Abstract
The ocular lens is the only organ that does not develop spontaneous tumor. The molecular mechanism for this phenomenon remains unknown. Through examination of the signaling pathways mediating stress-induced apoptosis, here we presented evidence to show that different from most other tissues in which the extracellular signal-regulated kinases (ERKs) pathway is generally implicated in mediation of survival signals activated by different factors, the RAF/MEK/ERK signaling pathway alone plays a key role in stress-activated apoptosis of lens epithelial cells. Treatment of N/N1003A cells with calcimycin, a calcium mobilizer, activates the RAF/MEK/ERK pathway through RAS, which is indispensable for the induced apoptosis because inhibition of this pathway by either pharmacological drug or dominant negative mutants greatly attenuates the induced apoptosis. Calcimycin also activates p38 kinase and JNK2, which are not involved in calcium-induced apoptosis. Downstream of ERK activation, p53 is essential. Activation of RAF/MEK/ERK pathway by calcimycin leads to distinct up-regulation of p53. Moreover, overexpression of p53 enhances calcimycin-induced apoptosis, whereas inhibition of p53 expression attenuates calcimycin-induced apoptosis. Up-regulation of p53 directly promotes Bax expression, which changes the integrity of mitochondria, leading to release of cytochrome c, activation of caspase-3 and eventually execution of apoptosis. Overexpression of αB-crystallin, a member of the small heat-shock protein family, blocks activation of RAS to inhibit ERK1/2 activation, and greatly attenuates calcimycin-induced apoptosis. Together, our results provide 1) a partial explanation for the lack of spontaneous tumor in the lens, 2) a novel signaling pathway for calcium-induced apoptosis, and 3) a novel antiapoptotic mechanism for αB-crystallin.
INTRODUCTION
Apoptosis is a programmed cell death (Kerr et al., 1972). It either occurs as a normal physiological process during animal development (Raff, 1992) or it can be triggered by a number of external signals such as hormone (Wyllie, 1980), γ-irradiation (Strasser et al., 1991), withdrawal of growth factors (Williams et al., 1990) and stress (Li et al., 1995a). Under normal physiological conditions, apoptosis helps to remove excess or unwanted cells or tissues such as vertebrate neurons (Cowan et al., 1984), and larval tissue during metamorphosis (Kerr et al., 1974). In contrast, induced apoptosis can have serious pathological consequences (Carson and Ribeiro, 1993; Thompson, 1995). For example, the bacterial pathogen Shigella flexneri can induce apoptotic death of macrophages, potentially leading to human dysentery (Zychlinsky et al., 1992). Human immunodeficiency virus-1 induces apoptosis of CD4+ T lymphocytes, contributing to development of AIDS (Meyaard et al., 1992).
The lens of the vertebrate eye is a unique organ in that it is nonvascularized and noninnervated (Bloemendal, 1981). It contains only a single layer of epithelial cells that remain quiescent in the central section, divide toward the equatorial area and terminally differentiate into fiber cells in the equatorial region (McAvoy, 1980; Piatigorsky, 1981). This single layer of lens epithelial cells is essential for maintaining the metabolic homeostasis and transparency of the entire lens (Spector, 1995). Induced apoptosis of these lens epithelial cells by various stress factors likely initiates noncongenital cataractogenesis (Li et al., 1995a,b; Li and Spector, 1996; Li, 1997). Another unique feature of the ocular lens is that it never develops spontaneous tumor (Veromann, 1994). The molecular mechanism underlining this phenomenon is largely unknown.
Both biochemical and genetic studies have shown that apoptosis is mediated by different signaling components and controlled by various regulators (Yuan, 1997; Wang, 2001). Among these signaling molecules and apoptotic regulators, the mitogen-activated protein kinases (MAPKs) (Franklin and McCubrey, 2000; Kyriakis and Avruch, 2001; Ballif and Blenis, 2001; Weston and Davis, 2002) and the heat-shock proteins play an important role in regulating stress-induced apoptosis (Xanthoudakis and Nicholson, 2000).
