Abstract
The TOM (translocase of the outer mitochondrial membrane) complex of the outer mitochondrial membrane is required for the import of proteins into the organelle. The core TOM complex contains five proteins, including three small components Tom7, Tom6, and Tom5. We have created single and double mutants of all combinations of the three small Tom proteins of Neurospora crassa. Analysis of the mutants revealed that Tom6 plays a major role in TOM complex stability, whereas Tom7 has a lesser role. Mutants lacking both Tom6 and Tom7 have an extremely labile TOM complex and are the only class of mutant to exhibit an altered growth phenotype. Although single mutants lacking N. crassa Tom5 have no apparent TOM complex abnormalities, studies of double mutants lacking Tom5 suggest that it also has a minor role in maintaining TOM complex stability. Our inability to isolate triple mutants supports the idea that the three proteins have overlapping functions. Mitochondria lacking either Tom6 or Tom7 are differentially affected in their ability to import different precursor proteins into the organelle, suggesting that they may play roles in the sorting of proteins to different mitochondrial subcompartments. Newly imported Tom40 was readily assembled into the TOM complex in mitochondria lacking any of the small Tom proteins.
INTRODUCTION
Import of mitochondrial precursor proteins from the cytosol to the various subcompartments of the organelle requires the action of several multiprotein complexes housed in the mitochondrial inner and outer membranes (Paschen and Neupert, 2001; Pfanner and Wiedemann, 2002; Endo et al., 2003; Hoppins et al., 2004; Koehler, 2004). The TOM complex (translocase of the outer mitochondrial membrane) is responsible for the initial recognition of virtually all mitochondrial precursors synthesized in the cytosol. The holo-TOM complex contains seven proteins. Tom70 and Tom20 contain large hydrophilic domains that extend into the cytosol and act as receptors for precursor proteins. The five additional proteins, Tom40, Tom22, Tom7, Tom6, and Tom5, make up the TOM core complex which acts as the general insertion pore (Dekker et al., 1998; Ahting et al., 1999; Meisinger et al., 2001).
Tom40 and Tom22 of the core complex have been studied extensively in both S. cerevisiae and Neurospora crassa. Tom40 is essential for viability in both organisms (Baker et al., 1990; Taylor et al., 2003) and is known to be the pore forming component of the complex (Hill et al., 1998; Ahting et al., 2001). The protein also plays a role in binding precursors at both the cis (cytosol facing side) and trans (intermembrane space facing side) sites of the TOM complex during the translocation process (Rapaport et al., 1997, 1998b). Tom22 is also essential in N. crassa (Nargang et al., 1995), but yeast strains lacking the protein can grow slowly on fermentable carbon sources (van Wilpe et al., 1999). Tom22 has several roles including a receptor function (Mayer et al., 1995), passage of precursor proteins from the receptors to the pore (Kiebler et al., 1993; van Wilpe et al., 1999), binding precursors at the trans site (Nakai et al., 1995; Court et al., 1996; Moczko et al., 1997; Kanamori et al., 1999), and as a general organizer of the complex (van Wilpe et al., 1999).
A single study on the N. crassa Tom6 and Tom7 proteins focused on their assembly into the TOM complex (Dembowski et al., 2001). However, the effects of knocking out the genes encoding the proteins have only been investigated in Saccharomyces cerevisiae. Disruption of the gene encoding yeast Tom6 resulted in a slightly reduced growth rate on nonfermentable carbon sources at 37°C (Kassenbrock et al., 1993; Alconada et al., 1995). Import of the ADP-ATP carrier protein (AAC) of the mitochondrial inner membrane and precursors destined to the mitochondrial matrix was reduced (Alconada et al., 1995). Mitochondria lacking Tom6 had a destabilized TOM complex that gave rise to subcomplexes containing Tom40 but not Tom22 in digitonin (DIG)-solubilized mitochondria. However, because subcomplexes of Tom40 and Tom22 that lack Tom6 were observed from wild-type mitochondria solubilized in Triton X-100, it was suggested that Tom6 is required to promote assembly of Tom40 and Tom22, but is not required for the maintenance of the interaction (Alconada et al., 1995; Dekker et al., 1998).
Yeast strains with a deletion of the gene encoding Tom7 were found to grow very slowly at 30°C on nonfermentable carbon sources and were inviable on any carbon source at 37°C (Hönlinger et al., 1996). Tom7 appears to be involved in preprotein sorting as import into mitochondria lacking the protein was only slightly affected for preproteins destined to interior compartments of the mitochondria, whereas the import of porin to the outer membrane was greatly reduced (Hönlinger et al., 1996). Absence of Tom7 resulted in more stable associations between Tom40, Tom20, and Tom22 so that the protein seems to play a role opposite that of Tom6 with respect to TOM complex stability (Hönlinger et al., 1996). Tom7 has also been found to have a function that overlaps with that of the C-terminal intermembrane space domain of Tom22. Both are involved in trans site binding and passage of precursors to the TIM23 complex (Esaki et al., 2004).
Mutant strains lacking Tom5 have been studied in both S. cerevisiae and N. crassa. Yeast strains lacking Tom5 were unable to grow at 37°C and had growth defects at 30°C. Furthermore, deletions of tom5 were found to be synthetically lethal in combination with any of the other TOM complex proteins that were known to be viable as single deletions (Tom6, Tom7, Tom20, Tom70; Dietmeier et al., 1997). Import of all classes of preproteins into yeast mitochondria lacking Tom5 was strongly reduced and it was proposed that the cytosolic domain of the protein played a role in the passage of preproteins from the TOM complex receptors to the general insertion pore (Dietmeier et al., 1997). The protein was also found to be a specific receptor for the small Tim proteins of the intermembrane space, which do not require the cytosolic domains of Tom20 or Tom70 for their import (Kurz et al., 1999). However, it has subsequently been shown that replacement of the cytosolic domain of Tom5 with either GFP (Horie et al., 2003) or the cytosolic domain of Fis1 (Habib et al., 2003), could restore the growth defects of tom5Δ strains. Thus, the role of the cytosolic domain of yeast Tom5 is unclear.
Surprisingly, the recent characterization of an N. crassa strain lacking Tom5 revealed no growth or import defects (Schmitt et al., 2005). Furthermore, no abnormalities in the TOM complex of the N. crassa mutant strain could be discerned, and the function of the protein in the organism was unclear. Further investigation of the TOM complex from a S. cerevisiae strain lacking Tom5 revealed altered structural characteristics. The structural abnormalities could be rescued by a Fis1-Tom5 fusion protein lacking the Tom5 N-terminus. Thus, it was suggested that the role of Tom5 in S. cerevisiae is to maintain the structure of the TOM complex rather than directly acting in preprotein transfer (Schmitt et al., 2005).
The role of each of the small Tom proteins in the assembly of newly imported Tom40 into the TOM complex has also been studied in yeast. Tom40 assembles via a complex pathway involving two intermediates and many factors including the TOB complex (topogenesis of outer membrane β-barrels), which is also known as the SAM complex (sorting and assembly complex; Kozjak et al., 2003; Paschen et al., 2003; Wiedemann et al., 2003; Gentle et al., 2004; Ishikawa et al., 2004; Milenkovic et al., 2004; Waizenegger et al., 2004), the Mdm10 protein (Meisinger et al., 2004), and the Tom13 or Mim1 protein (Ishikawa et al., 2004; Waizenegger et al., 2005). Tom5 was found to act at an early stage in the pathway because formation of the earliest intermediate in assembly was inhibited in mitochondria lacking the protein (Model et al., 2001). Tom6 was thought to function late in assembly because the early intermediates formed efficiently, whereas progression to the final assembled form was inhibited. The role of Tom7 in assembly was consistent with its action in destabilizing the interaction between Tom40 and the receptor proteins. Thus, the antagonistic roles of Tom6 and Tom7 were suggested to result in an ongoing cycling of the fully assembled TOM complex and a late assembly intermediate to promote efficient incorporation of newly imported Tom subunits into the complex (Model et al., 2001; Pfanner and Wiedemann, 2002).
