP-1 ( ), gramicidin D (
), and polymyxin B (▪) were used in this study. Each experiment was done in triplicate, and the values represent means ± standard deviations. (B) Kinetics of staphylocidal activity. Killing of S. aureus over time following exposure to tPMP-1 at 0.5 μg/ml (•), 1.0 μg/ml (▪), or 2.0 μg/ml (▴) and gramicidin D at 1× MIC (•), 2.5× MIC (▪), 5× MIC (▴), or 10× MIC (
), gramicidin D (
), and polymyxin B (▪) were used in this study. Each experiment was done in triplicate, and the values represent means ± standard deviations. (B) Kinetics of staphylocidal activity. Killing of S. aureus over time following exposure to tPMP-1 at 0.5 μg/ml (•), 1.0 μg/ml (▪), or 2.0 μg/ml (▴) and gramicidin D at 1× MIC (•), 2.5× MIC (▪), 5× MIC (▴), or 10× MIC ( ). (C) S. aureus whole-cell membrane permeabilization by antimicrobial peptides. Membrane permeabilization of S. aureus whole cells by antimicrobial peptides for 1-h exposure was detected via the leakage of calcein. hNP-1 (□), tPMP-1 (
). (C) S. aureus whole-cell membrane permeabilization by antimicrobial peptides. Membrane permeabilization of S. aureus whole cells by antimicrobial peptides for 1-h exposure was detected via the leakage of calcein. hNP-1 (□), tPMP-1 ( ), gramicidin D (
), and polymyxin B (▪) were used in this study. Each experiment was done in triplicate, and the values represent means ± standard deviations. (D) Kinetics of membrane permeabilization. Membrane permeabilization of S. aureus over time following exposure to tPMP-1 at 0.5 μg/ml (•), 1.0 μg/ml (▪), or 2.0 μg/ml (▴) and gramicidin D at 1× MIC (•), 2.5× MIC (▪), 5× MIC (▴), or 10× MIC (
), gramicidin D (
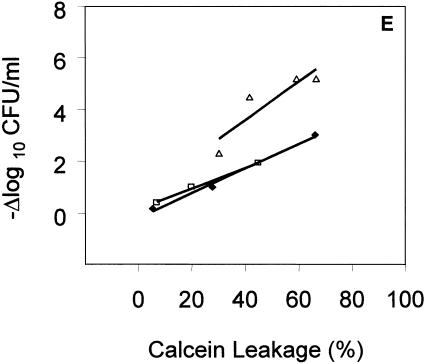
), and polymyxin B (▪) were used in this study. Each experiment was done in triplicate, and the values represent means ± standard deviations. (D) Kinetics of membrane permeabilization. Membrane permeabilization of S. aureus over time following exposure to tPMP-1 at 0.5 μg/ml (•), 1.0 μg/ml (▪), or 2.0 μg/ml (▴) and gramicidin D at 1× MIC (•), 2.5× MIC (▪), 5× MIC (▴), or 10× MIC ( ). (E) Correlation analysis of the relationship between killing (−Δlog10 CFU/ml) and membrane permeabilization (% calcein leakage). Linear regressions were determined, and correlation coefficients (r2) were calculated from mean results of three independent experiments. r2 for tPMP-1 (⧫), hNP-1 (□), and gramicidin D (▵) are 0.9925, 0.9961, and 0.8074, respectively (P < 0.05 for each).
). (E) Correlation analysis of the relationship between killing (−Δlog10 CFU/ml) and membrane permeabilization (% calcein leakage). Linear regressions were determined, and correlation coefficients (r2) were calculated from mean results of three independent experiments. r2 for tPMP-1 (⧫), hNP-1 (□), and gramicidin D (▵) are 0.9925, 0.9961, and 0.8074, respectively (P < 0.05 for each).
The comparative staphylocidal effects of the study APs are presented in Fig. 1A. hNP-1, tPMP-1, and gramicidin D exhibited concentration-dependent S. aureus killing, with gramicidin D exerting the greatest extent of killing. Figure 1B shows the time-dependent S. aureus killing for tPMP-1 and gramicidin D for all the concentrations studied. Similar time-dependent killing was also observed for hNP-1 (data not shown). As anticipated, polymyxin B exerted no microbicidal activity against S. aureus whole cells.
