Full text
PDF











































Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adrian R. H., Peachey L. D. The membrane capacity of frog twitch and slow muscle fibres. J Physiol. 1965 Nov;181(2):324–336. doi: 10.1113/jphysiol.1965.sp007764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aickin C. C., Brading A. F. Measurement of intracellular chloride in guinea-pig vas deferens by ion analysis, 36chloride efflux and micro-electrodes. J Physiol. 1982 May;326:139–154. doi: 10.1113/jphysiol.1982.sp014182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen D. G., Kurihara S. The effects of muscle length on intracellular calcium transients in mammalian cardiac muscle. J Physiol. 1982 Jun;327:79–94. doi: 10.1113/jphysiol.1982.sp014221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ammann D., Lanter F., Steiner R. A., Schulthess P., Shijo Y., Simon W. Neutral carrier based hydrogen ion selective microelectrode for extra- and intracellular studies. Anal Chem. 1981 Dec;53(14):2267–2269. doi: 10.1021/ac00237a031. [DOI] [PubMed] [Google Scholar]
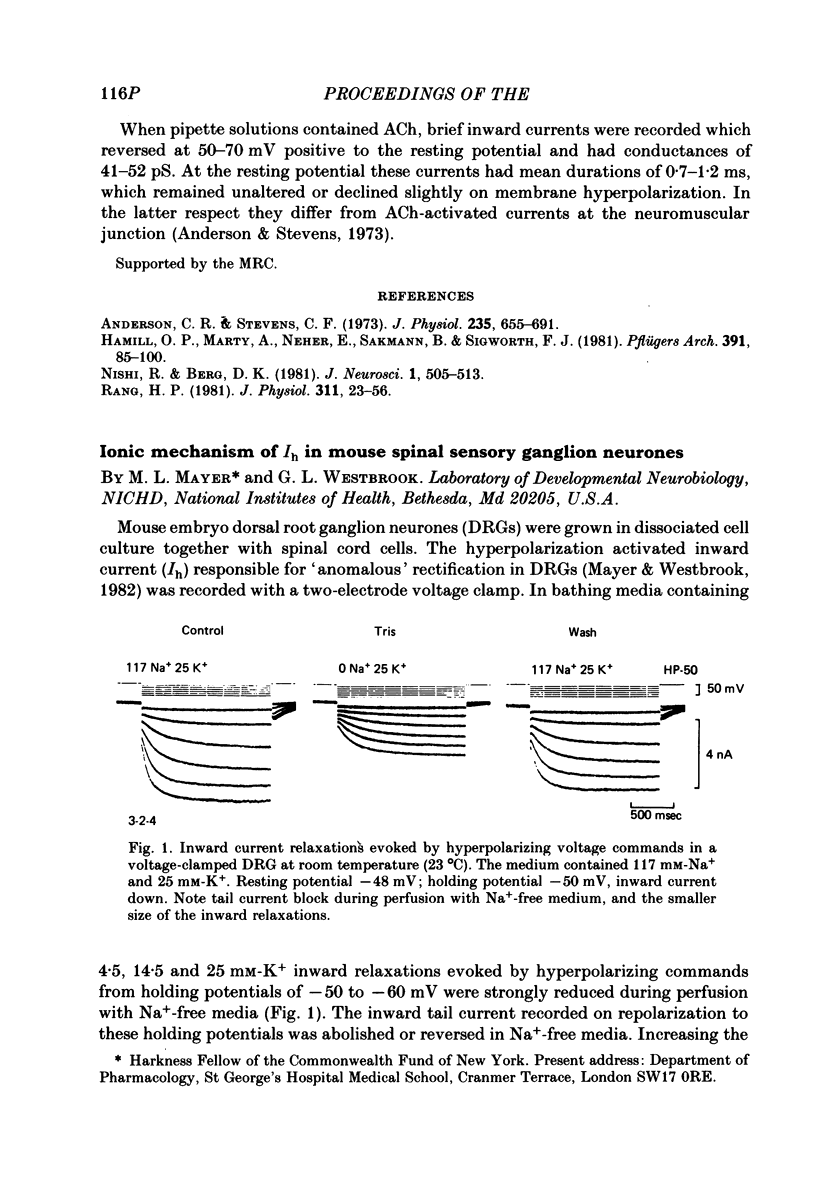
- Anderson C. R., Stevens C. F. Voltage clamp analysis of acetylcholine produced end-plate current fluctuations at frog neuromuscular junction. J Physiol. 1973 Dec;235(3):655–691. doi: 10.1113/jphysiol.1973.sp010410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderton B. H., Breinburg D., Downes M. J., Green P. J., Tomlinson B. E., Ulrich J., Wood J. N., Kahn J. Monoclonal antibodies show that neurofibrillary tangles and neurofilaments share antigenic determinants. Nature. 1982 Jul 1;298(5869):84–86. doi: 10.1038/298084a0. [DOI] [PubMed] [Google Scholar]
- Angel A., Dodd J., Gray J. D. Proceedings: Fluctuating anaesthetic state in the rat anaesthetized with urethane. J Physiol. 1976 Jul;259(1):11P–12P. [PubMed] [Google Scholar]
- Anner B., Ferrero J., Jirounek P., Straub R. W. Uptake of orthophosphate by rabbit vagus nerve fibres. J Physiol. 1975 Jun;247(3):759–771. doi: 10.1113/jphysiol.1975.sp010956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Axelsson S., Björklund A., Falck B., Lindvall O., Svensson L. A. Glyoxylic acid condensation: a new fluorescence method for the histochemical demonstration of biogenic monoamines. Acta Physiol Scand. 1973 Jan;87(1):57–62. doi: 10.1111/j.1748-1716.1973.tb05365.x. [DOI] [PubMed] [Google Scholar]
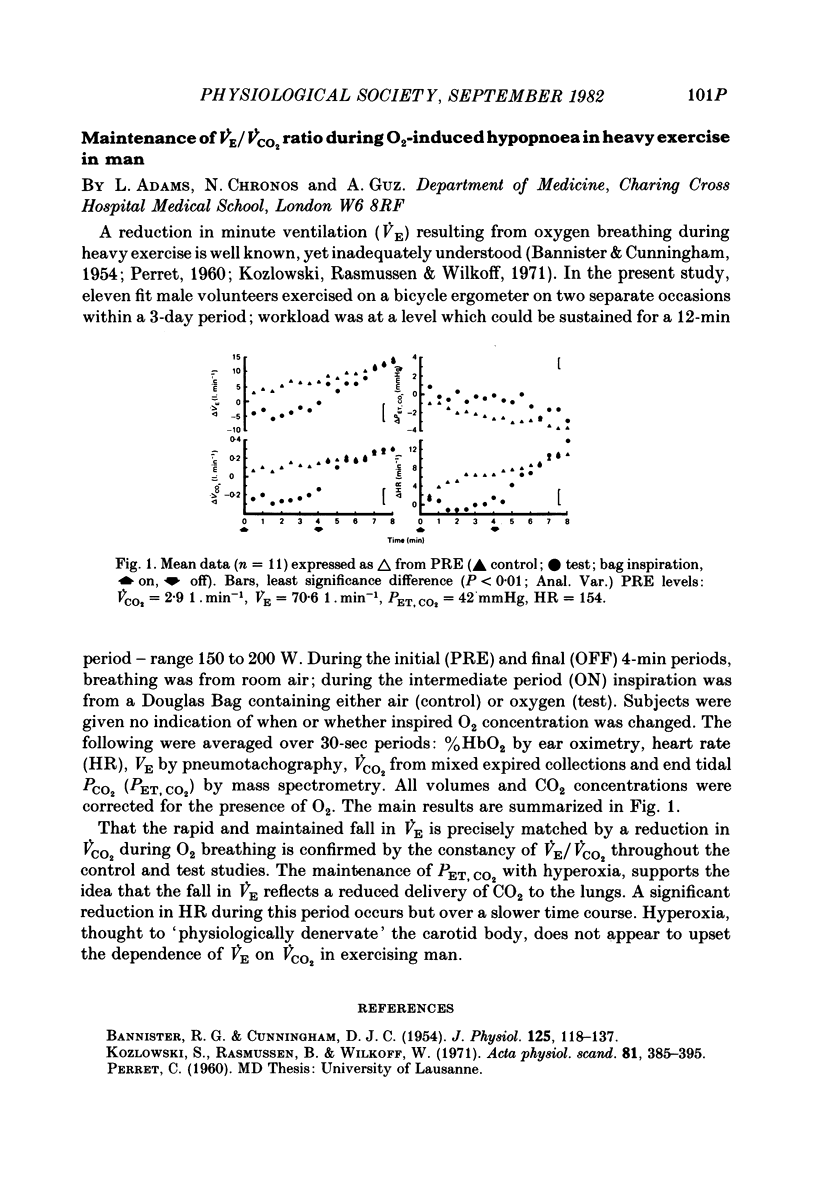
