Abstract
The H9N2 subtype avian influenza virus (AIV) continues to propagate and undergo evolution within China, thereby posing a significant threat to the poultry industry. This study encompassed the collection of 436 samples and swabs in East China over the period spanning 2018 to 2019, from which 31 strains of the H9N2 subtype viruses were isolated and purified. We revealed that the HA and NA genes of the 31 isolates categorized within the Y280 branch, while the PB2 and M genes were associated with the G1 branch, and the remaining genes aligned with the F/98 branch. Despite this alignment, antigenic mapping demonstrated differences between the 2018 and 2019 strains, with the early vaccine strains displaying low serological reactivity toward these isolates. Notably, the CK/SH/49/19 isolate exhibited lethality in mice, characterized by a PB2 E627V mutation and a HA deletion at amino acid position 217. Mechanistically, in vitro studies showed that the influenza virus CK/SH/49/19 carrying PB2 627V and HA 217M mutations displayed enhanced replication capacity, attributed to the heightened activity of the polymerase with PB2 627V. Moreover, the absence of the amino acid at the HA 217 site obstructed viral adsorption and internalization, resulted in lower activation pH, and impeded the virus budding process. Critically, in vivo experiments revealed that CK/SH/49/19 (PB2 627V, HA 217Δ) triggered a robust activation of interferon response and interferon-stimulated genes. This study furnished a theoretical foundation for the scientific prevention and control strategies against H9N2 subtype avian influenza.
Keywords: Avian influenza virus (AIV), H9N2, PB2 627V, HA 217M, Pathogenicity
Highlights
-
•
Genetic evolution analysis of H9N2 viruses revealed that all isolates in this study were classified within the G57 genotype.
-
•
The CK/SH/49/19 isolate exhibited lethality in mice, characterized by a PB2 E627V mutation and a deletion at HA 217.
-
•
The deletion of HA 217 hindered viral adsorption and internalization, leading to a lower activation pH and impeding budding.
Introduction
Influenza A virus (IAV) is a segmented negative-strand RNA virus enclosed by an envelope. The viral genome comprises eight single-stranded RNA segments enclosed within the virus particle. These segments are organized based on gene length, ranging from segment 1 to segment 8 (Noda et al., 2006). Each of these genetic fragments encodes a minimum of one protein, thereby totaling 10 core proteins alongside other accessory proteins. These include two surface glycoproteins, hemagglutinin (HA) and neuraminidase (NA), the nucleoprotein (NP), three polymerase proteins (PB2, PB1, PA), two matrix proteins (M1, M2), nonstructural protein 1 (NS1), and the nuclear export protein (NEP) (Gao et al., 2015; Krischuns et al., 2021). The H9N2 subtype of avian influenza virus (AIV) is distinguished by its rapid spread (Homme and Easterday, 1970; Fusaro et al., 2011). In 1994, the first instance of H9N2 in China was reported from infected chickens in Guangdong. Currently, H9N2 stands as the predominant subtype within China (Gu et al., 2017). According to HA phylogenetic analysis, the H9N2 subtype AIV can usually be divided into two major lineages: Eurasian and North American. The Eurasian lineage has been stably prevalent in poultry and is further divided into three different branches, A/quail/Hong Kong/G1/1997, i.e., the G1 branch; A/chicken/Beijing/1/1994, A/duck/Hong Kong/Y280/1997, i.e., the Y280/G9 (BJ94) branch; and A/duck/Hong Kong/Y439/1997, i.e., the Y439 branch (Sun and Liu, 2015). H9N2 lineages and sublineages can usually be subdivided based on kinship and geographic distribution. The G1 branch is divided into two sublineages, namely the Eastern sublineage and the Western sublineage. The G1 Eastern sublineage is more common in southern China, Vietnam, Cambodia and other Middle Eastern countries; the viruses of the G1 Western sublineage are mainly distributed in poultry in Africa and South Asia, and are currently prevalent in poultry in Egypt. Viruses from the Y280/G9 branch have been detected in various countries, including China, Vietnam, Cambodia, Myanmar, and Indonesia, with prevalence observed in northern and eastern regions of China. On the other hand, the Y439 branch is predominantly found in South Korea (Alexander, 2003, 2007; Zhang P. et al., 2009). Additionally, by analyzing the genetic evolution of the HA gene of the H9N2 virus, four distinct evolutionary branches, designated as h9.1–h9.4, have been identified through distinct categorizations. Currently, the majority of prevalent strains in China belong to the h9.4.2.5 and h9.4.2.6 branches, with the h9.4.2.5 branch showing dominance, and the h9.4.2.6 branch beginning to spread nationwide around 2010 (Jiang et al., 2012).
Unlike cellular DNA polymerase, viral RNA polymerase lacks proofreading capabilities, leading to a higher mutation rate and the likelihood of adaptive amino acid mutations. The segmented nature of the IAV viral genome allows for viral gene recombination, presenting an additional mechanism for adaptive mutations. Consequently, the mutation patterns of the H9N2 subtype AIV have garnered significant attention. The internal genes of the H9N2 virus exhibit strong compatibility with those of various IAV subtypes. Particularly, the H9N2 virus can contribute internal genes to human-infecting viruses such as H5N1, H5N6, H7N9, and H10N8 (Liu et al., 2014; Shi W. et al., 2014; Liu et al., 2022; Liu K. et al., 2024). Additionally, it is noted that single or multiple internal genes of the H9N2 virus may also originate from other IAV subtypes (Iqbal et al., 2009). This underscores the importance of monitoring the prevalence of the H9N2 subtype AIV and investigating its biological characteristics, including genetic evolution, antigenicity, and pathogenicity.
This study involved the collection of tissues and swabs from diseased poultry at breeding enterprises and farms in East China during the period of 2018–2019. The H9N2 subtype AIV was isolated, identified, and purified from these samples. Whole genome sequencing was conducted to analyze genetic evolution and amino acid variation characteristics at the gene level. Overall, this study elucidates the prevalence, genetic evolution, and pathogenicity of the H9N2 subtype AIV in East China. Significantly, it identifies the critical sites, PB2 627 and HA 217, within CK/SH/49/19 that contribute to its lethality in mice.
Results
Epidemiological and genetic evolution analysis of H9N2 influenza virus in eastern China from 2018 to 2019
To explore the genetic evolution of H9N2 AIV in East China, this study conducted a collection of 436 samples and swabs from various breeding enterprises and farmers in the region between 2018 and 2019. Among these samples, 50 tested positive for the H9 subtype, leading to the purification of 31 virus strains via limited dilution within chicken embryos. Detailed strain information can be found in Supplementary Table S1. The results of the phylogenetic analysis of the HA gene revealed that the HA genes of the 31 isolates were categorized within the Eurasian lineage, specifically clustering in the Y280 branch. This branch was notably distinct from the well-known vaccine strains CK/SD/6/96 and CK/SH/F/98, instead exhibiting closer proximity to strains originating from regions such as Jiangsu, Zhejiang, and Anhui (Fig. 1A). The receptor binding sites in the HA gene of the 31 isolates exhibited a relatively conservative pattern, with positions 91, 143, 145, 184, and 185 (H9 encoding) aligning with reference strains such as CK/BJ/1/94, CK/SD/6/96, DK/HK/Y280/97, and CK/SH/F/98. Notably, when compared to the classic vaccine strains CK/SD/6/96 and CK/SH/F/98, mutations T129I, K131 N, and K131T were identified at sites 128–132 on the receptor binding's right edge (Table 1). It is important to highlight that isolate CK/SH/49/19 showcased an amino acid deletion at position 217, along with the appearance of an R219I mutation at the more conserved site 219 (Table 1). The HA cleavage site spans from position 317 to 323, with the exception of CK/FY/869/19 that exhibits a cleavage site sequence of KSSR↓GLF, whereas the rest mostly feature an RSSR↓GLF sequence. This sequence includes only two non-adjacent basic amino acids, conforming to the fundamental characteristics of Low Pathogenic AIV (LPAIV) (Table 1). Regarding the statistical analysis of HA potential glycosylation sites, it was observed that 25 out of the 31 isolates possessed 7 potential glycosylation sites, notably consistent across positions 11, 123, 280, 287, 295, 474, and 533. The glycosylation pattern was found to be highly conserved. However, the isolate CK/SH/49/19 lacked glycosylation site 280. In addition, some isolates showed variations in potential glycosylation sites, with CK/FY/177/19 and CK/TA/146/18 increasing sites 54 and 127, respectively, CK/FY/338/18 and CK/HF/316/18 increasing sites 148, and CK/HZ/46/19 increasing sites 189 and 267 (Supplementary Table S2).
Fig. 1.
Phylogenetic tree of the HA (A) and NA (B) segment. The legend notes that the reliability of phylogenetic inference at each branch node was assessed using the bootstrap method with 1000 replications. The AIVs isolated in this study are highlighted in red.
Table 1.