In parallel with their staphylocidal profiles, hNP-1, tPMP-1, and gramicidin D also exerted concentration-dependent MP of S. aureus (Fig. 1C). Moreover, all three APs also demonstrated time-dependent MP of S. aureus whole cells at all concentrations studied (Fig. 1D; data for hNP-1 not shown). Polymyxin B caused no MP (Fig. 1C).
To further characterize the relationship between MP and the staphylocidal activity of APs, regression analyses were performed comparing these parameters (Fig. 1E). For all APs except polymyxin B, a significant and positive correlation existed between whole-cell killing and MP (r2 > 0.8; P < 0.05).
Staphylocidal activity and MP of APs against protoplasts.
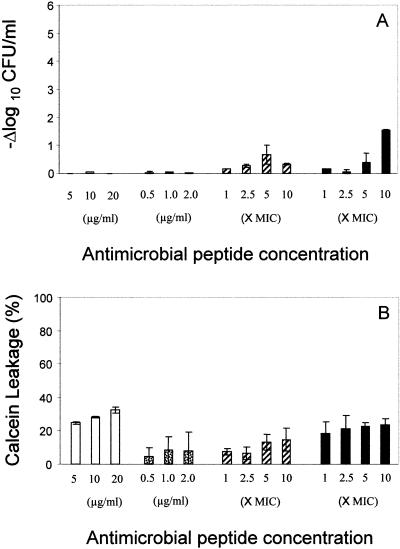
The staphylocidal effects of the study APs against protoplasts are presented in Fig. 2A. In contrast to their efficacies against whole cells, neither hNP-1 nor tPMP-1 exerted microbicidal activity against S. aureus protoplasts at any tested concentration. By comparison, gramicidin D and polymyxin B exerted minimal bactericidal effects against protoplasts. Paralleling the overall lack of AP-induced staphylocidal activity, protoplasts were relatively resistant to MP by all APs (Fig. 2B).
FIG. 2.
(A) Staphylocidal activity of antimicrobial peptides on S. aureus protoplasts. Killing of S. aureus protoplasts exposed to different concentrations of antimicrobial peptides for 1 h with respect to the untreated control is shown. (B) Membrane permeabilization by antimicrobial peptides on S. aureus protoplasts. Membrane permeabilization of S. aureus protoplasts by antimicrobial peptides for 1-h exposure was detected via the leakage of calcein. hNP-1 (□), tPMP-1 ( ), gramicidin D (
), and polymyxin B (▪) were used in this study. Each experiment was done in triplicate, and the values represent means ± standard deviations.
), gramicidin D (
), and polymyxin B (▪) were used in this study. Each experiment was done in triplicate, and the values represent means ± standard deviations.
Phospholipid composition of whole cells and protoplasts.
The relationships between CM phospholipid composition and relative susceptibility to AP-induced killing and/or MP are shown in Fig. 3A. Whole cells and protoplasts displayed differences in their relative phospholipid content. For example, whole cells contained an average of 67% PG compared with 48% for the protoplasts, while the CL content of the protoplasts was approximately twice that seen in whole cells (19% versus 9%, respectively). The LPG content was also relatively higher in protoplasts than that in whole cells (30% versus 20%, respectively).
FIG. 3.
Membrane characteristics of whole cells versus protoplasts versus liposomes. (A) Phospholipid composition of whole cells (□) and protoplasts (▪). Phospholipid contents were determined by 2D-TLC and expressed as percentages of total phospholipids as described in Materials and Methods. (B) Membrane fluidity of whole cells (□) and protoplasts (▪) and of liposomes of different composition (C) was determined by fluorescence polarization (p) measurements using fluorescent probe DPH as described in Materials and Methods. Each estimation was done at least in triplicate, and the values represent means ± SDs.
CM fluidity.
Protoplasts exhibited significantly higher fluorescence polarization (p) values than whole cells (Fig. 3B), indicating that their CMs are notably less fluid (i.e., more rigid) than those of whole cells (P < 0.005).
