Abstract
Toxoplasma gondii is an obligate intracellular parasite and an important human pathogen. Relatively little is known about the proteins that orchestrate host cell invasion by T. gondii or related apicomplexan parasites (including Plasmodium spp., which cause malaria), due to the difficulty of studying essential genes in these organisms. We have used a recently developed regulatable promoter to create a conditional knockout of T. gondii apical membrane antigen-1 (TgAMA1). TgAMA1 is a transmembrane protein that localizes to the parasite's micronemes, secretory organelles that discharge during invasion. AMA1 proteins are conserved among apicomplexan parasites and are of intense interest as malaria vaccine candidates. We show here that T. gondii tachyzoites depleted of TgAMA1 are severely compromised in their ability to invade host cells, providing direct genetic evidence that AMA1 functions during invasion. The TgAMA1 deficiency has no effect on microneme secretion or initial attachment of the parasite to the host cell, but it does inhibit secretion of the rhoptries, organelles whose discharge is coupled to active host cell penetration. The data suggest a model in which attachment of the parasite to the host cell occurs in two distinct stages, the second of which requires TgAMA1 and is involved in regulating rhoptry secretion.
INTRODUCTION
Toxoplasma gondii is one of the one most common protozoan parasites of humans, infecting approximately one-third of the world's population. The disease caused by an acute T. gondii infection, toxoplasmosis, can be life threatening in the congenitally infected fetus and immunocompromised hosts. Like all members of the Phylum Apicomplexa, including Plasmodium spp. (the causative agents of malaria) and Cryptosporidium parvum (a significant worldwide cause of water-borne illness), T. gondii is an obligate intracellular parasite. Host cell invasion is a critical step in the pathogenesis of the diseases caused by apicomplexan parasites, including toxoplasmosis. T. gondii represents an excellent model system for studying the relatively conserved process of apicomplexan invasion, because of the ease with which this parasite can be cultured and genetically manipulated (Roos et al., 1994; Black and Boothroyd, 2000; Kim and Weiss, 2004).
Host cell invasion by apicomplexan parasites is a complex, multistep process (reviewed in Black and Boothroyd, 2000; Chitnis and Blackman, 2000). In T. gondii, the asexual stage tachyzoites move over solid surfaces, including cells, by an unusual form of substrate-dependent gliding motility (Sibley et al., 1998; Hakansson et al., 1999). After the apical end of a parasite comes in contact with the host cell membrane, apical secretory organelles (micronemes and rhoptries) sequentially discharge their contents (Dubremetz et al., 1993; Carruthers and Sibley, 1997) and a zone of tight interaction forms between the two cells (Nichols and O'Connor, 1981; Dubremetz et al., 1985; Grimwood and Smith, 1995). An invagination in the host cell plasma membrane develops at the point of entry and progressively deepens, ultimately surrounding the fully internalized parasite (Suss-Toby et al., 1996).
Apical membrane antigen-1 (AMA1) is a microneme protein that is highly conserved among apicomplexan parasites (Waters et al., 1990; Donahue et al., 2000; Hehl et al., 2000; Gaffar et al., 2004). Since its initial discovery >15 years ago, AMA1 has received considerable attention as a malaria vaccine candidate (Deans et al., 1988; Collins et al., 1994; Anders et al., 1998; Kennedy et al., 2002; Stowers et al., 2002). AMA1 proteins are type I transmembrane proteins, with a short C-terminal cytoplasmic tail and a large N-terminal extracellular domain (ectodomain) containing 12-16 conserved cysteine residues (Waters et al., 1990; Hodder et al., 1996; Donahue et al., 2000; Hehl et al., 2000; Gaffar et al., 2004). Like other microneme proteins, AMA1 is secreted onto the parasite surface, where its ectodomain is proteolytically cleaved and shed (Narum and Thomas, 1994; Donahue et al., 2000; Hehl et al., 2000; Howell et al., 2003).
The recently determined crystal structure of Plasmodium vivax AMA1 demonstrates that the conserved cysteines divide the ectodomain into three distinct subdomains (Pizarro et al., 2005). Domains I and II belong to the PAN module superfamily, suggesting that they may function in adhesion to protein or carbohydrate receptors (Pizarro et al., 2005). Antibodies against AMA1 inhibit invasion (Thomas et al., 1984; Deans et al., 1988; Hehl et al., 2000; Kocken et al., 2000; Mitchell et al., 2004), as do phage-displayed peptides derived from (Urquiza et al., 2000) or with affinity for Plasmodium AMA1 (Li et al., 2002; Keizer et al., 2003). Together with trans-species complementation experiments (Triglia et al., 2000) and heterologous expression experiments (Fraser et al., 2001; Kato et al., 2005), these data suggest that AMA1 plays a role in host cell invasion, perhaps as an adhesin. However, it has not previously been possible to disrupt AMA1 for phenotypic analysis, in any apicomplexan parasite, presumably because AMA1 is an essential gene (Mital and Ward, unpublished results; Hehl et al., 2000; Triglia et al., 2000).
The recent development of a system for conditional gene expression in T. gondii has made it possible to study the function of essential genes by reverse genetics (Meissner et al., 2002). We have used the conditional expression system to generate a transgenic parasite line in which the levels of T. gondii AMA1 (TgAMA1) expression can be experimentally manipulated. Using these TgAMA1 conditional knockout parasites, we provide here direct genetic evidence that TgAMA1 functions in invasion. Systematic analysis of the individual steps in invasion shows that TgAMA1-depleted parasites are impaired in their ability to attach intimately to host cells and to regulate rhoptry secretion.
MATERIALS AND METHODS
Parasite and Host Cell Culture
Parasites were continually passaged in human foreskin fibroblast (HFF) cells as previously described (Roos et al., 1994). Parasites from fresh, spontaneously lysed HFF monolayers were added to new HFF monolayers and incubated at 37°C/5%CO2. One hour postinfection, fresh medium was added with or without 1.5 μg/ml anhydrotetracycline (Atc; Clontech, Palo Alto, CA). Thirty-six hours later, unless otherwise stated, parasites and infected cells were harvested with a cell scraper, passed through a 27-gauge needle, filtered through a 3-μm Nuclepore membrane (Whatman, Florham Park, NJ), and centrifuged (1000 × g, 4 min). Parasites were resuspended in Hanks' balanced salt solution containing 10 mM HEPES, pH 7.0 supplemented with 1% (vol/vol) fetal calf serum (HHFCS), and counted.
Generation of the Conditional Knockout
The TgAMA1 open reading frame with a C-terminal myc tag was inserted in p7TetOS1 downstream of the inducible promoter (Meissner et al., 2001), and a chloramphenicol acetyl transferase cassette was inserted upstream, generating p7TetOS1/AMA1-myc/CAT. Transactivator-expressing parasites (Meissner et al., 2002), designated here as “AMA1” parasites, were transfected with p7TetOS1/AMA1-myc/CAT. Parasites harboring the construct were selected with chloramphenicol and cloned by limiting dilution. Positive clones were identified by SDS-PAGE/Western blotting and immunofluorescence microscopy with monoclonal antibodies (mAbs) against TgAMA1 (B3.90, Donahue et al., 2000) and against myc (9E10, Clontech). The clone expressing the highest level of Atc-regulatable, properly localized TgAMA1-myc (designated “AMA1/AMA1-myc”) was used to generate the TgAMA1 knockout.