- BANNISTER R. G., CUNNINGHAM D. J. The effects on the respiration and performance during exercise of adding oxygen to the inspired air. J Physiol. 1954 Jul 28;125(1):118–137. doi: 10.1113/jphysiol.1954.sp005145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BRAUNWALD E., ROSS J., Jr, SONNENBLICK E. H., FROMMER P. L., BRAUNWALD N. S., MORROW A. G. SLOWING OF HEART RATE, ELECTROAUGMENTATION OF VENTRICULAR PERFORMANCE, AND INCREASE OF MYOCARDIAL OXYGEN CONSUMPTION PRODUCED BY PAIRED ELECTRICAL STIMULATION. Bull N Y Acad Med. 1965 May;41:481–497. [PMC free article] [PubMed] [Google Scholar]
- Baker P. F. Transport and metabolism of calcium ions in nerve. Prog Biophys Mol Biol. 1972;24:177–223. doi: 10.1016/0079-6107(72)90007-7. [DOI] [PubMed] [Google Scholar]
- Banks R. W. A histological study of the motor innervation of the cat's muscle spindle. J Anat. 1981 Dec;133(Pt 4):571–591. [PMC free article] [PubMed] [Google Scholar]
- Beckstead R. M. An autoradiographic examination of corticocortical and subcortical projections of the mediodorsal-projection (prefrontal) cortex in the rat. J Comp Neurol. 1979 Mar 1;184(1):43–62. doi: 10.1002/cne.901840104. [DOI] [PubMed] [Google Scholar]
- Beckstead R. M. Convergent thalamic and mesencephalic projections to the anterior medial cortex in the rat. J Comp Neurol. 1976 Apr 15;166(4):403–416. doi: 10.1002/cne.901660403. [DOI] [PubMed] [Google Scholar]
- Blaustein M. P. Sodium ions, calcium ions, blood pressure regulation, and hypertension: a reassessment and a hypothesis. Am J Physiol. 1977 May;232(5):C165–C173. doi: 10.1152/ajpcell.1977.232.5.C165. [DOI] [PubMed] [Google Scholar]
- Blikstad I., Markey F., Carlsson L., Persson T., Lindberg U. Selective assay of monomeric and filamentous actin in cell extracts, using inhibition of deoxyribonuclease I. Cell. 1978 Nov;15(3):935–943. doi: 10.1016/0092-8674(78)90277-5. [DOI] [PubMed] [Google Scholar]
- Brady A. J. Onset of contractility in cardiac muscle. J Physiol. 1966 Jun;184(3):560–580. doi: 10.1113/jphysiol.1966.sp007931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busby W. H., Jr, Youngblood W. W., Humm J., Kizer J. S. A reliable method for the quantification of thyrotropin-releasing hormone (TRH) in tissue and biological fluids. J Neurosci Methods. 1981 Dec;4(4):315–328. doi: 10.1016/0165-0270(81)90002-9. [DOI] [PubMed] [Google Scholar]
- Casteels R., Droogmans G. Exchange characteristics of the noradrenaline-sensitive calcium store in vascular smooth muscle cells or rabbit ear artery. J Physiol. 1981 Aug;317:263–279. doi: 10.1113/jphysiol.1981.sp013824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cochrane G. M., Prior J. G., Wolff C. B. Chronic stable asthma and the normal arterial pressure of carbon dioxide in hypoxia. Br Med J. 1980 Sep 13;281(6242):705–707. doi: 10.1136/bmj.281.6242.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohn P. F. Evaluation of inotropic contractile reserve in ischemic heart disease using postextrasystolic potentiation. Circulation. 1980 Jun;61(6):1071–1075. doi: 10.1161/01.cir.61.6.1071. [DOI] [PubMed] [Google Scholar]