Liposomal permeabilization by APs.
Liposomes exhibited < 5% spontaneous permeabilization in the absence of AP exposures (Fig. 4A through C). tPMP-1 and hNP-1 induced MP in all three liposomes, in an AP concentration-dependent manner, with a trend toward more extensive MP in more positively charged liposomes. In contrast, gramicidin D did not appreciably permeabilize PG:CL liposomes while inducing concentration-dependent MP in the more positively charged liposomes. In contrast, for polymyxin B, there was a decreasing extent of MP as liposomes became more positively charged.
FIG. 4.
Liposomal permeabilization by antimicrobial peptides. Liposomes with different phospholipid formulations (in molar ratio) of DPPG:CL (20:1) (A), SA:DPPG:CL (1:9:2) (B), and SA:DPPG:CL (1:4:1) (C) were incubated at 37°C with different APs, and permeabilization was measured via calcein leakage. hNP-1 (□), tPMP-1 ( ), gramicidin D (
), and polymyxin B (▪) were used in this study. Each experiment was done in triplicate, and the values represent means ± SDs.
), gramicidin D (
), and polymyxin B (▪) were used in this study. Each experiment was done in triplicate, and the values represent means ± SDs.
Fluidity of liposomal membranes.
In comparing the three liposomal model membranes, the PG:CL liposomes were the least fluid (i.e., the most rigid) (Fig. 3C). With increasing proportions of the positively charged species SA, liposomal membranes became progressively more fluid, reaching fluidity profiles similar to those of intact whole cells. There were significant differences in the extents of fluidity in comparing each of the three liposomal formulations (P < 0.05).
DISCUSSION
The mechanisms of AP action are mulitfactorial, and the relationships among MP, CM characteristics, the role of the CW, and the lethal pathways of APs have been difficult to define. Prior studies have implicated the microbial CM as an initial target for AP-induced killing (1, 25, 31, 49, 52). Thus, AP-induced perturbation of the CM, either by defined membrane poration or diffuse membrane permeabilization, has been felt to be required and sufficient to induce eventual microbial killing (25, 43, 49). However, recent investigations have also implicated intracellular targets and the autolytic enzyme system as participants in AP-induced lethal pathways (36, 39, 44, 45, 51). Moreover, several recent studies have documented that the composition of the CM or CW, as well as the overall microbial surface charge of certain pathogens (e.g., S. aureus), significantly influences the net antimicrobial activity of cationic APs (26, 28, 37, 38).
The present study was conducted to gain further insights into the relationships between AP-induced CM events, overall cell envelope properties, and killing of S. aureus. To do so, we examined MP, the presence or absence of the CW, CM fluidity, and phospholipid profiles in whole S. aureus cells versus CW-free protoplasts. We also studied model liposomes, formulated with a range of phospholipids to reflect the intact organism's CM properties. Understanding such interrelationships could delineate the relative contributions of CW or CM properties to the net lethal effects of diverse APs.
The panel of APs used in the current investigation was selected on the basis of their diverse sources, structures, antimicrobial spectra, and putative mechanisms of action. Of these, hNP-1 is perhaps the best characterized of the endogenous APs, particularly regarding structure-function correlations. Previous evidence indicates that formation of Δψ-independent pores, with subsequent disruption of CM integrity and energetics, is critical for hNP-1 activity (31, 49). In contrast, tPMP-1 appears to initiate its staphylocidal pathway by permeabilizing the CM, without global depolarization, in a Δψ-dependent manner, followed by the inhibition of intracellular targets (23, 24, 45, 49). Of note, increases in either the cell wall techoic acid alanylation or CM PG lysinylation of S. aureus cells (yielding a net increase in positive charge) correlate with reduced susceptibility to hNP-1 (37, 38) and tPMP-1 (Bayer et al., unpublished) in selected strains. Also, modifications of the fatty acid profile in the S. aureus CM appear to mitigate tPMP-1-induced permeabilization and killing (e.g., via altering CM fluidity characteristics) (1, 22, 49).