A knockout construct was engineered with the bleomycin-selectable marker (Soldati et al., 1995) flanked 5′ by 2361 base pairs of TgAMA1 genomic sequence (base pairs 29-2390, using the A of the start codon as base pair 1) and 3′ by 2521 base pairs of TgAMA1 genomic sequence (base pairs 2533-5054). This created both a 5′ truncation and a 142-base pair internal deletion that resulted in the loss of multiple conserved cysteines thought to be important for protein folding (Hodder et al., 1996). AMA1/AMA1-myc parasites were transfected with the knockout construct. Integrants were selected with phleomycin and cloned by limiting dilution. Parasites with a disrupted TgAMA1 gene (designated “Δama1/AMA1-myc”) were identified by PCR and Western blotting with mAb B3.90, and disruption of the wild-type locus was confirmed by Southern blot analysis. Western blotting with antisera against residues 21-36, 69-410, and 166-379 of TgAMA1 (Hehl et al., 2000; generously provided by Dr. John Boothroyd, Stanford University) confirmed the absence of partial TgAMA1 translation products in the Δama1/AMA1-myc parasites.
Quantitation of TgAMA1/TgAMA1-myc Expression
To determine the relative levels of TgAMA1 and TgAMA1-myc expression in the various parasite lines, total parasite extracts were resolved by SDS/PAGE and analyzed by Western blotting with mAb B3.90 using enhanced chemiluminescence detection reagents (Amersham Biosciences, Piscataway, NJ). Signal intensity was quantified, in three independent experiments, using a Fluor S MultiImager equipped with a CCD camera and Quant1 software (Bio-Rad, Hercules, CA). The sensitivity and linear range of the assay was determined by quantifying serially diluted extracts from AMA1/AMA1-myc parasites (-Atc), together with extracts from 7.5 × 107 AMA1/AMA1-myc and Δama1/AMA1-myc parasites (each grown with or without Atc). The maximum number of Δama1/AMA1-myc parasites (grown with Atc) that could be loaded on the gel with satisfactory resolution (7.5 × 107) showed no detectable TgAMA1 band on the Western blot. The minimum number of Δama1/AMA1-myc parasites (grown without Atc) producing a detectable band was used to determine the maximum relative amount of TgAMA1-myc that could be present in 7.5 × 107 parasites but undetectable after incubation with Atc (results from two independent experiments). All TgAMA1 signals were normalized to the corresponding actin signal in the same lane (antiactin antibody generously provided by Dr. David Sibley, Washington University).
Invasion and Attachment Assays
Host cell invasion and attachment were measured using laser scanning cytometer-based assays, as described elsewhere (Mital et al., manuscript in preparation). Briefly, for the invasion assay, parasites (AMA1, AMA1/AMA1-myc, and Δama1/AMA1-myc) grown with or without Atc were added to HFF monolayers and incubated at 37°C. At the specified times (10 min to 1 h), the coverslips were fixed, blocked, and labeled with an anti-SAG1 mAb (mAb GII-9; Argene, North Massapequa, NY) followed by an R-phycoerythrin-conjugated secondary antibody (“orange,” DAKO, Carpenteria, CA). Samples were then permeabilized, blocked, and labeled with anti-SAG1 followed by an Alexa647-conjugated secondary antibody (“red,” Molecular Probes, Eugene OR). Samples were analyzed on a CompuCyte laser scanning cytometer, and data were acquired and analyzed using Wincyte 3.4 Software (CompuCyte, Cambridge, MA). Red parasites were counted to determine the total number of parasites per field. The number of orange, extracellular parasites was counted and subtracted from the total to calculate the number of invaded parasites per field.
For the attachment assay, Δama1/AMA1-myc parasites grown with or without Atc were labeled for 15 min at 23°C with anti-SAG1 that had been directly conjugated to either Alexa488 (“green”) or Alexa647 (“red”). Fluorescently labeled parasites from each preparation (+/- Atc), labeled either green or red, were added pairwise to either fixed or unfixed HFF monolayers. After allowing the parasites to settle and attach for 15 min at 37°C, coverslips were washed three times in phosphate-buffered saline (PBS), permeabilized, and fixed. The number of green and red parasites in each sample was determined using the laser scanning cytometer, and the ratio was used as a measure of relative attachment.
Intracellular Replication Assay
Equal numbers of AMA1/AMA1-myc and Δama1/AMA1-myc parasites were plated onto confluent HFF monolayers on coverslips and allowed to invade for 1 h. Parasites were then incubated for an additional 12, 18, 24, or 30 h in the presence or absence of 1.5 μg/ml Atc. The coverslips were fixed with 2.5% (vol/vol) formaldehyde/0.05% (vol/vol) glutaraldehyde in PBS for 30 min and permeabilized with 0.25% (vol/vol) Triton-X 100 in PBS for 30 min. Samples were blocked with PBS containing 2% (wt/vol) bovine serum albumin (PBS-2% BSA). Parasites were labeled with anti-SAG1 diluted to 0.5 μg/ml in PBS-2% BSA followed by Alexa488-conjugated goat anti-mouse IgG. Coverslips were mounted and examined by immunofluorescence microscopy. The number of parasites/vacuole was counted blindly (200 vacuoles/sample), in each of two independent experiments.
Secondary Assays
Parasite motility was assayed by trail deposition as previously described (Dobrowolski and Sibley, 1996; Carey et al., 2004b). To observe gliding motility in real time, freshly harvested Δama1/AMA1-myc parasites, grown with or without Atc, were filtered, pelleted (2 min at 500 × g), and resuspended to 5 × 107 tachyzoites/ml in HHFCS. Parasites were added to eight-well chamber coverglasses (Nalge Nunc, Rochester, NY) that either had been pretreated (1 h at 37°C) with PBS containing 0.5% (wt/vol) BSA, or upon which HFF monolayers had been grown to confluence. Gliding motility was visualized at 37°C on a Nikon TE300 inverted microscope with Nomarski optics (Melville, NY). Video data were collected with a VE1000SIT camera (Dage-MTI, Michigan City, IN).
Conoid extension was assayed as previously described (Mondragon and Frixione, 1996; Carey et al., 2004b). At least 200 parasites from each parasite line (grown with or without Atc) were counted blindly, in each of two independent experiments.
Microneme secretion was assayed as previously described (Carruthers and Sibley, 1999; Carey et al., 2004b), except that induced secretion was assayed after 7 min rather than 5 min, and Western blots were probed with both anti-MIC5 and anti-MIC10 antibodies (generous gifts of Dr. Vern Carruthers, Johns Hopkins University). Western blotting for actin released into the assay supernatant was used as a control for loading and parasite lysis.
To assay evacuole formation (Hakansson et al., 2001), Δama1/AMA1-myc parasites, grown with or without Atc, were harvested, filtered, pelleted (1000 × g, 4 min) and resuspended (107 tachyzoites/ml) in 44.7 mM K2SO4, 10 mM Mg2SO4, 106 mM sucrose, 5 mM glucose, 20 mM Tris, 0.35% wt/vol BSA, pH 8.2 (Endo and Yagita, 1990; Kafsack et al., 2004) containing 1 μM cytochalasin D (CytD; Sigma-Aldrich, St. Louis, MO). After 10 min at 23°C, 3 × 106 pretreated parasites were added to confluent HFF monolayers on 12-mm coverslips and incubated for 20 min at 37°C. The buffer was then replaced with prewarmed HHFCS containing 1 μM CytD, and the samples incubated at 37°C for another 15 min. Monolayers were gently washed three times with PBS, fixed in 100% methanol on ice for 10 min, and labeled with mouse anti-ROP1 (mAb Tg49; a generous gift from Dr. Joseph Schwartzman, Dartmouth College) or mouse anti-ROP2,3,4 (Mab T3 4A7; a generous gift from Dr. Jean-Francois Dubremetz, Université Montpellier) followed by Alexa488-conjugated goat anti-mouse IgG. Coverslips were examined by immunofluorescence microscopy. For each set of parasites, the number of evacuoles associated with at least 500 parasites was counted blindly. It was also determined, for at least 500 parasites associated with evacuoles, whether the evacuole was greater or less than one parasite in length. All evacuole experiments were done in duplicate.