Receptor binding sites and cleavage site of HA gene.
| Strain | Receptor binding sites |
Cleavage site |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 91 | 128–132 | 143 | 145 | 173 | 180 | 184 | 185 | 214–219 | 317–323 | |
| CK/TZ/319/18 | Y | GTSTA | W | T | N | T | L | Y | NGLMGR | RSSR↓GLF |
| CK/XZ/875/18 | Y | GTSKA | W | T | N | V | L | Y | NGLMGR | RSSR↓GLF |
| CK/YZ/255/18 | Y | GTSKA | W | T | N | T | L | Y | NGLMGR | RSSR↓GLF |
| CK/WF/404/18 | Y | GTSTA | W | T | N | T | L | Y | NGLMGR | RSSR↓GLF |
| CK/FY/297/18 | Y | GTSTA | W | T | N | T | L | Y | NGLMGR | RSSR↓GLF |
| CK/FY/338/18 | Y | GTSKA | W | T | N | V | L | Y | NGLMGR | RSSR↓GLF |
| CK/XZ/968/18 | Y | GTSKA | W | T | N | V | L | Y | NGLMGR | RSSR↓GLF |
| CK/LY/977/19 | Y | GTSKA | W | T | N | A | L | Y | NGLMGR | RSSR↓GLF |
| CK/TA/146/18 | Y | GTSTA | W | T | N | T | L | Y | NGLMGR | RSSR↓GLF |
| CK/CZ/1806/19 | Y | GTSKA | W | T | N | V | L | Y | NGLMGR | RSSR↓GLF |
| CK/AH/1066/18 | Y | GTSTA | W | T | N | T | L | Y | NGLMGR | RSSR↓GLF |
| CK/CZ/325/18 | Y | GTSIA | W | T | N | T | L | Y | NGLMGR | RSSR↓GLF |
| CK/CZ/1020/19 | Y | GTSKA | W | T | N | A | L | Y | NGLMGR | RSSR↓GLF |
| CK/CZ/1149/18 | Y | GTSTA | W | T | N | T | L | Y | NGLMGR | RSSR↓GLF |
| CK/YZ/1025/19 | Y | GTSKA | W | T | N | V | L | Y | NGLMGR | RSSR↓GLF |
| CK/CZ/292/18 | Y | GTSKA | W | T | N | T | L | Y | NGLMGR | RSSR↓GLF |
| CK/FY/439/18 | Y | GTSKA | W | T | N | T | L | Y | NGLMGR | RSSR↓GLF |
| CK/CZ/424/18 | Y | GTSTA | W | T | N | T | L | Y | NGLMGR | RSSR↓GLF |
| CK/FY/901/19 | Y | GTSKA | W | T | N | T | L | Y | NGLMGR | RSSR↓GLF |
| CK/HF/316/18 | Y | GTSKA | W | T | N | V | L | Y | NGLMGR | RSSR↓GLF |
| CK/XZ/1078/19 | Y | GTSNA | W | T | N | T | L | Y | NGLMGR | RSSR↓GLF |
| CK/YZ/628/19 | Y | GTSTA | W | T | N | T | L | Y | NGLMGR | RSSR↓GLF |
| CK/FY/869/19 | Y | GTSKA | W | T | N | V | L | Y | NGLMGR | KSSR↓GLF |
| CK/XZ/1003/19 | Y | GTSTA | W | T | N | T | L | Y | NGLMGR | RSSR↓GLF |
| CK/FY/177/19 | Y | GISTA | W | T | N | T | L | Y | NGLMGR | RSSR↓GLF |
| CK/CZ/548/19 | Y | GTSTA | W | T | N | T | L | Y | NGLMGR | RSSR↓GLF |
| CK/HZ/46/19 | Y | GTSAA | W | F | N | T | L | Y | NGLMGR | RSSR↓GLF |
| CK/SD/S32/19 | Y | GTSTA | W | T | N | T | L | Y | NGLMGR | RSSR↓GLF |
| CK/CZ/913/19 | Y | GTSTA | W | T | N | T | L | Y | NGLMGR | RSSR↓GLF |
| CK/SH/49/19 | Y | GTSTA | W | T | N | T | L | Y | NGL-GI | RSSR↓GLF |
| CK/CZ/928/19 | Y | GTSTA | W | T | N | T | L | Y | NGLMGR | RSSR↓GLF |
| CK/BJ/1/94* | Y | GTSKA | W | T | N | V | L | Y | NGQQGR | RSSR↓GLF |
| CK/SD/6/96* | Y | GTSKA | W | T | N | A | L | Y | NGQQGR | RSSR↓GLF |
| DK/HK/Y280/97* | Y | GTSKA | W | T | N | T | L | Y | NGLQGR | RSSR↓GLF |
| QA/HK/G1/97* | Y | GISRA | W | T | H | E | L | Y | NDLQGR | RSSR↓GLF |
| CK/HK/G9/97* | Y | GTSKA | W | T | N | A | L | Y | NGLQGR | RSSR↓GLF |
| DK/HK/Y439/97* | Y | GTSRA | W | T | H | E | L | Y | NDQQGR | ASNR↓GLF |
| CK/SH/F/98* | Y | GTSKA | W | T | N | A | L | Y | NGQQGR | RSSR↓GLF |
An asterisk (*) indicates reference strains.
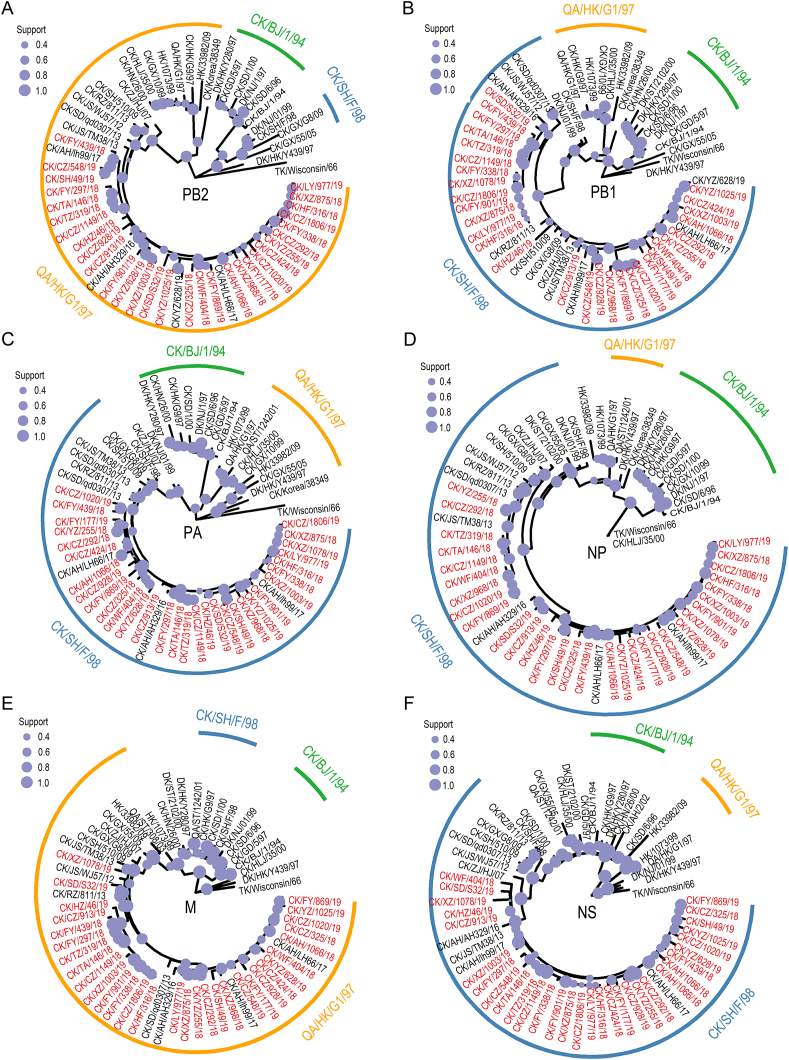
The NA genes of the 31 isolates are close to each other and belong to the Y280 branch, which is far away from the classic vaccine strains CK/SD/6/96, CK/SH/F/98, etc. (Fig. 1B). NA glycosylation sites are relatively conservative, with most strains containing sites 69, 86, 146, 200, 234, and 368. Among them, CK/CZ/325/18, CK/YZ/1025/19, and CK/YZ/628/19 lack potential glycosylation sites 69, 86, and 368, respectively, while CK/FY/901/19 increases site 330, CK/FY/297/18, CK/HZ/46/19, CK/SD/S32/19, and CK/CZ/913/19 add a potential glycosylation site at 264 (Supplementary Table S3). The internal genes of the 31 isolates are all from the Eurasian lineage, the PB2 and M genes belong to the G1 branch (Fig. 2A and E), PB1 (Fig. 2B), PA (Fig. 2C), NP (Fig. 2D), and NS (Fig. 2F) genes belong to the F/98 branch, which suggests that the above isolates were classified within the G57 genotype (Gu et al., 2014). The internal genes of the 31 isolates are relatively conservative, and the mutation rate between strains is low. Compared with the reference strains, there are certain differences. In this study, none of the 31 isolates had PB2 E627K and D701N mutations, but 3 isolates had E627V mutation (Table 2). In addition, compared with the reference strains, there were PB2 gene A588V and PA gene K356R mutations, and most isolates (22/31) had V at the site 292 of PB2.
Fig. 2.
Phylogenetic tree of the PB2 (A), PB1 (B), PA (C), NP (D), M (E) and NS (F) segment. The legend notes that the reliability of phylogenetic inference at each branch node was assessed using the bootstrap method with 1000 replications. The AIVs isolated in this study are marked in red.
Table 2.
