Abstract
The ArfGAP paxillin kinase linker (PKL)/G protein-coupled receptor kinase-interacting protein (GIT)2 has been implicated in regulating cell spreading and motility through its transient recruitment of the p21-activated kinase (PAK) to focal adhesions. The Nck-PAK-PIX-PKL protein complex is recruited to focal adhesions by paxillin upon integrin engagement and Rac activation. In this report, we identify tyrosine-phosphorylated PKL as a protein that associates with the SH3-SH2 adaptor Nck, in a Src-dependent manner, after cell adhesion to fibronectin. Both cell adhesion and Rac activation stimulated PKL tyrosine phosphorylation. PKL is phosphorylated on tyrosine residues 286/392/592 by Src and/or FAK and these sites are required for PKL localization to focal adhesions and for paxillin binding. The absence of either FAK or Src-family kinases prevents PKL phosphorylation and suppresses localization of PKL but not GIT1 to focal adhesions after Rac activation. Expression of an activated FAK mutant in the absence of Src-family kinases partially restores PKL localization, suggesting that Src activation of FAK is required for PKL phosphorylation and localization. Overexpression of the nonphosphorylated GFP-PKL Triple YF mutant stimulates cell spreading and protrusiveness, similar to overexpression of a paxillin mutant that does not bind PKL, suggesting that failure to recruit PKL to focal adhesions interferes with normal cell spreading and motility.
INTRODUCTION
Cell attachment, spreading, and motility are complex processes requiring the integration of diverse signaling networks and structural assemblies (Jockusch et al., 1995; Sastry and Burridge, 2000; Juliano, 2002). One of the earliest steps in transducing extracellular cues through integrins to the cytoskeleton is the activation of the tyrosine kinases Src and FAK (Schwartz et al., 1995; Giancotti and Tarone, 2003). FAK is an integrin-binding nonreceptor tyrosine kinase that upon integrin ligation is activated to autophosphorylate Y397 and bind to the Src SH2 domain (Schaller et al., 1994; Calalb et al., 1995). Src then phosphorylates FAK on multiple residues that increase FAK kinase activity and several downstream binding partners for Src/FAK are targeted for phosphorylation, including the focal adhesion proteins p130Cas and paxillin (reviewed in Brown and Turner, 2004; Playford and Schaller, 2004; Mitra et al., 2005). Phosphorylated paxillin binding to the SH2/SH3 adaptor protein Crk is implicated in Rac activation and stimulation of cell motility (Petit et al., 2000; Lamorte et al., 2003; Valles et al., 2004), whereas binding of paxillin to the p120RasGAP SH2 domain may displace and allow for the activation of p190RhoGAP and subsequent decrease in RhoA activity (Iwasaki et al., 2002).
The Cdc42, Rac1, and RhoA members of the Ras superfamily of p21 GTPases are central intermediaries in coordinating the defined temporal-spatial progression of signals emanating from initial integrin ligation, through acquisition of a polarized state, to initiation and maintenance of directed cellular migration. Downstream effector proteins that bind to the activated GTP-bound form of p21 GTPases propagate the signals in response to cellular stimulation. The p21 GTPases themselves are regulated by guanine nucleotide exchange factors (GEFs) that turn them on, GTPase activating proteins (GAPs) that turn them off, and guanine nucleotide dissociation inhibitors that can function as sequestering agents (Suetsugu and Takenawa, 2003; Burridge and Wennerberg, 2004; Raftopoulou and Hall, 2004). The activities of many of these proteins have been found to be regulated by FAK and Src-family kinases (Hildebrand et al., 1996; Han et al., 1997; Kiyono et al., 2000; Haskell et al., 2001; Tu et al., 2003; Zhai et al., 2003; Stacey et al., 2004).
The pathways of activation and signaling from the p21-activated protein kinase (PAK) typify the sophisticated mechanisms developed by cells to respond to extracellular cues (Bokoch, 2003). In response to cell attachment and/or growth factor stimulation, PAK is activated and signals to sites of cell-matrix interaction called focal adhesions, the cytoskeleton as well as the nucleus to promote appropriate changes in cell function (Manser and Lim, 1999). Several proteins have been shown to contribute to PAK activation and function within the cell through regulation of its subcellular distribution, including the SH3-SH2 adaptor protein Nck (Bokoch et al., 1996; Galisteo et al., 1996; Lu et al., 1997; Sells et al., 1997; Kiosses et al., 2002), the Cdc42/Rac GEF PIX (Bagrodia et al., 1998; Zhao et al., 2000a), the paxillin kinase linker (PKL)/G protein-coupled receptor kinase-interacting protein (GIT) family of ArfGAPs (Bagrodia et al., 1999; Premont et al., 2000; Brown et al., 2002; Manabe Ri et al., 2002), and the molecular scaffold protein paxillin (Turner et al., 1999; Zhao et al., 2000b; Hashimoto et al., 2001; West et al., 2001; Brown et al., 2002).
A complex of Nck-PAK-PIX-PKL exists within the cytosol (Turner et al., 1999; Zhao et al., 2000b) and/or endomembranes (Matafora et al., 2001; Paris et al., 2003). On cell stimulation, PAK undergoes and promotes conformation changes that induce association of the complex with paxillin (Turner et al., 1999; Premont et al., 2000; Zhao et al., 2000b; Brown et al., 2002; Manabe Ri et al., 2002). As a result, the complex is recruited to RhoA-type focal adhesions, where PAK is thought to trigger turnover (Zhao et al., 2000b) and also to nascent Cdc42/Rac adhesions at the leading edge, called focal complexes, where it may mediate maturation and transition to RhoA adhesions (Brown et al., 2002; Manabe Ri et al., 2002). Synchronization of adhesion formation and turnover is critical to the process of cell motility (Webb et al., 2004), and perturbation in the PKL-paxillin link results in enhanced spreading, combined with a significant inhibition of cell polarity, focal adhesion dynamics, and motility (Turner et al., 1999; Zhao et al., 2000b; West et al., 2001; Brown et al., 2002; Manabe Ri et al., 2002; Webb et al., 2004), in part through the misregulation of Rac (West et al., 2001). Similarly, fibroblasts derived from mice with germ-line deletions in Src/Yes/Fyn (SYF), FAK, paxillin, and Nck exhibit profound defects in cytoskeletal organization and the capacity to efficiently migrate, providing concrete evidence for the essential nature of these associations and signaling pathways (Ilic et al., 1995; Klinghoffer et al., 1999; Hagel et al., 2002; Bladt et al., 2003; Webb et al., 2004).
We have sought to further characterize the mechanism(s) by which the Nck-PAK-PIX-PKL complex becomes activated and competent to localize to focal adhesions and modulate adhesion and motility signaling. Protein phosphorylation is a common means of regulating protein function through allosteric modification as well as providing inducible protein recognition motifs (Cohen et al., 1995; Hubbard and Till, 2000). In fact, phosphorylation of paxillin, PAK, and PIX has been shown to modulate their activity, protein associations, and/or subcellular localization (Brown et al., 1998a; Zhao et al., 2000a; Howe, 2001; Zhou et al., 2003; Chahdi et al., 2005). We have identified PKL as a protein that is tyrosine phosphorylated and associates with Nck and Src after cell adhesion to fibronectin and in response to active Rac. Src and FAK cooperate to phosphorylate PKL, an event required for Rac1-dependent PKL localization to focal adhesions. Three major sites of PKL phosphorylation (Y286/392/592) were identified, and we show that their mutation inhibits PKL localization to focal adhesions in normal mouse embryo fibroblast (MEF) cells and also prevents adhesion-induced binding to paxillin. Furthermore, PKL is not tyrosine phosphorylated and fails to localize to focal adhesions efficiently in SYF and FAK null fibroblasts. Overexpression of the nontargeting PKL Triple YF mutant protein potentiates cell spreading and stimulates cell protrusiveness consistent with a critical role for PKL function at focal adhesions in regulating normal cell spreading and motility.
MATERIALS AND METHODS
Plasmids and Antibodies
Plasmids encoding avian PKL wild-type (WT; 1-757 amino acids), N terminus (1-576), C terminus (448-757), ArfGAP defective (R39A), SpaII homology domain (SHD) deletion (ΔSHD1; deletion 266-296), paxillin-binding subdomain (PBS) deletion (ΔPBS2; deletion 643-679), Y286F, Y392F, Y592F, and Triple YF (Y286/392/592F) were cloned into pEGFPC1 (BD Biosciences Clontech, Palo Alto, CA). pEGFPC1-paxillin WT (1-559) and ΔLD4 (deletion 263-282) have been described previously (West et al., 2001). T7-tagged G12VRac1 was a generous gift from Linda Van Aelst (Cold Spring Harbor Laboratory, Cold Spring, NY), pKH3 HA-FAK WT and kinase dead K454R were kindly provided by Jun-Lin Guan (Cornell University, Ithaca, NY), and pEGFPC1 GFP-GIT1 was provided by Rick Horwitz (University of Virginia, Charlottesville, VA). pLXSH WT Src and mutants were as described previously (Cary et al., 2002). cDNAs encoding WT Csk and p130Cas cDNAs were provided by Akira Imamoto (University of Chicago, Chicago, IL), WT Abl by Jean Wang (University of California, San Diego, CA), WT Nck from Bruce Mayer (University of Connecticut, Storrs, CT), WT PTP-PEST from Michel Tremblay (McGill University, Montreal, Quebec, Canada). SuperFAK K578/581E (Gabarra-Niecko et al., 2002) and PKL mutants were generated by QuikChange mutagenesis (Stratagene, La Jolla, CA) and sequenced in their entirety at the SUNY Upstate DNA Core Facility (Syracuse, NY).
Anti-PKL and anti-Hic-5 monoclonal antibodies were provided by BD Transduction Laboratories (Lexington, KY), anti-hemagglutinin (HA) monoclonal clone 12CA5 was obtained from Covance (Berkeley, CA), anti-Nck and anti-phosphotyrosine 4G10 were from Upstate Biotechnology (Charlottesville, VA), IgG-purified rabbit anti-green fluorescent protein (GFP) (Molecular Probes), and anti-T7 monoclonal was from Affinity Bioreagents (Golden, CO).
Cell Lines
All cells were cultured in DMEM containing 10% (vol/vol) certified fetal bovine serum (Atlas Biologicals, Norcross, GA), 50 U/ml penicillin, 50 μg/ml streptomycin and kanamycin (complete medium) at 37°C in a humidified chamber with 5% CO2. Normal mouse embryo fibroblasts (MEF) and paxillin null (Hagel et al., 2002), SYF null (Klinghoffer et al., 1999), and FAK null cells (Furuta et al., 1995) have been described previously. Nck1/2 null cells were provided by Tony Pawson (Samuel Lunenfeld Research Institute) (Bladt et al., 2003), Csk and p130Cas null cells were from Akira Imamoto (Imamoto and Soriano, 1993; Honda et al., 1998), Abl null cells from Jean Wang (Hardin et al., 1996) and PTP-PEST null cells from Michel Tremblay (Angers-Loustau et al., 1999). Human embryonic kidney (HEK)293A cells (AD-HEK) were from Stratagene, and FAK null cells were obtained from American Type Culture Collection (Manassas, VA).