Electron Microscopy
HFFs were grown overnight on 12-mm Millicell-PCF culture plate filter inserts (Millipore, Bedford, MA). Atc-treated or untreated Δama1/AMA1-myc parasites were added and allowed to settle for 20 min at 23°C. The inserts were placed in prewarmed media in 12-well plates at 37°C. After 1 min, the cells were washed with PBS and were fixed with Karnovsky's reagent (1% [wt/vol] paraformaldehyde, 2.5% [vol/vol] glutaraldehyde) for 1 h at 4°C. Filters were washed three times in Millonig's phosphate buffer (pH 7.2) and postfixed in 1% (wt/vol) OsO4 for 45 min at 4°C. After three washes in Millonig's, samples were sequentially dehydrated in 35, 50, 70, 85, 95% (vol/vol) EtOH for 10 min each and six times for 5 min each in 100% EtOH. Samples were then sequentially infiltrated with 3:1, 1:1, 1:3 (100% EtOH:100% Spurr's resin), and 100% Spurr's for 30, 30, 45, and 45 min, respectively. Samples were then embedded in 100% Spurr's and polymerized overnight at 65°C. Ultrathin sections were placed on copper or nickel grids, and contrasted with 2% (wt/vol) uranyl acetate in 50% (vol/vol) EtOH for 6 min and lead citrate for 4 min. Samples were analyzed on a JEOL 1210 transmission electron microscope (Peabody, MA). For CytD-treated samples, parasites were incubated with 1 μM CytD for 10 min at 23°C and allowed to settle for 12 min at 23°C before a 1-min incubation at 37°C.
RESULTS
Generation and Analysis of TgAMA1 Conditional Knockout Parasites
We and others have been unable to knock out TgAMA1 in wild-type parasites, suggesting that the gene is essential (Mital and Ward, unpublished results; Hehl et al., 2000). We therefore sought to introduce extra copies of TgAMA1, under the control of a conditional promoter, into the wild-type AMA1 background. If the wild-type allele could then be knocked out, expression of the remaining copies would be under experimental control, providing a means to determine the phenotype of TgAMA1-deficient parasites.
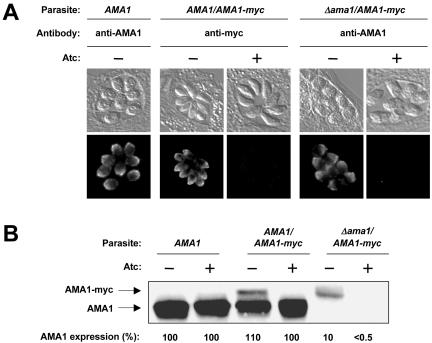
Parasites expressing an Atc-responsive transactivator protein (which express only endogenous AMA1 and are thus designated “AMA1” parasites) were transfected with a recombinant myc-tagged version of TgAMA1 under the control of a SAG1-based conditional promoter (Meissner et al., 2002). The addition of Atc to transactivator-expressing parasites results in a significant decrease in the activity of the conditional promoter (Meissner et al., 2002). Western blotting and immunofluorescence microscopy with antibodies directed against either TgAMA1 or the myc tag were used to identify a clone (designated “AMA1/AMA1-myc”) that stably expresses Atc-regulated TgAMA1-myc with an apical localization indistinguishable from endogenous TgAMA1 (see Figure 1A). Addition of the myc tag to TgAMA1 resulted in an electrophoretic mobility shift that allowed differentiation between endogenous TgAMA1 and TgAMA1-myc on Western blots (Figure 1B). Quantitative Western blotting of the AMA1/AMA1-myc parasites grown without or with Atc showed that the level of TgAMA1-myc expression is 10% and <0.5% that of endogenous TgAMA1 expression, respectively. A decrease in TgAMA1-myc expression is first observed by immunofluorescence microscopy and on Western blots 6-12 h after Atc addition (unpublished data) and expression is undetectable by 24 h (Figure 1, A and B).
Figure 1.
Conditional expression of TgAMA1-myc. (A) Immunofluorescence localization of TgAMA1 and TgAMA1-myc in wild-type (AMA1), AMA1/AMA1-myc, and conditional knockout (Δama1/AMA1-myc) parasites after a 24-h incubation with or without Atc. In the AMA1/AMA1-myc parasites, TgAMA1-myc shows Atc-regulated expression and an apical distribution that is indistinguishable from TgAMA1 in the AMA1 parasites. No TgAMA1, myc-tagged or endogenous, is detected in the knockout parasites after Atc treatment. (B) AMA1, AMA1/AMA1-myc, and Δama1/AMA1-myc parasites were each grown with or without Atc for 24 h, extracted, and probed on Western blots with an anti-TgAMA1 antibody. Only the relevant (60-70 kDa) portion of the blot is shown. The combined TgAMA1 and TgAMA1-myc expression levels in each of the parasite lines were determined by quantitative Western blotting; average results from multiple independent experiments (see Materials and Methods), relative to the AMA1 parasites, are shown below each lane.
AMA1/AMA1-myc parasites were transfected with a knockout plasmid to disrupt the endogenous, single-copy TgAMA1 locus. Sequences at the extreme 5′ and 3′ end of TgAMA1 that were excluded from the knockout construct allowed PCR-based screening for knockout plasmid integration into the genomic TgAMA1 locus. Generation of a viable TgAMA1 knockout clone (designated “Δama1/AMA1-myc”) was possible in parasites expressing inducible TgAMA1-myc, but not in the wild-type background, providing further evidence that TgAMA1 is an essential gene. Western blotting and immunofluorescence microscopy showed that the Δama1/AMA1-myc parasites do not express endogenous TgAMA1 and express detectable TgAMA1-myc only in the absence of Atc (Figure 1, A and B). Southern analysis confirmed disruption of the TgAMA1 locus and indicated that 4-5 copies of the regulatable TgAMA1-myc construct had integrated into the genome of this parasite (unpublished data). The sequential generation of the conditional knockout provided us with parasites expressing a range of TgAMA1 levels (Figure 1B) for phenotypic comparison.
TgAMA1 Plays No Detectable Role in Intracellular Replication
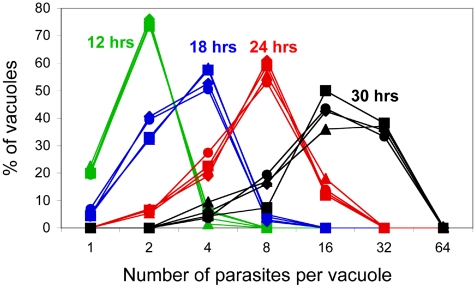
The intracellular replication rates of the AMA1/AMA1-myc and Δama1/AMA1-myc parasites were compared in the presence and absence of Atc (Figure 2). All parasites exhibited similar replication rates, demonstrating that TgAMA1 does not function in parasite intracellular replication. The similarity in the growth curves facilitates comparative functional assays of the different parasite populations (e.g., invasion assays; see below), by ensuring that each assay begins with parasites at equivalent stages of their life cycle.
Figure 2.
TgAMA1 plays no detectable role in parasite intracellular replication. The intracellular replication of AMA1/AMA1-myc parasites and Δama1/AMA1-myc parasites was monitored in the presence or absence of Atc (AMA1/AMA1-myc parasites -Atc ▴, +Atc ♦; Δama1/AMA1-myc parasites -Atc •, +Atc ▪). The number of parasites in each of 200 vacuoles was counted for each parasite line at various times after drug addition (green curves, 12 h; blue curves, 18 h; red curves, 24 h; black curves, 30 h).