MAPKs participate in diverse cellular functions such as cell proliferation, cell differentiation, and cell death (Kyriakis and Avruch, 2001; Pearson et al., 2001; Weston and Davis, 2002). The importance of MAPK signaling pathways in regulating apoptosis under stress conditions has been widely investigated (Xia et al., 1995; Dickens et al., 1997; Yang et al., 1997; Nemoto et al., 1998; Wang et al., 1998; Zechner et al., 1998; Behrens et al., 1999; Bonni et al., 1999; Hayakawa et al., 1999; Tournier et al., 2000; Liu et al., 2004). Much of the work has supported the general view that among the three types of MAPKs, the ERK1/2-mediated pathway plays an essential role in promoting cell cycle progression and are generally involved in proliferative signaling and cell survival (Xia et al., 1995; Wang and Reed, 1998; Blalock et al., 2000; Hoyle et al., 2000). On the other hand, c-Jun-NH2-terminal kinases (JNKs) and p38 kinase, activated by extracellular stress signals, are involved in apoptosis (Xia et al., 1995; Yang et al., 1997; Nemoto et al., 1998; Wang et al., 1998; Zechner et al., 1998; Behrens et al., 1999; Hayakawa et al., 1999; Tournier et al., 2000). The survival function of ERK1/2 is further demonstrated by the fact that inhibition of ERK signaling leads to increased sensitivity of ovarian cancer cell lines to cisplatin-induced apoptosis (Hayakawa et al., 1999; Persons et al., 1999). In contrast to these previous studies, we demonstrate here that activation of RAF/MEK/ERK signaling pathway alone mediates calcimycin-induced apoptosis of lens epithelial cells. Calcimycin also activates both p38 kinase and JNK2. However, activation of these two kinases does not seem to be involved in calcimycin-induced apoptosis. Downstream of ERK activation, p53 plays a critical role. It positively regulates expression of the proapoptotic factor Bax (Oltvai et al., 1993) and promotes the mitochondrial death pathway. Thus, our results provide a novel signaling pathway for calcium-triggered apoptosis. Our finding that the stress-activated RAF/MEK/ERK signaling pathway, instead of promoting survival, mediates stress-induced apoptosis also provides a partial explanation for the absence of the spontaneous tumor in the ocular lens.
The heat-shock proteins are important regulators against apoptosis (Xanthoudakis and Nicholson, 2000). These factors seem to protect cells from induction of apoptosis through different mechanisms. Hsp90 seems to inhibit apoptosome formation (Pandey et al., 2000a). Hsp70 can interact with BAG-1 (Takayama et al., 1997) and inhibit apoptosis by preventing recruitment of procaspase-9 to the Apaf-1 apoptosome complex (Beere et al., 2000). Hsp70 is also able to function downstream of caspase activation (Jaattela et al., 1998). Hsp27 prevents cell death at different signaling steps. First, it interacts with cytochrome c to prevent activation of procaspase-9 (Garrido et al., 1999; Bruey et al., 2000). It also binds to caspase-3 and modulates the activity of caspase-3 (Pandey et al., 2000b). Finally, a recent study demonstrates that Hsp27 can abrogate the apoptotic pathway through regulation of Bid intracellular distribution and protection of F-actin integrity (Paul et al., 2002). Both Hsp27 and α-crystallins are closely related family members (Ingolia and Craig, 1982). Compared with Hsp27, the antiapoptotic mechanisms for α-crystallins remain largely unknown until our recent study in which we showed that αB-crystallin is able to interact with the precursors of caspase-3 to repress its activation (Mao et al., 2001). Similar results are reported from another laboratory (Kamradt et al., 2001). More recently, we also have demonstrated that α-crystallin is able to sequester the translocation of Bax and Bcl-XS, proapoptotic members of the Bcl-2 family, from cytoplasm into mitochondria to prevent staurosporin-induced apoptosis (Mao et al., 2004). To further explore the antiapoptotic mechanism of α-crystallin, we have examined its ability to block calcimycin-induced apoptosis. Here, we demonstrate that αB-crystallin can repress calcimycin-induced activation of RAF/MEK/ERK pathway. By blocking RAS activation by RAS-GRF2, αB-crystallin suppresses the RAF/MEK/ERK pathway and abrogates calcimycin-induced apoptosis. Therefore, our results also provide a novel antiapoptotic mechanism for αB-crystallin.