- Colquhoun D., Sakmann B. Fluctuations in the microsecond time range of the current through single acetylcholine receptor ion channels. Nature. 1981 Dec 3;294(5840):464–466. doi: 10.1038/294464a0. [DOI] [PubMed] [Google Scholar]
- Conti F., Neher E. Single channel recordings of K+ currents in squid axons. Nature. 1980 May 15;285(5761):140–143. doi: 10.1038/285140a0. [DOI] [PubMed] [Google Scholar]
- Cox R. H. Influence of pentobarbital anesthesia on cardiovascular function in trained dogs. Am J Physiol. 1972 Sep;223(3):651–659. doi: 10.1152/ajplegacy.1972.223.3.651. [DOI] [PubMed] [Google Scholar]
- Cronly-Dillon J., Perry G. W. Effect of visual experience on tubulin synthesis during a critical period of visual cortex development in the hooded rat. J Physiol. 1979 Aug;293:469–484. doi: 10.1113/jphysiol.1979.sp012901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cull-Candy S. G., Parker I. Rapid kinetics of single glutamate-receptor channels. Nature. 1982 Feb 4;295(5848):410–412. doi: 10.1038/295410a0. [DOI] [PubMed] [Google Scholar]
- De Clerck N. M., Claes V. A., Van Ocken E. R., Brutsaert D. L. Sarcomere distribution patterns in single cardiac cells. Biophys J. 1981 Jul;35(1):237–242. doi: 10.1016/S0006-3495(81)84784-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiFrancesco D. A study of the ionic nature of the pace-maker current in calf Purkinje fibres. J Physiol. 1981 May;314:377–393. doi: 10.1113/jphysiol.1981.sp013714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EVERETT J. W., SAWYER C. H. A 24-hour periodicity in the "LH-release apparatus" of female rats, disclosed by barbiturate sedation. Endocrinology. 1950 Sep;47(3):198–218. doi: 10.1210/endo-47-3-198. [DOI] [PubMed] [Google Scholar]
- Eckert R., Tillotson D. L. Calcium-mediated inactivation of the calcium conductance in caesium-loaded giant neurones of Aplysia californica. J Physiol. 1981 May;314:265–280. doi: 10.1113/jphysiol.1981.sp013706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabiato A., Fabiato F. Effects of pH on the myofilaments and the sarcoplasmic reticulum of skinned cells from cardiace and skeletal muscles. J Physiol. 1978 Mar;276:233–255. doi: 10.1113/jphysiol.1978.sp012231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira S. H., Vane J. R. Prostaglandins: their disappearance from and release into the circulation. Nature. 1967 Dec 2;216(5118):868–873. doi: 10.1038/216868a0. [DOI] [PubMed] [Google Scholar]
