Abstract
Hepatic stellate cells (HSC) play a central role in the pathogenesis of liver fibrosis, transdifferentiating in chronic liver disease from “quiescent” HSC to fibrogenic myofibroblasts. Transforming growth factor-β (TGF-β), acting both directly and indirectly, is a critical mediator of this process. To characterize the function of the TGF-β signaling intermediates Smad2 and Smad3 in HSC, we infected primary rat HSC in culture with adenoviruses expressing wild-type and dominant negative Smads 2 and 3. Smad3-overexpressing cells exhibited increased deposition of fibronectin and type 1 collagen, increased chemotaxis, and decreased proliferation compared with uninfected cells and those infected with Smad2 or either dominant negative, demonstrating different biological functions for the two Smads. Additionally, coinfection experiments suggested that Smad2 and Smad3 signal via independent pathways. Smad3-overexpressing cells as well as TGF-β-treated cells demonstrated more focal adhesions and increased α-smooth muscle actin (α-SMA) organization in stress fibers, although all cells reached the same level of α-SMA expression, indicating that Smad3 also regulates cytoskeletal organization in HSC. We suggest that TGF-β, signaling via Smad3, plays an important role in the morphological and functional maturation of hepatic myofibroblasts.
INTRODUCTION
Hepatic stellate cells (HSC) are the major cell type responsible for abnormal matrix deposition in liver fibrosis. These cells undergo transdifferentiation from “quiescent” HSC into “activated” fibrogenic myofibroblasts both in chronic liver disease and when freshly isolated and plated on tissue culture plastic. This transdifferentiation is characterized by an increase in proliferation, changes in morphology with expression of α-smooth muscle actin (α-SMA), and deposition of extracellular matrix proteins, particularly fibrillar collagens (Friedman et al., 1992; Friedman, 2000).
Transforming growth factor-β (TGF-β) is the major fibrogenic cytokine in normal wound healing and in multiple fibrotic diseases, including fibrosis of the liver (Desmouliere et al., 1993; Wells, 2000; Gressner et al., 2002). The expression of TGF-β is up-regulated in the diseased liver (Nakatsukasa et al., 1990; Castilla et al., 1991; Roulot et al., 1999); exogenous expression of TGF-β in the liver induces liver fibrosis (Sanderson et al., 1995), and blockade of TGF-β signaling by multiple methods prevents progression of liver fibrosis in experimental animals (George et al., 1999; Qi et al., 1999; Ueno et al., 2000; Yata et al., 2002). Although the importance of TGF-β in the overall process of liver fibrosis has been clearly established, the direct and indirect mechanisms whereby TGF-β influences HSC transdifferentiation remain unclear. TGF-β is required for fibroblasts in culture to transdifferentiate to α-SMA-expressing myofibroblasts (Gabbiani, 2003), but the same is not true for HSC, which transdifferentiate to myofibroblasts as a function of the stiffness of their underlying matrix rather than the presence of TGF-β (Gaça and Wells, unpublished data). Additionally, primary HSC from TGF-β1 null mice cultured on plastic demonstrate the phenotypic appearance and α-SMA expression of wild-type cells (Hellerbrand et al., 1999), as do HSC treated with TGF-β receptor kinase inhibitors (unpublished data), although it has been reported that TGF-β antisense mRNA reduces α-SMA expression in HSC in culture (Arias et al., 2002).
TGF-β downstream signaling is mediated by Smads 2 and 3, structurally similar but functionally distinct proteins that are phosphorylated at C-terminal SSXS motifs by the activated type I TGF-β receptor (Heldin et al., 1997; Wells, 2000; Tomasek et al., 2002). Phosphorylated Smads 2 and 3 form heteromeric complexes with Smad4, a Smad common to multiple TGF-β superfamily signaling pathways, and translocate into the nucleus, where they regulate the activity of target genes. Nuclear Smad2/4 or Smad3/4 complexes interact with a variety of coactivators, corepressors, and transcription factors; Smad3/4 complexes also bind directly to cognate DNA consensus sites (Derynck et al., 1998; Massague and Wotton, 2000). Smad2 and Smad3 are functionally distinct, with different expression patterns. Smad2 null mice die before birth, whereas Smad3 null mice have defects in mucosal immunity but survive to adulthood (Weinstein et al., 1998; Datto et al., 1999; Yang et al., 1999); embryonic fibroblasts derived from the null mice also show distinct patterns of gene induction by TGF-β (Piek et al., 2001). Furthermore, expression of dominant negative Smad3 but not Smad2 prevents TGF-β-mediated inhibition of adipocyte differentiation (Choy et al., 2000).
We recently used rat primary HSC undergoing transdifferentiation in culture as a model system to demonstrate that 1) TGF-β causes phosphorylation and nuclear translocation of Smad2 primarily in quiescent HSC and Smad3 primarily in activated HSC; 2) TGF-β inhibits proliferation of quiescent but not transdifferentiated HSC; and 3) phosphorylated nuclear Smad2 is present constitutively in activated HSC (Liu et al., 2003a). We hypothesized that the two Smads have distinct roles in the activation and function of HSC. To test this hypothesis, we generated adenoviruses expressing wild-type and dominant negative Smad2 and Smad3. Infection of primary HSC demonstrated significant differences in the function of Smad2 and Smad3 at different stages of transdifferentiation. Surprisingly, we also observed that the two Smads do not seem to interact directly in HSC TGF-β signaling pathways and that Smad3 expression results in altered α-SMA organization and focal adhesion structure.
MATERIALS AND METHODS
HSC Isolation and Cell Culture
Primary HSC were isolated from male retired breeder Sprague-Dawley rats (450–750 g) by sequential digestion of the liver with pronase and collagenase, followed by density gradient centrifugation over 8.5% HistoDenz (Sigma-Aldrich, St. Louis, MO) as described previously (Friedman and Roll, 1987; Liu et al., 2003a). All animal work was approved by the University of Pennsylvania Institutional Animal Care and Use Committee. The purity of HSC was assessed microscopically and by autofluorescence of the stored retinoids in HSC lipid droplets. Cell viability was determined by trypan blue exclusion. HSC were consistently found to be 95–99% pure and >95% viable. HSC were seeded on uncoated polystyrene dishes and were maintained in M199 media (Invitrogen, Carlsbad, CA) as described previously (Friedman and Roll, 1987; Liu et al., 2003a). For culture on Matrigel, thin layers of Matrigel were prepared on tissue culture plates as described previously (Gaca et al., 2003) using growth factor-reduced Matrigel (BD Biosciences, San Jose, CA) mixed with serum free M199 media at a ratio of 2:1.