Several interesting observations emerged from our current studies related to hNP-1 and tPMP-1. Both APs killed and permeabilized whole S. aureus cells in a concentration- and time-dependent manner. These results parallel those in prior studies of whole-cell killing versus membrane permeabilization, using an S. aureus isogenic strain pair of genetic lineage distinct from SA 502A, which differed in their intrinsic tPMP-1 susceptibility profile (23, 25). In contrast, neither AP had activity against protoplasts, suggesting that the CW may contribute to the net lethal mechanisms of these APs. Since protoplasts grow and perform physiologic functions similar to whole cells (13, 14, 35), we investigated potential reasons for the disparity in AP efficiency against protoplasts. Two key differences between whole cells and protoplasts that correlated with these findings were identified: (i) protoplast CMs contained substantially more CL and LPG than whole cells and (ii) protoplast CMs were less fluid (more rigid) than those of whole cells. It is highly likely that these two latter observations are interrelated with protoplast resistance to AP killing. Previous studies of S. aureus and other bacterial protoplasts, as well as yeast mitochondria, confirmed the capacity of CL to stabilize spherical CM bilayers and preserve their osmotic stability (13, 14, 27, 33, 35). In addition to its CM-stabilizing property, CL has been ascribed a proton reservoir function, altering the CM ΔΨ (20). Thus, increases in CL content could potentially reduce the ΔΨ of protoplast CMs, negatively impacting ΔΨ-dependent lethal events for selected APs (22). Moreover, increased LPG content in the CM could contribute to an increase in net surface-positive charge and a repulsive effect against cationic APs (37).
We utilized liposomes differing in composition and biophysical properties to model AP-induced CM events in intact S. aureus cells. Distinct liposomes responded to APs in substantially different manners, depending on their CM properties. For example, addition of the positively charged phospholipid species stearylamine to PG and CL achieved a model liposome that paralleled the overall phospholipid composition and membrane fluidity properties of the whole S. aureus cell used in this study. Unexpectedly, such S. aureus-like liposomes exhibited an increased capacity to be permeabilized by tPMP-1 and hNP-1, compared to liposomes lacking positively charged phospholipids. Current pagadigms would predict that increasing the content of positively charged phospholipid species in the CM would increase its relative positive charge and adversely impact cationic AP-induced CM effects. The outcomes of our liposomal studies underscore the importance of phospholipid distribution, as well as overall content, within the target CM, relative to AP activities (21). Moreover, as noted above, the impact of an intact CW on AP-induced events cannot be underestimated.
Interactions of gramicidin D with target CMs exhibited both similarities and differences compared to hNP-1 and tPMP-1. This AP killed S. aureus cells in a time- and concentration-dependent manner (similar to prior data from our laboratory utilizing other S. aureus strains [25]) and had little activity against protoplasts, similar to hNP-1 and tPMP-1. However, in contrast to hNP-1 and tPMP-1, the two highest concentrations of gramicidin D only permeabilized 60 to 70% of whole S. aureus cells, despite complete staphylococcal killing. These data suggest that gramicidin D-induced MP may be required but is not sufficient to induce lethality. Moreover, gramicidin D was not able to permeabilize PG:CL liposomes, supporting the concept that it interacts with target CMs in a manner distinct from hNP-1 and tPMP-1.
The lack of polymyxin B activity against gram-positive bacteria is believed to relate to the absence of target lipopolysaccharides in their cell envelope, as well as the physical barrier posed by cell walls in accessing the CM target (29, 40). Our combined use of whole cells, protoplasts, and liposomal models in the present study provided insights into these hypotheses. As expected, polymyxin B did not induce significant MP or staphylocidal activity in whole S. aureus cells, even at large multiples above its MIC. In contrast, PG:CL liposomes were rapidly and completely permeabilized by polymyxin B. These data likely reflect the inability of polymyxin B to access the target cytoplasmic membrane of whole cells due to the complex staphylococcal cell wall (29, 40). Of note, PG:CL:SA liposomes were also permeabilized in a concentration-dependent manner, albeit to a lesser extent than PG:CL liposomes. The inverse relationship between polymyxin B-induced liposomal MP with increasing amounts of positively charged phospholipids suggests that the interaction of polymyxin B with target CMs of S. aureus is more related to electrostatic affinity than in the other APs tested. These hypotheses are being addressed in ongoing liposomal modeling in our laboratories.