MATERIALS AND METHODS
Chemicals
Various molecular biology reagents were purchased from Invitrogen (Carlsbad, CA), Stratagene (La Jolla, CA), and Promega (Madison, WI). DNA and protein size markers were purchased from Invitrogen. Mammalian expression vectors and constructs were purchased from BD Biosciences Clontech (Palo Alto, CA), Promega (Madison, WI), and Stratagene (La Jolla, CA). Various antibodies were obtained from Cell Signaling Technology (Beverely, MA), Santa Cruz Biotechnology (Santa Cruz, CA), Roche Diagnostics (India-napolis, IN), and BD Transduction Laboratories (San Diego, CA). The culture medium, and most other chemicals and antibiotics were purchased from Sigma (St. Louis, MO) and Invitrogen.
Cell Culture
The rabbit lens epithelial cells (RLECs), N/N1003A, were grown in Eagle's minimum essential medium containing 10% rabbit serum as we described previously (Mao et al., 2001).
Expression Constructs and Stable Cell Lines
The stable transfected cell lines, pEGFP-N/N1003A (expressing only the green fluorescence protein, GFP, from the vector), and pEGFP-mαB-N/N1003A (expressing the fusion protein of GFP and mouse αB-crystallin) were established and maintained as described previously (Li et al., 2001). The stable cell lines carrying either the p53 expression construct pCMV-p53 or its corresponding vector pCMV-neo were described previously (Miyashita and Reed, 1995). The antisense-p53 construct was created by first subcloning the HindIII-EcoRI fragment of p53 into pBluescript SK(+/-) vector and then isolate the EcoRI-SalI fragment and ligated back into pCMV-Neo vector. The mouse Bax cDNA was kindly provided by Dr. Stanley Korsmeyer (Oltvai et al., 1993). This cDNA was subcloned from pBluescript-SK into pCI-neo vector at the EcoRI site. The antisense-Bax construct was created with opposite insertion of the cDNA into pCI vector. The RAF and RAS dominant negative mutants (Rosen et al., 1994; Mischak et al., 1996) were obtained from the BD Biosciences Clontech. The ERK dominant negative mutant was described previously (Huang et al., 1999). These constructs were amplified in DH-5α and purified by two rounds of CsCl ultracentrifugation (Ausubel et al., 2002) or by Plasmid Maxiprep kit (catalog no. 732-6130; Bio-Rad, Hercules, CA). Transfection of N/N1003A cells was performed using electroporation with a BTX Electro Cell manipulator as we described previously (Xiang et al., 2002; Wang et al., 2005) or with Lipofectamine 2000 from the Invitrogen (Feng et al., 2004). The transfected cells were then subjected to neomycin (G418) selection for 4-6 wk before stable lines of individual clones were obtained.
Pretreatment or Treatment of Various Types of Cells by Dimethyl Sulfoxide (DMSO), Calcimycin, UO126, SP600125, PD169316, and Cyclosporine A
The parental N/N1003A and various transfected cells (Table 1) were grown to 100% confluence in minimal essential medium (MEM) containing 10% rabbit serum in the absence (for N/N1003A) or presence (for various transfected cell lines) of 400 μg/ml G418. Then, MEM containing 10% serum plus 0.01% DMSO (mock) or 5 μM calcimycin (experiment) was used to replace the culture media for the required period of incubation (as indicated in the figure legends and Table 1). For pretreatment, the MEM containing 10% serum plus 0.01% DMSO (mock), 50 μM UO126 (MEK1/2 inhibitor), 1 μM SP600125 (JNK1/2 inhibitor), 2 μM PD169316 (p38 inhibitor), or 250 nM cyclosporine A (PP-2B inhibitor) were used to replace the culture media, for a total of 6 h of incubation before subjected to treatment by 0.01% DMSO (mock) or 5 μM calcimycin (experiment) for the required time. After treatment, all samples were collected for analysis of apoptosis, protein and enzyme activity, or reporter gene activity.
Table 1.