- Ginsburg M., Greenstein B. D., MacLusky N. J., Morris I. D., Thomas P. J. An improved method for the study of high-affinity steroid binding:-oestradiol binding in brain and pituitary. Steroids. 1974 Jun;23(6):773–792. doi: 10.1016/0039-128x(74)90053-1. [DOI] [PubMed] [Google Scholar]
- Griffiths E. C., Kelly J. A. Mechanisms of inactivation of hypothalamic regulatory hormones. Mol Cell Endocrinol. 1979 Apr;14(1):3–17. doi: 10.1016/0303-7207(79)90054-6. [DOI] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Hamill O. P., Sakmann B. Multiple conductance states of single acetylcholine receptor channels in embryonic muscle cells. Nature. 1981 Dec 3;294(5840):462–464. doi: 10.1038/294462a0. [DOI] [PubMed] [Google Scholar]
- Hoffman P. N., Lasek R. J. Axonal transport of the cytoskeleton in regenerating motor neurons: constancy and change. Brain Res. 1980 Dec 8;202(2):317–333. doi: 10.1016/0006-8993(80)90144-4. [DOI] [PubMed] [Google Scholar]
- Horton E., Jones R., Thompson C., Poyser N. Release of prostaglandins. Ann N Y Acad Sci. 1971 Apr 30;180:351–362. doi: 10.1111/j.1749-6632.1971.tb53204.x. [DOI] [PubMed] [Google Scholar]
- Hotson J. R., Prince D. A. A calcium-activated hyperpolarization follows repetitive firing in hippocampal neurons. J Neurophysiol. 1980 Feb;43(2):409–419. doi: 10.1152/jn.1980.43.2.409. [DOI] [PubMed] [Google Scholar]
- Hubel D. H., Wiesel T. N., LeVay S. Plasticity of ocular dominance columns in monkey striate cortex. Philos Trans R Soc Lond B Biol Sci. 1977 Apr 26;278(961):377–409. doi: 10.1098/rstb.1977.0050. [DOI] [PubMed] [Google Scholar]
- Jackson I. M. Thyrotropin-releasing hormone. N Engl J Med. 1982 Jan 21;306(3):145–155. doi: 10.1056/NEJM198201213060305. [DOI] [PubMed] [Google Scholar]
- Jami L., Lan-Couton D., Malmgren K., Petit J. "Fast" and "slow" skeleto-fusimotor innervation in cat tenuissimus spindles; a study with the glycogen-depletion method. Acta Physiol Scand. 1978 Jul;103(3):284–298. doi: 10.1111/j.1748-1716.1978.tb06216.x. [DOI] [PubMed] [Google Scholar]
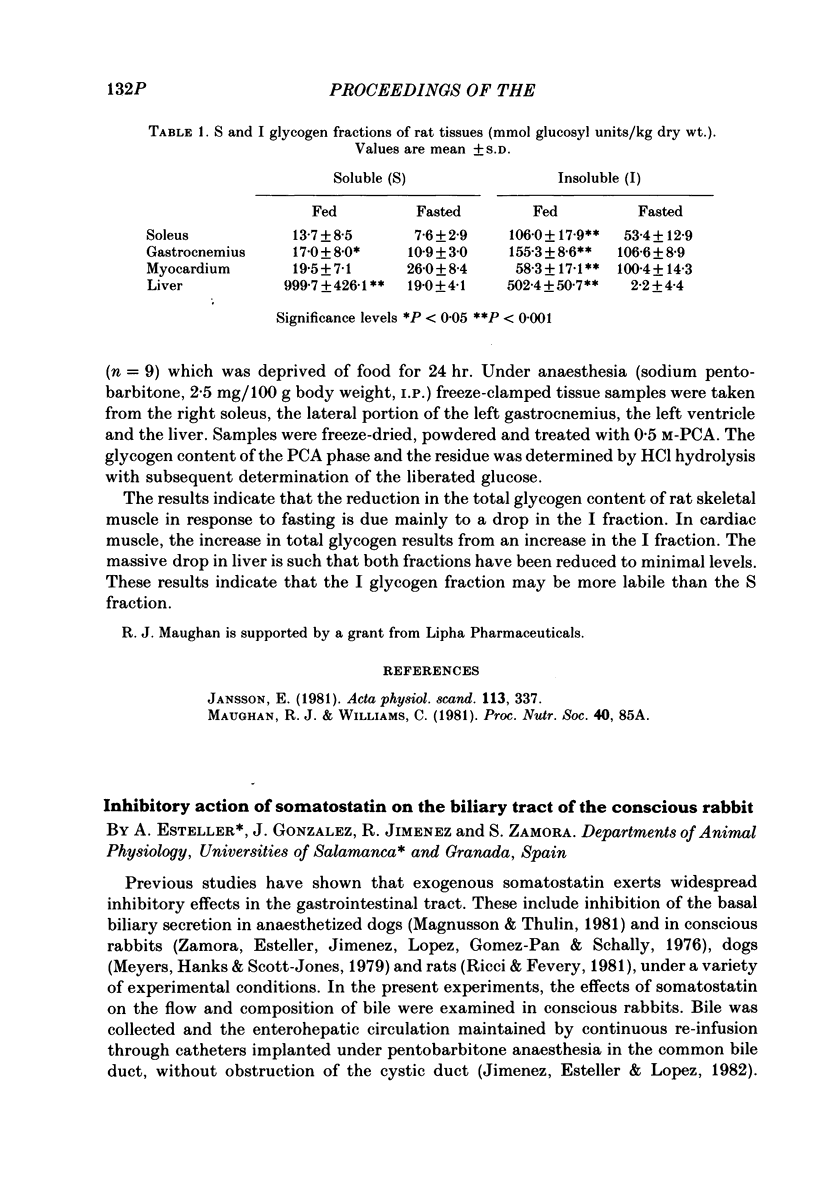
- Jansson E. Acid soluble and insoluble glycogen in human skeletal muscle. Acta Physiol Scand. 1981;113(3):337–340. doi: 10.1111/j.1748-1716.1981.tb06904.x. [DOI] [PubMed] [Google Scholar]
- Jessen K. R., Mirsky R., Dennison M. E., Burnstock G. GABA may be a neurotransmitter in the vertebrate peripheral nervous system. Nature. 1979 Sep 6;281(5726):71–74. doi: 10.1038/281071a0. [DOI] [PubMed] [Google Scholar]
- Jimenez R., Esteller A., Lopez M. A. Biliary secretion in conscious rabbits: surgical technique. Lab Anim. 1982 Apr;16(2):182–185. doi: 10.1258/002367782781110250. [DOI] [PubMed] [Google Scholar]
- Juraska J. M., Fifkova E. A Golgi study of the early postnatal development of the visual cortex of the hooded rat. J Comp Neurol. 1979 Jan 15;183(2):247–256. doi: 10.1002/cne.901830203. [DOI] [PubMed] [Google Scholar]