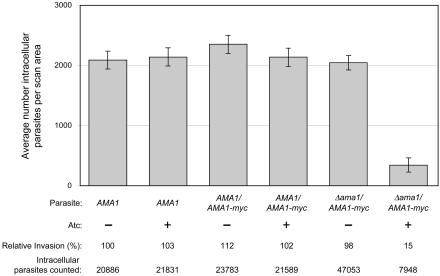
TgAMA1 Depletion Results in a Significant Decrease in Invasion
The invasiveness of the AMA1, AMA1/AMA1-myc, and Δama1/AMA1-myc parasites, each grown with or without Atc, was compared using a laser scanning cytometer-based invasion assay (Figure 3). In this assay, equal numbers of parasites are added to each coverslip and the numbers of intracellular parasites within identically sized scan areas are automatically counted to measure invasion (Mital et al., manuscript in preparation). There is an 83% decrease in the ability of the conditional knockout parasites grown in the presence of Atc to invade host cells, when compared with the same parasites grown in the absence of Atc (Figure 3; significant at p < 0.001), demonstrating that TgAMA1 plays a critical role in host cell invasion. The time allowed for invasion was varied (from 10 min to 1 h), as was the multiplicity of infection, and in all cases the results were similar: parasites lacking TgAMA1 are significantly less invasive. Interestingly, there was no difference at any time point in the invasiveness of parasites expressing wild-type levels of TgAMA1 (AMA1 parasites grown without Atc) and parasites expressing 90% less TgAMA1 (Δama1/AMA1-myc parasites grown without Atc; Figure 3). Wild-type parasites apparently express at least 10 times more TgAMA1 than is needed for efficient host cell invasion.
Figure 3.
A TgAMA1 deficiency results in significantly reduced invasion. Host cell invasion by the AMA1, AMA1/AMA1-myc, and Δama1/AMA1-myc parasites, each grown with or without Atc, was measured 1 h postinfection using the laser scanning cytometer-based invasion assay. The average number of intracellular parasites per scan area from at least five independent experiments is presented, with error bars representing the SE of the mean. The invasion levels for each parasite population (expressed relative to the AMA1 parasites, -Atc) are shown. The total number of intracellular parasites counted is also listed below each sample; parasite counts in the tens of thousands are readily attainable, illustrating the sampling power of the assay. Note that more samples comparing Δama1/AMA1-myc parasites -/+ Atc were assayed, resulting in higher numbers of total intracellular parasites counted in the -Atc sample. As previously reported (Meissner et al., 2002), Atc treatment itself has no effect on the invasiveness of parasites (compare AMA1 parasites, -/+ Atc).
A TgAMA1 Deficiency Does Not Affect the Early Steps of Invasion
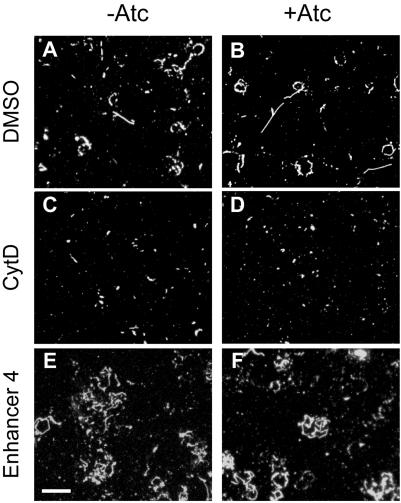
Motility. Gliding motility and parasite entry into the host cell are thought to be driven by the same myosin-based motor complex (Meissner et al., 2002; Gaskins et al., 2004; Soldati and Meissner, 2004). To determine whether TgAMA1 plays a role in parasite motility, the motility of the conditional knockout parasites was assayed both by examining the number and length of “slime trails” deposited by parasites as they glide on a glass coverslip (Dobrowolski and Sibley, 1996; Carey et al., 2004b) and by videomicroscopy (Hakansson et al., 1999; Carey et al., 2004b). No differences were observed in either assay between the Δama1/AMA1-myc parasites grown with or without Atc (Figure 4, A and B and unpublished data). The two parasite populations also showed similar decreases in trail deposition (Figure 4, C and D) after CytD treatment (Dobrowolski and Sibley, 1996), and increases in trail deposition (Figure 4, E and F) after treatment with a recently identified small molecule enhancer of motility (Enhancer 4; Carey et al., 2004b). Similar results were obtained when AMA1/AMA1-myc and Δama1/AMA1-myc parasites were compared (unpublished data). TgAMA1 therefore plays no detectable role in parasite gliding motility, and TgAMA1-deficient parasites remain responsive to drugs that affect motility.
Figure 4.
TgAMA1 depletion does not affect parasite motility. Δama1/AMA1-myc parasites, grown without (A, C, and E) or with (B, D, and F) Atc, were pretreated with dimethyl sulfoxide (A and B), the motility inhibitor CytD (C and D), or a motility enhancer (E and F; Enhancer 4; Carey et al., 2004b) and plated onto BSA-treated coverglasses. The trails deposited by moving parasites were visualized by immunofluorescence microscopy using an antibody against the surface protein SAG1. Scale bar (bottom left), 20 μm.
Conoid Extension. The conoid is a tubulin-based structure that extends and retracts from the apical tip of the parasite as the parasite moves (Hu et al., 2002; Morrissette and Sibley, 2002). The function of the conoid is unknown, but a recently described small molecule inhibitor of conoid extension inhibits invasion (Carey et al., 2004b). Conoid extension can also be inhibited by chelators of intracellular calcium, and extension can be induced by the calcium ionophore ionomycin (Mondragon and Frixione, 1996; Hu et al., 2002; Morrissette and Sibley, 2002). Conoid extension was compared in the AMA1/AMA1-myc and Δama1/AMA1-myc parasites, grown with or without Atc. The percentage of parasites with an extended conoid after ionomycin treatment was similar in all cases, as was the percentage of untreated parasites (Table 1), indicating that TgAMA1 does not play a role in conoid extension.
Table 1.
TgAMA1 depletion has no effect on either constitutive or ionomycin-induced conoid extension
| − Ionomycin
|
+ Ionomycin
|
|||
|---|---|---|---|---|
| Parasites | − Atc | + Atc | − Atc | + Atc |
| AMA1/AMA1-myc | 17.1 (4.8) | 18.1 (8.2) | 73.7 (6.9) | 75.9 (8.4) |
| Δama1/AMA1-myc | 15.3 (2.5) | 15.2 (7.4) | 67.7 (3.3) | 74.9 (0.4) |
Numbers indicate % of parasites with an extended conoid (average results from 2 independent experiments; SD in parentheses).
Microneme Secretion. Microneme secretion is critical to invasion by apicomplexan parasites (Carruthers et al., 1999; Soldati et al., 2001). We therefore investigated whether a TgAMA1 deficiency altered the secretion of other microneme proteins, using MIC5 as a marker. The amount of MIC5 secreted into the culture supernatant by Δama1/AMA1-myc parasites was analyzed by Western blotting. There was no observable difference in the amount of MIC5 secreted, either constitutively or in response to calcium ionophore (Carruthers and Sibley, 1999; Lovett et al., 2002), by parasites grown with or without Atc (Figure 5). Similar results were seen with a second microneme protein, MIC10, and when AMA1/AMA1-myc and Δama1/AMA1-myc parasites were compared (unpublished data). TgAMA1 therefore does not appear to function in the regulation of microneme secretion.
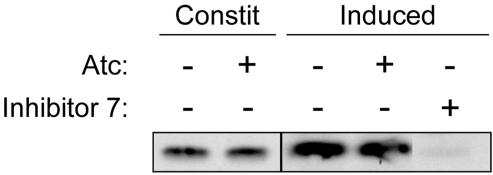
Figure 5.