Collectively, these findings support the following conclusions. (i) MP is an important event in the staphylocidal effects of many APs, although the requirement and scope of this effect may vary among distinct APs. Moreover, there may be a threshold extent of MP that is required to initiate the AP-induced lethal pathway. (ii) The presence of the CW likely impacts AP-induced lethality, potentially by acting as a mechanical barrier or electrostatic (repulsive) shield against AP access of the CM and/or via feedback effects that influence the composition or function of the CM; in contrast, certain APs (e.g., nisin) may exploit selected CW components as a secondary target in their lethal mechanism (39). (iii) Finally, in the absence of the CW (as in protoplasts), the CM can adaptively respond, in order to resist AP-induced killing, by changing its CM composition and biophysical properties; such adaptations may have potential in vivo relevance, for example, in circumstances where treatment with cell wall-active antibiotics may induce protoplast formation. Model liposomes may be valuable tools to investigate AP:CM interactions because of their versatility in representing individual or multiple CM parameters that may impact AP activity, such as phospholipid or fatty acid content, surface charge, or membrane fluidity.
Acknowledgments
This research was supported by grants from the National Institutes of Health (NIH) (AI39108 to A.S.B., AI48031 to M.R.Y., and S-06-GM053933 to J.A.-M.) and the American Heart Association (0265054Y and 0465142Y to Y.Q.X.).
We gratefully acknowledge Kimberly Gank for purifying tPMP-1, Molecular Express, Inc. (Los Angeles, CA) for their assistance in sizing the liposomes, and Supin Koo and Natalie Lucindo for technical assistance.
REFERENCES
- 1.Bayer, A. S., R. P. Prasad, J. Chandra, A. Koul, M. Smriti, A. Varma, R. A. Skurray, N. Firth, M. H. Brown, S.-P. Koo, and M. R. Yeaman. 2000. In vitro resistance of Staphylococcus aureus to thrombin-induced platelet microbicidal protein in associated with alterations in cytoplasmic membrane fluidity. Infect. Immun. 68:3548-3553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Burkhart, B. M., N. Li, D. A. Langs, W. A. Pangborn, and W. L. Duax. 1998. The conducting form of gramicidin A is a right-handed, double-stranded double helix. Proc. Natl. Sci. USA 95:12950-12955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Burkhart, B. M., R. M. Gassman, D. A. Langs, W. A. Pangborn, W. L. Duax, and V. Pletnev. 1999. Gramicidin D conformation, dynamics and membrane ion transport. Biopolymers 51:129-144. [DOI] [PubMed] [Google Scholar]
- 4.Chen, C.-C. H., and D. S. Feingold. 1973. The mechanism of polymyxin B action and selectivity towards biologic membranes. Biochemistry 12:2105-2111. [DOI] [PubMed] [Google Scholar]
- 5.Christensen, B., J. Fink, R. B. Merrifield, and D. Mauzerall. 1988. Channel-forming properties of cecropins and related model compounds incorporated into the planar lipid bilayer membranes. Proc. Natl. Acad. Sci. USA 85:5072-5076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clausell, A., M. A. Busquets, M. Pujol, A. Alsina, and Y. Cajal. 2004. Polymyxin B-lipid interactions in Langmuir-Blodgett monolayers of Escherichia coli lipids: a thermodynamic and atomic force microscopy study. Biopolymers 75:480-490. [DOI] [PubMed] [Google Scholar]