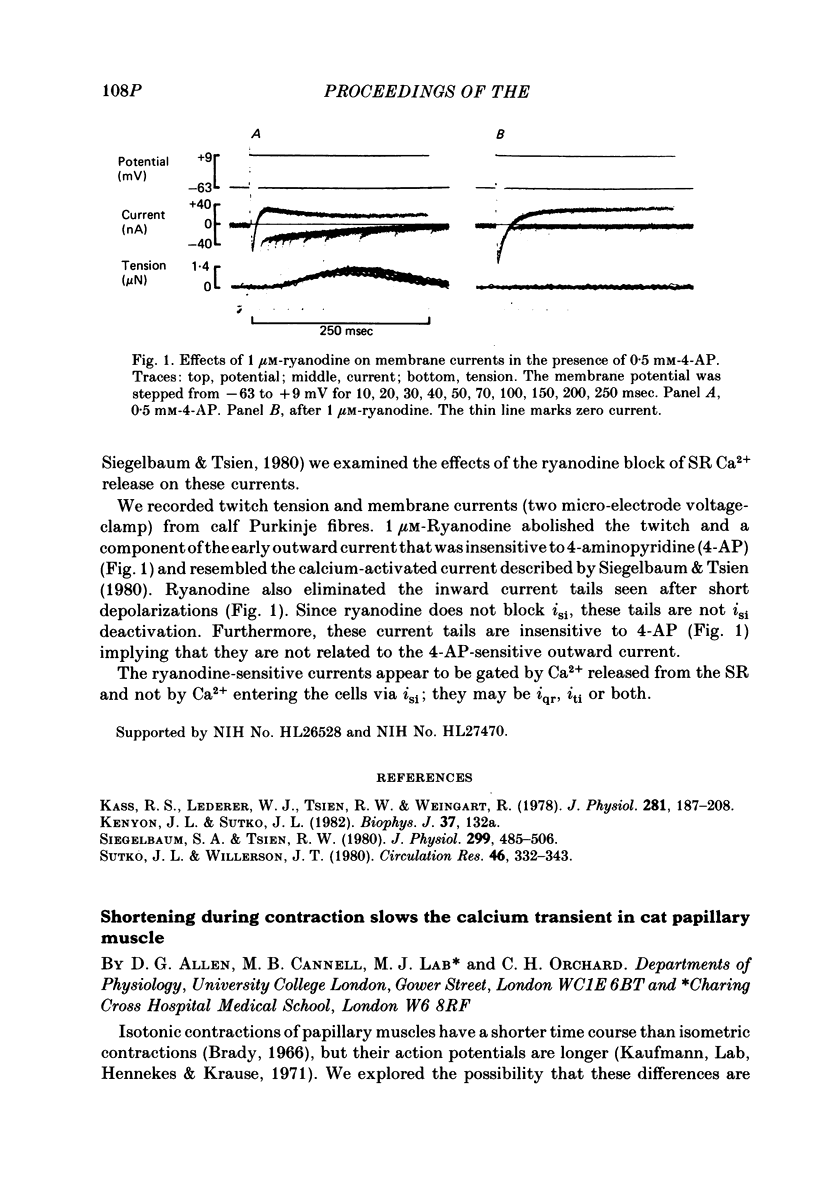
- Kass R. S., Lederer W. J., Tsien R. W., Weingart R. Role of calcium ions in transient inward currents and aftercontractions induced by strophanthidin in cardiac Purkinje fibres. J Physiol. 1978 Aug;281:187–208. doi: 10.1113/jphysiol.1978.sp012416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufmann R. L., Lab M. J., Hennekes R., Krause H. Feedback interaction of mechanical and electrical events in the isolated mammalian ventricular myocardium (cat papillary muscle). Pflugers Arch. 1971;324(2):100–123. doi: 10.1007/BF00592656. [DOI] [PubMed] [Google Scholar]
- Kozlowski S., Rasmussen B., Wilkoff W. G. The effect of high oxygen tensions on ventilation during severe exercise. Acta Physiol Scand. 1971 Mar;81(3):385–395. doi: 10.1111/j.1748-1716.1971.tb04913.x. [DOI] [PubMed] [Google Scholar]
- Kucera J. Histochemical study of long nuclear chain fibers in the cat muscle spindle. Anat Rec. 1980 Dec;198(4):567–580. doi: 10.1002/ar.1091980403. [DOI] [PubMed] [Google Scholar]
- LOGOTHETIS J., HARNER R., MORRELL F., TORRES F. The role of estrogens in catamenial exacerbation of epilepsy. Neurology. 1959 May;9(5):352–360. doi: 10.1212/wnl.9.5.352. [DOI] [PubMed] [Google Scholar]
- Lab M. J. Contraction-excitation feedback in myocardium. Physiological basis and clinical relevance. Circ Res. 1982 Jun;50(6):757–766. doi: 10.1161/01.res.50.6.757. [DOI] [PubMed] [Google Scholar]
- Lawson S. N. The postnatal development of large light and small dark neurons in mouse dorsal root ganglia: a statistical analysis of cell numbers and size. J Neurocytol. 1979 Jun;8(3):275–294. doi: 10.1007/BF01236123. [DOI] [PubMed] [Google Scholar]
- Login I. S., MacLeod R. M. The direct effect of reserpine in vitro on prolactin release from rat anterior pituitary glands. Brain Res. 1981 Jan 5;204(1):79–85. doi: 10.1016/0006-8993(81)90653-3. [DOI] [PubMed] [Google Scholar]
- Magnusson I., Thulin L. Effects of substance P and somatostatin on choleresis in anesthetized dogs. Acta Chir Scand. 1981;147(8):667–671. [PubMed] [Google Scholar]
- Matthews M. R., Cuello A. C. Substance P-immunoreactive peripheral branches of sensory neurons innervate guinea pig sympathetic neurons. Proc Natl Acad Sci U S A. 1982 Mar;79(5):1668–1672. doi: 10.1073/pnas.79.5.1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mefford I. N. Application of high performance liquid chromatography with electrochemical detection to neurochemical analysis: measurement of catecholamines, serotonin and metabolites in rat brain. J Neurosci Methods. 1981 Feb;3(3):207–224. doi: 10.1016/0165-0270(81)90056-x. [DOI] [PubMed] [Google Scholar]