In this study, we have investigated the characteristics of N. crassa mutants lacking Tom6 and Tom7. In addition, we constructed double mutants lacking each of the possible pairs of Tom5, Tom6, and Tom7. Our results show many differences with respect to the functions of these proteins in N. crassa when compared with the functions assigned to their S. cerevisiae homologues.
MATERIALS AND METHODS
Growth of N. crassa and Strains Used
Growth and handling of N. crassa followed standard procedures (Davis and De Serres, 1970). The strains used in this study are listed in Table 1. The mutants used in this study were derived from three parental strains: HI, NCN251 and 76-26 (Table 1). Comparison of TOM complex stability, mitochondrial protein import, and Tom40 assembly between these three parental strains revealed no differences. We chose NCN251 to be used as a control in experiments with the mutants.
Table 1.
Strains used in this study
| Strain | Genotype | Origin or source |
|---|---|---|
| NCN251 | A | Fungal Genetics Stock Center 2489 (74-OR23—1VA) |
| 7626 | his-3 mtrR a | R. L. Metzenberg |
| HI | nic-2 cyh-1 inl inv mei-2 | Fungal Genetics Stock Center 7251 |
| Tom5RIP-Hyg-6 | nic-2 cyh-1 inl inv mei-2 plus a single ectopic copy of tom5 and hygR | Transformation of HI with plasmid pTom5RIP-Hyg |
| tom5RIP (strain used: Tom5Rip-7) | nic-2 inl inv tom5RIP | RIP cross. NCN251 by Tom5RIP-Hyg-6 |
| Tom6RIP-Hyg-1 | his-3 mtrR a plus a single ectopic copy of tom6 and hygR | Transformation of 7626 with plasmid pTom6RIP-Hyg |
| tom6RIP (strain used: Tom6Rip-12) | his-3 tom6RIP | RIP cross. NCN251 by Tom6RIP-Hyg-1 |
| Δtom7 (strain used: Tom7KO-35) | Δtom7 hygR | Tom7 disruption in NCN251 |
| tom5RIPtom6RIP (strains used: Tom5/6Rip-9 and Tom5/6Rip-10) | nic-2 tom5RIPtom6RIP | Cross between tom5RIP and tom6RIP |
| tom5RIP Δtom7 (strain used: LV10—24) | nic-2 tom5RIP Δtom7 hygR | Cross between Δtom7 and tom5RIPtom6RIP |
| tom6RIP Δtom7 (strains used: LV10—11 and LV10—2) | nic-2 tom6RIP Δtom7 hygR | Cross between Δtom7 and tom5RIPtom6RIP |
Growth rates were measured by spotting 10 μl of suspensions containing 2 × 105, 2 × 104, 2 × 103, and 2 × 102 conidia in water onto plates containing standard sorbose medium with the appropriate supplements. The plates were incubated until growth was seen in the lowest dilution spot of the control strain.
Construction of N. crassa Mutant Strains
The tom5RIP mutant used in these studies was derived by crossing the tom5 duplication strain Tom5RIP-Hyg-6, described previously (Schmitt et al., 2005), with strain NCN 251. In this cross, the tom5 duplication served as a substrate for repeat induced point mutation (RIP), which affects duplicated sequences in a single nucleus that is undergoing the sexual phase of the N. crassa life cycle (Selker, 1990). Mitochondria were isolated from the progeny of the cross and examined for the presence of Tom5 by Western analysis. Strain Tom5Rip-7 was devoid of Tom5 and was used for further work. It should be noted that the tom5RIP strain used in this work was not the same one used in a previous study in which Tom5RIP-Hyg-6 was used in a sheltered RIP cross with strain MI to produce progeny lacking Tom5 (Schmitt et al., 2005).
The tom6RIP mutant was also generated via RIP mutagenesis. We wanted to create a plasmid carrying a copy of tom6 for transformation into an appropriate strain to generate a duplication to serve as a RIP substrate. We were unable to clone tom6 into a high copy plasmid. Therefore, a linker containing a NotI site flanked by EcoRI sticky ends was synthesized and inserted into the EcoRI site of the low copy plasmid pBR322 to give pBR322-NotI. To allow eventual selection in N. crassa transformations, the hygromycin resistance gene from plasmid pCSN44 (Staben et al., 1989) was then inserted into the SalI site of this vector to yield pBR322-NotI-HygR. Primers with NotI sites at their 5′ ends were then used to amplify a 2.5-kb tom6 containing fragment from N. crassa genomic DNA by PCR. The fragment was inserted into the NotI site of pBR322-NotI-Hyg to yield plasmid pTom6RIP-Hyg. A strain carrying a duplication of tom6 was generated by transforming strain 7626 with the latter plasmid. Strain Tom6RIP-Hyg-1 was identified by Southern analysis to contain one extra copy of the tom6 sequence inserted into the genome. This strain was crossed with NCN251 to generate progeny containing tom6RIP alleles. Strains lacking the Tom6 protein were identified by Western blot analysis of isolated mitochondria.
The Δtom7 strain (Tom7KO-35) was made by replacing the tom7 gene with a hygromycin resistance gene via homologous recombination using a split marker approach (Colot et al., unpublished results). Because no antibody is available for the N. crassa Tom7 protein, the presence of the replacement was demonstrated by PCR using primers outside the tom7 gene that resulted in amplification of either the wild-type tom7 allele or the larger, disrupted Δtom7 allele containing the hygromycin resistance gene. The absence of Tom7 in one of our mutant strains (tom5RIP Δtom7) was proven by isolation of purified TOM complex and silver staining of the components following SDS-PAGE (S. Schmitt, personal communication).
To obtain mutants lacking different combinations of the small Tom proteins, the tom5RIP strain (Tom5Rip-7) was crossed to the tom6RIP strain (Tom6Rip-12) and mitochondria from the progeny of this cross were screened for the absence of the Tom5 and Tom6 proteins by Western analysis. Two strains lacking both Tom5 and Tom6 were chosen (Tom5/6Rip-9 and Tom5/6Rip-10) and were shown to be sensitive to hygromycin. These were crossed to the Δtom7 strain, in which the hygromycin resistance marker had replaced the tom7 gene. Ascospores from the cross were plated onto medium containing hygromycin to select for strains containing the deletion of tom7. After 1- to 3-d growth on the plates, the spores were transferred to hygromycin containing slants. After growth and conidiation on these slants, the strains were subcultured to slants without hygromycin. The strains were then grown in liquid medium and mitochondria were isolated. These were screened for the absence of the Tom5 and Tom6 proteins by Western blot analysis. The absence of Tom7, which was selected on the basis of hygromycin resistance, was confirmed by PCR analysis of eight separate isolates.
In Vitro Import of Radiolabled Proteins into Isolated Mitochondria
For in vitro import studies, mitochondria were isolated (Mayer et al., 1993) and import of mitochondrial preproteins was performed as described (Harkness et al., 1994). Preproteins were produced by transcription and translation in rabbit reticulocyte lysate (Promega TnT reticulocyte lysate system, Madison, WI) in the presence of [35S]methionine (ICN Biomedicals, Costa Mesa, CA). Incubation time points were 1, 3 and 5 min. Import reactions were analyzed by SDS-PAGE and viewed by autoradiography or phosphorimaging. Quantification of the image from the latter was done using the image-quant program (version 5.2, Molecular Dynamics, Sunnyvale, CA). The precursors of the β-subunit of the F1 ATPase (F1β), the ATP-ADP carrier protein (AAC), and porin were used in import studies.