Cross-host transmission sites and pathogenic sites of internal genes.
| Strain | PB2 |
PB1 |
PA |
NP |
M2 |
NS1 |
|||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 292 | 588 | 627 | 701 | 577 | 356 | 404 | 672 | 105 | 31 | 106 | |
| CK/TZ/319/18 | V | V | E | D | K | R | A | L | V | N | I |
| CK/XZ/875/18 | V | V | E | D | K | R | A | L | V | N | I |
| CK/YZ/255/18 | V | V | E | D | K | R | A | L | V | N | I |
| CK/WF/404/18 | I | V | E | D | K | R | S | L | V | N | I |
| CK/FY/297/18 | V | V | E | D | K | R | A | L | V | N | I |
| CK/FY/338/18 | V | V | E | D | K | R | A | L | V | N | I |
| CK/XZ/968/18 | V | V | E | D | K | R | A | L | V | N | I |
| CK/LY/977/19 | V | V | E | D | K | R | A | L | V | N | I |
| CK/TA/146/18 | V | V | E | D | K | R | A | L | V | N | I |
| CK/CZ/1806/19 | V | V | E | D | K | R | A | L | V | N | I |
| CK/AH/1066/18 | V | V | A | D | K | R | A | L | V | N | I |
| CK/CZ/325/18 | I | V | A | D | K | R | S | L | V | N | I |
| CK/CZ/1020/19 | V | V | E | D | K | R | A | L | V | N | I |
| CK/CZ/1149/18 | V | V | E | D | K | R | A | L | V | N | I |
| CK/YZ/1025/19 | V | V | E | D | K | R | A | L | V | N | I |
| CK/CZ/292/18 | V | V | E | D | K | R | A | L | V | N | I |
| CK/FY/439/18 | V | V | V | D | K | R | A | L | V | N | I |
| CK/CZ/424/18 | V | V | E | D | K | R | A | L | M | N | I |
| CK/FY/901/19 | I | V | E | D | K | R | A | L | V | N | I |
| CK/HF/316/18 | V | V | E | D | K | R | A | L | V | N | I |
| CK/XZ/1078/19 | I | V | E | D | K | R | A | L | V | N | I |
| CK/YZ/628/19 | V | V | E | D | K | R | S | L | V | N | I |
| CK/FY/869/19 | I | V | E | D | K | R | A | L | V | N | I |
| CK/XZ/1003/19 | I | V | E | D | K | R | A | L | V | N | I |
| CK/FY/177/19 | V | V | E | D | K | R | A | L | V | N | I |
| CK/CZ/548/19 | I | I | V | D | K | R | A | L | V | N | I |
| CK/HZ/46/19 | V | V | E | D | K | R | A | L | V | N | I |
| CK/SD/S32/19 | V | V | E | D | K | R | A | L | V | N | I |
| CK/CZ/913/19 | I | V | E | D | K | R | A | L | V | N | I |
| CK/SH/49/19 | I | V | V | D | K | R | A | L | V | N | I |
| CK/CZ/928/19 | V | V | E | D | K | R | A | L | V | N | I |
| CK/BJ/1/94∗ | I | A | E | D | K | K | A | L | V | S | I |
| CK/SD/6/96∗ | I | A | E | D | K | K | A | F | V | S | I |
| DK/HK/Y280/97∗ | I | A | E | D | K | K | A | L | V | S | I |
| QA/HK/G1/97∗ | V | A | E | D | K | K | A | L | V | S | I |
| CK/HK/G9/97∗ | V | A | E | D | K | K | A | L | V | S | I |
| DK/HK/Y439/97∗ | I | A | E | D | K | K | A | L | M | S | M |
| CK/SH/F/98∗ | I | A | E | D | K | K | A | L | V | N | I |
An asterisk (∗) indicates reference strains.
In general, compared with the representative strains, there are differences in receptor binding sites, potential glycosylation sites, and pathogenicity-related sites.
Analysis of the pathogenicity and antigenic differences of H9N2 influenza virus isolates
Based on the genetic evolution analysis of the 31 H9N2 subtype AIV, nine representative viruses were selected for further study, considering HA receptor-binding sites, HA and NA potential glycosylation sites, and pathogenicity-related sites in internal genes. In order to analyze the antigenicity of the 9 strains of H9N2 subtype AIV isolated from 2018 to 2019, a cross-hemagglutination inhibition test was used for evaluation. The results showed that the HI titer (log2) of all strains with the self-prepared high immune serum was not less than 8, indicating that the immunization was successful and the chickens produced a high level of antibodies. There are differences in HI titers between different strains, reflecting the antigenic differences between strains (Supplementary Table S4). According to the position distribution of different strains in the antigen map, it can be intuitively seen that the strains present different antigenic groups. The two strains isolated in 2018 are far away from the strains isolated in 2019. Taking the six vaccine strains as reference, it was found that there were certain differences between the isolates in different periods. The serum reactivity between the early vaccine strains such as CK/HN/HP/98, CK/GX/55/05 and the isolates in this study was poor. In contrast, CK/FY/338/18 has a higher HI titer than the early vaccine strains (Fig. 3A).
Fig. 3.
Analysis of the pathogenicity of H9N2 influenza virus isolates. A The antigenic map was generated based on cross-hemagglutination inhibition (HI) results, where the distances between strains indicate antigenic differences among nine H9N2 avian influenza viruses and six reference strains. Solid circles represent viruses, while gray squares represent sera. Pathogenicity of nine strains of H9N2 subtype influenza virus in mice was detected. Female BALB/c mice were randomly divided into ten groups, with five mice per group, and were inoculated with the influenza virus isolates via intranasal drops. PBS was used as the control group. The infection kinetics in the mice were assessed based on body weight loss (B) and survival curve (C). Female BALB/c mice were randomly divided into four groups, each containing eight mice, and intranasally inoculated with different mutant strains. The rCK/SH/49/19 strain was generated via a reverse genetics system using the influenza virus isolate CK/SH/49/19, carrying the PB2 627V and an amino acid deletion in HA at position 217 (referred to as PB2 627V + HA 217Δ). Changes in body weight and survival rates were then evaluated (D). On the third day after infection, three mice in each group were randomly selected to be euthanized and lung tissue was harvested. The viral loads in the lungs of mice infected with influenza virus were measured by plaque assay (E). The results are presented as means ± standard deviations. Statistical variances between specific groups were determined using a two-tailed, unpaired t-test. Significance levels were indicated as follows: ∗∗∗∗, P < 0.0001.
The results of the pathogenicity of H9N2 subtype AIV to mammals showed that only one virus strain, CK/SH/49/19, was lethal to mice, causing mice to lose weight and show clinical symptoms such as depression, slow movement, shrinking head, and messy fur (Fig. 3B and C). Analysis of the CK/SH/49/19 sequence found that the 217th amino acid in the receptor binding region of the HA gene was missing, and the E627V mutation occurred in the key pathogenicity site of the PB2 gene, which are speculated to be the reasons for the enhanced pathogenicity to mammals. Subsequently, reverse genetics techniques were employed to rescue the wild-type virus rCK/SH/49/19 (PB2 627V, HA 217Δ) alongside mutant viruses rCK/SH/49/19 (PB2 627V, HA 217M), rCK/SH/49/19 (PB2 627E, HA 217Δ), and rCK/SH/49/19 (PB2 627E, HA 217M). The experimental outcomes revealed a significant decrease in body weight and a higher mortality rate in mice infected with rCK/SH/49/19 (PB2 627V, HA 217M) compared to those infected with rCK/SH/49/19 (PB2 627V, HA 217Δ). However, it is noteworthy that neither rCK/SH/49/19 (PB2 627E, HA 217Δ) nor rCK/SH/49/19 (PB2 627E, HA 217M) led to lethality in mice (Fig. 3D). Consistently, the lungs of mice infected with rCK/SH/49/19 (PB2 627V, HA 217M) harbored the highest levels of virus titers (Fig. 3E).
Based on the aforementioned results, it can be inferred that the pathogenicity of CK/SH/49/19 in mammals is influenced by both the amino acid at position 217 of the HA and the amino acid at position 627 of the PB2.
The amino acid located at position 217 within the receptor binding region of the HA gene had a significant impact on viral replication
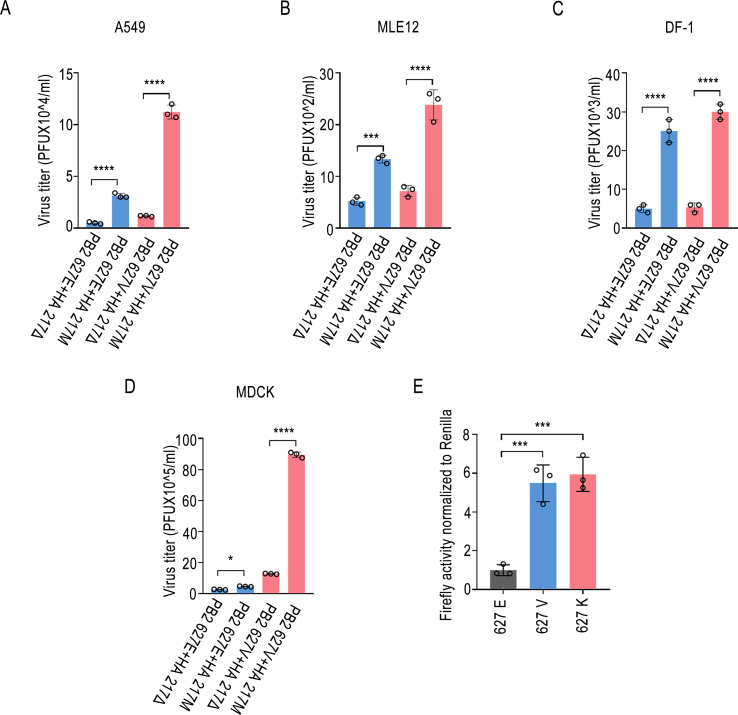
To further investigate the impact of the HA 217 amino acid deletion and the PB2 627 site on virus replication in cells, four recombinant viruses were utilized to infect A549, MLE12, DF-1, and MDCK cells. The results demonstrated that the titer of rCK/SH/49/19 carrying the PB2 627V variant was higher than that of the virus carrying PB2 627E in the different cell types. Additionally, it was observed that the virus containing the M variant at the HA 217 site exhibited a greater replication capability compared to the virus lacking the M variant at the HA 217 site (Fig. 4A–D). The viral polymerase activity serves as a key indicator of the replication capacity of AIV in mammalian cells (Long et al., 2016). By utilizing the PB2 gene of CK/SH/49/19 as a template, mutations were introduced at the 627 site to investigate the impact of these variations on viral polymerase activity. The findings revealed that the polymerase activity was significantly augmented when carrying the 627V and 627K mutations compared to the 627E mutation (Fig. 4E). These outcomes suggest a synergetic enhancement of CK/SH/49/19 virus replication with the presence of PB2 627V and HA 217M mutations.