Calcimycin-induced apoptosis in various cell lines or pretreatments
| Percentage of apoptosis
|
|||
|---|---|---|---|
| Row number | Different cell lines or pretreatment conditions | 0.01% DMSO 18 hr | 5 μM calcimycin 18 hr |
| 1 | N/N1003A | 3 ± 0.5 | 50 ± 3 |
| 2 | N/N1003A + UO126 | 3.6 ± 1 | 12 ± 4 |
| 3 | N/N1003A + PD 169316 | 3.5 ± 0.8 | 48 ± 4.8 |
| 4 | N/N1003A + SP600125 | 3 ± 1.2 | 49 ± 4.2 |
| 5 | pCMV-N/N1003A | 3 ± 1.6 | 50 ± 3.5 |
| 6 | pCMV-DNMRAS-N/N1003A | 3 ± 1.5 | 12.8 ± 3 |
| 7 | pCMV-DNMRAF-N/N1003A | 3 ± 1.8 | 11.2 ± 3.5 |
| 8 | pCMV-DNMERK-N/N1003A | 3 ± 1.9 | 10.8 ± 4 |
| 9 | pCMV-p53-N/N1003A | 4 ± 1.8 | 68 ± 4.4 |
| 10 | pCMV-antisense-p53-N/N1003A | 3 ± 1.6 | 24 ± 3.8 |
| 11 | pCI-Bax-N/N1003A | 4 ± 1.9 | 76 ± 6.2 |
| 12 | pCI-antisense-Bax-N/N1003A | 3 ± 1.4 | 22 ± 2.8 |
| 13 | pEGFP-N/N1003A | 3 ± 0.6 | 49.8 ± 4.1 |
| 14 | pEGFB-maB-N/N1003A | 3 ± 0.4 | 14.4 ± 2.4 |
| 15 | pEGFB-maB-N/N1003A + pCMV-Neo | 3 ± 0.6 | 15.2 ± 2.2 |
| 16 | pEGFB-maB-N/N1003A + pCMV-p53 | 4 ± 1 | 29.8 ± 4.2 |
Cell Flow Cytometry Analysis
The percentage of apoptotic cells was determined by cell flow cytometry, which was conducted as previously described (Ormerod, 1994) using the Annexin V-FITC apoptosis detection kit from MBL International (Watertown, MA). Briefly, cells were harvested with 0.025% trypsin/5 mM EDTA in phosphate-buffered saline (PBS), and 2.5% fetal bovine serum in PBS was added to the cells as soon as they were released from the culture dishes. Then, the cells were transferred to a centrifuge tube, washed with PBS, and incubated with Annexin V-FITC plus propidium iodide (PI) following the protocol included in the kit. Cells were analyzed on a BD Biosciences FACS Calibur flow cytometer (Beckman Coulter, Fullerton, CA), with the fluorescein isothiocyanate (FITC) signal in FL1 and the PI signal in FL2. Intact cells were gated in the FSC/SSC plot to exclude small debris. Cells in the lower right quadrant of the FL1/FL2 dot plot (labeled with annexin-FITC only) are considered to be in early apoptosis, and cells in the upper right quadrant (labeled with annexin-FITC and PI) are in late apoptosis. The quantitative results of apoptosis presented were averaged from three independent experiments. The SD was shown in each figure and Table 1.
DNA Fragmentation
The apoptotic nature of cell death was determined using Hoechst staining and DNA fragmentation as described previously (Li et al., 1998, 2001; Mao et al., 2001, 2004; Huang et al., 2005).
Membrane Protein Cross-linking
pEGFP-N/N1003A and pEGFP-mαB-N/N1003A cells were grown to 100% confluence and then treated with 5 μM calcimycin (experiment) or 0.01% DMSO (control) for 10 min and then further incubated in PBS with 5 μM calcimycin (experiment) or 0.01% DMSO (control) plus 2 mM dimethyl-3,3′-dithiobispropionimidate(2HCl) (the cross-linking agent; Pierce Chemical, Rockford, IL) for 30 min. After washing four times in PBS, cells were harvested in hypotonic buffer (50 mM HEPES, pH 7.4, 1 mM CaCl2, 1 mM phenylmethylsulfonyl fluoride, and 1× protease inhibitor cocktail) for extraction of both membrane and cytosol proteins as described previously (Brennan and Lin, 1996). Both membrane and cytosol proteins were used for Western blot as described below.
Isolation of Mitochondria
After treatment with 5 μM calcimycin (experiment), or 0.01% DMSO (control), N/N1003A (with or without UO126-pretreatment) and various transfected cells were harvested for isolation of cytosol and mitochondria as previously described previously (Eskes et al., 2000; Mao et al., 2004). The proteins from both mitochondria and cytosol were subjected to Western blot analysis as described below.
Protein Preparation and Western Blot Analysis
The total proteins were prepared from N/N1003A or various transfected cells as described previously (Mao et al., 2001 and 2004). Membrane proteins were isolated as described previously (Brennan and Lin, 1996). Western blot analysis was conducted as described previously (Li et al., 2001, 2003). Various antibodies and their concentrations used for Western blot analysis were described previously (Li et al., 2003; Mao et al., 2004; Feng et al., 2004; Wang et al., 2005).