Generation of Adenoviruses
A cDNA encoding rat Smad3 was a generous gift from Yun Chen (Indiana University, Bloomington, IN). Rat Smad2 was cloned by PCR as described previously (Liu et al., 2003a). Dominate negative Smads, with serine-to-alanine mutations in the phosphorylation site SSXS (to AAXA) were generated with the QuikChange mutagenesis kit (Stratagene, San Diego, CA) and confirmed by DNA sequencing. Recombinant E1-deleted adenoviral vectors carrying cDNAs under a constitutively active cytomegalovirus promoter/enhancer were prepared as described previously (Becker et al., 1994; Souchelnytskyi et al., 1997; Yu et al., 2002). Ad-βGal was used as a control virus throughout this study. HSC were infected for 2 h in M199 medium containing 10% fetal bovine serum (FBS). After infection, the medium was changed to fresh growth medium, and the cells were incubated at least 22 h before individual experiments. Recombinant adenoviruses were delivered to primary HSC with >95% efficiency (confirmed by infection with Ad-βGal) at 2 or 7 d after plating at a multiplicity of infection (MOI) of 100 or 50, respectively. For coinfection experiments, cells were infected simultaneously with two different viruses.
Antibodies and Reagents
The following antibodies were used: Smad2 (Cell Signaling Technology, Beverly, MA), Smad2/3 (clone 18; BD Biosciences), Smad3 (Zymed Laboratories, South San Francisco, CA), phospho-Smad2 [Ser465/467] (pSmad2) (Cell Signaling Technology; Liu et al., 2003a), phospho-Smad3 (pSmad3) (Liu et al., 2003a), α-SMA (clone 1A4; Sigma-Aldrich), fibronectin (for Western immunoblotting; clone 10, BD Biosciences; for immunofluorescence, Chemicon International [Temecula, CA] rat polyclonal), type I collagen (Accurate Chemical & Scientific, Westbury, NY), phospho-FAK [pY397] (pFAK) (BioSource International, Camarillo, CA), and heat shock protein (HSP)70 (clone W27; Santa Cruz Biotechnology, Santa Cruz, CA). TGF-β1 was obtained from R&D Systems (Minneapolis, MN) and used at 100 pM.
Western Blotting
Western blotting was performed as described previously (Liu et al., 2003a) with antibodies against Smad2 (1:200), Smad3 (1:200), pSmad2 (1:100), pSmad3 (1:200), αSMA (1:800), HSP70 (1:5000), fibronectin (1:10,000), and collagen (1:100). Horseradish peroxidase-conjugated secondary antibody was used at 1:5000 (Pierce Chemical, Rockford, IL), and signals were detected by SuperSignal West Pico chemiluminescence (Pierce Chemical). Images were quantitated using ImageJ software (National Institutes of Health).
Immunofluorescence
HSC were seeded onto culture slides and infected as described above. One to 5 d after infection, cells were fixed and permeabilized with cold methanol/acetone (1:1) for 10 min followed by 0.1% Triton X-100 in phosphate-buffered saline (PBS) for 3 min. Slides were washed with PBS and blocked with PBS containing 1% bovine serum albumin at room temperature for 1 h, and then they were incubated with primary antibodies against Smad2/3 (1:250), type I collagen (1:20), fibronectin (1:2500), αSMA (1:400), or pFAK (1:50). Slides were then incubated with Cy2- or Cy3-conjugated secondary antibodies (1:250; Jackson ImmunoResearch Laboratories, West Grove, PA) followed by 4,6-diamidino-2-phenylindole (DAPI) (1:10000; Molecular Probes, Eugene, OR) for nuclear staining.
Microscopy and Time-Lapse Imaging
Pictures of living cells were taken using an inverted microscope (model DM IRB; Leica Microsystems, Deerfield, IL) with CoolSNAP software and a CoolSNAP digital camera (model CF; Roper Scientific, Trenton, NJ). Images of fixed and stained cells were visualized with an inverted microscope (model eclipse E600; Nikon, Tokyo, Japan) and captured with a QI-CAM digital camera (QImaging), and processed by IPLab 3.9 (Scanalytics, Fairfax, VA) and Photoshop 7.0 (Adobe Systems, Mountain View, CA). Time-lapse images of the spreading behavior of passaged HSC were collected every 5 min for up to 16 h with an inverted microscope (model TE300; Nikon) equipped for time-lapse imaging with Image-Pro Plus 4.5 software and an Evolution QEi digital camera (Media Cybernetics, Silver Spring, MD). While on the microscope stage, Ad-βGal or Ad-wt3 infected HSC were cultured in 25-ml tissue culture flasks with regular medium containing 10 mM HEPES buffer. Throughout the experiment, cells were maintained at 37°C.
HSC Proliferation Assay
Proliferation was evaluated as described previously (Liu et al., 2003a). Briefly, 24 h after infection with adenoviruses, HSC were washed and incubated in M199 containing 0.5% FBS with or without 100 pM TGF-β1 for 24 h. [3H]Thymidine at 1 μCi/ml was added to the medium for the last 18 h of incubation. [3H]Thymidine incorporation into newly synthesized DNA was determined by scintillation counting of lysates.
Migration Assay
Migratory activity of HSC was quantitated by culturing cells on 12-well Transwell cell culture inserts with an 8-μm pore size (BD Biosciences). The lower side of each membrane was coated with type I collagen (100 μg/ml; BD Biosciences), and cross-linked under UV. Day 7 activated HSC were infected with adenoviruses. After 24 h, HSC were trypsinized and resuspended with M199 media supplemented with 0.5% FBS. Cells (1 × 105) were plated in each insert. Lower chambers were filled with M199 media supplemented with 10% FBS. Cells were incubated at 37°C for 15 h and then fixed and stained with Diff-Quik Staining Solutions (Dade Behring, Deerfield, IL) according to the manufacturer's instructions. Cells attached to the upper side of the membrane were wiped off gently. Cells that had migrated through and attached to the bottom of the membrane were photographed and counted.
Reproducibility and Statistical Analysis
All experiments were performed at least three times. Unless stated otherwise, figures show a representative experiment. Results are expressed as mean ± SEM and were tested by an unpaired Student's t test. Differences were considered to be statistically significant at p ≤ 0.05, as indicated by an asterisk (*) and marked with a double asterisk (**) for p ≤ 0.01.
RESULTS
Adenoviral Smads Were Effectively Expressed in Quiescent and Transdifferentiated HSC
To determine the role of Smad2 and Smad3 in rat HSC, we generated E1-deleted adenoviral vectors expressing rat wild-type Smad2, wild-type Smad3, dominant negative Smad2, dominant negative Smad3, and β-galactosidase (Ad-wt2, Ad-wt3, Ad-dn2, Ad-dn3, and Ad-βGal). These viruses were used to infect primary rat HSC, which typically undergo spontaneous transdifferentiation on uncoated plastic within 7 d of isolation, with associated expression of α-SMA and loss of vitamin A droplets (Friedman, 2000). Efficiency of infection with Ad-βGal at days 2 and 7 was evaluated by βGal staining of cells 24 h after infection and was >95% at MOI of 100 and 50, respectively. In quiescent (day 2) as well as activated (day 7) HSC, extrinsic Smad2 and Smad3 proteins were detected within 24 h of infection and were expressed in a time- and dose-dependent manner (our unpublished data). Infection with viruses encoding Smad2 and Smad3 resulted in increased expression and nuclear accumulation of the corresponding phosphorylated Smads, suggesting that the overexpressed proteins are functional and constitutively activated in HSC (Figure 1). Overexpression of Smad2, however, did not alter the expression or TGF-β1-induced phosphorylation of Smad3, and likewise overexpression of Smad3 did not alter Smad2 expression or TGF-β1-induced Smad2 phosphorylation. Dominant negative Smads were not phosphorylated, either constitutively or in response to TGF-β; in fact, infection with viruses encoding a dominant negative Smad blocked the phosphorylation of the corresponding endogenous Smad (Figure 1B). Expression of the dominant negative Smads, however, did not affect the phosphorylation of the opposing endogenous Smads.