Blue Native Gel Electrophoresis
Mitochondria (50 μg) were solubilized in 50 μl of buffer containing detergent (either 1% DIG or 1% n-Dodecyl-β-d-maltoside [DDM] in 20 mM Tris-Cl, pH 7.4; 0.1 mM EDTA; 50 mM NaCl; 1% glycerol; 1 mM phenylmethylsulfonyl fluoride). After gentle rocking at 4°C for 15 min and a clarifying spin (30 min, 4°C, 13,000 rpm), the supernatant was added to 5 μl of sample buffer (5% Coomassie Brilliant Blue G-250 in 100 mM Bis-Tris, 500 mM 6-aminocaproic acid, pH 7.0) and gently mixed at 4°C. Samples were analyzed on 6-13% gradient blue native gels as previously described (Schägger and von Jagow, 1991; Schägger et al., 1994) except that electrophoresis was performed overnight (∼16-20 h) at 4°C between 40 and 60 V before the excess Coomassie Blue was electrophoresed out for 1-1.5 h at 500 V.
Other Techniques
Standard techniques for agarose gel electrophoresis, transformation of Escherichia coli, isolation of bacterial plasmid DNA, cloning, restriction digests of plasmid DNA, and PCR using Taq polymerase were performed as described (Ausubel et al., 1992; Sambrook and Rusell, 2001). Separation of mitochondrial proteins by SDS-PAGE (Laemmli, 1970), Western blotting (Good and Crosby, 1989), genomic DNA isolation from N. crassa (Wendland et al., 1996), mitochondria isolation (Mayer et al., 1993), preparation of genomic DNA from conidiaspores for PCR analysis (Descheneau et al., 2005), and electroporation of N. crassa conidiaspores (Tanton et al., 2003) were as described previously.
The following procedures were performed as recommended by the supplier: Western blot detection by light emission using LumiGLO chemiluminescent substrate (KPL, Mandel, Guelph, Ontario, Canada), protein determination by the Coomassie dye binding assay (Bio-Rad, Hercules, CA), automated sequencing using a BigDye Terminator Cycle sequencing kit (version 3.1) with a Model 373 stretch sequencer separation system (Applied Biosystems, Foster City, CA), bacterial DNA plasmid isolation with Qiagen mini-prep spin kits (Qiagen, Santa Clarita, CA), and site-directed mutagenesis based on the Muta-Gene system (Bio-Rad).
Antiserum to Tom40 was raised in rabbits against a peptide composed of the C-terminal 12 amino acid residues. Tob55 antiserum was raised in rabbits against a hexahistidinyl-tagged DHFR fusion protein containing the N-terminal 69 residues of Tob55. All other antisera were a generous gift from the laboratory of W. Neupert.
RESULTS
Isolation and Growth Characteristics of N. crassa Strains Lacking the Small Tom Proteins
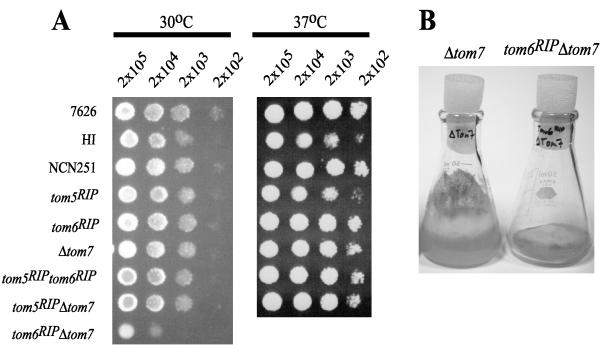
The Tom5, Tom6, and Tom7 proteins of S. cerevisiae have been assigned various roles based primarily on the study of mutants lacking the proteins. A recent study of a N. crassa strain lacking Tom5 revealed no detectable phenotype (Schmitt et al., 2005), in striking contrast to the severe phenotype of yeast cells lacking the protein (Dietmeier et al., 1997). Therefore, it was of interest to determine the phenotype of N. crassa tom6 and tom7 mutants. As described in Materials and Methods, a mutant lacking Tom6 was constructed by RIP mutagenesis. A tom7 knockout mutant (Δtom7) was isolated by replacing the Tom7 coding sequence with a gene for hygromycin resistance (Colot et al., unpublished results). Neither of these strains exhibited a growth phenotype (Figure 1A).
Figure 1.
Growth of strains lacking the small Tom proteins. (A) A 10-μl suspension containing the indicated number of conidia from each strain was applied to sorbose-containing medium. Plates were incubated at 30 or 37°C. We were unable to obtain sufficient numbers of conidia to test the tom6RIPΔtom7 strain at both temperatures. (B) Growth of the Δtom7 strain (left) in flasks containing solid medium is indistinguishable from any other strain used in this study except for the tom6RIPΔtom7 strain (right).
In an attempt to reveal synthetic defects in cells lacking two or more of the proteins, we created strains carrying different combinations of the three small Tom mutant alleles by genetic crossing. A cross of a tom5RIP strain with the tom6RIP strain produced tom5RIPtom6RIP double mutants, which had no obvious growth phenotype (Figure 1A). We then crossed the Δtom7 mutant with the tom5RIPtom6RIP double mutant and plated the resulting ascospores on medium containing hygromycin to select directly for spores carrying the Δtom7 allele (see Materials and Methods). When germinated spores were picked to slants containing hygromycin, “fast-growing” and “slow-growing” isolates were identified, based on the time required to cover the surface of the slant with mycelium. To ensure that plating on hygromycin had selected for spores carrying Δtom7, four isolates of each growth class were chosen randomly for analysis by PCR to determine whether the wild-type or disrupted allele of tom7 was present (see Materials and Methods). All eight were found to contain the Δtom7 allele (unpublished data). We then examined 30 of the fast growing strains by Western blot analysis for the presence of Tom5 and Tom6. This revealed that 18 strains were Δtom7 single mutants and 12 were tom5RIPΔtom7 double mutants. We also examined 20 slow growing strains for the presence of Tom5 and Tom6 and all were found to be tom6RIPΔtom7 double mutants. Thus, the predicted tom5RIPtom6RIPΔtom7 triple mutant was not present in either class. Because all three small Tom genes are located on separate chromosomes (Galagan et al., 2003), the triple mutants should have represented 25% of the Δtom7, hygromycin-resistant progeny. The absence of triple mutants among the progeny strongly suggests that loss of all three small Tom proteins is lethal in N. crassa.
The growth rate of each class of single and double mutant at 30 and 37°C is shown in Figure 1A. All mutant strains were indistinguishable from controls except for the tom6RIPΔtom7 double mutant which had a severe growth rate defect. Unlike all controls and other mutants examined in this study, the tom6RIPΔtom7 double mutants are also characterized by an inability to climb the walls of growth flasks containing solid medium and an inability to produce conidia (Figure 1B). Both the tom5RIP strain and its parent HI, displayed a minor growth rate defect at 37°C. Further genetic analysis proved that the growth defect did not segregate with the Tom5 deficiency (unpublished data).