GTP Loading Assays
GTP loading assays were conducted according to Rosen et al. (1994). pEGFP-N/N1003A and pEGFP-mαB-N/N1003A cells were metabolically labeled in PO4-free DMEM (DMEM) in the presence of 0.5 mCi/ml [32P]orthophosphate for 4 h. Then, 0.01% DMSO or 5 μM calcimycin was added into these labeling cells and incubated for 30 min. After stimulation, the cells in each dish were washed once with cold PBS, and lysed on the plate with 250 μl of lysis buffer (20 mM Tris-HCl, pH 7.4, 150 mM NaCl, 1 mM MgCl2, 1% Triton X-100) containing 2 μg of anti-RAS monoclonal antibody (Oncogene Science, Cambridge, MA). Extracts were scraped into Eppendorf tubes and drawn 15 times through a 0.22-gauge needle, and 1:10 volume of PBS/1% bovine serum albumin/10% charcoal (Sigma) slurry was added. Extracts were rocked at 4°C for 1 h and centrifuged 5 min at 12,000 × g, and 200 μl of the supernatants was mixed with 40 μl of proteins A/G agarose. The mixture was rocked at 4°C for another hour and then washed three times with lysis buffer and once with PBS. Pellets were resuspended in 40 μl of 1 M KH2PO4, pH 3.4, and incubated at 85°C for 3 min. Samples were centrifuged at 12,000 × g for 5 min, and 20 μl of supernatant was decanted to a new tube and stored at -20°C overnight. Samples were centrifuged again the next day, and 10 μl was decanted for spotting, 1 μl at a time, onto 20 × 20-cm PEI-cellulose thin layer chromatography plates (sigma). Plates were developed in 1 M KH2PO4, pH 3.4, for 2 h.
Assay of Caspase-3 Activity
The caspase-3 activity in various cell lines with or without pretreatment followed by treatment with calcimycin was assayed as we described previously (Li et al., 2001; Mao et al., 2001, 2004).
Analysis of Transient Gene Expression
For reporter gene activity, the construct of chloramphenicol acetyltransfeRASe (CAT) reporter gene driven by either the human bax gene promoter or the same promoter with the p53 sites mutated (Miyashita and Reed, 1995), together with the control construct expressing β-galactosidase (Xiang et al., 2002), was introduced into pCMV vector-, pCMV-p53- and pCMV-antisense-p53-transfected cells as described above. The transfected cells were grown in 100-mm culture dishes for 24 h and then treated with either 0.01% DMSO or 5 μM calcimycin for additional 6 h before harvested for assays of β-galactosidase and CAT activities as described previously (Ausubel et al., 2002; Xiang et al., 2002).
RESULTS
Calcimycin-induced Apoptosis Requires Activation of ERK1/2 MAP Kinases
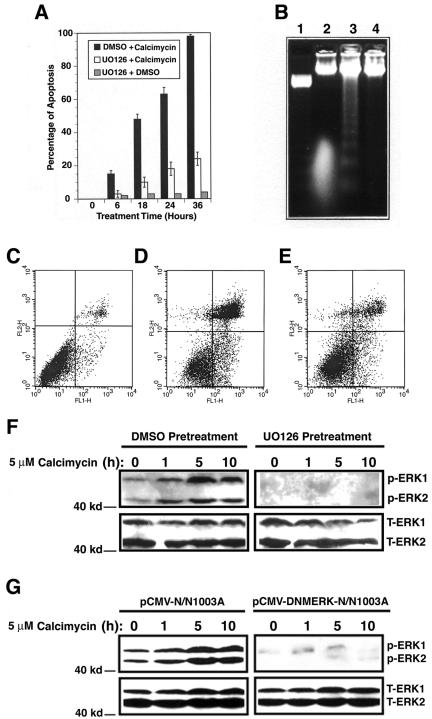
Our previous studies have demonstrated that lens epithelial cell apoptosis induced by various stress factors may be a common cellular mechanism mediating cataractogenesis (Li et al., 1995a,b; Li and Spector, 1996). To explore the signaling pathways for stress-induced apoptosis, we studied calcium-induced apoptosis in rabbit lens epithelial cells (RLECs), N/N1003A. Calcimycin, a calcium mobilizer, is known to induce calcium influx and apoptosis of different types of cells (McConkey et al., 1992; Orrenius et al., 2003). As shown in Figure 1, A and D, cell flow cytometry analysis revealed that treatment of N/N1003A cells with 5 μM calcimycin induced time-dependent apoptosis of N/N1003A cells. The apoptotic nature was verified by DNA fragmentation assay (Figure 1B, lane 3). N/N1003A cells treated with 0.01% DMSO (a solvent for calcimycin) displayed very few apoptotic cells (our unpublished data).