Fig. 4.
Mutations at amino acid position 217 of the HA gene and position 627 of the PB2 gene significantly affect viral replication. Cells from various species, such as A549 (A), MLE12 (B), DF-1 (C), and MDCK (D), were infected with influenza virus rCK/SH/49/19 mutants at an MOI of 0.01. Supernatants were collected 24 h post-infection, and virus titers were assessed using plaque assays. E The PB2 mutants (PB2 627E, PB2 627V, and PB2 627K) of the CK/SH/49/19 influenza virus were individually co-transfected with PB1, PA, and NP, along with PolI-Luc and RL-TK vectors, into HEK293T cells. The cells were then maintained at 37 °C. Forty-eight hours post-transfection, polymerase activity was assessed. The results are presented as means ± standard deviations. Statistical variances between specific groups were determined using a two-tailed, unpaired t-test (A–D) or one-way ANOVA (E) followed by Dunnett's multiple comparisons test. Significance levels were indicated as follows: ∗, P < 0.05; ∗∗∗, P < 0.001; ∗∗∗∗, P < 0.0001.
The deletion of amino acid 217 in the HA protein of the rCK/SH/49/19 markedly hindered viral adsorption and endocytosis
The influenza virus HA facilitates virus entry into host cells by binding to receptors and inducing virus-host membrane fusion (Hu et al., 2024). AIV specifically targets the α-2, 3 sialic acid receptor (avian receptor), while human influenza virus targets the α-2, 6 sialic acid receptor (human receptor) (Peacock et al., 2017). Our subsequent investigation focuses on understanding the influence of the HA 217 site in this process. To accomplish this, we selected human A549 cells abundant in α-2, 6 sialic acid receptors, canine MDCK cells containing both α-2, 3 and α-2, 6 sialic acid receptors, and avian DF-1 cells expressing higher levels of α-2, 3 sialic acid receptors (Chungu et al., 2021; Kamiki et al., 2022). These cell lines were utilized to examine the adsorption and endocytosis effects of rCK/SH/49/19. The findings indicated that the deletion of amino acids at the HA 217 site substantially impeded the infection of influenza virus rCK/SH/49/19 in cells from various species, along with the ensuing endocytosis process (Fig. 5A–C). Furthermore, these observations were corroborated in the MDCK cell line, which specifically expresses α-2,6 sialic acid receptors (Fig. 5D). Indirect immunofluorescence experiments also demonstrated that the diminished adsorption capacity of rCK/SH/49/19 (PB2 627V, HA 217Δ) notably hindered the nuclear localization of the virus (Fig. 5E and F). Solid-phase direct binding ELISA experiments demonstrated that both the rCK/SH/49/19 strain (PB2 627V, HA 217Δ) and the mutant strain rCK/SH/49/19 (PB2 627V, HA 217M) predominantly interacted with α−2,6 sialic acid (Fig. 6A–D). Analysis using the online SWISS-MODEL service predicted the HA protein structure of rCK/SH/49/19, revealing that the deletion of amino acid 217 had a minor impact on the conformation of the receptor binding region (Fig. 6E). These findings underscore the critical role of the HA 217 site in influenza virus adsorption and endocytosis processes.
Fig. 5.
The deletion of amino acid 217 in the HA protein of the rCK/SH/49/19 markedly hindered viral adsorption and endocytosis. Various cell lines, including A549 (A), MDCK (B), DF-1 (C), and MDCK cell lines engineered to exclusively express α-2,6-sialic acid glycans (D), were either untreated or infected with the wild-type strain rCK/SH/49/19 (PB2 627V, HA 217Δ) or the mutant strain rCK/SH/49/19 (PB2 627V, HA 217M) at an MOI of 5 in duplicate. The adsorption and internalization effects were assessed through Western blot analysis of viral NP content. A549 cells were infected with influenza virus rCK/SH/49/19 (PB2 627V, HA 217Δ) (E) or rCK/SH/49/19 (PB2 627V, HA 217M) (F) at an MOI of 1. At 1h, 2h, and 3h post-infection, the cells were fixed, permeabilized, and subjected to staining with anti-NP mouse monoclonal antibody, followed by immunostaining with donkey anti-mouse Alexa Fluor 555-labeled secondary antibody. The cells were additionally stained with 4,6-diamidino-2-phenylindole (DAPI) and examined using fluorescence microscopy.
Fig. 6.
Viral receptor-binding ability detection. Receptor specificity was assessed using a solid-phase direct binding assay. The binding of rCK/SH/49/19 (PB2 627V, HA 217Δ) (A) or the mutant strain rCK/SH/49/19 (PB2 627V, HA 217M) (B) to two different glycans (α-2,3 glycans, blue; α-2,6 glycans, red) was assessed. Influenza viruses A/swine/Jiangxi/261/2016 (H1N1) (C) and A/chicken/Chongqing/SD001/2021 (H5N6) (D) bind to human-like α-2,6 sialic acid and avian-like α-2,3 sialic acid, respectively, as references. E The three-dimensional structure of the HA protein from the rCK/SH/49/19 strain (with PB2 627V and HA 217Δ) or the mutant strain rCK/SH/49/19 (with PB2 627V and HA 217M) was predicted using the SWISS-MODEL service. The figures displaying both cartoon and surface models are presented.
The presence of HA (217M) in rCK/SH/49/19 exhibited an elevated activation pH and enhanced virus budding
To further investigate the impact of the influenza virus HA 217 site on HA-mediated virus-host membrane fusion, a syncytia assay was conducted to assess HA activation pH. The results revealed that HA 217M triggered extensive heterokaryon formation at pH 5.5–5.6, whereas HA 217Δ exhibited a similar effect at pH 5.4–5.3 (Fig. 7A). Additionally, an acid stability experiment involving influenza virus rCK/SH/49/19 displayed a subtle contrast between HA 217Δ and 217M. Notably, rCK/SH/49/19 (PB2 627V, HA 217M) exhibited a significantly reduced infectivity following treatment with pH 5.3 buffer, while rCK/SH/49/19 (PB2 627V, HA 217Δ) maintained robust infectivity levels (Fig. 7B).
Fig. 7.
The presence of HA (217M) in rCK/SH/49/19 exhibited an elevated activation pH and enhanced virus budding. A The determination of HA activation pH was conducted through a syncytium formation assay. Influenza viruses rCK/SH/49/19 (PB2 627V, HA 217Δ) or rCK/SH/49/19 (PB2 627V, HA 217M) were introduced to Vero cells at an MOI of 3. HA activation pH was characterized as the maximum pH value at which syncytia formation was observed. Assessment of the acid stability (B) and thermal stability (C) of influenza viruses rCK/SH/49/19 (PB2 627V, HA 217Δ) or rCK/SH/49/19 (PB2 627V, HA 217M) was conducted. The viral infectivity titers were determined through plaque assays. D A549 cells were infected with influenza viruses rCK/SH/49/19 (PB2 627V, HA 217Δ) or rCK/SH/49/19 (PB2 627V, HA 217M) at an MOI of 5 without TPCK-treated trypsin. Following 24 h of infection, cellular and supernatant lysates were collected, and the expression of HA protein was assessed via Western blot analysis to determine the virus's budding efficiency. E The HA 217Δ or 217M expression plasmid of influenza virus rCK/SH/49/19 was co-transfected into HEK293T cells with the M1 and NA expression plasmids, respectively. Following a 24 h-incubation, cells and supernatant lysates were harvested and subsequently subjected to analysis via Western blotting.
When IAV is exposed to specific temperatures, its surface HA protein undergoes denaturation and inactivation, leading to the loss of its ability to agglutinate red blood cells. The viral resistance to external temperatures correlates with its infectivity potential and poses a higher threat. Therefore, investigating the impact of the HA 217 site on the viral thermal stability can provide insights into the pathogenicity of CK/SH/49/19 and its mutant strains. The outcomes indicated that both rCK/SH/49/19 (PB2 627V, HA 217Δ) and rCK/SH/49/19 (PB2 627V, HA 217M) were swiftly and significantly inactivated at 56 °C across various time frames (Fig. 7C). This suggested that the mutation at the 217 site did not result in a notable alteration in the viral thermal stability.