For characterization of Src function in PKL phosphorylation, pooled clones of SYF cells expressing pLXSH vector only, and WT Src or Src mutants were generated by retroviral infection and selection with 0.4 mg/ml hygromycin B and used as described previously (Cary et al., 2002).
Immunoprecipitation and Pull-Down Assays
For anti-Nck and anti-phosphotyrosine 4G10 coimmunoprecipitation experiments, cells were placed in suspension and maintained for 1 h or replated on 5 μg/ml fibronectin-coated dishes for 20 min or the time indicated. Cells were washed in ice-cold phosphate-buffered saline followed by lysis in Triton buffer (50 mM Tris-HCl, pH 7.4, 150 mM NaCl, 10 mM MgCl2, 1 mM EDTA 1% Triton X (TX)-100, 10% glycerol, 20 μg/ml aprotinin, 10 μg/ml leupeptin, 1 mM phenylmethylsulfonyl fluoride [PMSF], and 0.2 mM sodium vanadate). After clarification at 21,000 × g for 10 min at 4°C, supernatants were taken as cell lysates. Immunoprecipitations were performed by incubating 250 μg of cell lysate end-over-end with the indicated primary antibody for 3 h at 4°C before adding protein A/G agarose beads (Santa Cruz Biotechnology, Santa Cruz, CA) for 1 h at 4°C. For anti-GFP (purified IgG; Molecular Probes, Eugene, OR) immunoprecipitation, null cells transiently transfected were lysed using radioimmunoprecipitation buffer (50 mM Tris-HCl, pH 7.4, 150 mM NaCl, 2 mM EDTA 1% TX-100, 1% sodium deoxycholate, 0.1% SDS, 10% glycerol, 20 μg/ml aprotinin, 10 μg/ml leupeptin, 1 mM PMSF, and 0.2 mM sodium vanadate) and then processed as described above. For paxillin coprecipitation, transiently transfected Chinese hamster ovary (CHO).K1 or HEK293A cells were replated on 10 μg/ml fibronectin for 60 min before processing. Immunoprecipitates were washed extensively with lysis buffer then prepared for SDS-PAGE analysis by bringing to 1× with dithiothreitol-based sample solubilization buffer containing sodium vanadate, and then proteins were separated on 10% polyacrylamide gels. After transfer to nitrocellulose, proteins were detected by standard Western immunoblotting procedures using enhanced chemiluminescence (Amersham Biosciences, Piscataway, NJ).
Glutathione S-transferase (GST)-fusion proteins were expressed and purified onto glutathione-Sepharose 4B beads (Amersham Biosciences) as described previously (Morris et al., 2002) with pull-down assays performed using 250 μg Triton lysis buffer cell lysates and 5 μg of GST or GST-fusion. After 2 h of sample incubation end-over-end at 4°C, proteins specifically bound to the GST-fusion coupled to the Sepharose beads were washed extensively with lysis buffer and processed as described above.
Fluorescence Microscopy
Cells were plated overnight onto ethanol washed no. 1 glass coverslips (Assistant, Sondheim, Germany) that had been coated with 10 μg/ml human plasma fibronectin (Sigma, St. Louis, MO) and blocked with bovine serum albumin (BSA). Next, cells were transfected overnight using FuGene6 (Roche Diagnostics, Indianapolis, IN) at a 3:1 ratio with 0.5 μg of the indicated plasmids and pcDNA3LacZ cDNA as necessary to normalize DNA content. The coverslips were fixed for 8 min with 3.7% formaldehyde in phosphate-buffered saline, washed for 10 min in Tris-buffered saline (TBS), and permeabilized for 2 min in 0.2% Triton X-100 in TBS. After washing for 10 min in TBS, focal adhesions were labeled for 1 h using a primary monoclonal antibody to Hic-5 (BD Biosciences, Palo Alto, CA) diluted in TBS containing 1% BSA. Next, the coverslips were incubated with a secondary donkey anti-mouse tetramethylrhodamine B isothiocyanate (TRITC) conjugate (Jackson ImmunoResearch Laboratories, West Grove, PA) and/or actin filaments were decorated with Alexa350- or TRITC-phalloidin (Molecular Probes) for 30 min at 37°C in TBS. Coverslips were then mounted in Gelvatol (AirProducts, Allentown, PA) and allowed to set overnight at 4°C.
Fluorescence was evaluated using a Nikon E800 fitted with a 60× oil immersion objective and photomicrographs were captured using a Hamamatsu Orca ER charge-coupled device (CCD) camera and Compix SimplePCI automated image capture software. Images were processed using Compix SimplePCI and Photoshop 6.0 (Adobe Systems, Mountain View, CA). For quantitation of GFP-PKL focal adhesion localization, 100-150 cotransfected cells were counted in at least three independent experiments.
Cell Spreading and Protrusion
For analyses related to cellular adhesion dynamics, 7.5 × 104 normal MEF cells were plated overnight in 35-mm dishes followed by transfection overnight using Metafectene (Biontex, Munich, Germany) at a 3:1 ratio with 1 μg of the indicated plasmids. Cells were placed in suspension using a PBS-1 mM EDTA solution with 0.025% trypsin and then transferred to complete medium containing 0.025% trypsin inhibitor and pelleted. After washing with DMEM containing 0.1% BSA, the cells were resuspended in the same medium at 100,000 cells/ml and maintained in suspension for 1 h. Next, 50,000 cells were plated into 35-mm dishes that had been coated with 5 μg/ml fibronectin and blocked with BSA. For cell spreading, cells were washed 10 min postattachment into prewarmed time-lapse medium (DMEM minus phenol red or sodium bicarbonate, supplemented with 25 mM HEPES, pH 7.5, and 0.1% BSA). The dishes were overlaid with mineral oil and then placed in a Harvard Apparatus PDMI 35-mm dish microincubation chamber mounted on a Nikon E600 and maintained at 37°C. GFP-expressing transfected cells were quickly identified by fluorescence and Hoffman Modulation Contrast time-lapse images were captured every 60 s for 2 h using a 20× extra-long working distance objective, Compix SimplePCI software and a Spot RT CCD camera. Average changes in cell area during 1 h of cell spreading were based on measurements of 25 cells.
For protrusion analysis, cells were processed as described above; however, to minimize the effect of cell spreading on apparent protrusiveness, cells were plated for ∼3 h before transfer to the microincubation chamber. Images were captured every 60 s for up to 3 h. Cell protrusion was quantitated spanning 1 h at 10-min intervals as reported previously (West et al., 2001; Kinley et al., 2003). To quantitate percentage of change in cell area due to extension of protrusions, two still images representing one 10-min interval were extracted, registered, thresholded, and subtracted to estimate the new area. These protruding areas were quantitated as percentage of cell protrusion area and averaged between 10 cells. Statistical analysis was performed using a paired Student's t test.
RESULTS
Identification of a 95-kDa Tyrosine Phosphorylated Nck-binding Protein
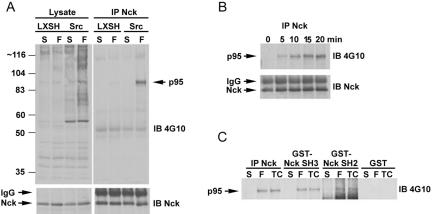
The SH3-SH3-SH3-SH2 adaptor protein Nck is a major downstream mediator of extracellular matrix and growth factor receptor signaling to the cytoskeleton through its ability to associate with receptor tyrosine kinases, PAK, and the WASP/WAVE complex (Buday et al., 2002). We sought to identify tyrosine phosphorylated proteins that associate with Nck, in a Src-dependent manner, after cell adhesion to fibronectin and establish their contribution to cell spreading and motility. MEF cells derived from SYF null mice (Klinghoffer et al., 1999) were reconstituted with either vector (pLXSH) or WT Src and left in suspension or replated on fibronectin-coated dishes for 15 min followed by lysis and anti-Nck immunoprecipitation. Immunoblotting with anti-phosphotyrosine 4G10 antibody revealed a major target of ∼95 kDa that was significantly enriched in Src-replete cells compared with vector (LXSH) rescued SYF cells (Figure 1A). The precipitation of the 95-kDa protein correlated with cell spreading, detectable within 5 min and increasing up to 20 min (Figure 1B). Furthermore, the 95-kDa protein was precipitated by GST-Nck SH2 and SH3 domains in addition to coimmunoprecipitating with anti-Nck antibody (Figure 1C), suggesting a multivalent interaction.
Figure 1.
Identification of a 95-kDa tyrosine phosphorylated protein that associates with Nck in a Src- and adhesion-dependent manner. (A) SYF null cells and SYF + Src replete cells were maintained in suspension or replated on fibronectin-coated dishes followed by anti-Nck immunoprecipitation (IP) and immunoblotting (IB) with anti-phosphotyrosine 4G10 antibody or anti-Nck antibody. A major adhesion induced tyrosine phosphorylated 95-kDa Nck binding partner was identified that was significantly enriched in Src replete versus vector control (LXSH) SYF null cells. (B) SYF + Src cells were plated on 5 μg/ml fibronectin for the indicated times followed by Nck IP and 4G10 anti-phosphotyrosine IB, demonstrating the phos-pho-p95 association with Nck correlates with cell spreading. (C) The tyrosine-phosphorylated p95 protein is precipitated by GST-Nck SH2 and SH3 domains but not GST.
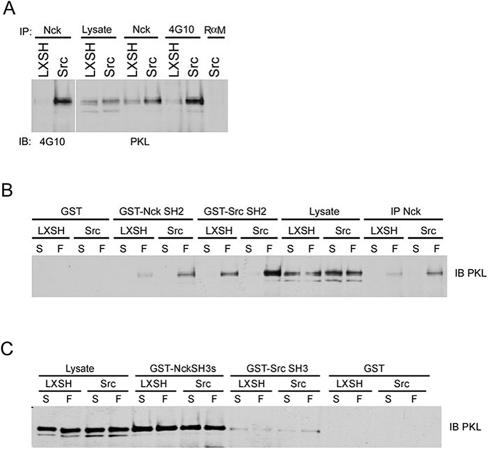
We used GST-Nck SH3 pull downs and mass spectrometry to identify the 95-kDa protein. A total of six independent PKL/GIT2 peptides were identified, accounting for 56 amino acids (approx 7% of PKL by sequence; our unpublished data). To confirm that PKL/GIT2 was the 95-kDa protein, anti-Nck, and anti-phosphotyrosine 4G10 immunoprecipitates were probed with anti-PKL antibodies (Figure 2A). Indeed, PKL was precipitated by the Nck and anti-phosphotyrosine antibodies. In addition, PKL was enriched in immunoprecipitates from SYF/c-Src replete cells relative to SYF null cells and migrated at the same size as the tyrosine phosphorylated Nck-associated anti-4G10 immunoreactive target (Figure 2A). We also found that PKL could associate with the Nck SH2 domain as well as the Src SH2 domain (Figure 2B) in an adhesion-dependent manner, consistent with the identity of the major Nck-associated phosphoprotein as PKL. Finally, PKL is precipitated by the Nck SH3 domain (and less effectively by the Src SH3 domain) (Figure 2C), although in this case the interaction is not influenced by adhesion.