Figure 1.
Calcimycin-induced apoptosis requires activation of ERK1/2. (A) Viability assays. N/N1003A cells were grown to 100% confluence and then either pretreated with 50 μM UO126 or 0.01% DMSO for 6 h followed by treatment with 5 μM calcimycin for 0-36 h in MEM (with 10% rabbit serum. The same serum conditions were used in all the studies described in Figures 1, 2, 3, 4, 5, 6, 7, 8). As a control, the UO126-pretreated N/N1003A cells were further treated by 0.01% DMSO for the same length of time. Viability of the three different cultures was determined as described previously (Li et al., 1998) and further confirmed by cell flow cytometry (C, D, and E). (B) DNA fragmentation assay. N/N1003A cells were grown to 100% confluence and then either pretreated with 50 μM UO126 (lane 4) or 0.01% DMSO (lane 3) for 6 h followed by treatment with 5 μM calcimycin for 18 h in MEM. Included as a control, the UO126-pretreated N/N1003A cells were further treated by 0.01% DMSO for 18 h (lane 2). Then, the cells from the different treatment conditions were harvested for isolation of genomic DNA, which were analyzed as described previously (Li et al., 1995a). The 123-base pair marker from Invitrogen was shown in lane 1. (C, D, and E) Cell flow cytometry. N/N1003A cells were grown to 100% confluence and then either pretreated with 50 μM UO126 (E) or 0.01% DMSO (D) for 6 h followed by treatment with 5 μM calcimycin for 18 h in MEM. (C) Control where the UO126-pretreated N/N1003A cells were further treated by 0.01% DMSO for 18 h. After treatment, the three different samples were harvested for cell flow cytometry as described in Materials and Methods. (F) Analysis of ERK1/2 activation with Western blot analysis. N/N1003A cells were grown to 100% confluence and then either pretreated with 50 μM UO126 (right) or 0.01% DMSO (left) for 6 h followed by treatment with 5 μM calcimycin for 0, 1, 5, or 10 h in MEM. Then, total proteins were isolated, resolved in 10% SDS-PAGE, and analyzed with antibodies either against total ERK1/2 (T-ERK1/2) or phospho-ERK1/2 (p-ERK1/2, activated form) as described previously (Li et al., 2003; Feng et al., 2004). (G) Confirmation that calcimycin-induced apoptosis requires activation of ERK1/2. Both vector and dominant negative mutant ERK (DNMERK) transfected cells were grown to 100% confluence and subsequently subjected to treatment with 5 μM calcimycin for 0, 1, 5, or 10 h in MEM. Then, total proteins were isolated, resolved in 10% SDS-PAGE and analyzed with antibodies against either total ERK1/2 (T-ERK1/2) or phospho-ERK1/2 (p-ERK1/2, activated form) as described in Figure 1F.
Because MAP kinases are actively mediating stress-induced apoptosis (Xia et al., 1995), we next examined whether these kinases are involved in calcimycin-induced apoptosis. As shown in Figure 1F, left, treatment of N/N1003A cells with calcimycin induces steady activation of ERK1/2 within the first 5 h of treatment, and by 10 h of treatment, the activation slightly decreased. To demonstrate that activation of ERK1/2 was necessary for calcimycin-induced apoptosis, N/N1003A cells were pretreated with 50 μM UO126, an inhibitor of MEK1/2 kinases, for 6 h followed by treatment with 5 μM calcimycin for 0-36 h. As shown in Figure 1F, right, pretreatment with UO126 completely blocked activation of ERK1/2. When ERK1/2 activation was blocked, the calcimycin-induced apoptosis was substantially blocked (Figure 1, A and E, and Table 1, rows 1 and 2). This inhibition of calcimycin-induced apoptosis was further verified by DNA fragmentation assay (Figure 1B, lane 4). As control, N/N1003A cells pretreated with 50 μM UO126 followed by treatment with 0.01% DMSO displayed very few apoptotic cells (Figure 1A, B, lane 2, and C). Together, these results indicate that activation of ERK1/2 is necessary for calcimycin-induced apoptosis.