HA protein clusters within lipid-rich areas of the cell membrane to create budding sites, induces membrane curvature at these sites, and initiates the budding process. Subsequently, we investigated this phenomenon under high multiplicity of infection and without TPCK-treated trypsin. The findings revealed that the supernatant from CK/SH/49/19 (PB2 627V, HA 217M)-infected cells contained a higher quantity of virus particles (Fig. 7D). To elucidate the impact of the HA 217 site on virus budding, we co-transfected the HA, NA, and M1 proteins of the influenza virus. This approach efficiently mediates virus budding and facilitates the formation of virus-like particles. The outcomes demonstrated that the presence of the M variant at the HA 217 site notably enhanced the generation of virus-like particles (Fig. 7E). These findings underscore the influence of the HA 217 site on the membrane fusion and budding processes of influenza virus rCK/SH/49/19.
rCK/SH/49/19 (PB2 627V, HA 217Δ) more strongly activated the production of interferon and interferon-stimulated genes in mice
For a more comprehensive understanding of the variation in lethality between rCK/SH/49/19 (PB2 627V, HA 217Δ) and rCK/SH/49/19 (PB2 627V, HA 217M) in mice, we utilized high-throughput sequencing to delve deeper into the differentially expressed genes in the infected mice. Intriguingly, our analysis revealed that compared to infection with rCK/SH/49/19 (PB2 627V, HA 217M), infection with rCK/SH/49/19 (PB2 627V, HA 217Δ) triggered a more robust immune response, as evidenced by elevated levels of interferon and inflammatory factors (Fig. 8A). GO terms analysis results showed that the differentially expressed genes were significantly enriched in biological processes such as response to virus, defense response to virus, response to interferon-beta, cellular response to interferon-beta, and cytokine-mediated signaling pathway (Fig. 8B). KEGG pathways analysis results showed that the differentially expressed genes were significantly enriched in pathways such as Cytokine-cytokine receptor interaction, NOD-like receptor signaling pathway, and Toll like receptor signaling pathway (Fig. 8C). Furthermore, genes in the enriched GO terms “response to virus” were significantly activated (Fig. 8D). To validate the variance in immune response triggered by the infections of rCK/SH/49/19 (PB2 627V, HA 217Δ) and rCK/SH/49/19 (PB2 627V, HA 217M), A549 cells were subjected to infect the aforementioned viruses. qPCR results showed that rCK/SH/49/19 (PB2 627V, HA 217Δ) induced higher levels of IFNβ and IL6 production (Fig. 8E), leading to the enhanced transcription of potent interferon-stimulated genes, including IFI16, OAS1, SP110, MLKL, IFIT3, IFI6, and IFITM1 (Fig. 8F). Overall, the above results suggested that HA 217M weakened the rCK/SH/49/19 immune response and caused higher lethality compared with HA 217Δ.
Fig. 8.
rCK/SH/49/19 (PB2 627V, HA 217Δ) more strongly activated the production of interferon and interferon-stimulated genes in mice. A The volcano plot depicts the mRNA expression profiles in mice infected with rCK/SH/49/19 (PB2 627V, HA 217Δ) compared to those infected with rCK/SH/49/19 (PB2 627V, HA 217M). In the plot, red dots indicate upregulated genes (adjusted p < 0.05 and log2FC > 1), blue dots denote downregulated genes (adjusted p < 0.05 and log2FC < −1), and gray dots represent genes that did not show significant differential expression. Functional enrichment analysis was conducted on differentially expressed genes. Significantly enriched GO terms (B) and KEGG pathways (C) were assessed. D The network relationship diagram shows the specific genes and differential expression folds in the enriched GO terms “response to virus”. A549 cells were either untreated or infected with rCK/SH/49/19 (PB2 627V, HA 217Δ) or rCK/SH/49/19 (PB2 627V, HA 217M) (MOI = 0.1) and lysed at 12 h post-infection. The mRNA levels of IFNβ, IL6 (E) or interferon-stimulated genes (F) were determined via qPCR. Experiments were independently repeated three times, with similar results. The results are presented as means ± standard deviations. Statistical differences between designated groups are noted according to two-way ANOVA with Dunnett's multiple comparisons test. ∗, P < 0.05; ∗∗∗∗, P < 0.0001.
Discussion
The H9N2 virus, although less pathogenic, exhibits a broad host range, rapid spread, numerous epidemic zones, and occasional interspecies transmission to infect humans directly. Additionally, the H9N2 virus can contribute some or all of its internal genes to highly pathogenic subtypes like H5N1 and H7N9, leading to the generation of more virulent recombinant viruses (Bi et al., 2015; Tian et al., 2023; Wang et al., 2023; Hou et al., 2024; Liu L. et al., 2024; Xing et al., 2024). In this study, 436 samples from diseased poultry and swabs were collected from breeding enterprises and farmers in Shandong, Jiangsu, Zhejiang, Anhui, and Shanghai between 2018 and 2019, including 50 samples testing positive for H9. Subsequently, 31 purified H9N2 viruses were obtained through three successive limiting dilutions in chicken embryos. Initially, the complete genomes of the 31 isolates were sequenced to investigate their genetic evolutionary patterns and sequence features, revealing that their HA genes were classified under the Y280 branch and h9.4.2.5. The genetic distance between these strains was relatively close, aligning with the overall trend of H9N2 virus circulation but forming distinct evolutionary subgroups. Analysis indicated that the HA and NA genes of the 31 isolates belonged to the Y280 branch, while the PB2 and M genes were grouped in the G1 branch, and the remaining genes were classified under the F/98 branch. This suggests that significant antigenic variations and gene recombination events have not arisen in H9N2 subtype AIV in East China in recent years. All isolates were identified as part of the G57 genotype, which has been prevalent in the country since 2013. While no notable variations have been observed in the overall trend, the homology of the HA and NA genes is comparatively lower when compared to classic vaccine strains. This indicates a decreasing homology between the H9N2 virus and vaccine strains over time, potentially leading to vaccine ineffectiveness. Consequently, there is a need for frequent updates to the vaccine to ensure adequate protection.
The receptor specificity of the HA gene is considered a crucial factor in determining the host range limitations, with its cleavage site serving as a significant indicator of virus pathogenicity (Suzuki, 2005). Research indicates a gradual increase in the prevalence of HA 216 L and 217 M forms since 2011, becoming dominant within the country by 2013. Zhang et al. identified that the H9N2 strains isolated in Anhui Province from 2013 to 2018 possessed an HA 216 site as L and 217 as M (Zhang S. et al., 2021). In contrast, our study revealed that among the 31H9N2 isolates, the HA 216 site was L, and 30 strains exhibited M at position 217. Notably, the impact of the Q217M mutation form has been relatively underexplored. Intriguingly, one viral strain was observed to lack amino acid 217, a rarity among natural avian H9N2 strains. Glycosylation plays a vital role in the infectivity, receptor binding capability, and immune response elicited by the H9N2 virus. In comparison to the reference strain, the isolates examined in this study displayed an increase in potential glycosylation sites at HA 295 and NA 368, while lacking potential glycosylation sites at HA 200 and NA 402. NP, M, and NS genes are also related to viral pathogenicity. Among the isolates, one strain NP had a V105M mutation, which may enhance pathogenicity to mice. Most strains of M2 evolved from S to N at position 31, which may lead to enhanced resistance of the virus to adamantane. Mutations occurring at specific amino acid sites within internal genes can modify the host range or influence the pathogenicity of avian influenza virus. Notably, the E627K and D701N mutations within the PB2 gene are recognized as molecular markers associated with cross-species transmission of the H9N2 virus. Within the cohort of 31 H9N2 isolates, common mutations such as PB2 A588V, PA K356R, and M2 S31N were observed, suggesting that H9N2 variants in East China have evolved towards heightened transmissibility, pathogenicity, and drug resistance in recent years. Furthermore, three strains exhibited PB2 E627V mutations, previously documented in H7 viruses to augment cross-species polymerase activity, consequently enhancing pathogenicity in mice.
Nine representative viruses were chosen for a detailed analysis of HA receptor binding sites, HA and NA glycosylation sites, and internal gene pathogenicity-related amino acid sites. Antigenic variations were identified among the nine strains studied, with distinct differences observed between the 2018 and 2019 isolates. Notably, these isolates displayed significant deviations from early vaccine strains like CK/HN/HP/98 and CK/GX/55/05, suggesting a potential new evolutionary path for H9N2 virus in East China in recent years. Subsequent in vivo experiments assessed the pathogenicity of the H9N2 isolates, revealing CK/SH/49/19 to be lethal to mice, a surprising finding. Further investigation identified mutations, including E627V in PB2 and a deficiency in amino acid 217 in HA, in the CK/SH/49/19 strain. To investigate the biological characteristics of CK/SH/49/19 isolates and the molecular basis of their lethality in mice, we generated rCK/SH/49/19 variants with specific mutations (PB2 627V, HA 217Δ), (PB2 627V, HA 217M), (PB2 627E, HA 217Δ), and (PB2 627E, HA 217M) using a reverse genetics system. In vivo experiments demonstrated that both rCK/SH/49/19 (PB2 627V, HA 217Δ) and rCK/SH/49/19 (PB2 627V, HA 217M) were lethal to mice, with rCK/SH/49/19 (PB2 627V, HA 217M) exhibiting higher lethality. Conversely, rCK/SH/49/19 (PB2 627E, HA 217Δ), and rCK/SH/49/19 (PB2 627E, HA 217M) did not induce lethality in mice, indicating that the mutation from E to V at position 627 in PB2 is the key determinant of the high pathogenicity of the CK/SH/49/19 isolate. Subsequent in vitro cell experiments demonstrated that the replication capacity of the influenza virus rCK/SH/49/19 with PB2 627V was significantly greater than that with PB2 627E across multiple cell lines, such as A549, MLE12, DF-1, and MDCK. Additionally, viral polymerase-minigenome assays indicated that the polymerase activity of the PB2 627V variant was markedly higher compared to the PB2 627E counterpart, providing a rationale for the heightened pathogenicity observed in rCK/SH/49/19 (PB2 627V, HA 217Δ) and rCK/SH/49/19 (PB2 627V, HA 217M). Importantly, the polymerase activity associated with PB2 627V was comparable to that of PB2 627K, suggesting that the mutation to PB2 627V in the H9N2 virus could serve as a critical marker for increased cross-host transmission and pandemic potential. The replication capacity of rCK/SH/49/19 with HA 217M is higher than that of HA 217Δ, irrespective of the presence of PB2 627E or PB2 627V. This suggests that the HA 217 site also significantly contributes to the pathogenicity of rCK/SH/49/19. HA has the capability to interact with sialic acid receptors situated on the surface of host cell membranes. The specificity of HA binding to receptors dictates the host range, while the binding strength of HA to these receptors influences the viral pathogenicity towards the host. The receptor binding domain is positioned in the top domain of HA and comprises a pocket-like structure mainly consisting of three secondary structures: the 130-loop, 190-helix, and 220-loop, with four highly conserved amino acids at its base (Shi Y. et al., 2014). The amino acid site within the 220-loop, which has been extensively studied, plays a crucial role in modulating receptor binding characteristics. Studies have revealed that the presence of HA at the 216 site can determine the virus's receptor affinity, where Q at 216 tends to bind with avian α-2, 3 SA receptors, while L at 216 prefers human α-2, 6 SA receptors; the 217 site is also situated within this region (Obadan et al., 2019). Subsequently, we examined the impact of the HA 217 site on adsorption, internalization, and nuclear entry during the early stages of the influenza virus replication cycle. Our findings revealed that the removal of amino acid 217 from the HA protein in rCK/SH/49/19 significantly impeded viral adsorption and endocytosis, and disrupted normal nuclear entry. To delve deeper into the underlying molecular mechanism by which the HA 217 site influences these processes, we assessed the activation pH, acid stability, and thermal stability of HA. Our analysis demonstrated that the presence of HA (217M) in rCK/SH/49/19 resulted in an increased activation pH and enhanced acid stability, while no substantial differences were observed in thermal stability. Subsequently, we conducted experiments to assess the budding process during the late stage of viral replication. Whether under conditions of viral infection or transfection to mimic the formation of virus-like particles, the inclusion of HA (217M) notably enhanced the production of viral particles in the cell supernatant. These outcomes affirm the impact of the HA 217 site on the membrane fusion and budding mechanisms of the influenza virus rCK/SH/49/19. In the final stage, we sought to further investigate the impact of the HA 217 site in vivo through the utilization of high-throughput sequencing technology. Our findings revealed that rCK/SH/49/19 (PB2 627V, HA 217Δ) elicited a more robust activation of interferon response and interferon-stimulated genes in mice, explaining the lower replication level observed in rCK/SH/49/19 (PB2 627V, HA 217Δ) due to heightened activation of antiviral factors. Moreover, the lethality observed in mice could be attributed to the induction of a cytokine storm. Consequently, the HA 217 site emerges as a critical target for monitoring influenza virus mutations and anticipating cross-host infections. This study offers a theoretical foundation for the scientific prevention and control of H9N2 subtype avian influenza.