Figure 1.
Adenoviral expression resulted in specific expression of functional Smads. Adenoviruses encoding wild-type and dominant negative Smads were used to infect HSC at day 2 (A) and day 7 (B) after isolation at MOI of 100 and 50, respectively. Cells were lysed 24 h after infection and immunoblotted with the antibodies specified. Blots were sequentially stripped and reprobed; the HSP70 signal demonstrates equal loading in all lanes. (A) Cells infected with the adenoviruses indicated (across the top) were immunoblotted with the antibodies indicated (on the left). The blots demonstrate that all Smads are expressed appropriately, that the wild-type and dominant negative Smads are expressed equally well, and that infection with one Smad did not alter expression of the other. There is constitutive phosphorylation of wild-type but not dominant negative Smads. The Smad2 antibody also recognizes Smad3, as shown by the asterisk (*). (B) Cells were treated with or without 100 pM TGF-β1 in 0.5% serum for 30 min before lysis. The blot demonstrates phosphorylation of the exogenous wild-type but not dominant negative Smads, partially constitutively and partially in response to TGF-β. Note that there is constitutive phosphorylation of endogenous Smad2, and TGF-β-mediated phosphorylation of endogenous Smad3 (“no virus” lanes), as we have observed previously in activated HSC (Liu et al., 2003a), and that infection with each dominant negative Smad resulted in decreased phosphorylation of the corresponding endogenous Smad. (C) Fluorescence micrographs of HSC confirm the findings from Western immunoblotting. HSC were infected with the adenoviruses indicated at day 2 after isolation. Cells were fixed on day 5 and stained with anti-Smad2/3 antibody. Wild-type Smads are localized in the nucleus, whereas dominant negative Smads are excluded. The nuclear localization of endogenous Smads (likely to be primarily Smad2) is seen in cells infected with Ad-βGal. Bars, 50 μm.
The distribution of the overexpressed Smad2 and Smad3 proteins was determined by immunofluorescence staining of infected HSC with an antibody recognizing both Smads. Wild-type Smad2 and Smad3 accumulated in the nucleus, whereas dominant negative Smad2 and Smad3 remained cytoplasmic (Figure 1C).
Smad2 and Smad3 Have Different Effects on HSC Proliferation
Proliferation of intermediate and transdifferentiated HSC was evaluated by [3H]thymidine incorporation. In intermediate cells, infection with Ad-wt3 but not Ad-wt2 caused a significant decrease in baseline thymidine incorporation, although neither Ad-dn3 nor Ad-dn2 had any effect (Figure 2A). In activated cells, wild-type Smad3 overexpression resulted in a significant decrease in baseline proliferation; overexpression of dominant negative Smad2, however, resulted in a marked increase in proliferation (Figure 2B). These data are consistent with our previous finding that constitutively active Smad2 is present at high levels in transdifferentiated HSC: the dominant negative Smad2 likely removes tonic growth inhibition caused by this constitutively active protein (Liu et al., 2003a). The large amounts of active endogenous Smad2 may saturate the system, such that additional expression of wild-type Smad2 has minimal incremental effect. Activated Smad3, on the other hand, is present at low levels in day 7 cells such that overexpression results in effective growth inhibition; there is minimal constitutively active protein available for inhibition by the dominant negative Smad3. Treatment of infected cells with TGF-β had little additional effect, likely because the overexpressed Smads are constitutively active (Figure 1) and because even cells at the early time point (Figure 2A) are intermediate in phenotype, corresponding to day 4 cells, which we have previously shown are not growth inhibited by TGF-β (Liu et al., 2003a). Unfortunately, it is not possible to evaluate the effects of adenoviral Smad2 or Smad3 on quiescent cells due to the need to infect cells at day 2 and to allow at least 24 h for protein expression.
Figure 2.
Smad2 and Smad3 had different effects on proliferation of HSC. HSC at day 2 (A) or day 7 after isolation (B–D) were infected with adenoviruses as indicated. Cells were treated with or without 100 pM TGF-β1 in 0.5% serum for 24 h after infection. Incorporation of [3H]thymidine during the last 18 h of incubation was measured. (A) Overexpression of Smad3 in intermediate cells (assayed at 3–4 d after isolation) resulted in a significant decrease in incorporation of [3H]thymidine, whereas overexpression of Smad2 and the dominant negative Smads had no effect. (B) Overexpression of Smad3 in transdifferentiated cells also decreased [3H]thymidine incorporation, although overexpression of dominant negative Smad2 increased [3H]thymidine incorporation in these cells. (C) Activated HSC were infected with a combination of adenoviruses expressing the wild-type and dominant negative forms of a given Smad, in variable ratios. The MOI of each virus is given in the table below; the total amount of virus was constant. The graph demonstrates that wild-type Smad2 effectively neutralizes the effects of dominant negative Smad2 and that dominant negative Smad3 effectively neutralizes wild-type Smad3. (D) Wild-type and dominant-negative forms of each Smad do not neutralize or enhance the effect of the opposing Smad. Activated HSC were infected with a combination of two adenoviruses, at an MOI of 50 for each, as indicated. Note that the Ad-wt3 effect is dominant when this virus is coinfected with Ad-dn2. All data (A–D) represent the mean ± SEM of at least three independent experiments performed in triplicate (*p < 0.05, **p <0.01 compared with control cells infected with Ad-βGal).
Interestingly, coinfection experiments suggested that Smad3 is the dominant Smad and that Smad2 and Smad3 function in independent pathways. Although control experiments demonstrated that each dominant negative Smad antagonized the function of the corresponding wild-type Smad (Figure 2C), the dominant negative Smads did not antagonize the function of the opposing wild-type Smads (Figure 2D). Overexpression of dominant negative Smad2 did not alter the growth inhibition observed with wild-type Smad3; likewise, overexpression of dominant negative Smad3 did not increase proliferation, as would be expected if it antagonized the function of the endogenous constitutively active Smad2. We observed a similar lack of interaction of the Smads in transdifferentiation experiments, in which Smad3 also seemed to be the dominant Smad (see below; Figure S1).
Smad3 Up-Regulates Matrix Protein Synthesis and Chemotaxis of Activated HSC
Immunostaining and Western immunoblotting demonstrated increased synthesis of type I collagen and fibronectin in Ad-wt3-infected cells. Cells were infected at day 2 after isolation and evaluated at day 7; cells overexpressing wild-type Smad3 but not Smad2 or either dominant negative Smad demonstrated by immunostaining a modest increase in collagen expression and a more marked increase in fibronectin expression compared with uninfected cells (Figure 3, A and B). These findings were confirmed by Western immunoblotting (Figure 3, C–E).
Figure 3.