TgAMA1 depletion does not affect microneme secretion. Δama1/AMA1-myc parasites were grown with or without Atc, and the amount of MIC5 secreted by each, either constitutively (Constit) or in response to the calcium ionophore ionomycin (Induced), was determined by Western blotting. Only the relevant (20-25 kDa) portion of the blot is shown. Inhibitor 7 significantly blocks secretion, as previously described (Carey et al., 2004b) and was included as a control. The lanes shown were from the same blot and were exposed and adjusted for contrast and brightness identically.
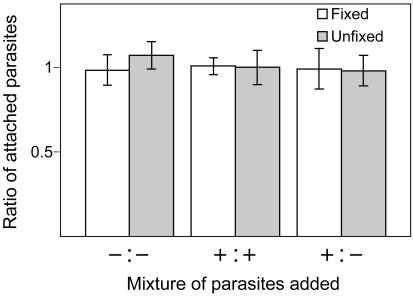
Attachment. Many microneme proteins are thought to function as host cell adhesins (Tomley and Soldati, 2001), and AMA1 has been postulated to play such a role (Fraser et al., 2001; Kato et al., 2005; Pizarro et al., 2005). The ability of AMA1/AMA1-myc and Δama1/AMA1-myc parasites to bind to host cells was therefore compared using a laser scanning cytometer-based attachment assay. In this assay, the two populations of parasites to be compared are differentially fluorescently labeled and mixed in a 1:1 ratio on the same coverslip. The ratio of bound parasites is then determined using the laser scanning cytometer, yielding a highly reproducible measure of relative attachment (Mital et al., manuscript in preparation). Both fixed and unfixed host cells (Mineo et al., 1993; Ortega-Barria and Boothroyd, 1999; Jacquet et al., 2001; Kieschnick et al., 2001) were tested in the assay, because there appear to be subtle differences in the way parasites attach to live versus fixed host cells (Mital et al., manuscript in preparation). Figure 6 shows that the conditional knockout parasites, grown with or without Atc, attach to either fixed or unfixed host cells in a 1:1 ratio, indicating equivalent binding. Coverslips containing mixtures of the same parasites (Figure 6, -Atc:-Atc or +Atc: +Atc) were used as controls to confirm the accuracy of the assay (Mital et al., manuscript in preparation). When Δama1/AMA1-myc parasites (+Atc), which express the minimal amount of TgAMA1 (<0.5% of wild-type levels; Figure 1B), were compared pairwise with AMA1/AMA1-myc parasites (-Atc), which express the maximal amount of TgAMA1 (110% of wild-type; Figure 1B), equivalent binding was again observed (unpublished data).
Figure 6.
TgAMA1 depletion does not affect binding to host cells. Δama1/AMA1-myc parasites, grown with (+) or without (-) Atc, were labeled with Alexa488 (green) or Alexa647 (red), mixed in a 1:1 ratio, allowed to bind to either fixed (white bars) or unfixed (gray bars) host cells for 15 min, and counted using the laser scanning cytometer. The ratio of red:green parasites is shown, with error bars representing the SD; a ratio of 1 indicates equivalent binding. Mixtures of the same parasites (-:- or +:+) were used as controls for parasite counting and labeling (Mital et al., manuscript in preparation).
Parasites Depleted of TgAMA1 Are Defective in Rhoptry Secretion
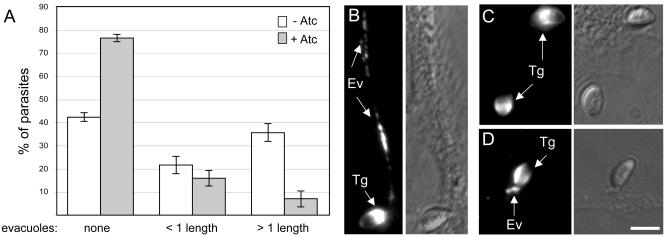
Secretion of proteins from the rhoptries occurs subsequent to microneme discharge and is coupled to active host cell penetration (Carruthers and Sibley, 1997). CytD treatment inhibits parasite motility and invasion but does not prevent attachment or rhoptry secretion (Carruthers and Sibley, 1997; Hakansson et al., 2001). CytD-arrested parasites secrete the contents of their rhoptries into host cells in the form of small vesicular clusters, termed evacuoles, which can be visualized by immunofluorescence microscopy with antibodies against ROP1 and other rhoptry proteins (Hakansson et al., 2001; Carey et al., 2004a). To determine whether TgAMA1 depletion affects rhoptry secretion, the percentage of CytD-arrested parasites associated with ROP1-containing evacuoles was compared for Δama1/AMA1-myc parasites grown with or without Atc. TgAMA1-depleted parasites form significantly fewer evacuoles (Figure 7A), and those evacuoles that do form in the TgAMA1-depleted parasites are significantly shorter (Figure 7A; see Figure 7, B-D, for representative images). The net result is a >80% decrease in the formation of long evacuoles (i.e., more than one parasite length) by the TgAMA1-depleted parasites. Fewer evacuoles were also observed with the TgAMA1-deficient parasites using an antibody that recognizes ROP2, 3, and 4, indicating a general effect on rhoptry secretion, rather than a specific defect in the secretion of ROP1.
Figure 7.
TgAMA1 depletion significantly decreases ROP1 secretion into evacuoles. Δama1/AMA1-myc parasites, grown with or without Atc, were incubated with host cells in the presence of CytD. The evacuoles formed were visualized by immunofluorescence microscopy using an antibody against ROP1. (A) Percentage of attached Δama1/AMA1-myc parasites, grown with (gray bars) or without (white bars) Atc, that are associated with: no evacuoles (left bars); evacuoles shorter than one parasite in length (less than ∼7 μm; middle bars); or evacuoles longer than one parasite in length (right bars). Error bars represent the SE of the mean. (B) Representative immunofluorescence image of a long evacuole generated by a Δama1/AMA1-myc parasite grown without Atc. (C and D) Representative immunofluorescence images of Δama1/AMA1-myc parasites grown in the presence of Atc, which, although attached, generate either no detectable evacuoles (C) or short evacuoles (D) within the host cell. The corresponding differential interference contrast images are shown to the right of each fluorescence image. Arrows indicate parasites (Tg) or evacuoles (Ev). Scale bar (bottom right), 5 μm.
TgAMA1-depleted Parasites Frequently Do Not Progress beyond the Initial, Distant Attachment Stage of Invasion
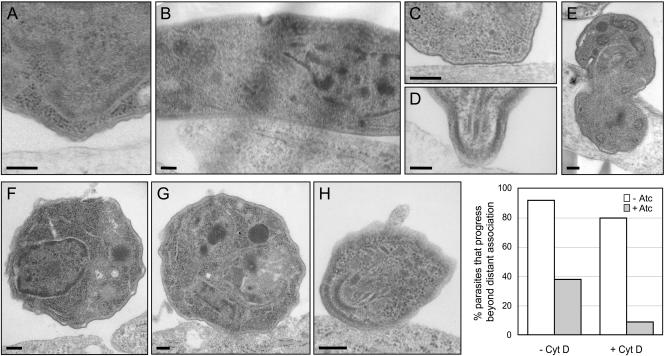
Invasion can be resolved into a series of distinct steps by transmission electron microscopy, including: initial attachment of the parasite to the host cell surface at a distance of >6 nm (typically >40 nm; Figure 8A); more intimate (<6 nm) and often extensive interaction between the membranes of the two cells (Figure 8B); intimate interaction between the apical end of the parasite and the host cell (Figure 8, C and D); and active penetration (Figure 8E). Two hundred profiles of Δama1/AMA1-myc parasites, grown either with or without Atc, were examined 1 min postinfection. The Atc-treated parasites (Figure 8A) were less frequently observed beyond the initial, distant attachment step, when compared with the same parasites grown in the absence of Atc (Figures 8, B-E, and graph). Similar differences between untreated and Atc-treated parasites were observed at 2 and 10 min postinfection. These differences were even greater for CytD-treated parasites: intimate attachment was observed in only 9% of the Δama1/AMA1-myc parasites grown in the presence of Atc, compared with 80% of the parasites grown in the absence of Atc (Figure 8, F-H, and graph).