The TOM Complex in the Small Tom Mutants
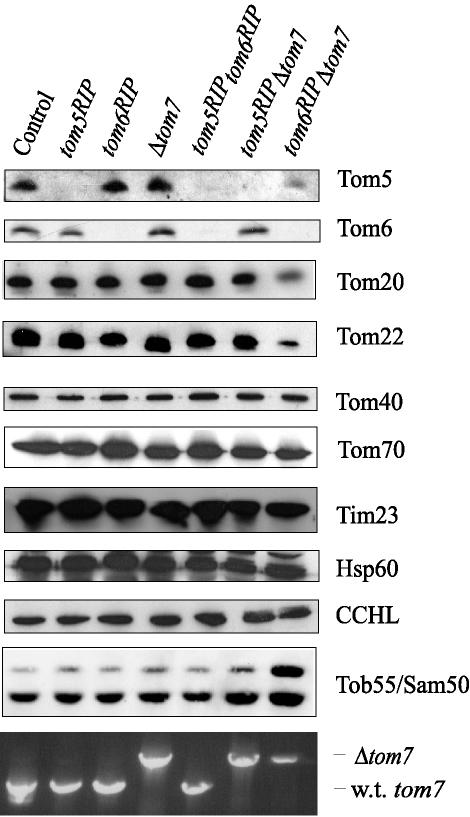
Because the small Tom proteins are known to be part of the TOM core complex (Dekker et al., 1998; Ahting et al., 1999; Meisinger et al., 2001), it was conceivable that the level of other components of the complex might be affected by the absence of one or more of the small proteins. We examined the levels of various mitochondrial proteins in each of the mutant strains by Western blot analysis. Unless directly affected by the mutated gene, the levels of Tom70, Tom40, Tom22, Tom20, Tom6, and Tom5 are unaltered in the individual mutants, the tom5RIPtom6RIP double mutant and the tom5RIPΔtom7 double mutant (Figure 2). The loss of both Tom6 and Tom7 dramatically reduced the levels of Tom5, Tom20, and Tom22. Interestingly, the amount of Tob55/Sam50, the major component of the β-barrel assembly complex of the outer membrane, appears to be slightly increased in the tom6RIPΔtom7 double mutant. Conceivably, this could be due to a response to compensate for the presence of a TOM complex, which is deficient in several of its usual components. For unknown reasons we consistently observe a double band in Western blots for Tob55/Sam50. Two observations suggest that both bands represent the Tob55/Sam50 protein. First, both bands disappear in Neurospora crassa tob55 mutants. Second, when a tob55 mutant is rescued with a gene encoding a His-tagged version of the protein, an antibody to the tag also detects two bands on Western blots (S. C. Hoppins and F. E. Nargang, unpublished results). The ratio of the higher molecular-weight band to the lower one appears to be increased in the tom6RIPΔtom7 mitochondria. The possible significance of this is not known. The levels of Tim23 of the inner membrane, CCHL of the intermembrane space, and Hsp60 of the matrix were not affected by the absence of any of the small Tom proteins. We were unable to measure effects caused by the lack of Tom5 or Tom6 on the level of Tom7 because an antibody to the N. crassa protein is not available.
Figure 2.
Levels of TOM complex and other mitochondrial proteins in strains lacking the small Tom proteins. Mitochondria, 30 μg, isolated from each strain was subjected to SDS-PAGE and transferred to nitrocellulose. Western blots were analyzed for each of the TOM complex proteins and for the matrix protein mitochondrial Hsp60, Tim23 of the inner membrane, and cytochrome c heme lyase (CCHL) of the intermembrane space. The presence or absence of Tom7 was determined by PCR analysis because no antibody to N. crassa Tom7 is available. Genomic DNA was isolated from each strain and PCR was performed using primers outside the tom7 gene that amplify both the disrupted allele (Δtom7), carrying the gene encoding hygromycin resistance, and the wild-type tom7 gene (w.t. tom7). Because the hygromycin resistance gene is larger than the gene encoding Tom7, the PCR product is larger for the Δtom7 allele. (The control is NCN251.)
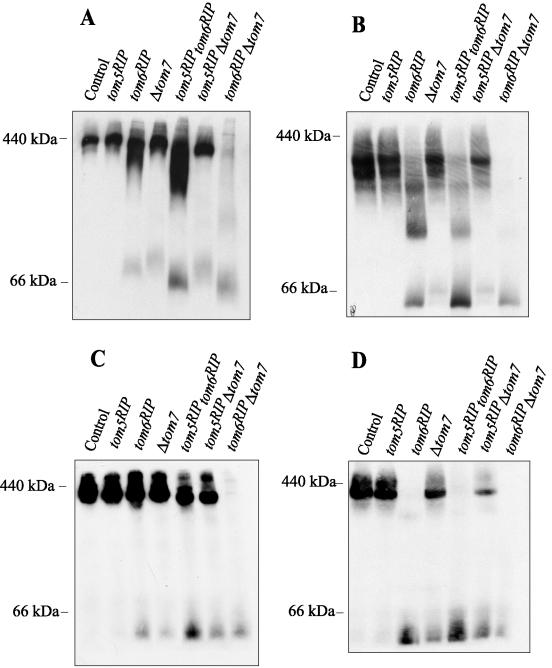
We examined the stability of the TOM complex in the mutants by lysing mitochondria in the presence of DIG or DDM and determining the location of Tom40 after blue native gel electrophoresis (BNGE; Figure 3, A and B). In control mitochondria, Tom40 is present with other TOM components in a 400-kDa complex after treatment with 1% DIG. However, this complex breaks down into smaller complexes after treatment with 1% DDM. As reported previously (Schmitt et al., 2005), the absence of Tom5 had no effect on the stability of the N. crassa TOM complex. The absence of Tom6 resulted in some Tom40 dissociating from the 400-kDa complex into a smaller subcomplex in 1% DIG and caused the TOM complex to completely dissociate to smaller Tom40 containing forms in 1% DDM. The stability of the N. crassa TOM complex appears to be less dependent than its yeast counterpart on the presence of Tom6 because yeast mitochondria lacking Tom6 show severe instability even in the presence of the mild detergent DIG (Dekker et al., 1998). The absence of N. crassa Tom7 resulted in a slight loss of Tom40 from the 400-kDa complex and its appearance in smaller subcomplexes in both detergents. The loss of Tom40 from the complex in the tom5RIP tom6RIP double mutant was slightly enhanced compared with the tom6RIP single mutant in both DIG and DDM, suggesting a role for N. crassa Tom5 in TOM complex stability. In the tom5RIP Δtom7 double mutant the behavior of the TOM complex was similar to the single Δtom7 mutant in DIG but was slightly less stable in DDM. The absence of both Tom6 and Tom7 resulted in severe destabilization of Tom40 in the TOM complex in both DIG and DDM (Figure 3, A and B). Similar results were observed when blue gels were examined for the position of Tom22, the other essential component of the core TOM complex in N. crassa (Nargang et al., 1995). In DIG (Figure 3C), at least some Tom22 was lost from the TOM complex of all single and double mutants except the mutant lacking Tom5 alone. In DDM (Figure 3D), most of Tom22 disassembles from the complex in the single tom6RIP mutant and the tom5RIP tom6RIP double mutant. In contrast to what was seen with Tom40, no high molecular-weight subcomplex containing Tom22 remains after lysis in DDM, demonstrating that the loss of Tom6 weakens the interactions between Tom40 and Tom22. Absence of Tom7 also resulted in loss of Tom22 from the TOM complex in the presence of DDM. The most severe effects were seen in the mutant lacking both Tom6 and Tom7, in which virtually all of Tom22 was seen in low-molecular-weight forms after lysis in either detergent.
Figure 3.
Stability of the TOM complex in strains lacking the small Tom proteins. Mitochondria (50 μg) lacking different small Tom proteins were dissolved in 1% DIG (A and C) or 1% DDM (B and D), subjected to BNGE, and blotted to PVDF membrane. Membranes were decorated with αTom40 antiserum (A and B) or αTom22 antiserum (C and D) and processed for detection of bands by light emission. The positions of 440- (apoferritin) and 66-kDa (bovine serum albumin) marker proteins are shown on the left. Although equal amounts of mitochondrial protein were loaded in all lanes, there is a striking loss of material in the tom6RIPΔtom7 lanes. We have previously suggested that this may be due to aggregation of disassembled components (Taylor et al., 2003; the control is NCN251.)