Conclusions
This study revealed that among the 31 isolates studied, the HA and NA genes were categorized under the Y280 branch, the PB2 and M genes under the G1 branch, and the remaining genes under the F/98 branch, which corresponds to the G57 genotype. Antigenic mapping revealed variations between the 2018 and 2019 strains, indicating low serological reactivity of early vaccine strains towards these isolates. Significantly, in vitro and in vivo experiments elucidated the molecular mechanisms of the CK/SH/49/19 isolate's pathogenicity in mice, marked by a PB2 E627V mutation and an HA deletion at amino acid position 217. This research contributes a theoretical basis for the surveillance, prevention, and management of H9N2 subtype avian influenza virus.
Materials and methods
Cells
HEK293T, A549, MDCK, DF-1, and Vero cells were cultured in Dulbecco's Modified Eagle's Medium (DMEM; Gibco, USA), while MLE12 cells were maintained in DMEM/Nutrient Mixture F-12 (DMEM/F12; Gibco). Both media were supplemented with 10% fetal bovine serum (FBS; Gibco), 0.2% sodium bicarbonate (NaHCO3), 100 μg/mL streptomycin, and 100 IU/mL penicillin (Beyotime, China) at 37 °C in a 5% CO2 atmosphere. Additionally, the MDCK cell line, which exclusively expresses α-2, 6-sialic acid glycans, was maintained in our laboratory (Kikuchi et al., 2023).
Virus isolation and purification
Tissue samples were homogenized using a tissue grinder, and swabs underwent three cycles of freezing and thawing at −20 °C. Supernatant samples were subsequently filtered and sterilized through a 0.22 μm filter before being inoculated into 9- to 10-day-old specific pathogen-free (SPF) chicken embryos via the allantoic cavity. The embryos were then incubated at 37 °C. After incubation, the allantoic fluid was harvested, and the hemagglutination titer was determined at the highest dilution. To purify the virus, three limiting dilutions were performed in chicken embryos, and aliquots were stored at −80 °C. In cases of negative samples, blind passages were conducted for an additional three generations. Additionally, six reference strains, provided by Dr. Zhang Haitao from Jiangsu Lihua Animal Husbandry Co., Ltd., were preserved in our laboratory: A/chicken/Jiangsu/18325/2018 (H9N2), A/chicken/Anhui/LH66/2017 (H9N2), A/chicken/Guangxi/55/2005 (H9N2), A/chicken/Henan/HP/1998 (H9N2), A/chicken/Jiangsu/WJ57/2012 (H9N2), and A/chicken/Anhui/LH99/2017 (H9N2).
Viral genome sequencing
Viral RNA was extracted using Trizol Reagent (Beyotime). A total of 1000 ng of viral RNA was used for first-strand cDNA synthesis with a universal primer (5′-CACACACGTCTCCGGGAGCAAAAGCAGG-3′), using the HiScript II 1st-strand cDNA synthesis kit (Vazyme, China). Specific primers for each segment of the H9N2 virus were designed based on the conserved regions of the sequences. The amplified DNA was sent for sequencing if it met the expected size criteria. The primers used in this study are listed in Supplementary Table S5.
Genetic evolution analysis
Nucleotide sequences of reference strains were downloaded from the NCBI database. The phylogenetic tree for each viral gene was constructed using the maximum likelihood method in Mega (version 7.0.26), with branch support assessed through 1000 bootstrap replicates. Next, the R packages ggtree and ggtreeExtra were utilized to visualize the phylogenetic tree. Information of representative H9N2 AIV strains are listed in Supplementary Table S6.
Preparation of high immune serum
SPF chickens were immunized with inspected inactivated vaccines, receiving subcutaneous injections of 0.5 mL per chicken. Blood samples were collected 14 days post-immunization, and antibody levels were measured using the hemagglutination inhibition (HI) test. Additional blood samples were collected three weeks later. The blood was incubated at 37 °C for 30 min, followed by overnight storage at 4 °C. After serum precipitation, the supernatant was collected and centrifuged at 12,000 rpm for 5–10 min. The resulting aliquots were labeled and stored at −20 °C for future use.
Antigenicity analysis
The prepared serum was tested against the H9N2 subtype influenza virus and six reference strains. The serum working concentration was diluted two-fold. HI titers were determined using a cross-HI test. The antigenic correlation coefficient (R value) was calculated based on the HI titers to assess the cross-protection capability among the different strains. The formula utilized to calculate R is as follows: R = (r1 × r2)1/2, where r1 represents the HI titer of virus A to serum B divided by the HI titer of virus A to serum A, and r2 represents the HI titer of virus B to serum A divided by the HI titer of virus B to serum B. An R value of 1 indicates identical antigenicity between the two strains, R ≥ 0.67 signifies no significant difference, 0.5 ≤ R < 0.67 reveals a minor difference, and R < 0.5 indicates a substantial difference. Data can be uploaded to generate an antigen map at https://acmacs-web.antigenic-cartography.org/.
Virus rescue
Virus rescues were conducted following the protocol described previously (Neumann et al., 1999). Point mutant plasmids were constructed using a site-directed mutagenesis kit (Beyotime). Specifically, mutant plasmids encoding HA 217M and PB2 627E amino acid sites were constructed to rescue the wild-type virus rCK/SH/49/19 (PB2 627V, HA 217Δ), the mutant viruses rCK/SH/49/19 (PB2 627V, HA 217M), rCK/SH/49/19 (PB2 627E, HA 217Δ), and rCK/SH/49/19 (PB2 627E, HA 217M) were rescued.
Quantitative real-time PCR (qPCR)
Cellular RNA was extracted, and 1000 ng of total RNA underwent first-strand cDNA synthesis using oligo(dT)20 or random hexamers with the HiScript II 1st Strand cDNA Synthesis Kit (Vazyme). Subsequently, qPCR was conducted with the synthesized cDNAs and gene-specific primer pairs using AceQ qPCR SYBR Green Master Mix (Vazyme) in a Roche LightCycler 96 system. GAPDH served as the endogenous control for mRNA. The relative levels of candidate genes were determined using the 2−ΔΔCt method. The primers are listed in Supplementary Table S5.
Viral polymerase-minigenome assay
The influenza polymerase activity was assessed through a viral polymerase-minigenome assay, following previously established protocols (Zhao, L. et al., 2022a). Briefly, HEK293T cells were transfected in 24-well plates with pCAGGS plasmids encoding the PB2, PB1, PA and NP proteins of influenza virus, together with minigenome reporter plasmids (PolI-Luc) and Renilla luciferase expression plasmids (RL-TK), using ExFect transfection reagent (Vazyme) according to manufacturers. The activity of Firefly and Renilla luciferase was quantified using a Dual-luciferase system from Promega.
Immunofluorescence
A549 cells were seeded on glass bottom cell culture dishes and either transfected with the specified plasmids or infected with the designated influenza viruses. After the incubation period, the cells were fixed using 4% paraformaldehyde and permeabilized with 0.2% Triton X-100 for 10 min. Subsequently, the cells were washed and subjected to primary antibody staining for 12 h, followed by staining with fluorescence secondary antibodies in a 5% skim milk solution (Beyotime). In this study, donkey anti-mouse Alexa Fluor 555-labeled secondary antibody (Beyotime) was utilized. The cells were then stained with 4,6-diamidino-2-phenylindole (DAPI) for 10 min. Finally, images were captured using confocal microscopy (Nikon, Japan) equipped with a micro-objective (Plan Apo 60×/1.40, oil immersion, Nikon, Japan) and a Microscope eyepiece (CFI, 10×/22, Nikon).