Overexpression of Smad3 increased matrix synthesis. Cells were infected with the adenoviruses indicated at day 2 after isolation. At day 7, they were immunostained using antibodies against type I collagen (A) or fibronectin (B). Cells processed without primary antibody are shown as a control (no 1° antibody). Nuclei were stained with DAPI. Immunofluorescence microscopy demonstrates a clear increase in collagen and fibronectin production in cells infected with Ad-wt3 compared with cells infected with other viruses. Cells infected with Ad-wt2, dominant negative Smads and Ad-βGal showed similar levels of expression of matrix proteins as uninfected control cells (our unpublished data). Bar, 50 μm. (C) Western immunoblotting of lysates from infected cells with an antifibronectin antibody (middle) and an anticollagen I antibody (top) confirmed increased matrix synthesis by Ad-wt3-infected cells. The blot was stripped and reprobed with an antibody against HSP70 (bottom) to confirm equal loading. One representative experiment of three is shown. (D and E) Western blots from three representative experiments were quantitated using ImageJ software and normalized to levels of HSP70. Percentage of expression of the uninfected cells is set at 100. Increased expression of both fibronectin and collagen I is statistically significant with p < 0.05, as shown.
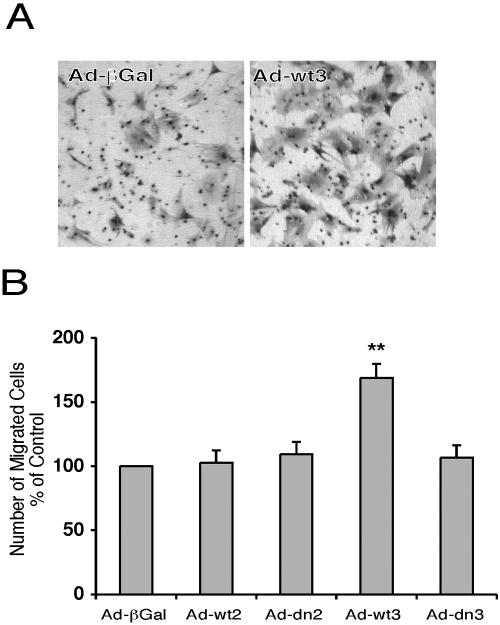
TGF-β stimulates the chemotaxis of activated HSC, enabling their accumulation in areas of injury (Fibbi et al., 2001; Yang et al., 2003). To assess the role of the Smads on chemotaxis of HSC in culture, cells infected at day 7 after isolation were passaged 24 h later and cultured on 8-μm-pore size Transwell inserts. Type I collagen and serum served as chemoattractants, and cells migrating through the inserts were counted. Overexpression of wild-type Smad3 resulted in a significant increase in migration toward serum and type I collagen, whereas dominant negative Smad3 and wild-type and dominant negative Smad2 had no effect (Figure 4).
Figure 4.
Smad3 enhances chemotaxis of transdifferentiated HSC. HSC were infected with adenoviruses at day 7 after isolation, as indicated. Cells were passaged 24 h later and cultured on 8-μm pore size Transwell inserts. Type I collagen and 10% serum served as chemoattractants. Cells that had migrated through the inserts 15 h later were stained, photographed, and counted manually. (A) Representative images of the undersides of the inserts, demonstrating an increase in chemotaxis by the Ad-wt3-infected cells (right) compared with Ad-βGal-infected cells (left). (B) Quantitation of mean ± SEM from three independent experiments performed in triplicate, showing a significant increase in chemotaxis in cells infected with Ad-wt3. **p ≤ 0.01 compared with Ad-βGal control.
Overexpression of Smad3 Accelerates HSC Transdifferentiation and Alters α-SMA Organization
Overexpression of wild-type Smad3, but not wild-type Smad2 or either dominant negative Smad, hastened the transdifferentiation of quiescent HSC infected at day 2 after isolation, as defined by expression of α-SMA. Twenty-four hours after infection (day 3), cells infected with Ad-wt3 showed increased expression of α-SMA by Western blotting (Figure 5A). By day 5, these cells seemed to be myofibroblasts, with greater spreading than cells infected with the other viruses. By days 7 and 8, however, all cells (uninfected or infected with any of the adenoviruses) had a spread out morphology and expressed similar amounts of α-SMA as assessed by Western blotting (Figure 5, A and B). This is consistent with the observation using HSC from Smad3 and TGF-β null mice that neither is required for in vitro transdifferentiation and α-SMA expression (Hellerbrand et al., 1999; Schnabl et al., 2001). Close examination of Ad-wt3-infected cells at day 8, however, demonstrated that they had a more angular shape, with increased spreading and fewer peripheral protrusions (Figure 5B; also shown in Figure 5D, top). Similar changes in morphology were observed when Ad-βGal-infected HSC were treated with TGF-β, suggesting that the changes reflected a physiological response to increased TGF-β signaling mediated by Smad3 and were unlikely to be an artifact of adenoviral overexpression (Figure 5C). Additionally, TGF-β treatment of Ad-dn3-infected cells failed to result in the phenotypic changes, providing additional evidence for the role of Smad3 in the process. Immunostaining with α-SMA showed that these cells had highly organized stress fibers compared with cells infected with the other viruses, in which α-SMA was distributed homogeneously. α-SMA and phospho-FAK were colocalized by immunostaining at the end of the stress fibers, indicating that the Ad-wt-Smad3-expressing cells also had increased focal adhesions (Figure 5D). Note in the top panels that the shape of infected cells is significantly different and that the entire population of infected cells is affected; in bottom panels, both an increase in size and number of focal adhesions is evident. Total levels of FAK were unchanged, as assayed by Western blotting (our unpublished data).
Figure 5.
Smad3 expression accelerated transdifferentiation of HSC and increased α-SMA organization. For analysis of transdifferentiation and α-SMA expression, HSC were infected with the indicated adenoviruses at day 2 after isolation. (A) Infected HSC were lysed at days 3, 5, and 7 after isolation, as labeled. Lysates were separated by SDS-PAGE and immunoblotted with antibodies against α-SMA, and, as a loading control, HSP70. Western blotting demonstrates that Ad-wt3-infected cells express α-SMA more rapidly than uninfected cells and those infected with other viruses, but that by day 7 after isolation (day 5 after infection), α-SMA expression is the same regardless of Smad expression. (B) Light microscopic analysis of cells at day 6 after isolation demonstrates significant morphological differences between cells infected with Ad-wt3 and cells infected with other viruses (or uninfected cells; our unpublished data). The Smad3-overexpressing cells display a more angular shape, fewer membrane protrusions, and an obvious increase in cytoskeletal organization. (C) Cells infected with Ad-βGal, Ad-dn3, or Ad-wt3 were treated (bottom) or not (top) with 100 pM TGF-β1 beginning at day 2 and were photographed at day 7. TGF-β-treated control and Ad-wt3-infected cells but not Ad-dn3-infected cells, demonstrated similar morphological changes. (D) Cells overexpressing Smad3 (right) demonstrate increased α-SMA organization in stress fibers and increased focal adhesions compared with control cells (left), as shown by immunofluorescence staining of cells at day 7 with α-SMA (top) or with α-SMA and phospho-FAK (bottom). Uninfected cells and those infected with Smad2 or either dominant negative Smad had the same appearance on staining as the Ad-βGal infected cells shown (our unpublished data). Nuclei were stained with DAPI; 20× magnification, top; 40× magnification, bottom. Bars, 50 μm.