Figure 8.
Ultrastructural characterization of the interaction between TgAMA1-depleted parasites and host cells. Δama1/AMA1-myc parasites were incubated with host cells for 1 min at 37°C, fixed, and examined by transmission electron microscopy. Representative images of parasites, grown with (A) or without (B-E) Atc and in various states of interaction with host cells, are shown. In most profiles of TgAMA1-depleted tachyzoites examined, the parasite had not progressed beyond the distant attachment step (A). In contrast, the same parasites grown in the absence of Atc were frequently observed in intimate lateral (B) or apical (C and D) association with the host cell membrane or were found partially (E) or fully (unpublished data) internalized. When the parasites were pretreated with CytD before addition to the host cells, the majority of the Atc-treated, TgAMA1-depleted parasites again had not progressed beyond the initial attachment phase in the profiles examined (e.g., F), whereas most of those grown in the absence of Atc progressed to the point of forming intimate (G), often apical (H), associations with the host cell. Scale bars, 200 nm. The percentage of Δama1/AMA1-myc parasites (+/- Atc) that progress beyond distant association with the host cell, with or without CytD pretreatment, is shown in the graph.
DISCUSSION
The development of a system for conditional gene expression in T. gondii (Meissner et al., 2002), also applied recently to P. falciparum (Meissner et al., 2005), has provided a means to study the function of essential genes in apicomplexan parasites. In the second successful application of this system we have shown directly that TgAMA1 functions in host cell invasion.
We consistently observed an ∼85% reduction in invasiveness of the Δama1/AMA1-myc parasites after growth in Atc, when compared with either the same parasites without Atc treatment or to wild-type parasites. The residual (∼15%) invasion seen in the conditional knockout parasites after Atc treatment may be due to incomplete shutoff of TgAMA1-myc expression. Given that the amount of TgAMA1 necessary for efficient invasion shows a relatively low threshold, i.e., somewhere between 10 and 0.5% that of wild-type levels, it is conceivable that a small percentage of the parasites in any given population have TgAMA1-myc levels above the threshold. Alternatively, the residual invasion could be due to a second, TgAMA1-independent pathway for parasite invasion. Such redundant invasion pathways are known to exist in Plasmodium (Gaur et al., 2004). The presence of two additional, less well-conserved AMA1 homologues in the genome of T. gondii (TgTwinScan 0701 and 5064; www.Toxodb.org) suggests potential candidates for future study.
One significant advantage to studying host cell invasion in T. gondii, compared with other apicomplexan parasites, is that assays are available to examine the individual steps of T. gondii invasion in isolation. Using these assays, we have shown that the TgAMA1-deficient parasites move, extrude their conoids, secrete the contents of their micronemes, and attach at a distance to host cells in a manner indistinguishable from wild-type parasites. However, the TgAMA1-depleted parasites appear to be impaired in their ability to form intimate (<6 nm) membrane contacts with the host cell. These data suggest a model in which TgAMA1 functions as a secondary adhesin, enabling an initial interaction—presumably mediated by some other adhesin—to be consolidated into a more intimate interaction between the two cells. This model is consistent with studies in P. knowlesi showing that an antibody against AMA1 inhibits close junctional contact of merozoites and erythrocytes (Mitchell et al., 2004) and with structural studies of P. vivax AMA1 revealing the presence of potentially adhesive PAN domains (Pizarro et al., 2005).
The TgAMA1-depleted parasites are also significantly impaired in rhoptry secretion, as assayed by evacuole formation. The data suggest a defect in rhoptry secretion, rather than an inability of rhoptry material secreted from distantly attached parasites to generate evacuoles, as ROP1 was not detectable at the parasite-host cell interface by immunofluorescence (unpublished data). In contrast to microneme and dense granule secretion, which can occur constitutively, rhoptry secretion is thought to be a tightly regulated process (Carruthers et al., 1999). Nothing is known about the signaling mechanisms underlying rhoptry secretion, although there may be a role for host cell cholesterol (Coppens and Joiner, 2003). Our data support a model in which TgAMA1-mediated intimate association with the host cell is a critical step in the pathway leading to rhoptry discharge, although we cannot currently rule out the converse, i.e., that rhoptry secretion is necessary for intimate attachment. Given the transmembrane topology and potential adhesive function of TgAMA1, TgAMA1 could be involved in consolidating interaction with the host cell, initiating the intracellular signaling events that lead to rhoptry secretion, or both.
In addition to providing insights into the function of a model AMA1 protein, the availability of a parasite background in which TgAMA1 expression can be experimentally controlled will facilitate mutational analysis of AMA1 functional domains, trans-species complementation studies and investigations into the functional significance of AMA1 cleavage and shedding from the parasite surface. Given the importance of AMA1 in invasion and the central role invasion plays in pathogenesis, these studies will likely have implications for vaccine design and for the development of new chemotherapeutic approaches to interfering with AMA1 function.
Acknowledgments
We thank Jeff Buzas and Alan Howard (University of Vermont Statistical Consulting Clinic) for their assistance with statistical analysis; Jan Schwarz and Nicole DeLance for assistance with electron microscopy and the laser scanning cytometer; Mariana Matrajt, Doug Johnson, Stacey Gilk, Kim Carey, and members of the Ward laboratory for helpful comments on the manuscript; and Mike Wichroski, John Boothroyd and David Alexander for many helpful discussions. This work was supported by Public Health Service Grants AI063276 and AI01719 (G.W.), the Burroughs Wellcome Fund (G.W.), a Kauffman fellowship from the University of Vermont College of Medicine (J.M.), the DFG and Wellcome Trust (D.S.), the Feodor-Lynen-Stipendium of the German Humboldt Gesellschaft and the BioFuture Programm of the Bundesmisnisterium für Bildung und Forschung (M.M.) and in part by Grant P30 CA22435 from the National Cancer Institute.
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E05-04-0281) on July 6, 2005.
Abbreviations used: TgAMA1, T. gondii apical membrane antigen-1; CytD, cytochalasin D; Atc, anhydrotetracycline; mAb, monoclonal antibody; HFF, human foreskin fibroblast.