To further confirm that activation of ERK1/2 is the key event in calcimycin-induced apoptosis, we next introduced a dominant negative mutant ERK2 (Huang et al., 1999) into N/N1003A cells. Western blot analysis of the total ERK1/2 proteins and their activities from either ERK mutant or vector-transfected cells revealed that the dominant negative mutant clearly interfered with calcimycin-induced activation of ERK1/2 (Figure 1G). Cell flow cytometry analysis demonstrated that inhibition of ERK activity by the dominant negative mutant substantially blocked calcimycin-induced apoptosis (Table 1, row 8). In contrast, the vector-transfected cells underwent similar apoptosis as parent cells did after calcimycin treatment (Table 1, row 5). The cells treated with 0.01% DMSO displayed only background apoptosis (our unpublished data). These results further confirm that activation of ERK1/2 is necessary in mediating calcimycin-induced apoptosis.
Calcimycin-induced Activation of MEK1/2 and RAF1 but Not p38 and JNK MAP Kinases Is Necessary for Calcimycin-induced Apoptosis
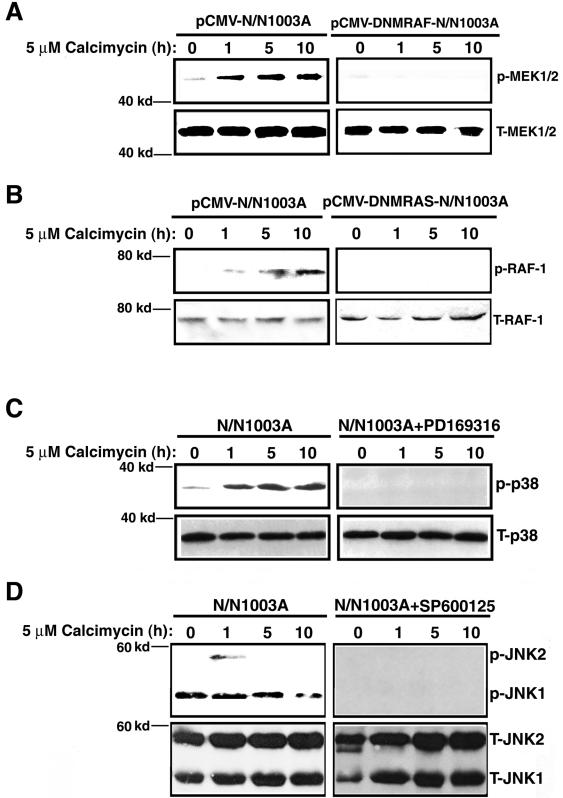
The UO126 inhibition experiments described above (Figure 1F) indicated that MEK1/2 activities were necessary for ERK1/2 activation and thus critical for calcimycin-induced apoptosis. Next, we determined the activation of MEK1/2 by calcimycin and also the importance of such activation in calcimycin-induced apoptosis. As shown in Figure 2A, left, calcimycin induced steady activation of MEK1/2. Inhibition of MEK1/2 activation by a dominant negative mutant RAF1 (Mischak et al., 1996) (Figure 2A, right) substantially suppressed calcimycin-induced apoptosis (Table 1, row 7). Next, we examined the role of RAF activation in calcimycin-induced apoptosis. The RAF kinase is normally activated by the small GTP binding protein, RAS (Hingorani and Tuveson, 2003). Under calcimycin treatment, RAF1 was steadily activated within the first 10 h (Figure 2B, left). Inhibition of RAF1 activation by a dominant negative mutant RAS (Rosen et al., 1994) (Figure 2B, right) also greatly suppressed calcimycin-induced apoptosis (Table 1, row 6). Together, our results showed that activation of RAF/MEK/ERK signaling pathway is necessary for calcimycin-induced apoptosis.
Figure 2.