- Meyers W. C., Hanks J. B., Jones R. S. Inhibition of basal and meal-stimulated choleresis by somatostatin. Surgery. 1979 Aug;86(2):301–306. [PubMed] [Google Scholar]
- Micro-electrode measurement of the intracellular pH and buffering power of mouse soleus muscle fibres. J Physiol. 1977 Jun;267(3):791–810. doi: 10.1113/jphysiol.1977.sp011838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson P. G., Henkart M. P. Oscillatory membrane potential changes in cells of mesenchymal origin: the role of an intracellular calcium regulating system. J Exp Biol. 1979 Aug;81:49–61. doi: 10.1242/jeb.81.1.49. [DOI] [PubMed] [Google Scholar]
- Nett T. M., Akbar A. M., Niswender G. D., Hedlund M. T., White W. F. A radioimmunoassay for gonadotropin-releasing hormone (Gn-RH) in serum. J Clin Endocrinol Metab. 1973 May;36(5):880–885. doi: 10.1210/jcem-36-5-880. [DOI] [PubMed] [Google Scholar]
- Nishi R., Berg D. K. Two components from eye tissue that differentially stimulate the growth and development of ciliary ganglion neurons in cell culture. J Neurosci. 1981 May;1(5):505–513. doi: 10.1523/JNEUROSCI.01-05-00505.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ovalle W. K., Smith R. S. Histochemical identification of three types of intrafusal muscle fibers in the cat and monkey based on the myosin ATPase reaction. Can J Physiol Pharmacol. 1972 Mar;50(3):195–202. doi: 10.1139/y72-030. [DOI] [PubMed] [Google Scholar]
- PLUMMER A. J., EARL A., SCHNEIDER J. A., TRAPOLD J., BARRETT W. Pharmacology of Rauwolfia alkaloids, including reserpine. Ann N Y Acad Sci. 1954 Apr 30;59(1):8–21. doi: 10.1111/j.1749-6632.1954.tb45914.x. [DOI] [PubMed] [Google Scholar]
- PUGH L. G., GILL M. B., LAHIRI S., MILLEDGE J. S., WARD M. P., WEST J. B. MUSCULAR EXERCISE AT GREAT ALTITUDES. J Appl Physiol. 1964 May;19:431–440. doi: 10.1152/jappl.1964.19.3.431. [DOI] [PubMed] [Google Scholar]
- Pardey-Borrero B., Gonzalez-Vegas J. A. Reciprocal connections between substantia nigra and medullary reticular formation in the rat. Experientia. 1980 Jun 15;36(6):665–666. doi: 10.1007/BF01970126. [DOI] [PubMed] [Google Scholar]
- Parsons D. S., Wade S. A. Sodium movements across the vascularly perfused anuran small intestine and colon. Q J Exp Physiol. 1982 Jan;67(1):121–131. doi: 10.1113/expphysiol.1982.sp002606. [DOI] [PubMed] [Google Scholar]
- Pearson G. T., Davison J. S., Collins R. C., Petersen O. H. Control of enzyme secretion by non-cholinergic, non-adrenergic nerves in guinea pig pancreas. Nature. 1981 Mar 19;290(5803):259–261. doi: 10.1038/290259a0. [DOI] [PubMed] [Google Scholar]