Import of Mitochondrial Precursor Proteins
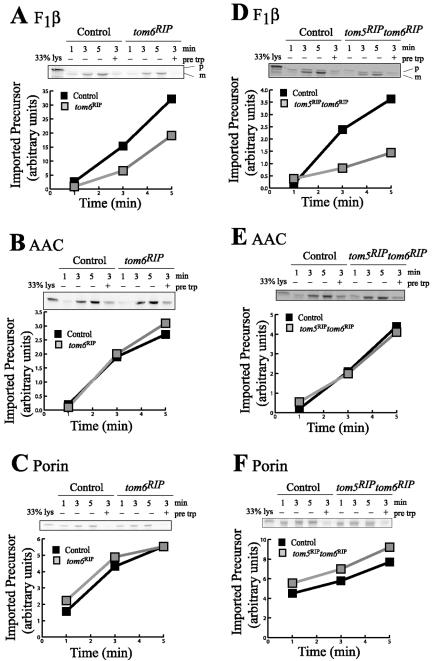
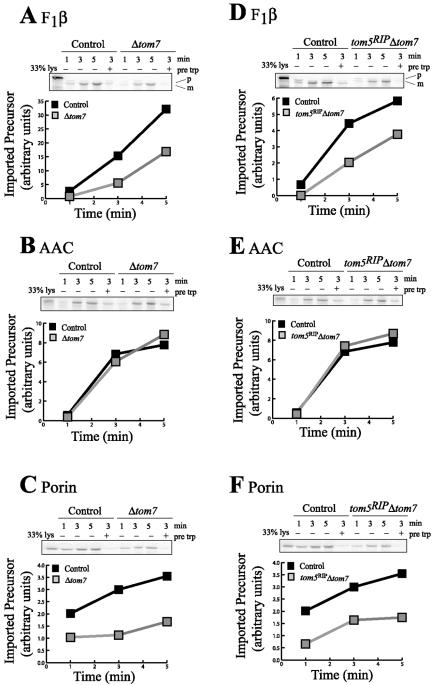
The ability of the small Tom mutant strains to import mitochondrial proteins was tested by in vitro import of radiolabeled preproteins into isolated mitochondria from each of the strains. In agreement with previous findings (Schmitt et al., 2005), we could not distinguish any difference between controls and the tom5RIP mutant for import of any precursor tested, including F1β, AAC, porin, and Tim10 (unpublished data). Mitochondria lacking Tom6 had a minor defect in importing F1β into the matrix but was similar to the control with respect to the import of AAC and porin, to the inner and outer mitochondrial membranes, respectively (Figure 4, A-C). Mitochondria lacking both Tom5 and Tom6 imported preproteins in a manner similar to those devoid of only Tom6 although the defect in F1β import was more pronounced (Figure 4). The strains lacking Tom7 or both Tom7 and Tom5 were defective in importing porin and F1β, but import of AAC was unaffected (Figure 5). We were unable to obtain enough conidia from the tom6RIP Δtom7 double mutant to grow cultures for analysis of mitochondrial protein import (see Figure 1B).
Figure 4.
In vitro import of preproteins into mitochondria lacking Tom6 or both Tom5 and Tom6. Radiolabeled mitochondrial preproteins F1β (A and D) and AAC (B and E) synthesized in rabbit reticulocyte lysate were incubated with mitochondria isolated from a control strain (NCN251) or from strains without Tom6 (tom6RIP) or both Tom5 and Tom6 (tom5RIPtom6RIP), for 1, 3, and 5 min at 25°C. Mitochondria were incubated at 15°C with lysate containing the labeled precursor of porin (C and F). A sample of mitochondria from each strain was treated with trypsin before import (pre trp), to demonstrate that import was receptor dependent. After the import reaction, mitochondria were treated with proteinase K to remove excess radiolabeled protein. Mitochondria were then reisolated and analyzed by SDS-PAGE. Proteins were transferred to nitrocellulose and exposed to x-ray film (insets) and then a Phosphorimager screen for visualization and quantification. “33% lysate” shows a lane in which one-third of the amount of radiolabeled lysate used in each import reaction was electrophoresed.
Figure 5.
In vitro import of preproteins into mitochondria lacking Tom7 or both Tom5 and Tom7. As in Figure 4, except that mitochondria were isolated from a control strain (NCN251) and from strains without Tom7 (Δtom7) or both Tom5 and Tom7 (tom5RIPΔtom7).
Effects of the Small Tom Proteins on Tom40 Assembly into the TOM Complex
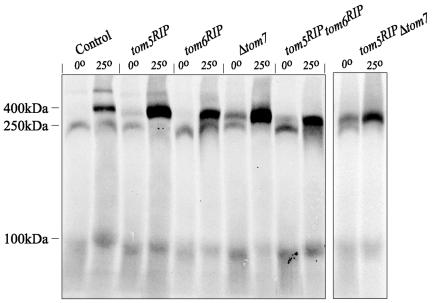
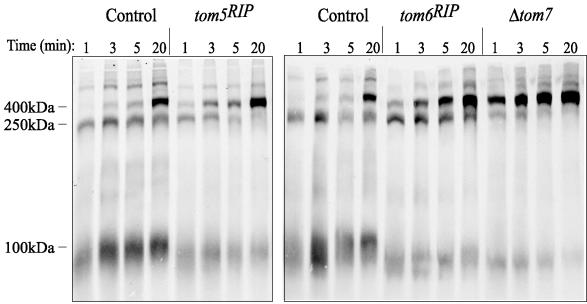
As shown previously, in both yeast and N. crassa, a newly imported Tom40 molecule is first observed in a 250-kDa intermediate, progresses to a 100-kDa intermediate, and then appears in the final assembled 400-kDa TOM core complex (Model et al., 2001; Taylor et al., 2003). Mitochondria from each of the small Tom mutant strains were examined for their ability to assemble newly imported Tom40. In the control strain, Tom40 accumulates in the 250-kDa intermediate when import is performed at 0°C (Figure 6). This represents a monomer of Tom40 associated with the TOB/SAM complex, which is required for the assembly of β-barrel proteins such as Tom40 into the mitochondrial outer membrane (Kozjak et al., 2003; Paschen et al., 2003). When import is performed at 25°C with control mitochondria, Tom40 can progress past the TOB/SAM complex to the 100-kDa intermediate, and then to the fully assembled 400 kDa complex. Membrane integration occurs at the 100-kDa form and assembly of the incoming precursor to the 400-kDa complex occurs within the membrane (Model et al., 2001; Rapaport, 2002). Each of the single and double mutant strains was able to assemble Tom40 into the 400-kDa complex at 25°C. In all cases this appeared to be somewhat more efficient than in the control strain (Figure 6). Mitochondria lacking either Tom5 or Tom7 even appeared to assemble a small amount of Tom40 into the 400-kDa form after 20 min of import at 0°C. Furthermore, very little Tom40 was observed in the 250-kDa intermediate following 20 min of import at 25°C in mitochondria from any of the mutant strains except the tom6RIP single mutant. These data suggest that loss of the small Tom proteins affects the kinetics of progression of Tom40 assembly through the TOB/SAM complex. To further investigate this possibility we performed time course assays of Tom40 import at 25°C using mitochondria isolated from each of the single mutants (Figure 7). In each case it is apparent that Tom40 assembly into the final 400-kDa complex occurs more rapidly than in the control. Unlike the wild-type control, significant amounts of Tom40 have reached the 400-kDa stage after only 1 min of import in all three mutant strains. Assembly to the final form in the mutants continues to be more efficient than in the control at each time point. This was particularly striking in the mitochondria lacking Tom7. The rapid disappearance of Tom40 from the initial 250-kDa intermediate in mitochondria from the tom5RIP and the Δtom7 strains was also confirmed in the time course experiments.
Figure 6.