Figure 2.
Tyrosine phosphorylated PKL binds to Nck and Src. (A) Cultured SYF vector control and Src replete MEFs were lysed followed by anti-Nck or anti-phosphotyrosine 4G10 IP and anti-PKL or 4G10 immunoblotting (IB) revealing PKL is tyrosine phosphorylated and coimmunoprecipitates with Nck. (B) PKL binds to the Nck and Src SH2 domains in an adhesion-dependent manner. SYF or SYF + Src cells were maintained in suspension or replated on fibronectin for 20 min followed by anti-Nck immunoprecipitation (IP), incubation with GST, GST-NckSH2, or GST-SrcSH2 followed by PKL IB. (C) PKL can also bind constitutively to the SH3 domains of Nck and Src.
Identification of the Principal Sites of PKL Phosphorylation and Their Requirement for PKL Focal Adhesion Localization
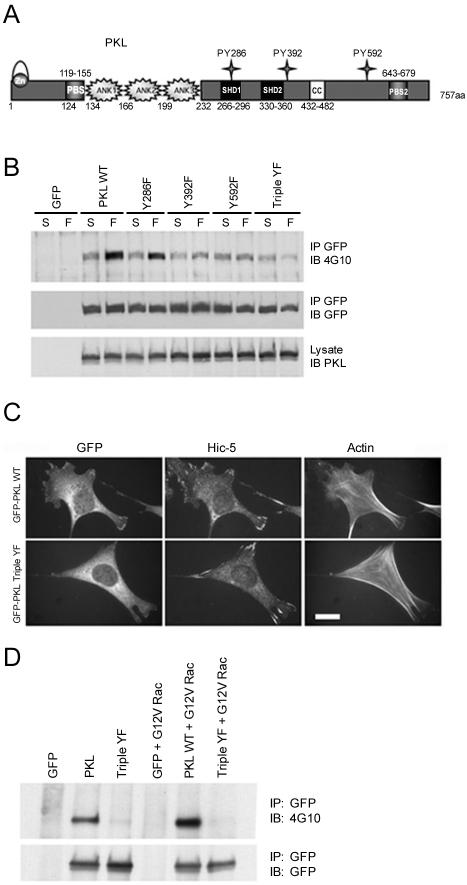
Sequence gazing was used to identify likely candidates for residues that could function in Nck and Src SH2 binding (Songyang et al., 1993). Amino acids Y286 (YDEV), Y392 (YDSV), and Y592 (YDNT) are conserved across species in both GIT1 and PKL/GIT2 (our unpublished data). These three residues of PKL were targeted for mutagenesis followed by expression in the context of GFP-PKL, and tyrosine phosphorylation after cell attachment to fibronectin was analyzed (Figure 3A). Elimination of Y286 resulted in a modest reduction in PKL phosphorylation, whereas mutation of either Y392 or Y592 significantly reduced the adhesion-induced tyrosine phosphorylation (Figure 3B). Mutation of all three sites (Triple YF) resulted in a loss of adhesion-stimulated phosphorylation, indicating these were the major targets of tyrosine phosphorylation (Figure 3B).
Figure 3.
Identification of PKL amino acid sites of phosphorylation and their requirement for PKL localization to focal adhesions. (A) The PKL family of Arf-GAP domain-containing proteins is characterized by an amino-terminal consensus zinc-finger Arf-GAP domain followed by three ankyrin repeat elements. PKL also has two consensus PBSs of which PBS2 has been demonstrated to bind paxillin, two SHDs that may mediate FAK binding, and a coiled-coil (CC) motif that may be involved in dimerization. The potential phosphorylation of three tyrosine residues (286/392/592) that conform with SH2-binding consensus prerequisites was evaluated. (B) SYF + Src cells expressing GFP-PKL tyrosine to phenylalanine point mutants were maintained in suspension or replated on fibronectin for 20 min followed by GFP IP and anti-phosphotyrosine immunoblotting (IB) and reprobing with anti-GFP for loading control. (C) PKL tyrosine phosphorylation is required for localization to focal adhesions. Normal MEFs were plated on fibronectin followed by transfection with GFP-PKL or GFP-PKL TripleYF and constitutively active G12V Rac1. GFP-PKL TripleYF is unable to localize efficiently to focal adhesions (only 25% of transfectants) unlike wild-type GFP-PKL (87% of transfectants). The existence of focal adhesions in GFP-PKL TripleYF transfectants was confirmed by double-labeling with anti-Hic-5. Actin organization was revealed by Alexa350 phalloidin staining. Bar, 20 μm. (D) Active Rac induces tyrosine phosphorylation of GFP-PKL but not the GFP-PKL Triple YF mutant. HEK cells were transfected with GFP-PKL or GFP-PKL Triple YF with or without active G12V Rac. The PKL constructs were precipitated with an anti-GFP antibody and blotted for phosphotyrosine by using 4G10.
PKL localization to focal adhesions is tightly regulated, occurring after cell adhesion to fibronectin (Turner et al., 1999) and activation of Cdc42 and Rac1 but not RhoA (Brown et al., 2002). To test whether adhesion-stimulated tyrosine phosphorylation of PKL contributes to its focal adhesion localization, GFP-PKL WT or GFP-PKL Y286/392/592F (Triple YF) cDNAs were introduced into normal MEF cells with constitutively active G12V Rac1. Consistent with previous reports, WT PKL localized efficiently to focal adhesions in 87 ± 4% of transfected cells (Figure 3C). Significantly, PKL Triple YF was unable to localize efficiently. GFP-PKL Triple YF localized in only 25 ± 3.5% of transfected cells providing compelling evidence PKL tyrosine phosphorylation is critical for its distribution and function. Nonphosphorylatable PKL Triple YF mutant also failed to localize efficiently to focal adhesions in NIH3T3 and CHO.K1 cells (our unpublished data), indicating PKL tyrosine phosphorylation is a general mechanism of regulating PKL localization.
Importantly, in addition to stimulating focal adhesion targeting of PKL, cotransfection with active G12V Rac also resulted in an increase in the tyrosine phosphorylation of GFP-PKL. In contrast, no phosphorylation of the nontargeting GFP-PKL Triple YF mutant was observed (Figure 3D).
PKL Tyrosine Phosphorylation Is Required for Paxillin Binding
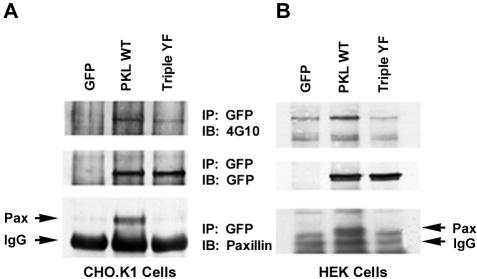
We have previously reported that PKL recruitment to focal adhesions during cell spreading and in response to Rac activation is dependent on an interaction with the LD4 motif of paxillin (West et al., 2001). To evaluate the importance of PKL tyrosine phosphorylation in regulating the interaction with paxillin, CHO.K1 cells were transiently transfected with GFP-PKL and GFP-PKL Triple YF mutant. The cells were then replated on fibronectin for 60 min and the PKL recovered by GFP-immunoprecipitation. Western blotting with 4G10 confirmed that tyrosine phosphorylation of WT PKL but not the Triple YF mutant was stimulated in response to adhesion. Importantly, paxillin only coprecipitated with the WT PKL (Figure 4A). This experiment was repeated in HEK293A cells and yielded similar results (Figure 4B). Together, these results suggest that adhesion and Rac induced tyrosine phosphorylation of PKL (Figure 3) is required for functional unmasking of the paxillin binding site, which in turn is necessary for PKL recruitment to focal adhesions.
Figure 4.
Tyrosine phosphorylation of PKL is required for binding to paxillin. (A) GFP-PKL or the GFP-PKL Triple YF mutant were expressed in CHO.KI cells and plated on FN for 60 min and then immunoprecipitated with anti-GFP antibody. The precipitates were subjected to anti-phosphotyrosine 4G10, GFP, and paxillin immunoblotting (IB). Only the tyrosine phosphorylated GFP-PKL coprecipitated with paxillin, consistent with a requirement for tyrosine phosphorylation of PKL in focal adhesion targeting via an interaction with Paxillin. (B) The experiment was repeated in HEK293A cells with similar results.
Relationship of PKL Structure/Function for Phosphorylation
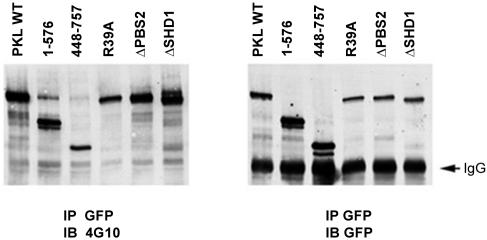
PKL is a multidomain protein including a catalytic ArfGAP domain, three ankyrin repeats, two SHD sequences, a dimerization motif, and two PBS sequences, of which the carboxyl-terminal PBS mediates its subcellular localization (Turner et al., 1999) (Figure 3A). The requirement for the various PKL domains for its tyrosine phosphorylation was examined using GFP-PKL mutant proteins transiently transfected into HEK293A cells that had been replated on fibronectin-coated dishes for 20 min. GFP-PKL was immunoprecipitated and analyzed using anti-phosphotyrosine 4G10 immunoblotting. Full-length WT PKL was efficiently phosphorylated (Figure 5). The PKL amino terminus (1-576), that is unable to localize to focal adhesions (Brown et al., 2002), as well as the targeting-competent carboxy terminus (448-757), were each phosphorylated. In addition, a mutation eliminating ArfGAP activity (R39A) failed to block PKL phosphorylation (Figure 5). Last, PKLΔPBS2 (643-679), in which the paxillin binding site is deleted and focal adhesion localization eliminated (West et al., 2001; Brown et al., 2002), was phosphorylated. These results show that PKL tyrosine phosphorylation does not require subcellular localization to focal adhesions and that PKL tyrosine phosphorylation in the absence of a functional paxillin binding site is not sufficient for localization. They further show that lack of tyrosine phosphorylation of Triple YF mutant PKL causes absence from focal adhesions rather than vice versa
Figure 5.
PKL targeting to focal adhesions is not required for tyrosine phosphorylation. GFP-PKL constructs were expressed in HEK293A cells followed by replating on fibronection, GFP immunoprecipitation (IP) and anti-phosphotyrosine 4G10 immunoblotting (IB). The nontargeting amino terminus (1-576) and PKLΔPBS2 were phosphorylated demonstrating localization to focal adhesions is not essential for phosphorylation.