- Pearson G. T., Singh J., Daoud M. S., Davison J. S., Petersen O. H. Control of pancreatic cyclic nucleotide levels and amylase secretion by noncholinergic, nonadrenergic nerves. A study employing electrical field stimulation of guinea pig segments. J Biol Chem. 1981 Nov 10;256(21):11025–11031. [PubMed] [Google Scholar]
- Rang H. P. The characteristics of synaptic currents and responses to acetylcholine of rat submandibular ganglion cells. J Physiol. 1981 Feb;311:23–55. doi: 10.1113/jphysiol.1981.sp013571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinhardt H. W., Kaczmarczyk G., Fahrenhorst K., Blendinger I., Gatzka M., Kuhl U., Riedel J. Postprandial changes of renal blood flow. Studies on conscious dogs on a high and low sodium intake. Pflugers Arch. 1975;354(4):287–297. doi: 10.1007/BF00587848. [DOI] [PubMed] [Google Scholar]
- Reiser S., Fitzgerald J. F., Christiansen P. A. Kinetics of the accelerated intestinal valine transport in 2-day-old rats. Biochim Biophys Acta. 1970 Apr 21;203(2):351–353. doi: 10.1016/0005-2736(70)90153-7. [DOI] [PubMed] [Google Scholar]
- Ricci G. L., Fevery J. Cholestatic action of somatostatin in the rat: effect on the different fractions of bile secretion. Gastroenterology. 1981 Sep;81(3):552–562. [PubMed] [Google Scholar]
- Sakmann B., Patlak J., Neher E. Single acetylcholine-activated channels show burst-kinetics in presence of desensitizing concentrations of agonist. Nature. 1980 Jul 3;286(5768):71–73. doi: 10.1038/286071a0. [DOI] [PubMed] [Google Scholar]
- Saller C. F., Zigmond M. J. A radioenzymatic assay for catecholamines and dihydroxyphenylacetic acid. Life Sci. 1978 Sep 18;23(11):1117–1130. doi: 10.1016/0024-3205(78)90345-4. [DOI] [PubMed] [Google Scholar]
- Sandberg D. E., Segal M. Pharmacological analysis of analgesia and self-stimulation elicited by electrical stimulation of catecholamine nuclei in the rat brain. Brain Res. 1978 Sep 8;152(3):529–542. doi: 10.1016/0006-8993(78)91108-3. [DOI] [PubMed] [Google Scholar]
- Schwartzkroin P. A., Stafstrom C. E. Effects of EGTA on the calcium-activated afterhyperpolarization in hippocampal CA3 pyramidal cells. Science. 1980 Dec 5;210(4474):1125–1126. doi: 10.1126/science.6777871. [DOI] [PubMed] [Google Scholar]
- Seyama I. Characteristics of the anion channel in the sino-atrial node cell of the rabbit. J Physiol. 1979 Sep;294:447–460. doi: 10.1113/jphysiol.1979.sp012940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shalton P. M., Wareham A. C. Some factors affecting spontaneous transmitter release in dystrophic mice. Muscle Nerve. 1980 Mar-Apr;3(2):120–127. doi: 10.1002/mus.880030204. [DOI] [PubMed] [Google Scholar]
- Shaw G., Osborn M., Weber K. Arrangement of neurofilaments, microtubules and microfilament-associated proteins in cultured dorsal root ganglia cells. Eur J Cell Biol. 1981 Apr;24(1):20–27. [PubMed] [Google Scholar]