Tom40 assembly in mitochondria lacking the small Tom proteins. Radiolabeled Tom40 protein was incubated with isolated mitochondria from a control (NCN251) and each of the indicated small Tom protein mutant strains for 20 min at either 0 or 25°C. After the incubation, mitochondria were pelleted, dissolved in 1% DIG, subjected to BNGE, transferred to PVDF membrane, and exposed to x-ray film. The positions of the 400-kDa assembled TOM complex, the 250-kDa intermediate, and the 100-kDa intermediate in the control mitochondria are indicated on the left.
Figure 7.
Time course of Tom40 assembly in mitochondria lacking the small Tom proteins. The experiment was performed as in Figure 6 except that no import was done at 0°C and imports at 25°C were done for each of the indicated time points.
DISCUSSION
Our study has revealed that the roles of the small Tom proteins in N. crassa differ in a variety of ways from the roles assigned to these proteins from studies in S. cerevisiae. It was previously shown that tom5 mutants of N. crassa displayed no observable phenotype, whereas yeast cells lacking the protein have growth defects, import defects, and decreased TOM complex stability (Dietmeier et al., 1997; Schmitt et al., 2005). Our findings support the conclusion that Tom5 plays a minor role in the N. crassa TOM complex compared with the more crucial role of the protein in S. cerevisiae. For example, yeast tom5 mutants display synthetic lethality when present in double mutants with knockouts of any other Tom protein (Dietmeier et al., 1997). In N. crassa, we observed no synthetic effects on growth when the tom5RIP mutation was combined with either the tom6RIP or Δtom7 mutation. Like the single mutants for each gene, the growth rate of these double mutants was indistinguishable from controls. However, the slight increase in loss of Tom40 and Tom22 from the TOM complex observed in the tom5RIPtom6RIP double mutant compared with the tom6RIP mutant alone offers the first insight into a function for N. crassa Tom5 and suggests that the protein plays a minor role in maintaining complex stability. This notion is supported by the fact that we were unable to isolate a tom5RIPtom6RIPΔtom7 triple mutant. Thus, the function of Tom5 in complex stability appears to be necessary for viability in the tom6RIPΔtom7 double mutant, which already has a severely destabilized complex and a severe growth phenotype. All three small Tom proteins may have overlapping or redundant roles in maintaining TOM complex stability in N. crassa.
Some aspects of Tom6 function are similar in yeast and N. crassa. The absence of Tom6 in both organisms leads to destabilization of the TOM complex. In addition, the subcomplexes of Tom40 formed in the N. crassa tom6 mutant in the presence of DDM do not contain Tom22, suggesting that Tom6 serves to stabilize the interaction between the two proteins. These findings are consistent with previous suggestions for the role of Tom6 in modulating the interactions of the Tom proteins during preprotein translocation (Rapaport et al., 1998a; Dembowski et al., 2001). N. crassa mitochondria lacking Tom6 showed a mild defect in the import of F1β to the matrix, whereas the import of AAC to the inner membrane and porin to the outer membrane was not affected. Import of precursors destined for the mitochondrial matrix, and of AAC, were reduced in yeast mitochondria devoid of Tom6. It seems likely that this difference between the organisms is due to the greater instability of the yeast TOM complex imparted by loss of Tom6.
The role of Tom7 appears to be quite different in N. crassa compared with its S. cerevisiae counterpart. In N. crassa the absence of Tom7 partially destabilizes the TOM complex. In contrast, the presence of yeast Tom7 is thought to have a destabilizing affect on the Tom complex so that loss of the protein results in stronger interactions between TOM complex components (Hönlinger et al., 1996). In keeping with this function, absence of yeast Tom7 provides a partial stabilizing effect on the subcomplexes present in solubilized yeast mitochondria lacking the stabilizing protein, Tom6 (Dekker et al., 1998). However, the opposite is observed in N. crassa in which the TOM complex is destabilized to a much greater degree in the tom6RIP Δtom7 double mutant compared with either single mutant. In yeast mitochondria lacking Tom7, the import of mitochondrial precursors to all subcompartments is reduced, with the effects greatest on the outer membrane protein porin. A severe effect on porin import is also seen in N. crassa mitochondria lacking Tom7 and a slight decrease in the import of F1β to the matrix is also observed. However, no effect was seen on import of AAC to the inner membrane. A decrease in the import of porin compared with the apparently increased efficiency of Tom40 import and assembly in the Δtom7 mutant suggests that the protein has different roles with respect to the precursors of these two β-barrel proteins. Tom7 may be more directly involved in porin import at the level of recognition or in the passage of the protein to post-TOM complex stages of import. On the other hand, absence of Tom7 may increase the ability of Tom40 to assemble into the 400-kDa core TOM complex. In support of this interpretation, Tom7 in yeast has been shown to have a role in forming the trans binding site along with the C-terminus of Tom22 on the intermembrane space side of the outer membrane (Esaki et al., 2004). Mutants lacking these trans site components were found to have a significant deficiency of porin, whereas Tom40 levels were unaffected.
The effects of the small Tom proteins on TOM complex stability make it difficult to establish whether they have a direct role in the import of mitochondrial preproteins or if the effects are the result of destablilization of other components. Regardless of the reason, absence of Tom6 or Tom7 has a differential effect on the import of different classes of precursor. As mentioned above, the role of yeast Tom7 in the trans site suggests a more direct role in import for this protein (Esaki et al., 2004).
Differences in the assembly of Tom40 into the TOM complex are also apparent in the small Tom mutant strains of N. crassa and S. cerevisiae. In yeast (Model et al., 2001), Tom5 is needed for Tom40 to reach the initial 250-kDa intermediate. Tom6 is needed for Tom40 to reach the fully assembled complex of 400 kDa, but the 250- and 100-kDa intermediates are efficiently formed. Tom7 is required for the Tom40 precursor to be released from the receptor subunits. Thus, when Tom40 is imported into yeast mitochondria lacking Tom7, a series of additional intermediates is observed that represent complexes containing extra receptor protein subunits. However, in N. crassa, import and assembly of Tom40 into mitochondria lacking one or more of the small Tom proteins appears to be more efficient as the appearance of an incoming Tom40 monomer in the final 400-kDa assembled complex occurs more rapidly in the mutants than wild-type mitochondria. Furthermore, the time course experiments revealed that the duration of association of the Tom40 precursor with the TOB/SAM complex is decreased in the tom5RIP and the Δtom7 strains. A decreased level of Tom40 accumulation in the 250-kDa intermediate is reminiscent of the situation in human mitochondria in which the Tom40 precursor does not accumulate at the 250-kDa TOB/SAM complex intermediate stage, even though the complex is required for Tom40 assembly (Humphries et al., 2005). It was suggested that in yeast, the requirement for newly imported Tom40 to interact with the Tom5 and Tom6 proteins, which may be absent from the mammalian TOM complex, might be the reason for Tom40 accumulation at the 250-kDa stage (Humphries et al., 2005). Because the 250-kDa intermediate is observed in N. crassa, but the requirement for the small Tom proteins in Tom40 assembly does not appear to be crucial, it is conceivable that the function of these proteins in N. crassa may be evolving away from their important roles in yeast toward a state in which they may be eventually lost, as may have happened in mammalian cells.
Acknowledgments
We are grateful to Hildur Colot and Jay Dunlap for supplying procedures and materials for knockout construction before publication. We also thank Simone Schmitt for examining the TOM complex in a Δtom7 strain. This work was supported by a grant from the Canadian Institutes of Health Research to F.E.N.
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E05-03-0187) on June 29, 2005.
Abbreviations used: AAC, ADP-ATP carrier protein; BNGE, blue native gel electrophoresis; DIG, digitonin; DDM, n-dodecyl-β-d-maltoside; F1β, beta subunit of the F1 ATP synthase; RIP, repeat induced point mutation; TOM, translocase of the outer mitochondrial membrane.