- 7.Daugelavicius, R., E. Bakiene, and D. H. Bamford. 2000. Stages of polymyxin B interaction with the Escherichia coli cell envelope. Antimicrob. Agents Chemother. 44:2969-2978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dogra, S., S. Krishnamurthy, V. Gupta, B. L. Dixit, C. M. Gupta, D. Sanglard, and R. Prasad. 1999. Asymmetric distribution of phosphatidylethanolamine in C. albicans: possible mediation by CDR1, a multidrug transporter belonging to ATP binding cassette (ABC) superfamily. Yeast 15:111-121. [DOI] [PubMed] [Google Scholar]
- 9.Essodaigui, M., H. J. Broxterman, and A. Garnier-Suillerot. 1998. Kinetic analysis of calcein and calcein-acetoxymethylester efflux mediated by the multidrug-resistance protein and P-glycoprotein. Biochemistry 37:2243-2250. [DOI] [PubMed] [Google Scholar]
- 10.Folch, J., M. Less, and G. H. Sloane-Stanley. 1957. A simple method for the isolation and purification of total lipids from animal tissue. J. Biol. Chem. 226:497-509. [PubMed] [Google Scholar]
- 11.Ganz, T., M. E. Selsted, and R. I. Lehrer. 1990. Defensins. Eur. J. Haematol. 44:1-8. [DOI] [PubMed] [Google Scholar]
- 12.Gao, F. H., T. Abee, and W. N. Konings. 1991. Mechanism of action of the peptide antibiotic nisin in liposomes and cytochrome c oxidase-containing proteoliposomes. Appl. Environ. Microbiol. 57:2164-2170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hoischen, C., W. Ihn, K. Gura, and J. Gumpert. 1997. Structural characterization of molecular phospholipid species in cytoplasmic membranes of the cell wall-less Streptomyces hygroscopicus L form by use of electrospray ionization coupled with collision-induced dissociation mass spectrometry. J. Bacteriol. 179:3437-3442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hoischen, C., K. Gura, C. Luge, and J. Gumpert. 1997. Lipid and fatty acid composition of cytoplasmic membranes from Streptomyces hygroscopicus and its stable protoplast-type L form. J. Bacteriol. 179:3430-3436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Holl, Z., L. Homolya, C. W. Davis, and B. Sarkadi. 1994. Calcein accumulation as a fluorometric functional assay of the multidrug transporter. Biochim. Biophys. Acta 1191:384-388. [DOI] [PubMed] [Google Scholar]
- 16.Hristova, K., M. E. Selsted, and S. H. White. 1997. Critical role of lipid composition in membrane permeabilization by rabbit neutrophil defensins. J. Biol. Chem. 272:24224-24233. [DOI] [PubMed] [Google Scholar]
- 17.Ibrahim, A. S., and M. A. Ghannoum. 1996. Chromatographic analysis of lipids, p. 52-79. In R. Prasad (ed.), Manual on membrane lipids. Springer-Verlag, Berlin, Germany.
- 18.Jones, G. R., and A. R. Cossins. 1990. Physical methods of study, p. 183-220. In R. R. C. New (ed.), Liposomes: a practical approach. IRL press, Oxford, United Kingdom.
- 19.Kagan, B. L., M. E. Selsted, T. Ganz, and R. I. Lehrer. 1990. Antimicrobial defensin peptides form voltage-dependent, ion-permeable channels in planar lipid bilayer membranes. Proc. Natl. Acad. Sci. USA 87:210-214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kates, M., J. Y. Syz, D. Gosser, and T. H. Haines. 1993. pH-dissociation characteristics of cardiolipin and its 2′-deoxy analogue. Lipids 28:877-882. [DOI] [PubMed] [Google Scholar]