PKL Tyrosine Phosphorylation Is Dependent on Src and FAK
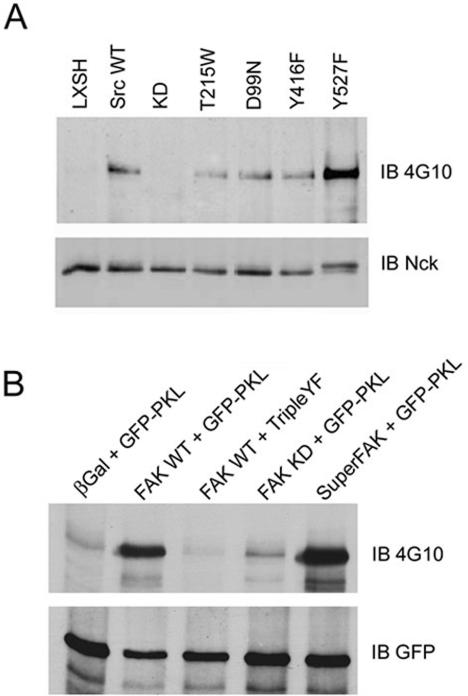
Efficient adhesion-induced PKL tyrosine phosphorylation is clearly Src-dependent (Figure 1). However, Src has both scaffold and kinase functions (Kaplan et al., 1994, 1995; Fincham and Frame, 1998; Cary et al., 2002; Li et al., 2002). To identify the basis of the Src requirement, we introduced a variety of Src mutants into SYF null cells followed by analysis of PKL tyrosine phosphorylation and association with Nck (Figure 6A). Reintroduction of kinase dead K259R (KD) Src failed to rescue the SYF defect demonstrating Src kinase activity is essential for PKL phosphorylation. However, expression of Src kinase competent SH2 (T215W) and SH3 (D99N) domain mutants and the Src activation loop mutant (Y416F) mutant that retains basal kinase functionality (Cary et al., 2002) restored PKL phosphorylation. Finally, constitutively active Src (Y527F) demonstrated a significant potentiation in PKL phosphorylation (Figure 6A).
Figure 6.
PKL-Nck association is dependent upon Src and FAK. (A) Nck immunoprecipitates from SYF cells expressing Src mutants were prepared followed by 4G10 anti-phosphotyrosine immunoblotting (IB). PKL phosphotyrosine signal was absent in cells expressing kinase dead Src (KD) but not SH2 (T215W), SH3 (D99N), or autophosphorylation (Y416F) mutants, demonstrating Src kinase activity was required for the association. Notably, cells expressing activated Y527F Src exhibited a stronger phosphotyrosine signal. (B) FAK null MEFs were transfected with GFP-PKL and WT or FAK mutants and plated on fibronectin followed by GFP immunoprecipitation (IP) and 4G10 IB. PKL was not tyrosine phosphorylated in FAK null cells but phosphorylation was rescued upon expression of WT FAK and to a lesser extent with kinase dead FAK (KD). No phosphorylation of GFP-PKL TripleYF was observed upon reexpression of FAK. Cells expressing constitutively active FAK, Super-FAK, had a demonstrably more intense signal.
Src function is closely linked to that of FAK (Playford and Schaller, 2004). The proteins interact through the Src SH3 domain (Thomas et al., 1998), and Src binds to the FAK autophosphorylation site (Y397F) through its SH2 domain, whereupon Src phosphorylates FAK residues 577/578 to further activate FAK (Calalb et al., 1995). In addition, reexpression of the various Src mutants mentioned above, except for KD Src, restores FAK activation in SYF null fibroblasts (Cary et al., 2002). The two kinases also share a number of binding partners and substrates. Consequently, it is often difficult to ascertain which kinase is the mediator of tyrosine phosphorylation. However, for paxillin and p130Cas it seems that FAK functions primarily as a Src scaffold with Src as the principal kinase (Richardson et al., 1997; Cary et al., 1998; Schaller et al., 1999; Ruest et al., 2001).
To determine whether FAK was functioning as an adaptor for Src-dependent phosphorylation of PKL, FAK null cells were transfected with GFP-PKL and either β-gal vector control or WT FAK. Cells were replated on fibronectin-coated dishes for 20 min followed by GFP immunoprecipitation and anti-phosphotyrosine 4G10 immunoblotting. PKL was not phosphorylated in FAK null cells expressing β-gal (Figure 6B). Importantly, reintroduction of WT FAK rescued WT PKL phosphorylation but not PKL Triple YF phosphorylation, consistent with these sites being the major targets of phosphorylation. A modest amount of PKL phosphorylation was detectable upon expression of kinase dead FAK, possibly due to transphosphorylation of Y397 by endogenous PYK2 (Li et al., 1999) allowing for some Src recruitment to KD FAK, or perhaps Src itself (Calalb et al., 1995). Constitutively active FAK, also called SuperFAK (Gabarra-Niecko et al., 2002), whose activity is independent of Src, efficiently restored PKL phosphorylation (Figure 6B). This was similar to constitutively active Src (Y527F) rescuing the SYF null PKL phosphorylation defect (Figure 6A). Thus, we conclude that either FAK or Src kinase activity is sufficient for adhesion-mediated PKL phosphorylation.
Loss of Src or FAK but Not Nck Results in a Profound Inhibition of PKL, but Not GIT1, Focal Adhesion Localization
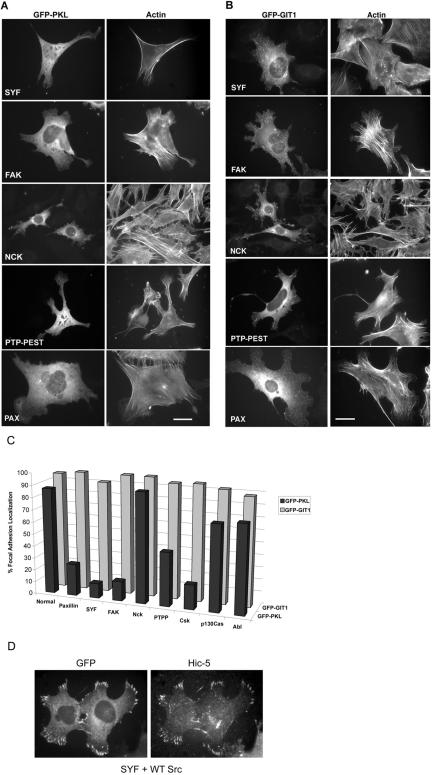
Because PKL tyrosine phosphorylation is required for its localization to focal adhesions (Figure 3C), and PKL is not phosphorylated in SYF or FAK null cells (Figure 6), one would predict that PKL would fail to localize to focal adhesions in these cells. We examined PKL localization in SYF and FAK null cells after transfection of GFP-PKL and constitutively active G12VRac1 (Figure 7, A and C). Consistent with our hypothesis, GFP-PKL was unable to localize efficiently to focal adhesions in either the SYF (12 ± 6%) or FAK (16 ± 8%) null cells (Figure 7, A and C). Although tyrosine phosphorylated PKL interacts with Nck (Figure 1), PKL localization to focal adhesions in Nck1/2 null cells was unaffected (90 ± 6%). Notably, PKL localization to focal adhesions was impaired in both PTP-PEST null cells (44 ± 2.5%) and Csk null cells (20 ± 5%) (Figure 7, A and C). PTP-PEST is a tyrosine phosphatase that interacts with FAK and paxillin (Shen et al., 1998; Cote et al., 1999; Shen et al., 2000). Csk is a tyrosine kinase that negatively regulates Src by phosphorylation on tyrosine 527 (Nada et al., 1991). The diminished capacity of PKL to localize in PTP-PEST and Csk null fibroblasts suggests a balance in cellular phosphorylation states is important for PKL localization. Finally, PKL localization in p130Cas and Abl null cell lines was only marginally reduced, 70 ± 9% and 72 ± 8%, respectively (Figure 7C). Reintroduction of WT cDNAs of the respective null protein restored PKL focal adhesion localization in each case of compromised PKL localization as demonstrated for Src reconstituted SYF cells (Figure 7D; our unpublished data).
Figure 7.
PKL, but not GIT1, requires paxillin, Src and FAK for efficient localization to focal adhesion in MEFs. (A) MEF null cells were plated on fibronectin followed by transfection with GFP-PKL and constitutively active G12VRac1. (A) The capacity of GFP-PKL to localize to focal adhesions was examined by fluorescence microscopy. The actin cytoskeleton was visualized by labeling with rhodamine phalloidin. A significant attenuation in the localization of PKL to focal adhesions in Src, FAK, PTP-PEST, and paxillin but not Nck null cells was observed. (B) The null MEF cells were plated on fibronectin followed by transfection with GFP-GIT1 and G12VRac1 revealing the efficient localization of GIT1 to focal adhesions in all null cells examined. (C) Quantitation of cells, processed as described above, showing focal adhesion localization of GFP-PKL or GFP-GIT1 (expressed as percentage of transfected cells). Unlike PKL, GIT1 retains the capacity to localize efficiently to G12VRac1 focal adhesions irrespective of the loss of Paxillin, Src, or FAK. Bars, 20 μm. (D) Reintroduction of WT Src into the SYF null cells rescues GFP-PKL targeting to focal adhesions.
Previously, we demonstrated that PKL required association with the LD4 motif of paxillin for localization in CHO.K1 cells (Brown et al., 2002). To confirm the necessity of paxillin as the PKL focal adhesion anchor, and further validate the model for characterization in MEF cells, we introduced GFP-PKL WT into paxillin null MEF cells (Figure 7A). Consistent with our dominant negative paxillin (ΔLD4) study, loss of paxillin blocked the capacity of PKL to localize efficiently to focal adhesions (only 24 ± 4.5%) of cells versus 87 ± 4% in normal MEF cells (Figures 3C and 7C).
PKL and its family member GIT1 share many characteristics, including tyrosine phosphorylation (Bagrodia et al., 1999). Consequently, the ability of GFP-GIT1 to localize to focal adhesions was examined in the various null cell lines (Figure 7B). Interestingly, no defect in GIT1 localization (≥88%) was observed (Figure 7C). Thus, despite the reported ability of PKL/GIT (and PIX) to form heterooligomers (Kim et al., 2003; Paris et al., 2003; Premont et al., 2004), focal adhesion localized GIT1 cannot substitute for the specific requirement for PKL tyrosine phosphorylation in its focal adhesion localization providing additional evidence for the structural, regulatory and functional divergence of PKL/GIT2 and GIT1.