References
- Ahting, U., Thieffry, M., Engelhardt, H., Hegerl, R., Neupert, W., and Nussberger, S. (2001). Tom40, the pore-forming component of the protein-conducting TOM channel in the outer membrane of mitochondria. J. Cell Biol. 153, 1151-1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahting, U., Thun, C., Hegerl, R., Typke, D., Nargang, F. E., Neupert, W., and Nussberger, S. (1999). The TOM core complex: the general protein import pore of the outer membrane of mitochondria. J. Cell Biol. 147, 959-968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alconada, A., Kübrich, M., Moczko, M., Hönlinger, A., and Pfanner, N. (1995). The mitochondrial receptor complex: the small subunit Mom8b/Isp6 supports association of receptors with the general insertion pore and transfer of preproteins. Mol. Cell. Biol. 15, 6196-6205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ausubel, R. A., Brent, R., Kingston, R. E., Moore, D. D., and Seidman, J. G. (1992). Current Protocols in Molecular Biology, New York: Greene and Wiley Interscience.
- Baker, K. P., Schaniel, A., Vestweber, D., and Schatz, G. (1990). A yeast mitochondrial outer membrane protein is essential for protein import and cell viability. Nature 348, 605-609. [DOI] [PubMed] [Google Scholar]
- Court, D. A., Nargang, F. E., Steiner, H., Hodges, R. S., Neupert, W., and Lill, R. (1996). Role of the intermembrane space domain of the preprotein receptor Tom22 in protein import into mitochondria. Mol. Cell. Biol. 16, 4035-4042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis, R. H., and De Serres, F. J. (1970). Genetic and microbiological research techniques for Neurospora crassa. Methods Enzymol. 17, 79-143. [Google Scholar]
- Dekker, P.J.T., Ryan, M. T., Brix, J., Müller, H., Hönlinger, A., and Pfanner, N. (1998). Preprotein translocase of the outer mitochondrial membrane: molecular dissection and assembly of the general import pore complex. Mol. Cell. Biol. 18, 6515-6524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dembowski, M., Künkele, K.-P., Nargang, F., Neupert, W., and Rapaport, D. (2001). Assembly of Tom6 and Tom7 into the TOM core complex of Neurospora crassa. J. Biol. Chem. 276, 17679-17685. [DOI] [PubMed] [Google Scholar]
- Descheneau, A. T., Cleary, I. A., and Nargang, F. E. (2005). Genetic evidence for a regulatory pathway controlling alternative oxidase production in Neurospora crassa. Genetics 169, 123-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietmeier, K., Hönlinger, A., Bömer, U., Dekker, P.J.T., Eckerskorn, C., Lottspeich, F., Kübrich, M., and Pfanner, N. (1997). Tom5 functionally links mitochondrial preprotein receptors to the general import pore. Nature 388, 195-200. [DOI] [PubMed] [Google Scholar]
- Endo, T., Yamamoto, H., and Esaki, M. (2003). Functional cooperation and separation of translocators in protein import into mitochondria, the double-membrane bounded organelles. J. Cell Sci. 116, 3259-3267. [DOI] [PubMed] [Google Scholar]
- Esaki, M., Shimizu, H., Ono, T., Yamamoto, H., Kanamori, T., Nishikawa, S., and Endo, T. (2004). Mitochondrial protein import. Requirement of presequence elements and Tom components for precursor binding of the TOM complex. J. Biol. Chem. 279, 45701-45707. [DOI] [PubMed] [Google Scholar]
- Galagan, J. E. et al. (2003). The genome sequence of the filamentous fungus Neurospora crassa. Nature 422, 859-868. [DOI] [PubMed] [Google Scholar]
- Gentle, I., Gabriel, K., Beech, P., Waller, R., and Lithgow, T. (2004). The Omp85 family of proteins is essential for outer membrane biogenesis in mitochondria and bacteria. J. Cell Biol. 164, 19-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Good, A. G., and Crosby, W. L. (1989). Anaerobic induction of alanine aminotransferase in barley root tissue. Plant Physiol. 90, 1305-1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habib, S. J., Vasiljev, A., Neupert, W., and Rapaport, D. (2003). Multiple functions of tail-anchor domains of mitochondrial outer membrane proteins. FEBS Lett. 555, 511-515. [DOI] [PubMed] [Google Scholar]
- Harkness, T.A.A., Nargang, F. E., Van der Klei, I., Neupert, W., and Lill, R. (1994). A crucial role of the mitochondrial protein import receptor MOM19 for the biogenesis of mitochondria. J. Cell Biol. 124, 637-648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill, K., Modell, K., Ryan, M., Dietmeier, K., Martin, F., Wagner, R., and Pfanner, N. (1998). Tom40 forms the hydrophilic channel of the mitochondrial import pore for preproteins. Nature 395, 516-521. [DOI] [PubMed] [Google Scholar]
- Hönlinger, A., Bömer, U., Alconada, A., Eckerskorn, C., Lottspeich, F., Dietmeier, K., and Pfanner, N. (1996). Tom7 modulates the dynamics of the mitochondrial outer membrane translocase and plays a pathway-related role in protein import. EMBO J. 15, 2125-2137. [PMC free article] [PubMed] [Google Scholar]
- Hoppins, S. C., Taylor, R. D., and Nargang, F. E. (2004). Import of proteins into mitochondria. In: The Mycota III. Biochemistry and Molecular Biology, eds. R. Brambl and G. A. Marzluf, Heidelberg: Springer-Verlag, 33-51.
- Horie, C., Suzuki, H., Sakaguchi, M., and Mihara, K. (2003). Targeting and assembly of mitochondrial tail-anchored protein Tom5 to the TOM complex depend on a signal distinct from that of tail-anchored proteins dispersed in the membrane. J. Biol. Chem. 278, 41462-41471. [DOI] [PubMed] [Google Scholar]
- Humphries, A. D., Streimann, I. C., Stojanovski, D., Johnston, A. J., Yano, M., Hoogenraad, N. J., and Ryan, M. T. (2005). Dissection of the mitochondrial import and assembly pathway for human Tom40. J. Biol. Chem. 280, 11535-11543. [DOI] [PubMed] [Google Scholar]
- Ishikawa, D., Yamamoto, H., Tamura, Y., Moritoh, K., and Endo, T. (2004). Two novel proteins in the mitochondrial outer membrane mediate β-barrel protein assembly. J. Cell Biol. 166, 621-627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanamori, T., Nishikawa, S., Nakai, M., Shin, I., Schultz, P. G., and Endo, T. (1999). Uncoupling of transfer of the presequence and unfolding of the mature domain in precursor translocation across the mitochondrial outer membrane. Proc. Natl. Acad. Sci. USA 96, 3634-3639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kassenbrock, C. K., Cao, W., and Douglas, M. G. (1993). Genetic and biochemical characterization of ISP6, a small mitochondrial outer membrane protein associated with the protein translocation complex. EMBO J. 12, 3023-3034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiebler, M., Keil, P., Schneider, H., van der Klei, I., Pfanner, N., and Neupert, W. (1993). The mitochondrial receptor complex: a central role of MOM22 in mediating transfer of preproteins from receptors to the general insertion pore. Cell 74, 483-492. [DOI] [PubMed] [Google Scholar]
- Koehler, C. M. (2004). New developments in mitochondrial assembly. Annu. Rev. Cell Dev. Biol. 20, 309-335. [DOI] [PubMed] [Google Scholar]
- Kozjak, V., Wiedemann, N., Milenkovic, D., Lohaus, C., Meyer, H. E., Guiard, B., Meisinger, C., and Pfanner, N. (2003). An essential role of Sam50 in the protein sorting and assembly machinery of the mitochondrial outer membrane. J. Biol. Chem. 278, 48520-48523. [DOI] [PubMed] [Google Scholar]