- 21.Kol, M. A., A. I. de Kroon, J. A. Killian, and B. de Kruijff. 2004. Transbilayer movement of phospholipids in biogenic membrane. Biochemistry 43:2673-2681. [DOI] [PubMed] [Google Scholar]
- 22.Koo, S.-P., A. S. Bayer, H.-G. Sahl, R. A. Proctor, and M. R. Yeaman. 1996. Staphylocidal action of thrombin-induced platelet microbicidal protein is not solely dependent on transmembrane potential. Infect. Immun. 64:1070-1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Koo, S.-P., M. R. Yeaman, C. C. Nast, and A. S. Bayer. 1997. The cytoplasmic membrane is a primary target for the staphylocidal action of thrombin-induced platelet microbicidal protein. Infect. Immun. 65:4795-4800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Koo, S.-P., A. S. Bayer, B. L. Kagan, and M. R. Yeaman. 1999. Membrane permeabilization by thrombin-induced platelet microbicidal protein 1 is modulated by transmembrane voltage polarity and magnitude. Infect. Immun. 67:2475-2481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Koo, S.-P., A. S. Bayer, and M. R. Yeaman. 2001. Diversity in antistaphylococcal mechanisms among membrane-targeting antimicrobial peptides. Infect. Immun. 69:4916-4922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Koprivnjak, T., A. Peschel, M. H. Gelb, N. S. Liang, and J. P. Weiss. 2002. Role of charge properties of bacterial envelope in bactericidal action of human group IIA phospholipase A2 against Staphylococcus aureus. J. Biol. Chem. 277:47636-47644. [DOI] [PubMed] [Google Scholar]
- 27.Koshkin, V., and M. L. Greenberg. 2002. Cardiolipin prevents rate-dependent uncoupling and provides osmotic stability in yeast mitochondria. Biochem. J. 364:317-322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kristian, S. A., M. Durr, J. A. Van Strijp, B. Neumeister, and A. Peschel. 2003. MprF-medicated lysinylation of phospholipids in Staphylococcus aureus leads to protection against oxygen-independent neutrophil killing. Infect. Immun. 71:546-549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.LaPorte, D. C., K. S. Rosenthal, and D. R. Storm. 1977. Inhibition of Escherichia coli growth and respiration by polymyxin B covalently attached to agarose beads. Biochemistry 16:1642-1648. [DOI] [PubMed] [Google Scholar]
- 30.Lehrer, R. I., A. Barton, and T. Ganz. 1988. Concurrent assessment of inner and outer membrane permeabilization and bacteriolysis in E. coli by multiple-wavelength spectrophotometry. J. Immunol. Methods 108:153-158. [DOI] [PubMed] [Google Scholar]
- 31.Lehrer, R. I., A. Barton, K. A. Daher, S. S. L. Harwig, T. Ganz, and M. E. Selsted. 1989. Interaction of human defensins with Escherichia coli. Mechanisms of bactericidal activity. J. Clin. Investig. 84:553-561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Matsuzaki, K., M. Harada, S. Funakoshi, N. Fujii, and K. Miyajima. 1991. Physiochemical determinants for the interactions of magainins 1 and 2 with acidic lipid bilayers. Biochim. Biophys. Acta 1063:162-170. [DOI] [PubMed] [Google Scholar]
- 33.Nagamachi, E., Y. Hirai, K. Tomochika, and Y. Kanemasa. 1992. Studies on osmotic stability of liposomes prepared with bacterial membrane lipids by carboxyfluorescein release. Microbiol. Immunol. 36:231-234. [DOI] [PubMed] [Google Scholar]
- 34.National Committee for Clinical Laboratory Standards. 1993. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically, 2nd ed. Approved standard M7-A3. NCCLS, Villanova, Pa.