The difference in α-SMA organization and cell shape was stable and had functional consequences for cell spreading. When activated HSC were infected with Ad-wt3 or Ad-βGal and were passaged, all cells spread rapidly after replating. Ad-wt3 infected cells, however, maintained their differences in shape, and, by 10 h after replating, demonstrated fewer protrusions and more stable interaction with the underlying support than the Ad-βGal-infected cells (Figure 6 and Videos 1 and 2).
Figure 6.
Smad3-infected cells demonstrate different spreading behavior. HSC at day 7 after isolation were infected with Ad-βGal or Ad-wt3. After an additional 24 h, cells were trypsinized and replated and then fixed at the times indicated (1–30 h) and photographed with an inverted microscope. Ad-wt3-infected cells maintained their differences in cell shape compared with Ad-βGal-infected cells, with formation of fewer protrusions, as is seen at 10 h and at later time points. Bars, 50 μm.
When activated HSC were infected at day 7, they remained activated for at least 10 d, even when infected with the dominant negative Smads. Infection of HSC with Ad-wt3 at day 7 resulted in enhanced organization of α-SMA in stress fibers and increased the numbers of focal adhesions, as was seen when cells were infected at earlier time points. All other HSC (uninfected or infected with any of the other adenoviruses) had phenotypic appearances identical to each other, with prominent membrane protrusions and without the angular shape that characterized the Ad-wt3-infected-cells. Cells coinfected with two viruses demonstrated either no change from uninfected or, if infected with Ad-wt3, demonstrated increased focal adhesions and stress fiber organization (Figure S1).
Effect of wt-Smad3 in Transdifferentiation Was Enhanced When Cultured on Matrigel
To determine whether Smad3 expression alone is sufficient to induce activation of HSC, primary HSC were plated on the basement membrane-like matrix Matrigel immediately after isolation and were then infected with adenoviruses at day 2. Culture on Matrigel is a well established model for maintaining HSC in the quiescent state and for causing reversion of activated cells to quiescence (Friedman et al., 1989; Schnabl et al., 2002; Gaça et al., 2003). All cells remained quiescent except cells infected with Ad-wt3, which surprisingly seemed to migrate through the thin layer of Matrigel, and, after reaching the glass or plastic support below, underwent activation (Figure 7 and Video 3). A similar phenomenon also was observed when activated HSC were infected with adenoviruses on day 7 and passaged onto Matrigel on day 8 (our unpublished data). The mechanism whereby Ad-wt3-infected HSC migrates through Matrigel is not known although it likely involves a protease. We have demonstrated that HSC transdifferentiate as a function of matrix stiffness (Gaça and Wells, unpublished data) and that they remain quiescent on Matrigel due to the high compliance of this substrate in gel form; it is thus consistent that the Ad-wt3-infected cells underwent transdifferentiation when they migrated and encountered the stiff plastic or glass underlying the Matrigel.
Figure 7.
Differences in transdifferentiation were enhanced when infected cells were cultured on Matrigel. Primary HSC were seeded on tissue culture plastic coated with a thin layer of Matrigel. Cells were infected with the indicated adenoviruses at day 2 after isolation, at an MOI of 800 (necessary for efficient infection of these quiescent cells). At day 9 (7 d after infection), all cells seemed phenotypically quiescent, with a rounded shape and prominent lipid droplets (note the yellow droplets in the cells), except Ad-wt3-infected cells. These cells had migrated through the Matrigel and were adherent to the underlying plastic. They demonstrated the spread out shape, prominent protrusions, and decreased lipid droplets typical of cells in the midst of transdifferentiation. Note the granularity apparent in the four smaller panels; this reflects the appearance of the surface of the Matrigel. The larger panel lacks this granular appearance because the focal plane is below the surface. Bar, 50 μm.
DISCUSSION
Our data support the following conclusions: 1) Smad2 and Smad3 have different functions in HSC, particularly in matrix protein deposition, chemotaxis, and cytoskeletal organization; 2) Smad2 and Smad3 may signal via independent pathways in HSC; and 3) Smad3 enhances focal adhesion formation and α-SMA organization in transdifferentiated HSC.
We overexpressed wild-type and dominant negative Smads2 and 3 in primary HSC using adenoviral vectors. The validity of this method is supported by the coincidence between our results with the dominant negative Smad3 and those observed with HSC from Smad3-null mice; additionally, the similarity in phenotype between TGF-β-treated cells and Smad3- (but not Smad2-) overexpressing HSC suggests that we have observed physiological responses and not the effects of Smad overexpression in general.
Our data demonstrate that Smad3 is a direct mediator of HSC matrix production and matrix interactions. Smad3 increased expression of collagen and fibronectin, without a significant effect on proliferation. Smad2, on the other hand, seemed to be primarily antiproliferative, with a minimal role in HSC fibrogenesis. Evidence from other model systems also implicates Smad3 as the dominant Smad in extracellular matrix deposition, wound healing, and fibrosis (Verrecchia et al., 2001; Flanders, 2004). Smad3 null mice are less susceptible to bleomycin-induced lung fibrosis and radiation-induced skin fibrosis than wild-type mice (Flanders et al., 2002; Zhao et al., 2002). Similarly, in the liver, collagen expression in response to an acute fibrogenic stimulus was decreased by about one-half in Smad3 null mice compared with their wild-type counterparts (Schnabl et al., 2001). A number of genes important in fibrogenesis, including several collagen genes and regulators of matrix degradation, seem to be Smad3- but not Smad2-dependent (Verrecchia et al., 2001; Yuan and Varga, 2001). Additionally, in an in vitro model of tissue fibrosis and remodeling, TGF-β augmented the fibroblast-mediated contraction of collagen gels when wild-type and Smad2 null embryonic fibroblasts, but not Smad3 null cells, were examined (Liu et al., 2003b); overexpression of Smad3 in fibroblasts augmented collagen gel contraction (Sumiyoshi et al., 2003). These findings are in contrast to one report that Smad2, not Smad3, is important in the transdifferentiation of pancreatic stellate cells, which are similar to HSC. In their study, however, Ohnishi et al. (2004) used passaged cells and thus did not evaluate the effects of the two Smads on the initial expression or organization of α-SMA; expression of matrix proteins was not addressed.
In addition to mediating matrix deposition, Smad3 may mediate other key matrix-related functions of transdifferentiated HSC, including chemotaxis and basement membrane degradation. We have shown that Smad3-overexpressing HSC migrate in response to type I collagen and a serum gradient, an in vitro observation that may be relevant to the in vivo finding that HSC migrate toward type I collagen-containing regions of injury. Monocytes from the Smad3 null mouse demonstrate impaired migration and chemotaxis, suggesting that Smad3 is a general regulator of these processes in wound healing (Ashcroft et al., 1999). We also observed that when Ad-wt3-infected cells overexpressing Smad3 were cultured on the basement membrane-like substrate Matrigel, they seemed to digest the matrix and migrate through to the plastic below. The ability to digest basement membrane may be an important pathophysiological mechanism in liver fibrosis and also may facilitate migration, as has been shown in vitro in a Transwell system (Yang et al., 2003). The protease induced by Smad3 overexpression is unknown; it may be related to a previously reported neutral metalloproteinase from HSC that degrades basement membrane collagen (Arthur et al., 1989).