- Kurz, M., Martin, H., Rassow, J., Pfanner, N., and Ryan, M. T. (1999). Biogenesis of Tim proteins of the mitochondrial carrier import pathway: differential targeting mechanisms and crossing over with the main import pathway. Mol. Biol. Cell 10, 2461-2474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli, U. K. (1970). Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227, 680-685. [DOI] [PubMed] [Google Scholar]
- Mayer, A., Lill, R., and Neupert, W. (1993). Translocation and insertion of precursor proteins into isolated outer membranes of mitochondria. J. Cell Biol. 121, 1233-1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer, A., Nargang, F. E., Neupert, W., and Lill, R. (1995). MOM22 is a receptor for mitochondrial targeting sequences and cooperates with MOM19. EMBO J. 14, 4204-4211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meisinger, C. et al. (2004). The mitochondrial morphology protein Mdm10 functions in assembly of the preprotein translocase of the outer membrane. Dev. Cell 7, 61-71. [DOI] [PubMed] [Google Scholar]
- Meisinger, C., Ryan, M. T., Hill, K., Model, K., Lim, J. H., Sickmann, A., Müller, H., Meyer, H. E., Wagner, R., and Pfanner, N. (2001). Protein import channel of the outer mitochondrial membrane: a highly stable Tom40-Tom22 core structure differentially interacts with preproteins, small tom proteins, and import receptors. Mol. Cell. Biol. 21, 2337-2348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milenkovic, D., Kozjak, V., Wiedemann, N., Lohaus, C., Meyer, H. E., Guiard, B., Pfanner, N., and Meisinger, C. (2004). Sam35 of the mitochondrial protein sorting and assembly machinery is a peripheral outer membrane protein essential for cell viability. J. Biol. Chem. 279, 22781-22785. [DOI] [PubMed] [Google Scholar]
- Moczko, M., Bömer, U., Kübrich, M., Zufall, N., Hönlinger, A., and Pfanner, N. (1997). The intermembrane space domain of mitochondrial Tom22 functions as a trans binding site for preproteins with N-terminal targeting sequences. Mol. Cell. Biol. 17, 6574-6584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Model, K., Meisinger, C., Prinz, T., Wiedemann, N., Truscott, K. N., Pfanner, N., and Ryan, M. T. (2001). Multistep assembly of the protein import channel of the mitochondrial outer membrane. Nat. Struct. Biol. 8, 361-370. [DOI] [PubMed] [Google Scholar]
- Nakai, M., Kinoshita, K., and Endo, T. (1995). Mitochondrial receptor complex protein. The intermembrane space domain of yeast Mas17 is not essential for its targeting or function. J. Biol. Chem. 270, 30571-30575. [DOI] [PubMed] [Google Scholar]
- Nargang, F. E., Künkele, K.-P., Mayer, A., Ritzel, R. G., Neupert, W., and Lill, R. (1995). “Sheltered disruption” of Neurospora crassa MOM22, an essential component of the mitochondrial protein import complex. EMBO J. 14, 1099-1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paschen, S., and Neupert, W. (2001). Protein import into mitochondria. Life 52, 101-112. [DOI] [PubMed] [Google Scholar]
- Paschen, S. A., Waizenegger, T., Stan, T., Preuss, M., Cyrklaff, M., Hell, K., Rapaport, D., and Neupert, W. (2003). Evolutionary conservation of biogenesis of β-barrel membrane proteins. Nature 426, 862-866. [DOI] [PubMed] [Google Scholar]
- Pfanner, N., and Wiedemann, N. (2002). Mitochondrial protein import: two membranes, three translocases. Curr. Opin. Cell Biol. 14, 400-411. [DOI] [PubMed] [Google Scholar]
- Rapaport, D. (2002). Biogenesis of the mitochondrial TOM complex. Trends Biochem. Sci. 27, 191-197. [DOI] [PubMed] [Google Scholar]
- Rapaport, D., Kunkele, K. P., Dembowski, M., Ahting, U., Nargang, F. E., Neupert, W., and Lill, R. (1998a). Dynamics of the TOM complex of mitochondria during binding and translocation of preproteins. Mol. Cell. Biol. 18, 5256-5262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapaport, D., Mayer, A., Neupert, W., and Lill, R. (1998b). Cis and trans sites of the TOM complex of mitochondria in unfolding and initial translocation of preproteins. J. Biol. Chem. 273, 8806-8813. [DOI] [PubMed] [Google Scholar]
- Rapaport, D., Neupert, W., and Lill, R. (1997). Mitochondrial protein import: Tom40 plays a major role in targeting and translocation of preproteins by forming a specific binding site for the presequence. J. Biol. Chem. 272, 18725-18731. [DOI] [PubMed] [Google Scholar]
- Sambrook, J., and Rusell, D. W. (2001). Molecular Cloning. A Laboratory Manual, Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press.
- Schägger, H., Cramer, W. A., and von Jagow, G. (1994). Analysis of molecular masses and oligomeric states of protein complexes by blue native electrophoresis and isolation of membrane protein complexes by two-dimensional native electrophoresis. Anal. Biochem. 217, 220-230. [DOI] [PubMed] [Google Scholar]
- Schägger, H., and von Jagow, G. (1991). Blue native electrophoresis for isolation of membrane complexes in enzymatically active form. Anal. Biochem. 199, 223-231. [DOI] [PubMed] [Google Scholar]
- Schmitt, S., Ahting, U., Eichacker, L., Granvogl, B., Go, N. E., Nargang, F. E., Neupert, W., and Nussberger, S. (2005). Role of Tom5 in maintaining the structural stability of the TOM complex of mitochondria. J. Biol. Chem. 280, 14499-14506. [DOI] [PubMed] [Google Scholar]
- Selker, E. U. (1990). Premeiotic instability of repeated sequences in Neurospora crassa. Annu. Rev. Genet. 24, 579-613. [DOI] [PubMed] [Google Scholar]
- Staben, C., Jensen, B., Singer, M., Pollock, J., and Schechtman, M. (1989). Use of bacterial hygromycin B resistance gene as a dominant selectable marker in Neurospora crassa transformation. Fungal Genet. Newsl. 36, 79-81. [Google Scholar]
- Tanton, L. L., Nargang, C. E., Kessler, K. E., Li, Q., and Nargang, F. E. (2003). Alternative oxidase expression in Neurospora crassa. Fungal Genet. Biol. 39, 176-190. [DOI] [PubMed] [Google Scholar]
- Taylor, R., McHale, B., and Nargang, F. E. (2003). Characterization of Neurospora crassa Tom40-deficient mutants and effect of specific mutations on Tom40 assembly. J. Biol. Chem. 278, 765-775. [DOI] [PubMed] [Google Scholar]
- van Wilpe, S. et al. (1999). Tom22 is a multifunctional organizer of the mitochondrial preprotein translocase. Nature 401, 485-489. [DOI] [PubMed] [Google Scholar]
- Waizenegger, T., Habib, S. J., Lech, M., Mokranjac, D., Paschen, S. A., Hell, K., Neupert, W., and Rapaport, D. (2004). Tob38, a novel essential component in the biogenesis of β-barrel proteins of mitochondria. EMBO Rep. 5, 704-709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waizenegger, T., Schmitt, S., Zivkovic, J., Neupert, W., and Rapaport, D. (2005). Mim1, a protein required for the assembly of the TOM complex of mitochondria. EMBO Rep. 6, 57-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wendland, J., Lengeler, K., and Kothe, E. (1996). An instant preparation method for nucleic acids of filamentous fungi. Fungal Genet. Newslett. 43, 54-55. [Google Scholar]
- Wiedemann, N., Kozjak, V., Chacinska, A., Schönfisch, B., Rospert, S., Ryan, M. T., Pfanner, N., and Meisinger, C. (2003). Machinery for protein sorting and assembly in the mitochondrial outer membrane. Nature 424, 565-571. [DOI] [PubMed] [Google Scholar]