- Siegelbaum S. A., Tsien R. W. Calcium-activated transient outward current in calf cardiac Purkinje fibres. J Physiol. 1980 Feb;299:485–506. doi: 10.1113/jphysiol.1980.sp013138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman H., Atwood H. L. Increase in oxidative capacity of muscle fibers in dystrophic mice and correlation with overactivity in these fibers. Exp Neurol. 1980 Apr;68(1):97–113. doi: 10.1016/0014-4886(80)90069-2. [DOI] [PubMed] [Google Scholar]
- Stephens N. L., Kroeger E. A., Loh W. Intracellular pH in hypoxic smooth muscle. Am J Physiol. 1977 Mar;232(3):E330–E335. doi: 10.1152/ajpendo.1977.232.3.E330. [DOI] [PubMed] [Google Scholar]
- Sutko J. L., Willerson J. T. Ryanodine alteration of the contractile state of rat ventricular myocardium. Comparison with dog, cat, and rabbit ventricular tissues. Circ Res. 1980 Mar;46(3):332–343. doi: 10.1161/01.res.46.3.332. [DOI] [PubMed] [Google Scholar]
- Taegtmeyer H., Hems R., Krebs H. A. Utilization of energy-providing substrates in the isolated working rat heart. Biochem J. 1980 Mar 15;186(3):701–711. doi: 10.1042/bj1860701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terasawa E., Timiras P. S. Electrical activity during the estrous cycle of the rat: cyclic changes in limbic structures. Endocrinology. 1968 Aug;83(2):207–216. doi: 10.1210/endo-83-2-207. [DOI] [PubMed] [Google Scholar]
- Teräväinen H. Anatomical and physiological studies on muscles of lamprey. J Neurophysiol. 1971 Nov;34(6):954–973. doi: 10.1152/jn.1971.34.6.954. [DOI] [PubMed] [Google Scholar]
- Thomas R. C., Cohen C. J. A liquid ion-exchanger alternative to KCl for filling intracellular reference microelectrodes. Pflugers Arch. 1981 Apr;390(1):96–98. doi: 10.1007/BF00582719. [DOI] [PubMed] [Google Scholar]
- Thornton V. F. Stimulation of calcium-dependent release of labelled protein from pulse-labelled mouse pituitary intermediate lobe tissue. J Physiol. 1982 Aug;329:425–437. doi: 10.1113/jphysiol.1982.sp014311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Breemen C., McNaughton E. The separation of cell membrane calcium transport from extracellular calcium exchange in vascular smooth muscle. Biochem Biophys Res Commun. 1970 May 22;39(4):567–574. doi: 10.1016/0006-291x(70)90241-x. [DOI] [PubMed] [Google Scholar]
- West J. B., Wagner P. D. Predicted gas exchange on the summit of Mt. Everest. Respir Physiol. 1980 Oct;42(1):1–16. doi: 10.1016/0034-5687(80)90100-0. [DOI] [PubMed] [Google Scholar]
- Yudilevich D. L., Alvarez O. A. Water, sodium, and thiourea transcapillary diffusion in the dog heart. Am J Physiol. 1967 Aug;213(2):308–314. doi: 10.1152/ajplegacy.1967.213.2.308. [DOI] [PubMed] [Google Scholar]