Our data raise the interesting possibility that Smad2 and Smad3 signal independently, because the dominant negative of one Smad cannot antagonize the function of the other Smad. There is now increasing evidence that the Smads undergo activation in endosomal compartments, and it is conceivable that their signaling pathways are thereby physically separate (Hayes et al., 2002; Hu et al., 2002; Panopoulou et al., 2002; Di Guglielmo et al., 2003). Subcellular distribution of the type II TGF-β receptor differs markedly between quiescent and transdifferentiated HSC, suggesting a possible mechanism for differential activation of the two Smad pathways in wild-type HSC (Gaça and Wells, unpublished observations). The finding that Smad2 and Smad3 signal independently and with different effects implies that the selective inhibition of Smad3 could decrease fibrogenesis while maintaining Smad2-mediated growth inhibition, a potentially important consideration for long-term anti-TGF-β treatment.
Our most intriguing result is that Smad3 overexpression alters α-SMA organization and enhances focal adhesion formation. Interestingly, HSC overexpressing the inhibitory Smad, Smad7, had levels of α-SMA that were quantitatively equivalent to those of wild-type cells but with decreased fibrillar organization (Dooley et al., 2003). Although Dooley et al. (2003) did not report staining for focal adhesions, we would predict based on our data that the numbers and size of focal adhesions in the Smad7-expressing cells were decreased compared with wild-type. We fail, however, to observe differences between Ad-dn3-expressing cells and wild-type HSC, which we would expect if Smad7 antagonized Smad3 signaling in the same way as the dominant negative Smad3.
The mechanism whereby Smad3 alters actin organization is not clear. It has recently been reported using bone marrow mesenchymal stem cells that TGF-β decreases expression of the actin assembly protein gelsolin, resulting in decreased α-SMA organization despite equivalent α-SMA expression (Wang et al., 2004). Alternatively, there is evidence in fibroblasts that the formation of focal adhesions and the organization of α-SMA in stress fibers are interregulated processes; it is therefore possible that Smad3 regulates focal adhesion assembly that in turns regulates α-SMA organization (Hinz et al., 2003).
The organization of α-SMA into stress fibers has significant implications for HSC function. Increased stress fiber organization increases HSC contractility, a feature that may be critical in some of the local liver hemodynamic alterations in liver fibrosis. In addition, as the time-lapse photography of HSC clearly demonstrates, Ad-wt3-infected cells have different interactions with the matrix than Ad-βGal-infected cells. Although the implications of these behaviors are not completely clear, our observations suggest differences in the way cells sense the local environment, with potentially significant effects on cell phenotype and function.
A two-step model for the myofibroblastic transdifferentiation of fibroblasts has been proposed, with the first step being the mechanical tension-dependent emergence of protomyofibroblasts, which lack α-SMA but demonstrate stress fibers composed of cytoplasmic actins, and the second step being the TGF-β- and fibronectin ED-A-dependent transdifferentiation of the protomyofibroblast to an α-SMA-expressing differentiated myofibroblast (Tomasek et al., 2002). There is a significant amount of data supporting this model for fibroblasts. HSC, however, are different, because they spontaneously acquire α-SMA expression in culture. We have recently demonstrated that this is dependent on mechanical tension (Gaça and Wells, unpublished observations). In combination with the data reported here, this suggests that a modification of the model of Tomasek et al. (2002) applies to HSC (Figure 8). For HSC, as for fibroblasts, there are two phases of myofibroblastic transdifferentiation, also controlled sequentially by mechanical tension and TGF-β signaling, but the intermediate step is an α-SMA-expressing cell lacking organized stress fibers, and the final step is a differentiated myofibroblast with fully organized stress fibers. The role of fibronectin ED-A in this second step has not been determined, and the role of Smad3 in mediating its production is not known.
Figure 8.
Model for the sequential transdifferentiation of HSC. As described in the text, we propose that HSC transdifferentiate by a two-step model, with step 1 being the mechanical stiffness-dependent acquisition of α-SMA expression and step 2 being the Smad3-dependent development of focal adhesions and organization of α-SMA in stress fibers. Reversion of HSC transdifferentiation has been demonstrated for cells cultured on stiff plastic, but it has not been demonstrated in vivo or for cells after the second step in transdifferentiation.
In summary, we have demonstrated that Smad3 plays a critical role in multiple important functions of transdifferentiated HSC in vitro, including matrix deposition, chemotaxis, basement membrane degradation, contractility, and matrix interactions. Our data suggest that Smad3 is an attractive potential target for antifibrosis therapies, because specific targeting of Smad3 would enable maintenance of Smad2-mediated signaling pathways, in particular growth inhibitory pathways. Work is ongoing to determine the in vivo correlates of the Smad3-mediated behaviors we observe in vitro.
Supplementary Material
Acknowledgments
We are grateful to Min Li and Jia-Ji Hui for hepatic stellate cell isolation, to Reed Hickey for assistance with adenovirus construction, to Erick Chan for helpful discussions, to Gary Swain for technical support with microscopy, and to James E. Hayden (Microscopy Core Faculty, Wistar Institute, Philadelphia, PA) for assistance with videomicroscopy. This work was supported by Grant R01–58123 from the National Institute of Diabetes and Digestive and Kidney Diseases (to R.G.W.) and by the core facilities of the University of Pennsylvania National Institute of Diabetes and Digestive and Kidney Diseases Center for Molecular Studies in Digestive and Liver Disease (P30-DK050306). M. U. is a recipient of an American Liver Foundation 2004 Postdoctoral Research Fellowship.
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E05-02-0149) on June 29, 2005.
Abbreviations used: α-SMA, α-smooth muscle actin; βGal, β-galactosidase; HSC, hepatic stellate cells; HSP, heat shock protein; MOI, multiplicity of infection; pSmad, phosphorylated Smad; pFAK, phosphorylated FAK; TGF-β, transforming growth factor-β.
The online version of this article contains supplemental material at MBC Online (http://www.molbiolcell.org).