References
- Anders, R. F., Crewther, P. E., Edwards, S., Margetts, M., Matthew, M. L., Pollock, B., and Pye, D. (1998). Immunisation with recombinant AMA-1 protects mice against infection with Plasmodium chabaudi. Vaccine 16, 240-247. [DOI] [PubMed] [Google Scholar]
- Black, M. W., and Boothroyd, J. C. (2000). Lytic cycle of Toxoplasma gondii. Microbiol. Mol. Biol. Rev. 64, 607-623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey, K. L., Jongco, A. M., Kim, K., and Ward, G. E. (2004a). The Toxoplasma gondii rhoptry protein ROP4 is secreted into the parasitophorous vacuole and becomes phosphorylated in infected cells. Eukaryot Cell 3, 1320-1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey, K. L., Westwood, N. J., Mitchison, T. J., and Ward, G. E. (2004b). A small-molecule approach to studying invasive mechanisms of Toxoplasma gondii. Proc. Natl. Acad. Sci. USA 101, 7433-7438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carruthers, V. B., Giddings, O. K., and Sibley, L. D. (1999). Secretion of micronemal proteins is associated with Toxoplasma invasion of host cells. Cell. Microbiol. 1, 225-235. [DOI] [PubMed] [Google Scholar]
- Carruthers, V. B., and Sibley, L. D. (1997). Sequential protein secretion from three distinct organelles of Toxoplasma gondii accompanies invasion of human fibroblasts. Eur. J. Cell Biol. 73, 114-123. [PubMed] [Google Scholar]
- Carruthers, V. B., and Sibley, L. D. (1999). Mobilization of intracellular calcium stimulates microneme discharge in Toxoplasma gondii. Mol. Microbiol. 31, 421-428. [DOI] [PubMed] [Google Scholar]
- Chitnis, C. E., and Blackman, M. J. (2000). Host cell invasion by malaria parasites. Parasitol Today 16, 411-415. [DOI] [PubMed] [Google Scholar]
- Collins, W. E. et al. (1994). Protective immunity induced in squirrel monkeys with recombinant apical membrane antigen-1 of Plasmodium fragile. Am. J. Trop. Med. Hyg. 51, 711-719. [DOI] [PubMed] [Google Scholar]
- Coppens, I., and Joiner, K. A. (2003). Host but not parasite cholesterol controls Toxoplasma cell entry by modulating organelle discharge. Mol. Biol. Cell 14, 3804-3820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deans, J. A., Knight, A. M., Jean, W. C., Waters, A. P., Cohen, S., and Mitchell, G. H. (1988). Vaccination trials in rhesus monkeys with a minor, invariant, Plasmodium knowlesi 66 kD merozoite antigen. Parasite Immunol. 10, 535-552. [DOI] [PubMed] [Google Scholar]
- Dobrowolski, J. M., and Sibley, L. D. (1996). Toxoplasma invasion of mammalian cells is powered by the actin cytoskeleton of the parasite. Cell 84, 933-939. [DOI] [PubMed] [Google Scholar]
- Donahue, C. G., Carruthers, V. B., Gilk, S. D., and Ward, G. E. (2000). The Toxoplasma homolog of Plasmodium apical membrane antigen-1 (AMA-1) is a microneme protein secreted in response to elevated intracellular calcium levels. Mol. Biochem. Parasitol 111, 15-30. [DOI] [PubMed] [Google Scholar]
- Dubremetz, J. F., Achbarou, A., Bermudes, D., and Joiner, K. A. (1993). Kinetics and pattern of organelle exocytosis during Toxoplasma gondii/host-cell interaction. Parasitol. Res. 79, 402-408. [DOI] [PubMed] [Google Scholar]
- Dubremetz, J. F., Rodriguez, C., and Ferreira, E. (1985). Toxoplasma gondii: redistribution of monoclonal antibodies on tachyzoites during host cell invasion. Exp. Parasitol. 59, 24-32. [DOI] [PubMed] [Google Scholar]
- Endo, T., and Yagita, K. (1990). Effect of extracellular ions on motility and cell entry in Toxoplasma gondii. J. Protozool. 37, 133-138. [DOI] [PubMed] [Google Scholar]
- Fraser, T. S., Kappe, S. H., Narum, D. L., VanBuskirk, K. M., and Adams, J. H. (2001). Erythrocyte-binding activity of Plasmodium yoelii apical membrane antigen-1 expressed on the surface of transfected COS-7 cells. Mol. Biochem. Parasitol. 117, 49-59. [DOI] [PubMed] [Google Scholar]
- Gaffar, F. R., Yatsuda, A. P., Franssen, F. F., and de Vries, E. (2004). Erythrocyte invasion by Babesia bovis merozoites is inhibited by polyclonal antisera directed against peptides derived from a homologue of Plasmodium falciparum apical membrane antigen 1. Infect. Immun. 72, 2947-2955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaskins, E., Gilk, S., DeVore, N., Mann, T., Ward, G., and Beckers, C. (2004). Identification of the membrane receptor of a class XIV myosin in Toxoplasma gondii. J. Cell Biol. 165, 383-393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaur, D., Mayer, D. C., and Miller, L. H. (2004). Parasite ligand-host receptor interactions during invasion of erythrocytes by Plasmodium merozoites. Int. J. Parasitol. 34, 1413-1429. [DOI] [PubMed] [Google Scholar]
- Grimwood, J., and Smith, J. E. (1995). Toxoplasma gondii: redistribution of tachyzoite surface protein during host cell invasion and intracellular development. Parasitol. Res. 81, 657-661. [DOI] [PubMed] [Google Scholar]
- Hakansson, S., Charron, A. J., and Sibley, L. D. (2001). Toxoplasma evacuoles: a two-step process of secretion and fusion forms the parasitophorous vacuole. EMBO J. 20, 3132-3144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hakansson, S., Morisaki, H., Heuser, J., and Sibley, L. D. (1999). Time-lapse video microscopy of gliding motility in Toxoplasma gondii reveals a novel, biphasic mechanism of cell locomotion. Mol. Biol. Cell 10, 3539-3547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hehl, A. B., Lekutis, C., Grigg, M. E., Bradley, P. J., Dubremetz, J. F., Ortega-Barria, E., and Boothroyd, J. C. (2000). Toxoplasma gondii homologue of Plasmodium apical membrane antigen 1 is involved in invasion of host cells. Infect. Immun. 68, 7078-7086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodder, A. N., Crewther, P. E., Matthew, M. L., Reid, G. E., Moritz, R. L., Simpson, R. J., and Anders, R. F. (1996). The disulfide bond structure of Plasmodium apical membrane antigen-1. J. Biol. Chem. 271, 29446-29452. [DOI] [PubMed] [Google Scholar]
- Howell, S. A., Well, I., Fleck, S. L., Kettleborough, C., Collins, C. R., and Blackman, M. J. (2003). A single malaria merozoite serine protease mediates shedding of multiple surface proteins by juxtamembrane cleavage. J. Biol. Chem. 278, 23890-23898. [DOI] [PubMed] [Google Scholar]
- Hu, K., Roos, D. S., and Murray, J. M. (2002). A novel polymer of tubulin forms the conoid of Toxoplasma gondii. J. Cell Biol. 156, 1039-1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacquet, A., Coulon, L., De Neve, J., Daminet, V., Haumont, M., Garcia, L., Bollen, A., Jurado, M., and Biemans, R. (2001). The surface antigen SAG3 mediates the attachment of Toxoplasma gondii to cell-surface proteoglycans. Mol. Biochem. Parasitol. 116, 35-44. [DOI] [PubMed] [Google Scholar]
- Kafsack, B., Beckers, C., and Carruthers, V. B. (2004). Synchronous invasion of host cells by Toxoplasma gondiii. Mol. Biochem. Parasitol. 136, 309-311. [DOI] [PubMed] [Google Scholar]
- Kato, K., Mayer, D.C.G., Singh, S., Reid, M., and Miller, L. H. (2005). Domain III of Plasmodium falciparum apical membrane antigen 1 binds to the erythrocyte membrane protein Kx. Proc. Natl. Acad. Sci. USA 102, 5552-5557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keizer, D. W., Miles, L. A., Li, F., Nair, M., Anders, R. F., Coley, A. M., Foley, M., and Norton, R. S. (2003). Structures of phage-display peptides that bind to the malarial surface protein, apical membrane antigen 1, and block erythrocyte invasion. Biochemistry 42, 9915-9923. [DOI] [PubMed] [Google Scholar]