Virus adsorption and internalization
For virus adsorption detection, A549 cells were seeded in 12-well plates and infected with AIV at an MOI of 5. The infected cells were then incubated at 4 °C for 1 h, with gentle shaking every 10 min. After incubation, the cells were washed 3 to 5 times with pre-chilled PBS at pH 1.5, followed by two washes with PBS at pH 7.2 to neutralize the acidic environment. Subsequently, the cells were lysed, and Western blotting was performed to quantify the NP content and analyze the viral adsorption. To detect virus internalization, the procedure outlined for virus adsorption was replicated. Following the 4 °C incubation, the viral solution was removed, and Opti-MEM medium was introduced. The cells were then relocated to a 37 °C, 5% CO2 cell culture incubator for 1 h to facilitate virus internalization. Next, any virus particles adhering to the cell surface without internalization were eliminated by washing with PBS at pH 1.5. Post cell lysis, a Western blot assay was carried out to quantify NP protein levels and evaluate viral internalization. Poteins were detected using specific antibodies according to previously described methods (Zhao, L. et al., 2022b). The antibodies used included: anti-GAPDH (10494-1-AP; Proteintech, China), anti-Flag MAb (F1804; Sigma-Aldrich, USA). Additionally, the anti-NP antibodies were generously provided by Prof. Chengjun Li from the Harbin Veterinary Research Institute, Chinese Academy of Agricultural Sciences. Mouse monoclonal antibodies against HA protein of influenza virus were prepared and stored in our laboratory.
Syncytia assay to measure HA activation pH
Vero cells were seeded in 24-well tissue culture plates and allowed to form confluent monolayers, subsequently infected with IAV (MOI = 3) for 1 h. Following infection, the medium was aspirated, and the cells were overlaid with viral growth medium devoid of TPCK-treated trypsin. The culture plates were then placed in a 5% CO2 incubator at 37 °C for 17 h. Post-incubation, the cells were treated with 5 μg/mL of TPCK-treated trypsin for 5 min, after which the trypsin activity was neutralized using DMEM supplemented with 5% FBS. Following this, Vero cells were overlaid with pH-adjusted PBS buffer and incubated at 37 °C for 5 min. Subsequently, DMEM containing 5% FBS was added to the cells and further incubated for 3 h at 37 °C. The cells were then fixed with 70% ethanol and stained with Giemsa stain for 15 min. Images were captured using a Nikon optical microscope. The pH at which heterokaryon formation was first observed is reported as the HA activation pH.
Virus acid and thermal inactivation assays
To assess the viral stability under different pH conditions, 5 μL of virus stock (usually at a concentration of 105 PFU/mL) was mixed with 495 μL of buffer adjusted to the desired pH. Following acid treatment, 90 μL of the virus-containing solution was then combined with 810 μL of virus infection medium to restore neutral pH. In heat inactivation experiments, virus samples were pipetted into 1 mL Eppendorf tubes and incubated at 56 °C until reaching the specified time points, followed by rapid cooling. The infectious titers of influenza viruses were determined using plaque assays. The infectious titers of influenza viruses were determined using plaque assays, following established protocols (Zhao et al., 2020).
Viral receptor-binding ability detection
Receptor specificity was evaluated through a solid-phase direct binding assay, following established protocols (Fan et al., 2022), utilizing two distinct glycopolymers: α-2,3 sialic acid glycopolymer [Neu5Acα2-3Galb1-4GlcNAcb1-pAP(p-aminophenyl)-α-polyglutamic acid (α-PGA)] and α-2,6 sialic acid glycopolymer [Neu5Acα2-6Galb1-4GlcNAcb1-pAP(p-aminophenyl)-α-polyglutamic acid (α-PGA)]. For the assay, Horseradish peroxidase (HRP)-conjugated goat anti-chicken antibody (Sigma-Aldrich) was utilized. The optical density at 490 nm was then measured with a microplate reader. Each reported value represents the mean ± standard deviation (SD) from three independent experiments, each of which was performed in triplicate.
Mouse pathogenicity assays
To assess the pathogenicity of the isolated strains in mice, female BALB/c mice aged 4–6 weeks (sourced from Shanghai BK/KY Biotechnology Co.) were randomly divided into groups of 5 or 8 mice each. The mice were anesthetized with isoflurane and inoculated intranasally with the virus at a dose of 105 PFU (50 μL per mouse). PBS was administered intranasally as the control. Both the control and challenge groups were fed normally and weighed at the same time daily for 14 days, during which the survival status of the mice was recorded.
High-throughput sequencing
BALB/c mice, aged 4–6 weeks, were randomly allocated into three groups, with three mice in each group. They were intranasally infected with rCK/SH/49/19 (PB2 62V, HA 217Δ) and rCK/SH/49/19 (PB2 62V, HA 217M) at a dose of 105 PFU per mouse. The control group received an equal dose of PBS via the same nasal cavity route. After 20 h, the mice were euthanized, and fresh lung tissue was aseptically obtained and homogenized in a sterile mortar with liquid nitrogen. The homogenized tissue was then mixed with lysis solution, transferred to a clean 1.5 mL centrifuge tube, flash-frozen with liquid nitrogen, and stored at −80 °C. Libraries were prepared using the TruSeq RNA Sample Preparation Kit (Illumina, USA) following the manufacturer's protocol and sequenced on the Illumina HiSeq XTen platform. Library construction and sequencing were conducted by Shanghai OE Biotech Co. (Shanghai, China). Initially, differentially expressed genes (DEGs) were determined utilizing the limma package (Ritchie et al., 2015). Volcano plots displaying the DEGs were created using the ggplot2 package. DEGs were identified based on adjusted p-value < 0.05 and |log2FC| > 1 as the specified cutoff criteria. The high-throughput sequencing data generated for this study are provided in Supplementary Table S7.
Statistical analysis
Statistical analyses were performed using Prism 8 (GraphPad). The data were evaluated using either a two-tailed student's t-test or one-way/two-way ANOVA, as appropriate. Statistical significance was indicated as follows: ∗, P < 0.05; ∗∗, P < 0.01; ∗∗∗, P < 0.001; ∗∗∗∗, P < 0.0001; and “ns” for non-significant.
Data availability
Data supporting the findings of this research are reported in the main text and figures and in the Supplementary Data. Results from whole genome sequencing of the isolated viruses are available in ScienceDB (https://doi.org/10.57760/sciencedb.13859).
Ethics statement
All research studies involving the use of animals were reviewed and approved by Experimental Animal Welfare and Ethics Committee of Nanjing Agricultural University and were carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals. The ethical approval number is NJAULLSC2022002.
Author contributions
Lingcai Zhao: conceptualization, writing-original draft preparation, writing-review and editing, data curation; Miao Tian: data curation, visualization; Xifeng Hu: data curation, visualization; Menglu Fan: data curation, visualization; Chenglin Hou: data curation, visualization; Jihui Ping: conceptualization, supervision, project administration, funding acquisition, writing-review and editing.
Conflict of interest
The authors declare no conflict of interest.
Acknowledgements
This work was supported by National Natural Science Foundation of China [Grant number: 32272992 (JP); 31772775 (JP)], National Key Research and Development Program of China [Grant number: 2021YFD1800205 (JP)].