References
- Arias, M., Lahme, B., Van de Leur, E., Gressner, A. M., and Weiskirchen, R. (2002). Adenoviral delivery of an antisense RNA complementary to the 3′ coding sequence of transforming growth factor-β1 inhibits fibrogenic activities of hepatic stellate cells. Cell Growth Differ. 13, 265-273. [PubMed] [Google Scholar]
- Arthur, M. J., Friedman, S. L., Roll, F. J., and Bissell, D. M. (1989). Lipocytes from normal rat liver release a neutral metalloproteinase that degrades basement membrane (type IV) collagen. J. Clin. Investig. 84, 1076-1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashcroft, G. S., et al. (1999). Mice lacking Smad3 show accelerated wound healing and an impaired local inflammatory response. Nat. Cell Biol. 1, 260-266. [DOI] [PubMed] [Google Scholar]
- Becker, T. C., Noel, R. J., Coats, W. S., Gomez-Foix, A. M., Alam, T., Gerard, R. D., and Newgard, C. B. (1994). Use of recombinant adenovirus for metabolic engineering of mammalian cells. Methods Cell Biol. 43, 161-189. [DOI] [PubMed] [Google Scholar]
- Castilla, A., Prieto, J., and Fausto, N. (1991). Transforming growth factors β 1 and alpha in chronic liver disease. Effects of interferon alfa therapy. N. Engl. J. Med. 324, 933-940. [DOI] [PubMed] [Google Scholar]
- Choy, L., Skillington, J., and Derynck, R. (2000). Roles of autocrine TGF-β receptor and Smad signaling in adipocyte differentiation. J. Cell Biol. 149, 667-682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datto, M. B., Frederick, J. P., Pan, L., Borton, A. J., Zhuang, Y., and Wang, X. F. (1999). Targeted disruption of Smad3 reveals an essential role in transforming growth factor β-mediated signal transduction. Mol. Cell. Biol. 19, 2495-2504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derynck, R., Zhang, Y., and Feng, X. H. (1998). Smads: transcriptional activators of TGF-β responses. Cell 95, 737-740. [DOI] [PubMed] [Google Scholar]
- Desmouliere, A., Geinoz, A., Gabbiani, F., and Gabbiani, G. (1993). Transforming growth factor-β 1 induces alpha-smooth muscle actin expression in granulation tissue myofibroblasts and in quiescent and growing cultured fibroblasts. J. Cell Biol. 122, 103-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Guglielmo, G. M., Le Roy, C., Goodfellow, A. F., and Wrana, J. L. (2003). Distinct endocytic pathways regulate TGF-β receptor signalling and turnover. Nat. Cell Biol. 5, 410-421. [DOI] [PubMed] [Google Scholar]
- Dooley, S., Hamzavi, J., Breitkopf, K., Wiercinska, E., Said, H. M., Lorenzen, J., Ten Dijke, P., and Gressner, A. M. (2003). Smad7 prevents activation of hepatic stellate cells and liver fibrosis in rats. Gastroenterology 125, 178-191. [DOI] [PubMed] [Google Scholar]
- Fibbi, G., Pucci, M., D'Alessio, S., Grappone, C., Pellegrini, G., Salzano, R., Casini, A., Milani, S., and Del Rosso, M. (2001). Transforming growth factor β-1 stimulates invasivity of hepatic stellate cells by engagement of the cell-associated fibrinolytic system. Growth Factors 19, 87-100. [DOI] [PubMed] [Google Scholar]
- Flanders, K. C., et al. (2002). Mice lacking Smad3 are protected against cutaneous injury induced by ionizing radiation. Am. J. Pathol. 160, 1057-1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flanders, K. C. (2004). Smad3 as a mediator of the fibrotic response. Int. J. Exp. Pathol. 85, 47-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman, S. L., and Roll, F. J. (1987). Isolation and culture of hepatic lipocytes, Kupffer cells, and sinusoidal endothelial cells by density gradient centrifugation with Stractan. Anal. Biochem. 161, 207-218. [DOI] [PubMed] [Google Scholar]
- Friedman, S. L., Roll, F. J., Boyles, J., Arenson, D. M., and Bissell, D. M. (1989). Maintenance of differentiated phenotype of cultured rat hepatic lipocytes by basement membrane matrix. J. Biol. Chem. 264, 10756-10762. [PubMed] [Google Scholar]
- Friedman, S. L., Rockey, D. C., McGuire, R. F., Maher, J. J., Boyles, J. K., and Yamasaki, G. (1992). Isolated hepatic lipocytes and Kupffer cells from normal human liver: morphological and functional characteristics in primary culture. Hepatology 15, 234-243. [DOI] [PubMed] [Google Scholar]
- Friedman, S. L. (2000). Molecular regulation of hepatic fibrosis, an integrated cellular response to tissue injury. J. Biol. Chem. 275, 2247-2250. [DOI] [PubMed] [Google Scholar]
- Gabbiani, G. (2003). The myofibroblast in wound healing and fibrocontractive diseases. J. Pathol. 200, 500-503. [DOI] [PubMed] [Google Scholar]
- Gaca, M. D., Zhou, X., Issa, R., Kiriella, K., Iredale, J. P., and Benyon, R. C. (2003). Basement membrane-like matrix inhibits proliferation and collagen synthesis by activated rat hepatic stellate cells: evidence for matrix-dependent deactivation of stellate cells. Matrix Biol. 22, 229-239. [DOI] [PubMed] [Google Scholar]
- George, J., Roulot, D., Koteliansky, V. E., and Bissell, D. M. (1999). In vivo inhibition of rat stellate cell activation by soluble transforming growth factor β type II receptor: a potential new therapy for hepatic fibrosis. Proc. Natl. Acad. Sci. USA 96, 12719-12724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gressner, A. M., Weiskirchen, R., Breitkopf, K., and Dooley, S. (2002). Roles of TGF-β in hepatic fibrosis. Front. Biosci. 7, d793-d807. [DOI] [PubMed] [Google Scholar]
- Hayes, S., Chawla, A., and Corvera, S. (2002). TGF β receptor internalization into EEA1-enriched early endosomes: role in signaling to Smad2. J. Cell Biol. 158, 1239-1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heldin, C. H., Miyazono, K., and ten Dijke, P. (1997). TGF-β signalling from cell membrane to nucleus through SMAD proteins. Nature 390, 465-471. [DOI] [PubMed] [Google Scholar]
- Hellerbrand, C., Stefanovic, B., Giordano, F., Burchardt, E. R., and Brenner, D. A. (1999). The role of TGFβ1 in initiating hepatic stellate cell activation in vivo. J. Hepatol. 30, 77-87. [DOI] [PubMed] [Google Scholar]
- Hinz, B., Dugina, V., Ballestrem, C., Wehrle-Haller, B., and Chaponnier, C. (2003). α-Smooth muscle actin is crucial for focal adhesion maturation in myofibroblasts. Mol. Biol. Cell 14, 2508-2519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu, Y., Chuang, J. Z., Xu, K., McGraw, T. G., and Sung, C. H. (2002). SARA, a FYVE domain protein, affects Rab5-mediated endocytosis. J. Cell Sci. 115, 4755-4763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, C., Gaca, M. D., Swenson, E. S., Vellucci, V. F., Reiss, M., and Wells, R. G. (2003a). Smads 2 and 3 are differentially activated by transforming growth factor-β (TGF-β) in quiescent and activated hepatic stellate cells. Constitutive nuclear localization of Smads in activated cells is TGF-β-independent. J. Biol. Chem. 278, 11721-11728. [DOI] [PubMed] [Google Scholar]