Rescue of PKL Localization in SYF Cells by SuperFAK (K578/581E)
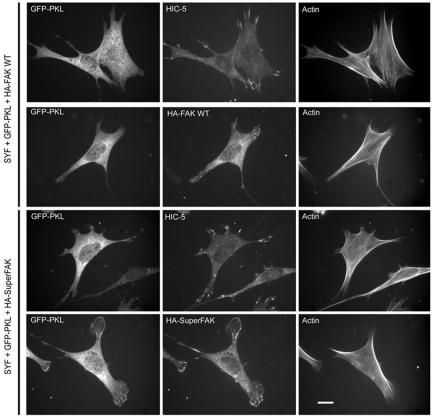
SYF cells have been reported to exhibit a defect in integrin-mediated FAK activation (Klinghoffer et al., 1999). To further explore the potential that Src functions primarily to activate FAK to stimulate PKL tyrosine phosphorylation, we introduced WT FAK and SuperFAK into the SYF null cells with GFP-PKL and constitutively active G12V Rac1 followed by analysis of PKL localization (Figure 8). Introduction of WT FAK was without effect (12 ± 4 versus 12 ± 6%); however, introduction of SuperFAK resulted in a substantial rescue in PKL localization (50 ± 2.5 from 12 ± 4%). These data provide evidence that Src activates FAK to directly phosphorylate PKL thereby stimulating its focal adhesion localization. The lack of complete restoration of PKL function by Super-FAK expression suggests direct Src phosphorylation of PKL is also a major mechanism of PKL activation.
Figure 8.
Introduction of SuperFAK(K578/581E) into SYF cells rescues the ability of PKL to localize to focal adhesions. SYF cells were plated on fibronectin, transfected with GFP-PKL, G12VRac1, and either HA-FAK WT or HA-SuperFAK followed by labeling with Al-exa350-phalloidin and Anti-Hic-5 or HA 12CA5 to detect focal adhesion localization of Hic-5 and exogenous FAK, respectively. The capacity of PKL to localize to focal adhesions was then quantified as percentage focal adhesion localization. In the presence of WT-FAK only 12% exhibited GFP-PKL focal adhesion localization, whereas with SuperFAK, 50% of transfectants demonstrated PKL focal adhesion localization. Bar, 10 μm.
GFP-PKL Triple YF Increases Cell Spreading and Alters Cell Morphology
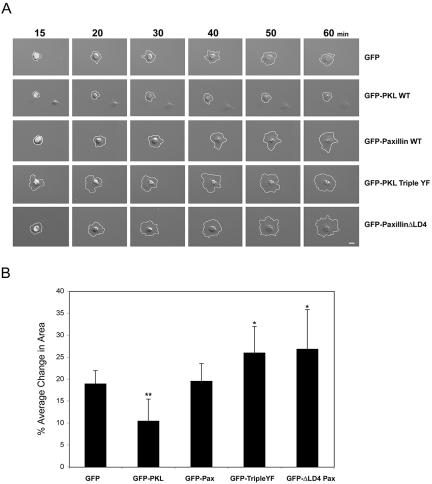
We have previously found that perturbing PKL localization to focal adhesions, through either overexpression of paxillinΔLD4 or PKLΔPBS2, results in aberrant cell spreading associated with increased protrusiveness and inhibition of directed movement (West et al., 2001). We sought to determine the effects of overexpression of PKL Triple YF on cell adhesion and spreading. Normal MEF cells were transiently transfected with GFP, GFP-PKL WT, GFP-PKL Triple YF, GFP-Paxillin WT, or GFP-PaxillinΔLD4 followed by plating on fibronectin and tracking cell spreading for 1 h by time-lapse microscopy (Figure 9A). Expression of GFP or GFP-Paxillin WT had no significant effect on cell spreading. GFP-PKL WT expression led to an attenuation in cell spreading, whereas GFP-PKL Triple YF and GFP-PaxillinΔLD4 stimulated cell spreading (Figure 9B). Percentage of change in cell area between 20 and 60 min of cell spreading were measured at 10-min intervals and averaged (Figure 9B). GFP expressing cells exhibited an average change of 18 ± 3% similar to expression of GFP-Paxillin WT (19 ± 4%). However, GFP-PKL Triple YF (26 ± 6%) and GFP-Paxillin ΔLD4 (27 ± 7%) cell spreading was increased ∼40% relative to GFP; whereas expression of GFP-PKL WT inhibited cell spreading by 40% (10 ± 5%).
Figure 9.
Expression of GFP-PKL TripleYF increases cell spreading similar to cells expressing paxillin ΔLD4. (A) Normal MEFs were transiently transfected with GFP, GFP-PKL WT, GFP-PKL TripleYF, GFP-Paxillin WT, or GFP-Paxillin ΔLD4 followed by plating on 5 μg/ml fibronectin and acquisition of time-lapse images for 60 min. Both GFP-PKL TripleYF and GFP-Paxillin ΔLD4 increased cell spreading, whereas GFP-PKL WT inhibited cell spreading. GFP or GFP-Paxillin expression was without significant effect. (B) The average change in cell area at 10-min intervals between 20 and 60 min postspreading was calculated. Bars, 10 μm. **p < 0.01, *p < 0.05
GFP-PKL TripleYF and GFP-Paxillin Δ LD4 Increase Cell Protrusion
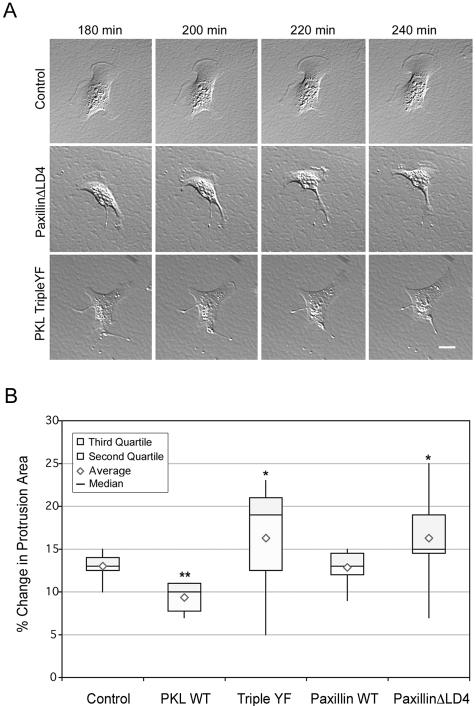
As spreading progressed beyond 3 h and cells reached a “steady state,” the GFP-PaxillinΔLD4 and GFP-PKL Triple YF-expressing cells developed exaggerated retraction fibers and protrusions (Figure 10A) similar to that described for GFP-PaxillinΔLD4 and GFP-PKLΔPBS2 in CHO.K1 cells (West et al., 2001). Changes in cell shape and protrusion dynamics of MEF cells transiently transfected as described above were examined after cell spreading 3 h postplating on fibronectin. Protrusion area changes were quantified as described in West et al. (2001) and Materials and Methods and expressed as a Box-and-Whiskers Plot (Figure 10B). The attenuation in early spreading, observed upon expression of GFP-PKL WT, continued at later time points as evidenced by reduced changes in protrusion areas. GFP-Paxillin WT-expressing cells functioned essentially the same as GFP control cells. Notably, both GFP-PKL Triple YF and GFP-PaxillinΔLD4-expressing cells displayed greater fluctuations in protrusion area changes, prominently indicated by the span (minimum and maximum value) of the “whiskers.” In addition, the median changes in protrusion area were increased relative to GFP control. Thus, abrogation of proper temporal-spatial regulation of PKL profoundly impairs normal cell adhesion dynamics consistent with a critical role for this protein and its binding partners.
Figure 10.
Expression of GFP-PKL TripleYF increases cell protrusiveness similar to cells expressing paxillin ΔLD4. (A) Normal MEF cells were transfected and plated on 5 μg/ml fibronectin for 180 min followed by acquisition of time-lapse images every 10 min. Both GFP-PKL TripleYF and GFP-Paxillin ΔLD4 exhibited increased cell protrusion and exaggerated retraction fiber formation relative to GFP, suggesting PKL localization is essential for proper integrin-mediated cell function. (B) Changes in cell protrusion areas of at least 10 normal MEF cells transiently transfected and plated as described above were quantitated at 10-min intervals for 1 h as detailed in Materials and Methods, demonstrating both GFP-PKL TripleYF and GFP-Paxillin ΔLD4 exhibited enhanced protrusiveness. Bars, 20 μm. **indicates p < 0.01, *p < 0.05.
DISCUSSION
We have identified Src/FAK- and Rac-dependent phosphorylation of the ArfGAP PKL and determined that phosphorylation is required for PKL focal adhesion localization, paxillin binding and normal cell adhesion dynamics. The related protein GIT1 is tyrosine phosphorylated in a Src-dependent manner (Bagrodia et al., 1999; Van Nieuw Amerongen et al., 2004; Yin et al., 2004). However, unlike GIT1, PKL tyrosine phosphorylation is essential for its localization to focal adhesions.
Tyrosine phosphorylated PKL coimmunoprecipitates with Nck and is capable of interacting with the Nck and Src SH2 domains (Figure 2). Interestingly, several groups have described an ∼100-kDa tyrosine-phosphorylated protein that associates with Nck after growth factor stimulation (Galisteo et al., 1996; Voisin et al., 1999; Schmitz et al., 2001). GIT1 is phosphorylated by Src after angiotensin II stimulation and is capable of binding to the phospholipase C (PLC)γ1 SH2 domain (Haendeler et al., 2003). However, GIT1 failed to be precipitated by Nck or the Nck SH2 domain in angiotensin II-stimulated vascular smooth muscle cells (Schmitz et al., 2004). Thus, it remains to be determined whether PKL represents the unidentified Nck binding partner described in these reports and whether PKL phosphorylation is regulated by growth factor receptor ligation similarly to integrin activation.
Amino acid residues Y286/392/592 (YDEV, YDSV, YDNT) of PKL were found to be the principal sites of adhesion-stimulated phosphorylation (Figure 3). These sequences are conserved among the PKL/GIT/CAT family, except for the absence of Y592 from GIT2short (Premont et al., 2000; Vitale et al., 2000), and their sequences resemble the consensus sequence for Nck SH2 binding (Songyang et al., 1993). In addition to binding the SH2 domains of Nck and Src (Figure 2), tyrosine-phosphorylated PKL also binds to the Grb2 and Crk SH2 domains (Brown and Turner, unpublished observation). Further work will characterize potential specificity between the sites of PKL phosphorylation and SH2 domain binding and their role in programming distinct cell responses to extracellular matrix and growth factor signals. PKL binding to the Nck SH3 domain was also identified. Using GST-Nck SH3 pull downs and mass spectrometry, a total of six independent PKL/GIT2 peptides were identified, but peptides from the related GIT1, which can bind to the PLCγ1 SH3 domain (Haendeler et al., 2003), were not detected. Two type I SH3 domain binding consensus sequences (K/RXXPXXP) (Feng et al., 1994; Lim et al., 1994) are present within PKL, 52KHTPWPP58 and 540RLQPFPP546. Future studies will evaluate the potential regulation and roles for the PKL-Nck SH3 association.