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.virs.2024.12.003.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- Alexander D.J. Report on avian influenza in the eastern hemisphere during 1997-2002. Avian Dis. 2003;47:792–797. doi: 10.1637/0005-2086-47.s3.792. [DOI] [PubMed] [Google Scholar]
- Alexander D.J. Summary of avian influenza activity in Europe, Asia, Africa, and Australasia, 2002-2006. Avian Dis. 2007;51:161–166. doi: 10.1637/7602-041306R.1. [DOI] [PubMed] [Google Scholar]
- Bi Y., Xie Q., Zhang S., Li Y., Xiao H., Jin T., Zheng W., Li J., Jia X., Sun L., et al. Assessment of the internal genes of influenza A (H7N9) virus contributing to high pathogenicity in mice. J. Virol. 2015;89:2–13. doi: 10.1128/JVI.02390-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chungu K., Park Y.H., Woo S.J., Lee S.B., Rengaraj D., Lee H.J., Han J.Y. Establishment of a genetically engineered chicken DF-1 cell line for efficient amplification of influenza viruses in the absence of trypsin. BMC Biotechnol. 2021;21:2. doi: 10.1186/s12896-020-00663-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan M., Liang B., Zhao Y., Zhang Y., Liu Q., Tian M., Zheng Y., Xia H., Suzuki Y., Chen H., et al. Mutations of 127, 183 and 212 residues on the HA globular head affect the antigenicity, replication and pathogenicity of H9N2 avian influenza virus. Transbound Emerg Dis. 2022;69:e659–e670. doi: 10.1111/tbed.14363. [DOI] [PubMed] [Google Scholar]
- Fusaro A., Monne I., Salviato A., Valastro V., Schivo A., Amarin N.M., Gonzalez C., Ismail M.M., Al-Ankari A.R., Al-Blowi M.H., et al. Phylogeography and evolutionary history of reassortant H9N2 viruses with potential human health implications. J. Virol. 2011;85:8413–8421. doi: 10.1128/JVI.00219-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao H., Sun H., Hu J., Qi L., Wang J., Xiong X., Wang Y., He Q., Lin Y., Kong W., et al. Twenty amino acids at the C-terminus of PA-X are associated with increased influenza A virus replication and pathogenicity. J. Gen. Virol. 2015;96:2036–2049. doi: 10.1099/vir.0.000143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu M., Chen H., Li Q., Huang J., Zhao M., Gu X., Jiang K., Wang X., Peng D., Liu X. Enzootic genotype S of H9N2 avian influenza viruses donates internal genes to emerging zoonotic influenza viruses in China. Vet. Microbiol. 2014;174:309–315. doi: 10.1016/j.vetmic.2014.09.029. [DOI] [PubMed] [Google Scholar]
- Gu M., Xu L., Wang X., Liu X. Current situation of H9N2 subtype avian influenza in China. Vet. Res. 2017;48:49. doi: 10.1186/s13567-017-0453-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homme P.J., Easterday B.C. Avian influenza virus infections. I. Characteristics of influenza A-Turkey-Wisconsin-1966 virus. Avian Dis. 1970;14:66–74. [PubMed] [Google Scholar]
- Hou Y., Deng G., Cui P., Zeng X., Li B., Wang D., He X., Yan C., Zhang Y., Li J., et al. Evolution of H7N9 highly pathogenic avian influenza virus in the context of vaccination. Emerg. Microb. Infect. 2024;13 doi: 10.1080/22221751.2024.2343912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Y., Jiang L., Wang G., Song Y., Shan Z., Wang X., Deng G., Shi J., Tian G., Zeng X., et al. M6PR interacts with the HA2 subunit of influenza A virus to facilitate the fusion of viral and endosomal membranes. Sci. China Life Sci. 2024;67:579–595. doi: 10.1007/s11427-023-2471-4. [DOI] [PubMed] [Google Scholar]
- Iqbal M., Yaqub T., Reddy K., Mccauley J.W. Novel genotypes of H9N2 influenza A viruses isolated from poultry in Pakistan containing NS genes similar to highly pathogenic H7N3 and H5N1 viruses. PLoS One. 2009;4 doi: 10.1371/journal.pone.0005788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang W., Liu S., Hou G., Li J., Zhuang Q., Wang S., Zhang P., Chen J. Chinese and global distribution of H9 subtype avian influenza viruses. PLoS One. 2012;7 doi: 10.1371/journal.pone.0052671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamiki H., Murakami S., Nishikaze T., Hiono T., Igarashi M., Furuse Y., Matsugo H., Ishida H., Katayama M., Sekine W., et al. Influenza A virus agnostic receptor tropism revealed using a novel biological system with terminal sialic acid knockout cells. J. Virol. 2022;96 doi: 10.1128/jvi.00416-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikuchi C., Antonopoulos A., Wang S., Maemura T., Karamanska R., Lee C., Thompson A.J., Dell A., Kawaoka Y., Haslam S.M., et al. Glyco-engineered MDCK cells display preferred receptors of H3N2 influenza absent in eggs used for vaccines. Nat. Commun. 2023;14:6178. doi: 10.1038/s41467-023-41908-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krischuns T., Lukarska M., Naffakh N., Cusack S. Influenza virus RNA-dependent RNA polymerase and the host transcriptional apparatus. Annu. Rev. Biochem. 2021;90:321–348. doi: 10.1146/annurev-biochem-072820-100645. [DOI] [PubMed] [Google Scholar]
- Liu D., Shi W., Gao G.F. Poultry carrying H9N2 act as incubators for novel human avian influenza viruses. Lancet. 2014;383:869. doi: 10.1016/S0140-6736(14)60386-X. [DOI] [PubMed] [Google Scholar]
- Liu K., Ding P., Pei Y., Gao R., Han W., Zheng H., Ji Z., Cai M., Gu J., Li X., et al. Emergence of a novel reassortant avian influenza virus (H10N3) in Eastern China with high pathogenicity and respiratory droplet transmissibility to mammals. Sci. China Life Sci. 2022;65:1024–1035. doi: 10.1007/s11427-020-1981-5. [DOI] [PubMed] [Google Scholar]
- Liu K., Qi X., Bao C., Wang X., Liu X. Novel H10N3 avian influenza viruses: a potential threat to public health. Lancet Microbe. 2024;5 doi: 10.1016/S2666-5247(23)00409-3. [DOI] [PubMed] [Google Scholar]
- Liu L., Wang F., Wu Y., Mi W., Zhang Y., Chen L., Wang D., Deng G., Shi J., Chen H., et al. The V223I substitution in hemagglutinin reduces the binding affinity to human-type receptors while enhancing the thermal stability of the H3N2 canine influenza virus. Front. Microbiol. 2024;15 doi: 10.3389/fmicb.2024.1442163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long J.S., Giotis E.S., Moncorge O., Frise R., Mistry B., James J., Morisson M., Iqbal M., Vignal A., Skinner M.A., et al. Species difference in ANP32A underlies influenza A virus polymerase host restriction. Nature. 2016;529:101–104. doi: 10.1038/nature16474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann G., Watanabe T., Ito H., Watanabe S., Goto H., Gao P., Hughes M., Perez D.R., Donis R., Hoffmann E., et al. Generation of influenza A viruses entirely from cloned cDNAs. Proc. Natl. Acad. Sci. U. S. A. 1999;96:9345–9350. doi: 10.1073/pnas.96.16.9345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noda T., Sagara H., Yen A., Takada A., Kida H., Cheng R.H., Kawaoka Y. Architecture of ribonucleoprotein complexes in influenza A virus particles. Nature. 2006;439:490–492. doi: 10.1038/nature04378. [DOI] [PubMed] [Google Scholar]
- Obadan A.O., Santos J., Ferreri L., Thompson A.J., Carnaccini S., Geiger G., Gonzalez Reiche A.S., Rajao D.S., Paulson J.C., Perez D.R. Flexibility in vitro of amino acid 226 in the receptor-binding site of an H9 subtype influenza A virus and its effect in vivo on virus replication, tropism, and transmission. J. Virol. 2019;93:e02011–e02018. doi: 10.1128/JVI.02011-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peacock T.P., Benton D.J., Sadeyen J.R., Chang P., Sealy J.E., Bryant J.E., Martin S.R., Shelton H., Mccauley J.W., Barclay W.S., et al. Variability in H9N2 haemagglutinin receptor-binding preference and the pH of fusion. Emerg. Microb. Infect. 2017;6 doi: 10.1038/emi.2016.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritchie M.E., Phipson B., Wu D., Hu Y., Law C.W., Shi W., Smyth G.K. Limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 2015;43:e47. doi: 10.1093/nar/gkv007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi W., Li W., Li X., Haywood J., Ma J., Gao G.F., Liu D. Phylogenetics of varied subtypes of avian influenza viruses in China: potential threat to humans. Protein Cell. 2014;5:253–257. doi: 10.1007/s13238-014-0036-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y., Wu Y., Zhang W., Qi J., Gao G.F. Enabling the 'host jump': structural determinants of receptor-binding specificity in influenza A viruses. Nat. Rev. Microbiol. 2014;12:822–831. doi: 10.1038/nrmicro3362. [DOI] [PubMed] [Google Scholar]
- Sun Y., Liu J. H9N2 influenza virus in China: a cause of concern. Protein Cell. 2015;6:18–25. doi: 10.1007/s13238-014-0111-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki Y. Sialobiology of influenza: molecular mechanism of host range variation of influenza viruses. Biol. Pharm. Bull. 2005;28:399–408. doi: 10.1248/bpb.28.399. [DOI] [PubMed] [Google Scholar]
- Tian J., Bai X., Li M., Zeng X., Xu J., Li P., Wang M., Song X., Zhao Z., Tian G., et al. Highly pathogenic avian influenza virus (H5N1) clade 2.3.4.4b introduced by wild birds, China, 2021. Emerg. Infect. Dis. 2023;29:1367–1375. doi: 10.3201/eid2907.221149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X., Liu K., Guo Y., Pei Y., Chen X., Lu X., Gao R., Chen Y., Gu M., Hu J., et al. Emergence of a new designated clade 16 with significant antigenic drift in hemagglutinin gene of H9N2 subtype avian influenza virus in eastern China. Emerg. Microb. Infect. 2023;12 doi: 10.1080/22221751.2023.2249558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing X., Shi J., Cui P., Yan C., Zhang Y., Zhang Y., Wang C., Chen Y., Zeng X., Tian G., et al. Evolution and biological characterization of H5N1 influenza viruses bearing the clade 2.3.2.1 hemagglutinin gene. Emerg. Microb. Infect. 2024;13 doi: 10.1080/22221751.2023.2284294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang P., Tang Y., Liu X., Liu W., Zhang X., Liu H., Peng D., Gao S., Wu Y., Zhang L., et al. A novel genotype H9N2 influenza virus possessing human H5N1 internal genomes has been circulating in poultry in eastern China since 1998. J. Virol. 2009;83:8428–8438. doi: 10.1128/JVI.00659-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S., Yu J.L., He L., Gong L., Hou S., Zhu M., Wu J.B., Su B., Liu J., Wu G., et al. Molecular characteristics of the H9N2 avian influenza viruses in live poultry markets in Anhui Province, China, 2013 to 2018. Health Sci. Rep. 2021;4 doi: 10.1002/hsr2.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao L., Li Y., Zhao Y., Liu Q., Lu Y., Ping J. SRSF3 facilitates replication of influenza A virus via binding and promoting the transport of viral mRNA. Vet. Microbiol. 2022;266 doi: 10.1016/j.vetmic.2022.109343. [DOI] [PubMed] [Google Scholar]
- Zhao L., Liu Q., Huang J., Lu Y., Zhao Y., Ping J. TREX (transcription/export)-NP complex exerts a dual effect on regulating polymerase activity and replication of influenza A virus. PLoS Pathog. 2022;18 doi: 10.1371/journal.ppat.1010835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao L., Xia H., Huang J., Zheng Y., Liu C., Su J., Ping J. Features of nuclear export signals of NS2 protein of influenza D virus. Viruses. 2020;12:1100. doi: 10.3390/v12101100. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data supporting the findings of this research are reported in the main text and figures and in the Supplementary Data. Results from whole genome sequencing of the isolated viruses are available in ScienceDB (https://doi.org/10.57760/sciencedb.13859).