- Kennedy, M. C., Wang, J., Zhang, Y., Miles, A. P., Chitsaz, F., Saul, A., Long, C. A., Miller, L. H., and Stowers, A. W. (2002). In vitro studies with recombinant Plasmodium falciparum apical membrane antigen 1 (AMA1): production and activity of an AMA1 vaccine and generation of a multiallelic response. Infect. Immun. 70, 6948-6960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kieschnick, H., Wakefield, T., Narducci, C. A., and Beckers, C. (2001). Toxoplasma gondii attachment to host cells is regulated by a calmodulin-like domain protein kinase. J. Biol. Chem. 276, 12369-12377. [DOI] [PubMed] [Google Scholar]
- Kim, K., and Weiss, L. M. (2004). Toxoplasma gondii: the model apicomplexan. Int. J. Parasitol. 34, 423-432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kocken, C. H., Narum, D. L., Massougbodji, A., Ayivi, B., Dubbeld, M. A., van der Wel, A., Conway, D. J., Sanni, A., and Thomas, A. W. (2000). Molecular characterisation of Plasmodium reichenowi apical membrane antigen-1 (AMA-1), comparison with P. falciparum AMA-1, and antibody-mediated inhibition of red cell invasion. Mol. Biochem. Parasitol. 109, 147-156. [DOI] [PubMed] [Google Scholar]
- Li, F., Dluzewski, A., Coley, A. M., Thomas, A., Tilley, L., Anders, R. F., and Foley, M. (2002). Phage-displayed peptides bind to the malarial protein apical membrane antigen-1 and inhibit the merozoite invasion of host erythrocytes. J. Biol. Chem. 277, 50303-50310. [DOI] [PubMed] [Google Scholar]
- Lovett, J. L., Marchesini, N., Moreno, S. N., and Sibley, L. D. (2002). Toxoplasma gondii microneme secretion involves intracellular Ca(2+) release from inositol 1,4,5-triphosphate (IP(3))/ryanodine-sensitive stores. J. Biol. Chem. 277, 25870-25876. [DOI] [PubMed] [Google Scholar]
- Meissner, M., Brecht, S., Bujard, H., and Soldati, D. (2001). Modulation of myosin A expression by a newly established tetracycline repressor-based inducible system in Toxoplasma gondii. Nucleic Acids Res. 29, E115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meissner, M., Krejany, E., Gilson, P. R., de Koning-Ward, T. F., Soldati, D., and Crabb, B. S. (2005). Tetracycline analogue-regulated transgene expression in Plasmodium falciparum blood stages using Toxoplasma gondii transactivators. Proc. Natl. Acad. Sci. USA 102, 2980-2985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meissner, M., Schluter, D., and Soldati, D. (2002). Role of Toxoplasma gondii myosin A in powering parasite gliding and host cell invasion. Science 298, 837-840. [DOI] [PubMed] [Google Scholar]
- Mineo, J. R., McLeod, R., Mack, D., Smith, J., Khan, I. A., Ely, K. H., and Kasper, L. H. (1993). Antibodies to Toxoplasma gondii major surface protein (SAG-1, P30) inhibit infection of host cells and are produced in murine intestine after peroral infection. J. Immunol. 150, 3951-3964. [PubMed] [Google Scholar]
- Mitchell, G. H., Thomas, A. W., Margos, G., Dluzewski, A. R., and Bannister, L. H. (2004). Apical membrane antigen 1, a major malaria vaccine candidate, mediates the close attachment of invasive merozoites to host red blood cells. Infect. Immun. 72, 154-158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mondragon, R., and Frixione, E. (1996). Ca(2+)-dependence of conoid extrusion in Toxoplasma gondii tachyzoites. J. Eukaryot. Microbiol. 43, 120-127. [DOI] [PubMed] [Google Scholar]
- Morrissette, N. S., and Sibley, L. D. (2002). Cytoskeleton of apicomplexan parasites. Microbiol. Mol. Biol. Rev. 66, 21-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narum, D. L., and Thomas, A. W. (1994). Differential localization of full-length and processed forms of PF83/AMA-1 an apical membrane antigen of Plasmodium falciparum merozoites. Mol. Biochem. Parasitol. 67, 59-68. [DOI] [PubMed] [Google Scholar]
- Nichols, B. A., and O'Connor, G. R. (1981). Penetration of mouse peritoneal macrophages by the protozoon Toxoplasma gondii. New evidence for active invasion and phagocytosis. Lab. Invest. 44, 324-335. [PubMed] [Google Scholar]
- Ortega-Barria, E., and Boothroyd, J. C. (1999). A Toxoplasma lectin-like activity specific for sulfated polysaccharides is involved in host cell infection. J. Biol. Chem. 274, 1267-1276. [DOI] [PubMed] [Google Scholar]
- Pizarro, J. C. et al. (2005). Crystal structure of the malaria vaccine candidate apical membrane antigen 1. Science 308, 408-411. [DOI] [PubMed] [Google Scholar]
- Roos, D. S., Donald, R. G., Morrissette, N. S., and Moulton, A. L. (1994). Molecular tools for genetic dissection of the protozoan parasite Toxoplasma gondii. Methods Cell Biol. 45, 27-63. [DOI] [PubMed] [Google Scholar]
- Sibley, L. D., Hakansson, S., and Carruthers, V. B. (1998). Gliding motility: an efficient mechanism for cell penetration. Curr. Biol. 8, R12-R14. [DOI] [PubMed] [Google Scholar]
- Soldati, D., Dubremetz, J. F., and Lebrun, M. (2001). Microneme proteins: structural and functional requirements to promote adhesion and invasion by the apicomplexan parasite Toxoplasma gondii. Int. J. Parasitol. 31, 1293-1302. [DOI] [PubMed] [Google Scholar]
- Soldati, D., Kim, K., Kampmeier, J., Dubremetz, J. F., and Boothroyd, J. C. (1995). Complementation of a Toxoplasma gondii ROP1 knock-out mutant using phleomycin selection. Mol. Biochem. Parasitol. 74, 87-97. [DOI] [PubMed] [Google Scholar]
- Soldati, D., and Meissner, M. (2004). Toxoplasma as a novel system for motility. Curr. Opin. Cell Biol. 16, 32-40. [DOI] [PubMed] [Google Scholar]
- Stowers, A. W., Kennedy, M. C., Keegan, B. P., Saul, A., Long, C. A., and Miller, L. H. (2002). Vaccination of monkeys with recombinant Plasmodium falciparum apical membrane antigen 1 confers protection against blood-stage malaria. Infect. Immun. 70, 6961-6967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suss-Toby, E., Zimmerberg, J., and Ward, G. E. (1996). Toxoplasma invasion: the parasitophorous vacuole is formed from host cell plasma membrane and pinches off via a fission pore. Proc. Natl. Acad. Sci. USA 93, 8413-8418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas, A. W., Deans, J. A., Mitchell, G. H., Alderson, T., and Cohen, S. (1984). The Fab fragments of monoclonal IgG to a merozoite surface antigen inhibit Plasmodium knowlesi invasion of erythrocytes. Mol. Biochem. Parasitol. 13, 187-199. [DOI] [PubMed] [Google Scholar]
- Tomley, F. M., and Soldati, D. S. (2001). Mix and match modules: structure and function of microneme proteins in apicomplexan parasites. Trends Parasitol. 17, 81-88. [DOI] [PubMed] [Google Scholar]
- Triglia, T., Healer, J., Caruana, S. R., Hodder, A. N., Anders, R. F., Crabb, B. S., and Cowman, A. F. (2000). Apical membrane antigen 1 plays a central role in erythrocyte invasion by Plasmodium species. Mol. Microbiol. 38, 706-718. [DOI] [PubMed] [Google Scholar]
- Urquiza, M., Suarez, J. E., Cardenas, C., Lopez, R., Puentes, A., Chavez, F., Calvo, J.C., and Patarroyo, M. E. (2000). Plasmodium falciparum AMA-1 erythrocyte binding peptides implicate AMA-1 as erythrocyte binding protein. Vaccine 19, 508-513. [DOI] [PubMed] [Google Scholar]
- Waters, A. P., Thomas, A. W., Deans, J. A., Mitchell, G. H., Hudson, D. E., Miller, L. H., McCutchan, T. F., and Cohen, S. (1990). A merozoite receptor protein from Plasmodium knowlesi is highly conserved and distributed throughout Plasmodium. J. Biol. Chem. 265, 17974-17979. [PubMed] [Google Scholar]