To identify a function for PKL phosphorylation, we characterized its effect on localization to focal adhesions. A PKL mutant lacking the three major sites of phosphorylation (GFP-PKL Triple YF) failed to localize to focal adhesions (Figure 3C). In addition, PKL phosphorylation was abrogated in SYF and FAK null cells (Figure 6) and not surprisingly, PKL was unable to localize efficiently to focal adhesions in these cells (Figure 7A). Importantly, the PKL Triple YF mutant, unlike WT PKL, failed to coprecipitate paxillin from CHO.K1 or HEK293A cells (Figure 4), suggesting that tyrosine phosphorylation of PKL induces focal adhesion targeting by enhancing the PKL-paxillin interaction. In contrast, GIT1 localization was unimpaired in Src, FAK, and paxillin null cells (Figure 7B), consistent with the observation that elimination of GIT1 phosphorylation after PP2 blockade of Src did not affect GIT1 localization to focal adhesions (Van Nieuw Amerongen et al., 2004). This provides further evidence for the specific, nonredundant function for the PKL/GIT family members. The capacity of GIT1 to localize to focal adhesions in paxillin null fibroblasts may be explained by the robust expression of Hic-5 in these cells and the fact that GIT1 binds more efficiently to Hic-5 relative to paxillin (Nishiya et al., 2002).
Paxillin, Src-family kinases, and FAK are critical for cell spreading and cell motility (Ilic et al., 1995; Klinghoffer et al., 1999; Hagel et al., 2002; Webb et al., 2004). Inhibition or elimination of these proteins causes a stabilization of focal adhesions and a resultant impairment in the coordination of protrusion, focal adhesion remodeling, and tail release thereby inhibiting cell migration (Webb et al., 2004). Localization of a PAK-GIT1-FAK complex to focal adhesions through paxillin, with a consequent elimination of paxillin, has been suggested to be required for focal adhesion disassembly (Zhao et al., 2000b). Our determination that GIT1 localizes efficiently to focal adhesions in paxillin, SYF and FAK null fibroblasts, whereas PKL localization is significantly attenuated, may indicate that PKL rather than GIT1 is a principal mediator of focal adhesion turnover.
Analysis of PKL mutants showed that tyrosine phosphorylation does not require localization to focal adhesions (Figure 5), unlike the proposed mode of GIT1 phosphorylation (Van Nieuw Amerongen et al., 2004). Neither ArfGAP activity nor the FAK-binding SHD1 domain was essential for PKL phosphorylation (Figure 5). The precise role for PKL Arf-GAP activity in regulating cell protrusiveness, spreading and motility is unclear, although it is intriguing that FAK- and Src-family kinases interact with and phosphorylate a number of Arf-GAP proteins including ASAP1/2, ARAP3 and GIT1/GIT2 (Brown et al., 1998b; Andreev et al., 1999; Bagrodia et al., 1999; Zhao et al., 2000b; Haendeler et al., 2003; Randazzo and Hirsch, 2004; Stacey et al., 2004; Van Nieuw Amerongen et al., 2004; Yin et al., 2004). Additional work will be required to determine the precise role for PKL phosphorylation in generating a focal adhesion competent molecule. Efforts will focus on the mechanism(s) by which the cell regulates the PKL-paxillin bridge in this complex of proteins. Whether a conformational change occurs to expose the paxillin binding site (PBS2 domain) on PKL (Turner et al., 1999; Brown et al., 2002) and/or the recruitment of SH2/SH3 domain containing binding partner(s) influences the organization of a PKL complex remains to be determined.
We have previously demonstrated that perturbing the PKL-paxillin link and blocking PAK localization to focal adhesions causes significant alterations in cellular adhesive behavior, including sustained Rac activity (West et al., 2001; Brown et al., 2002; Brown and Turner, 2004). Expression of paxillinΔLD4, which does not bind PKL, or PKLΔPBS2, which does not bind paxillin, causes the generation of multiple aberrant lamellipodia and inefficient tail retraction, resulting in increased random motility and loss of microtubule organizing center reorganization and directional migration (West et al., 2001; Brown and Turner, 2004). We substantiate and extend these observations by showing that impeding PKL focal adhesion localization through expression of the nonphosphorylatable PKL Triple YF mutant potentiates spreading and increases extension of exaggerated membrane protrusions, similar to expression of paxillinΔLD4 (Figures 9 and 10).
Together, these results suggest a model in which PKL in the cytosol is tyrosine phosphorylated at one or more phosphorylation sites by FAK and/or Src family kinases following integrin and Rac activation. The phosphorylation of PKL promotes binding to paxillin and thus translocation of the Nck-PAK-PIX-PKL complex to focal adhesions. If the complex fails to associate with focal adhesions, either because PKL is not phosphorylated or because of mutations in either the PKL or paxillin binding sites, cell spreading is accelerated and unstable membrane projections develop due to sustained Rac activity. Recruitment of the complex to focal adhesions may thus act to coordinate protrusive activity and dampen excessive actin polymerization around the leading edge by suppressing further Rac-induced lamellipodia formation (West et al., 2001; Nishiya et al., 2005). In addition, Nck can associate with PAK, and, as we show here, with PKL. However, PKL localization in focal contacts does not require Nck. Thus, the Nck SH2 domain is unlikely to be the cause of the PKL complex localization. Rather, we propose that tyrosine phosphorylation of PKL may induce conformational changes or partner swapping within the Nck-PAK-PIX-PKL complex, which expose the PKL PBS2 region and allow paxillin binding. Further dissection of protein-protein interactions within the complex will be required to fully understand the mechanism.
Acknowledgments
We thank Brian Bouverat and Abby Racette for outstanding technical support, David LaLonde for critical evaluation of the manuscript, Bryan Ballif for contributions to identifying Nck-associated proteins, and Phil Gafken and the Proteomics Resource. We thank Drs. Jun-Lin Guan, Rick Horwitz, Akira Imamoto, Bruce Mayer, Tony Pawson, Michel Tremblay, Jean Wang, and Linda Van Aelst for valuable plasmids and cell lines. This study was supported by National Institutes of Health Grants GM-477607 (to C.E.T) and CA-41072 (to J.A.C).
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E05-02-0131) on July 6, 2005.
Abbreviations used: FAK, focal adhesion kinase; GAP, GTPase-activating protein; GFP, green fluorescent protein; GIT, G protein-coupled receptor kinase-interacting protein; GST, glutathione S-transferase; MEF, mouse embryo fibroblast; PAK, p21-activated kinase; PBS, paxillin binding subdomain; PKL, paxillin kinase linker; SHD, SpaII homology domain; SYF, Src/Yes/Fyn; WT, wild-type.
References
- Andreev, J., Simon, J. P., Sabatini, D. D., Kam, J., Plowman, G., Randazzo, P. A., and Schlessinger, J. (1999). Identification of a new Pyk2 target protein with Arf-GAP activity. Mol. Cell. Biol. 19, 2338-2350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angers-Loustau, A., Cote, J. F., Charest, A., Dowbenko, D., Spencer, S., Lasky, L. A., and Tremblay, M. L. (1999). Protein tyrosine phosphatase-PEST regulates focal adhesion disassembly, migration, and cytokinesis in fibroblasts. J. Cell Biol. 144, 1019-1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagrodia, S., Bailey, D., Lenard, Z., Hart, M., Guan, J. L., Premont, R. T., Taylor, S. J., and Cerione, R. A. (1999). A tyrosine-phosphorylated protein that binds to an important regulatory region on the cool family of p21-activated kinase-binding proteins. J. Biol. Chem. 274, 22393-22400. [DOI] [PubMed] [Google Scholar]
- Bagrodia, S., Taylor, S. J., Jordon, K. A., Van Aelst, L., and Cerione, R. A. (1998). A novel regulator of p21-activated kinases. J. Biol. Chem. 273, 23633-23636. [DOI] [PubMed] [Google Scholar]
- Bladt, F., Aippersbach, E., Gelkop, S., Strasser, G. A., Nash, P., Tafuri, A., Gertler, F. B., and Pawson, T. (2003). The murine Nck SH2/SH3 adaptors are important for the development of mesoderm-derived embryonic structures and for regulating the cellular actin network. Mol. Cell. Biol. 23, 4586-4597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bokoch, G. M. (2003). Biology of the p21-activated kinases. Annu. Rev. Biochem. 72, 743-781. [DOI] [PubMed] [Google Scholar]
- Bokoch, G. M., Wang, Y., Bohl, B. P., Sells, M. A., Quilliam, L. A., and Knaus, U. G. (1996). Interaction of the Nck adapter protein with p21-activated kinase (PAK1). J. Biol. Chem. 271, 25746-25749. [DOI] [PubMed] [Google Scholar]
- Brown, M. C., Perrotta, J. A., and Turner, C. E. (1998a). Serine and threonine phosphorylation of the paxillin LIM domains regulates paxillin focal adhesion localization and cell adhesion to fibronectin. Mol. Biol. Cell 9, 1803-1816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown, M. C., and Turner, C. E. (2004). Paxillin: adapting to change. Physiol. Rev. 84, 1315-1339. [DOI] [PubMed] [Google Scholar]
- Brown, M. C., West, K. A., and Turner, C. E. (2002). Paxillin-dependent paxillin kinase linker and p21-activated kinase localization to focal adhesions involves a multistep activation pathway. Mol. Biol. Cell 13, 1550-1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown, M. T., Andrade, J., Radhakrishna, H., Donaldson, J. G., Cooper, J. A., and Randazzo, P. A. (1998b). ASAP1, a phospholipid-dependent Arf GTPase-activating protein that associates with and is phosphorylated by Src. Mol. Cell. Biol. 18, 7038-7051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buday, L., Wunderlich, L., and Tamas, P. (2002). The Nck family of adapter proteins: regulators of actin cytoskeleton. Cell Signal 14, 723-731. [DOI] [PubMed] [Google Scholar]
- Burridge, K., and Wennerberg, K. (2004). Rho and Rac take center stage. Cell 116, 167-179. [DOI] [PubMed] [Google Scholar]