- 35.Okabe, A., Y. Hirai, H. Hayashi, and Y. Kanemasa. 1980. Alteration in phospholipid composition of Staphylococcus aureus during formation of autoplast. Biochim. Biophys. Acta 617:28-35. [DOI] [PubMed] [Google Scholar]
- 36.Park, C. B., H. S. Kim, and S. C. Kim. 1998. Mechanism of action of the antimicrobial peptide buforin II: buforin II kills microorganisms by penetrating the cell membrane and inhibiting cellular functions. Biochem. Biophys. Res. Commun. 244:253-257. [DOI] [PubMed] [Google Scholar]
- 37.Peschel, A., R. W. Jack, M. Otto, L. V. Collins, P. Staubitz, G. Nicholson, H. Kalbacher, W. F. Nieuwenhuizen, G. Jung, A. Tarkowski, K.P. van Kessel, and J. A. van Strijp. 2001. Staphylococcus aureus resistance to human defensins and evasion of neutrophil killing via the novel virulence factor MprF is based on modification of membrane lipids with l-lysine. J. Exp. Med. 193:1067-1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Peschel, A. 2002. How do bacteria resist human antimicrobial peptides? Trends Microbiol. 10:179-186. [DOI] [PubMed] [Google Scholar]
- 39.Sahl, H.-G, U. Pag, S. Bonness, S. Wagner, N. Antcheva, and A. Tossi. 2005. Mammalian defensins: structures and mechanism of antibiotic activity. J. Leukoc. Biol. 77:466-475. [Online.] doi: 10.1189/jlb.0804452. [DOI] [PubMed] [Google Scholar]
- 40.Storm, D. R., K. S. Rosenthal, and P. E. Swanson. 1977. Polymyxin and related peptide antibiotics. Annu. Rev. Biochem. 46:723-763. [DOI] [PubMed] [Google Scholar]
- 41.Takemura, H., M. Kaku, S. Kohno, Y. Hirakata, H. Tanaka, R. Yoshida, K. Tomono, H. Koga, A. Wada, T. Hirayama, and S. Kamihira. 1996. Evaluation of susceptibility of gram-positive and -negative bacteria to human defensins by using radial diffusion assay. Antimicrob. Agents Chemother. 40:2280-2284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vial, F., S. Rabhi, and C. Tribet. 2005. Association of octyl-modified poly(acrylic acid) onto unilamellar vesicles of lipids and kinetics of vesicle disruption. Langmuir 21:853-862. [DOI] [PubMed] [Google Scholar]
- 43.White, S. H., W. C. Wimley, and M. E. Selsted. 1995. Structure, function, and membrane integration of defensins. Curr. Opin. Struct. Biol. 5:521-527. [DOI] [PubMed] [Google Scholar]
- 44.Xiong, Y. Q., M. R. Yeaman, and A. S. Bayer. 1999. In vitro antibacterial activities of platelet microbicidal protein and neutrophil defensin against Staphylococcus aureus are influenced by antibiotics differing in mechanism of action. Antimicrob. Agents Chemother. 43:1111-1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Xiong, Y. Q., A. S. Bayer, and M. R. Yeaman. 2002. Inhibition of intracellular macromolecular synthesis in Staphylococcus aureus by thrombin-induced platelet microbicidal proteins. J. Infect. Dis. 185:348-356. [DOI] [PubMed] [Google Scholar]
- 46.Yeaman, M. R., D. C. Norman, and A. S. Bayer. 1992. Platelet microbicidal protein enhances antibiotic-induced killing of and postantibiotic effect in Staphylococcus aureus. Antimicrob. Agents Chemother. 36:1665-1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yeaman, M. R., Y.-Q. Tang, A. J. Shen, A. S. Bayer, and M. E. Selsted. 1997. Purification and in vitro activities of rabbit platelet microbicidal proteins. Infect. Immun. 65:1023-1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yeaman, M. R. 1997. The role of platelets in antimicrobial host defense. Clin. Infect. Dis. 25:951-970. [DOI] [PubMed] [Google Scholar]
- 49.Yeaman, M. R., A. S. Bayer, S.-P. Koo, W. Foss, and P. M. Sullam. 1998. Platelet microbicidal protein and neutrophil defensin disrupt the Staphylococcus aureus cytoplasmic membrane by distinct mechanism of action. J. Clin. Investig. 101:178-187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yeaman, M. R., and N. Y. Yount. 2003. Mechanisms of antimicrobial peptide action and resistance. Pharmacol. Rev. 55:27-55. [DOI] [PubMed] [Google Scholar]
- 51.Yonezawa, A., J. Kuwahare, N. Fujii, and Y. Sugiura. 1992. Binding of tachyplesin I to DNA revealed by footprinting analysis: significant contribution of secondary structure to DNA binding and implication for biological action. Biochemistry 31:2998-3004. [DOI] [PubMed] [Google Scholar]
- 52.Yount, N. Y., K. D. Gank, Y. Q. Xiong, A. S. Bayer, T. Pender, W. H. Welch, and M. R. Yeaman. 2004. Platelet microbicidal protein 1: Structural themes of a multifunctional antimicrobial peptide. Antimicrob. Agents Chemother. 48:4395-4404. [DOI] [PMC free article] [PubMed] [Google Scholar]