- Liu, X., Wen, F. Q., Kobayashi, T., Abe, S., Fang, Q., Piek, E., Bottinger, E. P., Roberts, A. B., and Rennard, S. I. (2003b). Smad3 mediates the TGF-β-induced contraction of type I collagen gels by mouse embryo fibroblasts. Cell Motil. Cytoskeleton 54, 248-253. [DOI] [PubMed] [Google Scholar]
- Massague, J., and Wotton, D. (2000). Transcriptional control by the TGF-β/Smad signaling system. EMBO J. 19, 1745-1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakatsukasa, H., Nagy, P., Evarts, R. P., Hsia, C. C., Marsden, E., and Thorgeirsson, S. S. (1990). Cellular distribution of transforming growth factor-β 1 and procollagen types I, III, and IV transcripts in carbon tetrachloride-induced rat liver fibrosis. J. Clin. Investig. 85, 1833-1843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohnishi, H., et al. (2004). Distinct roles of Smad2-, Smad3-, and ERK-dependent pathways in transforming growth factor-β1 regulation of pancreatic stellate cellular functions. J. Biol. Chem. 279, 8873-8878. [DOI] [PubMed] [Google Scholar]
- Panopoulou, E., Gillooly, D. J., Wrana, J. L., Zerial, M., Stenmark, H., Murphy, C., and Fotsis, T. (2002). Early endosomal regulation of Smad-dependent signaling in endothelial cells. J. Biol. Chem. 277, 18046-18052. [DOI] [PubMed] [Google Scholar]
- Piek, E., Ju, W. J., Heyer, J., Escalante-Alcalde, D., Stewart, C. L., Weinstein, M., Deng, C., Kucherlapati, R., Bottinger, E. P., and Roberts, A. B. (2001). Functional characterization of transforming growth factor β signaling in Smad2- and Smad3-deficient fibroblasts. J. Biol. Chem. 276, 19945-19953. [DOI] [PubMed] [Google Scholar]
- Qi, Z., Atsuchi, N., Ooshima, A., Takeshita, A., and Ueno, H. (1999). Blockade of type β transforming growth factor signaling prevents liver fibrosis and dysfunction in the rat. Proc. Natl. Acad. Sci. USA 96, 2345-2349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roulot, D., Sevcsik, A. M., Coste, T., Strosberg, A. D., and Marullo, S. (1999). Role of transforming growth factor β type II receptor in hepatic fibrosis: studies of human chronic hepatitis C and experimental fibrosis in rats. Hepatology 29, 1730-1738. [DOI] [PubMed] [Google Scholar]
- Sanderson, N., Factor, V., Nagy, P., Kopp, J., Kondaiah, P., Wakefield, L., Roberts, A. B., Sporn, M. B., and Thorgeirsson, S. S. (1995). Hepatic expression of mature transforming growth factor β 1 in transgenic mice results in multiple tissue lesions. Proc. Natl. Acad. Sci. USA 92, 2572-2576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnabl, B., Kweon, Y. O., Frederick, J. P., Wang, X. F., Rippe, R. A., and Brenner, D. A. (2001). The role of Smad3 in mediating mouse hepatic stellate cell activation. Hepatology 34, 89-100. [DOI] [PubMed] [Google Scholar]
- Schnabl, B., Choi, Y. H., Olsen, J. C., Hagedorn, C. H., and Brenner, D. A. (2002). Immortal activated human hepatic stellate cells generated by ectopic telomerase expression. Lab Invest 82, 323-333. [DOI] [PubMed] [Google Scholar]
- Souchelnytskyi, S., Tamaki, K., Engstrom, U., Wernstedt, C., ten Dijke, P., and Heldin, C. H. (1997). Phosphorylation of Ser465 and Ser467 in the C terminus of Smad2 mediates interaction with Smad4 and is required for transforming growth factor-β signaling. J. Biol. Chem. 272, 28107-28115. [DOI] [PubMed] [Google Scholar]
- Sumiyoshi, K., Nakao, A., Setoguchi, Y., Okumura, K., Tsuboi, R., and Ogawa, H. (2003). Smads regulate collagen gel contraction by human dermal fibroblasts. Br. J. Dermatol. 149, 464-470. [DOI] [PubMed] [Google Scholar]
- Tomasek, J. J., Gabbiani, G., Hinz, B., Chaponnier, C., and Brown, R. A. (2002). Myofibroblasts and mechano-regulation of connective tissue remodelling. Nat. Rev. Mol. Cell. Biol. 3, 349-363. [DOI] [PubMed] [Google Scholar]
- Ueno, H., Sakamoto, T., Nakamura, T., Qi, Z., Astuchi, N., Takeshita, A., Shimizu, K., and Ohashi, H. (2000). A soluble transforming growth factor β receptor expressed in muscle prevents liver fibrogenesis and dysfunction in rats. Hum. Gene Ther. 11, 33-42. [DOI] [PubMed] [Google Scholar]
- Verrecchia, F., Chu, M. L., and Mauviel, A. (2001). Identification of novel TGF-β/Smad gene targets in dermal fibroblasts using a combined cDNA microarray/promoter transactivation approach. J. Biol. Chem. 276, 17058-17062. [DOI] [PubMed] [Google Scholar]
- Wang, D., Park, J. S., Chu, J. S., Krakowski, A., Luo, K., Chen, D. J., and Li, S. (2004). Proteomic profiling of bone marrow mesenchymal stem cells upon transforming growth factor β1 stimulation. J. Biol. Chem. 279, 43725-43734. [DOI] [PubMed] [Google Scholar]
- Weinstein, M., Yang, X., Li, C., Xu, X., Gotay, J., and Deng, C. X. (1998). Failure of egg cylinder elongation and mesoderm induction in mouse embryos lacking the tumor suppressor smad2. Proc. Natl. Acad. Sci. USA 95, 9378-9383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells, R. G. (2000). Fibrogenesis. V. TGF-β signaling pathways. Am. J. Physiol. 279, G845-G850. [DOI] [PubMed] [Google Scholar]
- Yang, C., Zeisberg, M., Mosterman, B., Sudhakar, A., Yerramalla, U., Holthaus, K., Xu, L., Eng, F., Afdhal, N., and Kalluri, R. (2003). Liver fibrosis: insights into migration of hepatic stellate cells in response to extracellular matrix and growth factors. Gastroenterology 124, 147-159. [DOI] [PubMed] [Google Scholar]
- Yang, X., Letterio, J. J., Lechleider, R. J., Chen, L., Hayman, R., Gu, H., Roberts, A. B., and Deng, C. (1999). Targeted disruption of SMAD3 results in impaired mucosal immunity and diminished T cell responsiveness to TGF-β. EMBO J. 18, 1280-1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yata, Y., Gotwals, P., Koteliansky, V., and Rockey, D. C. (2002). Dose-dependent inhibition of hepatic fibrosis in mice by a TGF-β soluble receptor: implications for antifibrotic therapy. Hepatology 35, 1022-1030. [DOI] [PubMed] [Google Scholar]
- Yu, Q., Que, L. G., and Rockey, D. C. (2002). Adenovirus-mediated gene transfer to nonparenchymal cells in normal and injured liver. Am. J. Physiol. 282, G565-G572. [DOI] [PubMed] [Google Scholar]
- Yuan, W., and Varga, J. (2001). Transforming growth factor-β repression of matrix metalloproteinase-1 in dermal fibroblasts involves Smad3. J. Biol. Chem. 276, 38502-38510. [DOI] [PubMed] [Google Scholar]
- Zhao, J., Shi, W., Wang, Y. L., Chen, H., Bringas, P., Jr., Datto, M. B., Frederick, J. P., Wang, X. F., and Warburton, D. (2002). Smad3 deficiency attenuates bleomycin-induced pulmonary fibrosis in mice. Am. J. Physiol. 282, L585-L593. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.