- Calalb, M. B., Polte, T. R., and Hanks, S. K. (1995). Tyrosine phosphorylation of focal adhesion kinase at sites in the catalytic domain regulates kinase activity: a role for Src family kinases. Mol. Cell. Biol. 15, 954-963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cary, L. A., Han, D. C., Polte, T. R., Hanks, S. K., and Guan, J. L. (1998). Identification of p130Cas as a mediator of focal adhesion kinase-promoted cell migration. J. Cell Biol. 140, 211-221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cary, L. A., Klinghoffer, R. A., Sachsenmaier, C., and Cooper, J. A. (2002). SRC catalytic but not scaffolding function is needed for integrin-regulated tyrosine phosphorylation, cell migration, and cell spreading. Mol. Cell. Biol. 22, 2427-2440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chahdi, A., Miller, B., and Sorokin, A. (2005). Endothelin 1 induces beta 1Pix translocation and Cdc42 activation via protein kinase A-dependent pathway. J. Biol. Chem. 280, 578-584. [DOI] [PubMed] [Google Scholar]
- Cohen, G. B., Ren, R., and Baltimore, D. (1995). Modular binding domains in signal transduction proteins. Cell 80, 237-248. [DOI] [PubMed] [Google Scholar]
- Cote, J. F., Turner, C. E., and Tremblay, M. L. (1999). Intact LIM 3 and LIM 4 domains of paxillin are required for the association to a novel polyproline region (Pro 2) of protein-tyrosine phosphatase-PEST. J. Biol. Chem. 274, 20550-20560. [DOI] [PubMed] [Google Scholar]
- Feng, S., Chen, J. K., Yu, H., Simon, J. A., and Schreiber, S. L. (1994). Two binding orientations for peptides to the Src SH3 domain: development of a general model for SH3-ligand interactions. Science 266, 1241-1247. [DOI] [PubMed] [Google Scholar]
- Fincham, V. J., and Frame, M. C. (1998). The catalytic activity of Src is dispensable for translocation to focal adhesions but controls the turnover of these structures during cell motility. EMBO J. 17, 81-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuta, Y., Ilic, D., Kanazawa, S., Takeda, N., Yamamoto, T., and Aizawa, S. (1995). Mesodermal defect in late phase of gastrulation by a targeted mutation of focal adhesion kinase, FAK. Oncogene 11, 1989-1995. [PubMed] [Google Scholar]
- Gabarra-Niecko, V., Keely, P. J., and Schaller, M. D. (2002). Characterization of an activated mutant of focal adhesion kinase: `SuperFAK.' Biochem. J. 365, 591-603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galisteo, M. L., Chernoff, J., Su, Y. C., Skolnik, E. Y., and Schlessinger, J. (1996). The adaptor protein Nck links receptor tyrosine kinases with the serine-threonine kinase Pak1. J. Biol. Chem. 271, 20997-21000. [DOI] [PubMed] [Google Scholar]
- Giancotti, F. G., and Tarone, G. (2003). Positional control of cell fate through joint integrin/receptor protein kinase signaling. Annu. Rev. Cell. Dev. Biol. 19, 173-206. [DOI] [PubMed] [Google Scholar]
- Haendeler, J., Yin, G., Hojo, Y., Saito, Y., Melaragno, M., Yan, C., Sharma, V. K., Heller, M., Aebersold, R., and Berk, B. C. (2003). GIT1 mediates Src-dependent activation of phospholipase Cγ by angiotensin II and epidermal growth factor (EGF). J. Biol. Chem. 278, 49936-49944. [DOI] [PubMed] [Google Scholar]
- Hagel, M., George, E. L., Kim, A., Tamimi, R., Opitz, S. L., Turner, C. E., Imamoto, A., and Thomas, S. M. (2002). The adaptor protein paxillin is essential for normal development in the mouse and is a critical transducer of fibronectin signaling. Mol. Cell. Biol. 22, 901-915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han, J., Das, B., Wei, W., Van Aelst, L., Mosteller, R. D., Khosravi-Far, R., Westwick, J. K., Der, C. J., and Broek, D. (1997). Lck regulates Vav activation of members of the Rho family of GTPases. Mol. Cell. Biol. 17, 1346-1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardin, J. D., Boast, S., Schwartzberg, P. L., Lee, G., Alt, F. W., Stall, A. M., and Goff, S. P. (1996). Abnormal peripheral lymphocyte function in c-abl mutant mice. Cell Immunol. 172, 100-107. [DOI] [PubMed] [Google Scholar]
- Hashimoto, S., Tsubouchi, A., Mazaki, Y., and Sabe, H. (2001). Interaction of paxillin with p21-activated Kinase (PAK). Association of paxillin alpha with the kinase-inactive and the Cdc42-activated forms of PAK3. J. Biol. Chem. 276, 6037-6045. [DOI] [PubMed] [Google Scholar]
- Haskell, M. D., Slack, J. K., Parsons, J. T., and Parsons, S. J. (2001). Chinese hamster ovary-Src tyrosine phosphorylation of EGF receptor, P190 RhoGAP, and focal adhesion kinase regulates diverse cellular processes. Chem. Rev. 101, 2425-2440. [DOI] [PubMed] [Google Scholar]
- Hildebrand, J. D., Taylor, J. M., and Parsons, J. T. (1996). An SH3 domain-containing GTPase-activating protein for Rho and Cdc42 associates with focal adhesion kinase. Mol. Cell. Biol. 16, 3169-3178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honda, H., et al. (1998). Cardiovascular anomaly, impaired actin bundling and resistance to Src-induced transformation in mice lacking p130Cas. Nat. Genet. 19, 361-365. [DOI] [PubMed] [Google Scholar]
- Howe, A. K. (2001). Cell adhesion regulates the interaction between Nck and p21-activated kinase. J. Biol. Chem. 276, 14541-14544. [DOI] [PubMed] [Google Scholar]
- Hubbard, S. R., and Till, J. H. (2000). Protein tyrosine kinase structure and function. Annu. Rev. Biochem. 69, 373-398. [DOI] [PubMed] [Google Scholar]
- Ilic, D., Furuta, Y., Kanazawa, S., Takeda, N., Sobue, K., Nakatsuji, N., Nomura, S., Fujimoto, J., Okada, M., and Yamamoto, T. (1995). Reduced cell motility and enhanced focal adhesion contact formation in cells from FAK-deficient mice. Nature 377, 539-544. [DOI] [PubMed] [Google Scholar]
- Imamoto, A., and Soriano, P. (1993). Disruption of the csk gene, encoding a negative regulator of Src family tyrosine kinases, leads to neural tube defects and embryonic lethality in mice. Cell 73, 1117-1124. [DOI] [PubMed] [Google Scholar]
- Iwasaki, T., et al. (2002). Involvement of phosphorylation of Tyr-31 and Tyr-118 of paxillin in MM1 cancer cell migration. Int. J. Cancer 97, 330-335. [DOI] [PubMed] [Google Scholar]
- Jockusch, B. M., Bubeck, P., Giehl, K., Kroemker, M., Moschner, J., Rothkegel, M., Rudiger, M., Schluter, K., Stanke, G., and Winkler, J. (1995). The molecular architecture of focal adhesions. Annu. Rev. Cell. Dev. Biol. 11, 379-416. [DOI] [PubMed] [Google Scholar]
- Juliano, R. L. (2002). Signal transduction by cell adhesion receptors and the cytoskeleton: functions of integrins, cadherins, selectins, and immunoglobulin-superfamily members. Annu. Rev. Pharmacol. Toxicol. 42, 283-323. [DOI] [PubMed] [Google Scholar]
- Kaplan, K. B., Bibbins, K. B., Swedlow, J. R., Arnaud, M., Morgan, D. O., and Varmus, H. E. (1994). Association of the amino-terminal half of c-Src with focal adhesions alters their properties and is regulated by phosphorylation of tyrosine 527. EMBO J. 13, 4745-4756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan, K. B., Swedlow, J. R., Morgan, D. O., and Varmus, H. E. (1995). Chinese hamster ovary-Src enhances the spreading of src-/-fibroblasts on fibronectin by a kinase-independent mechanism. Genes Dev. 9, 1505-1517. [DOI] [PubMed] [Google Scholar]
- Kim, S., et al. (2003). The GIT family of proteins forms multimers and associates with the presynaptic cytomatrix protein Piccolo. J. Biol. Chem. 278, 6291-6300. [DOI] [PubMed] [Google Scholar]
- Kinley, A. W., Weed, S. A., Weaver, A. M., Karginov, A. V., Bissonette, E., Cooper, J. A., and Parsons, J. T. (2003). Cortactin interacts with WIP in regulating Arp2/3 activation and membrane protrusion. Curr. Biol. 13, 384-393. [DOI] [PubMed] [Google Scholar]
- Kiosses, W. B., Hood, J., Yang, S., Gerritsen, M. E., Cheresh, D. A., Alderson, N., and Schwartz, M. A. (2002). A dominant-negative p65 PAK peptide inhibits angiogenesis. Circ. Res. 90, 697-702. [DOI] [PubMed] [Google Scholar]
- Kiyono, M., Kaziro, Y., and Satoh, T. (2000). Induction of Rac-guanine nucleotide exchange activity of Ras-GRF1/CDC25(Mm) following phosphorylation by the nonreceptor tyrosine kinase Src. J. Biol. Chem. 275, 5441-5446. [DOI] [PubMed] [Google Scholar]
- Klinghoffer, R. A., Sachsenmaier, C., Cooper, J. A., and Soriano, P. (1999). Src family kinases are required for integrin but not PDGFR signal transduction. EMBO J. 18, 2459-2471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamorte, L., Rodrigues, S., Sangwan, V., Turner, C. E., and Park, M. (2003). Crk associates with a multimolecular Paxillin/GIT2/beta-PIX complex and promotes Rac-dependent relocalization of Paxillin to focal contacts. Mol. Biol. Cell 14, 2818-2831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, L., Okura, M., and Imamoto, A. (2002). Focal adhesions require catalytic activity of Src family kinases to mediate integrin-matrix adhesion. Mol. Cell. Biol. 22, 1203-1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, X., Dy, R. C., Cance, W. G., Graves, L. M., and Earp, H. S. (1999). Interactions between two cytoskeleton-associated tyrosine kinases: calcium-dependent tyrosine kinase and focal adhesion tyrosine kinase. J. Biol. Chem. 274, 8917-8924. [DOI] [PubMed] [Google Scholar]
- Lim, W. A., Richards, F. M., and Fox, R. O. (1994). Structural determinants of peptide-binding orientation and of sequence specificity in SH3 domains. Nature 372, 375-379. [DOI] [PubMed] [Google Scholar]
- Lu, W., Katz, S., Gupta, R., and Mayer, B. J. (1997). Activation of Pak by membrane localization mediated by an SH3 domain from the adaptor protein Nck. Curr. Biol. 7, 85-94. [DOI] [PubMed] [Google Scholar]
- Manabe Ri, R., Kovalenko, M., Webb, D. J., and Horwitz, A. R. (2002). GIT1 functions in a motile, multi-molecular signaling complex that regulates protrusive activity and cell migration. J. Cell Sci. 115, 1497-1510. [DOI] [PubMed] [Google Scholar]
- Manser, E., and Lim, L. (1999). Roles of PAK family kinases. Prog. Mol. Subcell. Biol. 22, 115-133. [DOI] [PubMed] [Google Scholar]
- Matafora, V., Paris, S., Dariozzi, S., and de Curtis, I. (2001). Molecular mechanisms regulating the subcellular localization of p95-APP1 between the endosomal recycling compartment and sites of actin organization at the cell surface. J. Cell Sci. 114, 4509-4520. [DOI] [PubMed] [Google Scholar]
- Mitra, S. K., Hanson, D. A., and Schlaepfer, D. D. (2005). Focal adhesion kinase: in command and control of cell motility. Nat. Rev. Mol. Cell. Biol. 6, 56-68. [DOI] [PubMed] [Google Scholar]
- Morris, S. M., Arden, S. D., Roberts, R. C., Kendrick-Jones, J., Cooper, J. A., Luzio, J. P., and Buss, F. (2002). Myosin VI binds to and localises with Dab2, potentially linking receptor-mediated endocytosis and the actin cytoskeleton. Traffic 3, 331-341. [DOI] [PubMed] [Google Scholar]
- Nada, S., Okada, M., MacAuley, A., Cooper, J. A., and Nakagawa, H. (1991). Cloning of a complementary DNA for a protein-tyrosine kinase that specifically phosphorylates a negative regulatory site of p60c-src. Nature 351, 69-72. [DOI] [PubMed] [Google Scholar]
- Nishiya, N., Kiosses, W. B., Han, J., and Ginsberg, M. H. (2005). An alpha4 integrin-paxillin-Arf-GAP complex restricts Rac activation to the leading edge of migrating cells. Nat. Cell Biol. 7, 343-352. [DOI] [PubMed] [Google Scholar]
- Nishiya, N., Shirai, T., Suzuki, W., and Nose, K. (2002). Hic-5 interacts with GIT1 with a different binding mode from paxillin. J. Biochem. 132, 279-289. [DOI] [PubMed] [Google Scholar]
- Paris, S., Longhi, R., Santambrogio, P., and de Curtis, I. (2003). Leucinezipper-mediated homo- and hetero-dimerization of GIT family p95-ARF GTPase-activating protein, PIX-, paxillin-interacting proteins 1 and 2. Biochem. J. 372, 391-398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petit, V., Boyer, B., Lentz, D., Turner, C. E., Thiery, J. P., and Valles, A. M. (2000). Phosphorylation of tyrosine residues 31 and 118 on paxillin regulates cell migration through an association with CRK in NBT-II cells. J. Cell Biol. 148, 957-970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Playford, M. P., and Schaller, M. D. (2004). The interplay between Src and integrins in normal and tumor biology. Oncogene 23, 7928-7946. [DOI] [PubMed] [Google Scholar]
- Premont, R. T., Claing, A., Vitale, N., Perry, S. J., and Lefkowitz, R. J. (2000). The GIT family of ADP-ribosylation factor GTPase-activating proteins. Functional diversity of GIT2 through alternative splicing. J. Biol. Chem. 275, 22373-22380. [DOI] [PubMed] [Google Scholar]
- Premont, R. T., Perry, S. J., Schmalzigaug, R., Roseman, J. T., Xing, Y., and Claing, A. (2004). The GIT/PIX complex: an oligomeric assembly of GIT family ARF GTPase-activating proteins and PIX family Rac1/Cdc42 guanine nucleotide exchange factors. Cell Signal 16, 1001-1011. [DOI] [PubMed] [Google Scholar]
- Raftopoulou, M., and Hall, A. (2004). Cell migration: Rho GTPases lead the way. Dev. Biol. 265, 23-32. [DOI] [PubMed] [Google Scholar]
- Randazzo, P. A., and Hirsch, D. S. (2004). Arf GAPs: multifunctional proteins that regulate membrane traffic and actin remodelling. Cell Signal 16, 401-413. [DOI] [PubMed] [Google Scholar]
- Richardson, A., Malik, R. K., Hildebrand, J. D., and Parsons, J. T. (1997). Inhibition of cell spreading by expression of the C-terminal domain of focal adhesion kinase (FAK) is rescued by coexpression of Src or catalytically inactive FAK: a role for paxillin tyrosine phosphorylation. Mol. Cell. Biol. 17, 6906-6914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruest, P. J., Shin, N. Y., Polte, T. R., Zhang, X., and Hanks, S. K. (2001). Mechanisms of CAS substrate domain tyrosine phosphorylation by FAK and Src. Mol. Cell. Biol. 21, 7641-7652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sastry, S. K., and Burridge, K. (2000). Focal adhesions: a nexus for intracellular signaling and cytoskeletal dynamics. Exp. Cell Res. 261, 25-36. [DOI] [PubMed] [Google Scholar]
- Schaller, M. D., Hildebrand, J. D., and Parsons, J. T. (1999). Complex formation with focal adhesion kinase: a mechanism to regulate activity and subcellular localization of Src kinases. Mol. Biol. Cell 10, 3489-3505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaller, M. D., Hildebrand, J. D., Shannon, J. D., Fox, J. W., Vines, R. R., and Parsons, J. T. (1994). Autophosphorylation of the focal adhesion kinase, pp125FAK, directs SH2-dependent binding of pp60src. Mol. Cell. Biol. 14, 1680-1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz, U., Thommes, K., Beier, I., Dusing, R., and Vetter, H. (2004). Identification of Nck interacting proteins in vascular smooth muscle cells. Clin. Exp. Hypertens. 26, 267-275. [DOI] [PubMed] [Google Scholar]
- Schmitz, U., Thommes, K., Beier, I., Wagner, W., Sachinidis, A., Dusing, R., and Vetter, H. (2001). Angiotensin II-induced stimulation of p21-activated kinase and c-Jun NH2-terminal kinase is mediated by Rac1 and Nck. J. Biol. Chem. 276, 22003-22010. [DOI] [PubMed] [Google Scholar]
- Schwartz, M. A., Schaller, M. D., and Ginsberg, M. H. (1995). Integrins: emerging paradigms of signal transduction. Annu. Rev. Cell Dev. Biol. 11, 549-599. [DOI] [PubMed] [Google Scholar]
- Sells, M. A., Knaus, U. G., Bagrodia, S., Ambrose, D. M., Bokoch, G. M., and Chernoff, J. (1997). Human p21-activated kinase (Pak1) regulates actin organization in mammalian cells. Curr. Biol. 7, 202-210. [DOI] [PubMed] [Google Scholar]
- Shen, Y., Lyons, P., Cooley, M., Davidson, D., Veillette, A., Salgia, R., Griffin, J. D., and Schaller, M. D. (2000). The noncatalytic domain of protein-tyrosine phosphatase-PEST targets paxillin for dephosphorylation in vivo. J. Biol. Chem. 275, 1405-1413. [DOI] [PubMed] [Google Scholar]
- Shen, Y., Schneider, G., Cloutier, J. F., Veillette, A., and Schaller, M. D. (1998). Direct association of protein-tyrosine phosphatase PTP-PEST with paxillin. J. Biol. Chem. 273, 6474-6481. [DOI] [PubMed] [Google Scholar]
- Songyang, Z., et al. (1993). SH2 domains recognize specific phosphopeptide sequences. Cell 72, 767-778. [DOI] [PubMed] [Google Scholar]
- Stacey, T. T., Nie, Z., Stewart, A., Najdovska, M., Hall, N. E., He, H., Randazzo, P. A., and Lock, P. (2004). ARAP3 is transiently tyrosine phosphorylated in cells attaching to fibronectin and inhibits cell spreading in a RhoGAP-dependent manner. J. Cell Sci. 117, 6071-6084. [DOI] [PubMed] [Google Scholar]
- Suetsugu, S., and Takenawa, T. (2003). Regulation of cortical actin networks in cell migration. Int. Rev. Cytol. 229, 245-286. [DOI] [PubMed] [Google Scholar]
- Thomas, J. W., Ellis, B., Boerner, R. J., Knight, W. B., White, G. C., 2nd, and Schaller, M. D. (1998). SH2- and SH3-mediated interactions between focal adhesion kinase and Src. J. Biol. Chem. 273, 577-583. [DOI] [PubMed] [Google Scholar]
- Tu, S., Wu, W. J., Wang, J., and Cerione, R. A. (2003). EGF-dependent regulation of Cdc42 is mediated by the Src tyrosine kinase. J. Biol. Chem. 278, 49293-49300. [DOI] [PubMed] [Google Scholar]
- Turner, C. E., Brown, M. C., Perrotta, J. A., Riedy, M. C., Nikolopoulos, S. N., McDonald, A. R., Bagrodia, S., Thomas, S., and Leventhal, P. S. (1999). Paxillin LD4 motif binds PAK and PIX through a novel 95-kD ankyrin repeat, ARF-GAP protein: a role in cytoskeletal remodeling. J. Cell Biol. 145, 851-863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valles, A. M., Beuvin, M., and Boyer, B. (2004). Activation of Rac1 by paxillin-Crk-DOCK180 signaling complex is antagonized by Rap1 in migrating NBT-II cells. J. Biol. Chem. 279, 44490-44496. [DOI] [PubMed] [Google Scholar]
- Van Nieuw Amerongen, G. P., Natarajan, K., Yin, G., Hoefen, R. J., Osawa, M., Haendeler, J., Ridley, A. J., Fujiwara, K., Van Hinsbergh, V. W., and Berk, B. C. (2004). GIT1 mediates thrombin signaling in endothelial cells. Role in turnover of RhoA-type focal adhesions. Circ Res. 94, 1041-1049. [DOI] [PubMed] [Google Scholar]
- Vitale, N., Patton, W. A., Moss, J., Vaughan, M., Lefkowitz, R .J., and Premont, R. T. (2000). GIT proteins, A novel family of phosphatidylinositol 3,4, 5-trisphosphate-stimulated GTPase-activating proteins for ARF6. J. Biol. Chem. 275, 13901-13906. [DOI] [PubMed] [Google Scholar]
- Voisin, L., Larose, L., and Meloche, S. (1999). Angiotensin II stimulates serine phosphorylation of the adaptor protein Nck: physical association with the serine/threonine kinases Pak1 and casein kinase I. Biochem. J. 341, 217-223. [PMC free article] [PubMed] [Google Scholar]
- Webb, D. J., Donais, K., Whitmore, L. A., Thomas, S. M., Turner, C. E., Parsons, J. T., and Horwitz, A. F. (2004). FAK-Src signalling through paxillin, ERK and MLCK regulates adhesion disassembly. Nat. Cell Biol. 6, 154-161. [DOI] [PubMed] [Google Scholar]
- West, K. A., Zhang, H., Brown, M. C., Nikolopoulos, S. N., Riedy, M. C., Horwitz, A. F., and Turner, C. E. (2001). The LD4 motif of paxillin regulates cell spreading and motility through an interaction with paxillin kinase linker (PKL). J. Cell Biol. 154, 161-176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin, G., Haendeler, J., Yan, C., and Berk, B. C. (2004). GIT1 functions as a scaffold for MEK1-extracellular signal-regulated kinase 1 and 2 activation by angiotensin II and EGF. Mol. Cell. Biol. 24, 875-885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhai, J., Lin, H., Nie, Z., Wu, J., Canete-Soler, R., Schlaepfer, W. W., and Schlaepfer, D. D. (2003). Direct interaction of focal adhesion kinase with p190RhoGEF. J. Biol. Chem. 278, 24865-24873. [DOI] [PubMed] [Google Scholar]
- Zhao, Z. S., Manser, E., and Lim, L. (2000a). Interaction between PAK and Nck: a template for Nck targets and role of PAK autophosphorylation. Mol. Cell. Biol. 20, 3906-3917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao, Z. S., Manser, E., Loo, T. H., and Lim, L. (2000b). Coupling of PAK-interacting exchange factor PIX to GIT1 promotes focal complex disassembly. Mol. Cell. Biol. 20, 6354-6363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou, G. L., Zhuo, Y., King, C. C., Fryer, B. H., Bokoch, G. M., and Field, J. (2003). Akt phosphorylation of serine 21 on Pak1 modulates Nck binding and cell migration. Mol. Cell. Biol. 23, 8058-8069. [DOI] [PMC free article] [PubMed] [Google Scholar]