Calcimycin-induced activation of MEK1/2 and RAF1 but not p38 and JNK2 is important for calcimycin-induced apoptosis. (A) Calcimycin-induced activation of MEK1/2 is blocked by DNM RAF1. N/N1003A cells transfected with pCMV-Neo vector or pCMV-DNMRAF1 were grown to 100% confluence and subsequently subjected to treatment with 5 μM calcimycin for 0, 1, 5, or 10 h in MEM. Then, total proteins were isolated, and resolved in 10% SDS-PAGE and analyzed with antibodies against either total MEK1/2 (T-MEK1/2) or phospho-MEK1/2 (p-MEK1/2, activated form) as described previously (Li et al., 2003; Liu et al., 2004). (B) Calcimycin-induced activation of RAF1 is blocked by DNM RAS. N/N1003A cells transfected with pCMV-Neo vector or pCMV-DNMRAS were processed as described in Figure 2A. Western blots were analyzed with antibodies against either total RAF-1 (T-RAF-1) or phospho-RAF-1 (p-RAF-1, activated form) as described Figure 2A. (C) Analysis of p38 kinase activation with Western blot analysis. N/N1003A cells were grown to 100% confluence and then either pretreated with 2 μM PD169316 (right, IC50 = 89 nM) or 0.01% DMSO (left) for 6 h followed by treatment with 5 μM calcimycin for 0, 1, 5, or 10 h in MEM. Then total proteins were isolated, resolved in 10% SDS-PAGE, and analyzed with antibodies against either total p38 (T-p38) or phospho-p38 kinase (p-p38, activated form) as described previously (Li et al., 2003). (D) Analysis of JNK2 kinase activation with Western blot analysis. N/N1003A cells were grown to 100% confluence and then either pretreated with 1 μM SP600125 (right, IC50 = 40 nM) or 0.01% DMSO (left) for 6 h followed by treatment with 5 μM calcimycin for 0, 1, 5, or 10 h in MEM. Then, total proteins were isolated, resolved in 10% SDS-PAGE and analyzed with antibodies against either total JNK1/2 (T-JNK1/2) or phospho-JNK1/2 (p-JNK1/2, activated form) as described previously (Li et al., 2003; Feng et al., 2004; Wang et al., 2005).
Because an early study (Lotem et al., 1999) revealed that activation of p38 kinase was necessary for calcimycin-induced apoptosis of mouse myeloid leukemic cells, we next examined the possible involvement of p38 kinase activation in calcimycin-induced apoptosis of N/N1003A cells. As shown in Figure 2C, left, calcimycin induced a steady activation of p38 kinase within the first 10 h of treatment. However, inhibition of p38 kinase activation by 2 μM PD169316 (Figure 2C, right) displayed little effects on calcimycin-induced apoptosis (Table 1, row 3). Calcimycin also induced a transient activation of JNK2 after 1-h treatment (Figure 2D, left). Inhibition of JNK2 activation by the pharmacological drug SP600125 (Figure 2D, right) did not interfere with calcimycin-induced apoptosis (Table 1, row 4). Together, these data demonstrated that calcimycin-induced RAF/MEK/ERK pathway alone mediates calcium-induced apoptosis in RLECs.
The Tumor Suppressor p53 Is the Key Regulator Downstream of ERK1/2 Activation during Calcimycin-induced Apoptosis
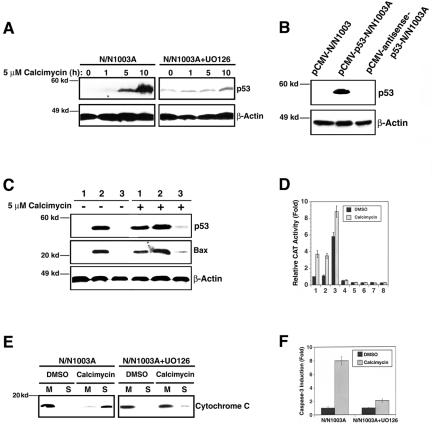
Previous studies have shown that p53 is an important regulator of apoptosis in the ocular lens. Knockout of p53 (-/-) completely suppresses the Rb-deficiency-induced apoptosis in mouse lens (Morgenbesser et al., 1994). To explore the role of p53 in calcimycin-induced apoptosis, we first examined the possible change in p53 expression after calcimycin treatment. As shown in Figure 3A, left, Western blot analysis revealed the steady up-regulation of p53 protein after treatment with 5 μM calcimycin for 5-10 h. Inhibition of ERK1/2 activation by UO126 almost completely abrogated calcimycin-induced up-regulation of p53 although UO126 pretreatment increased the background p53 expression (Figure 3A, right). These results suggest that downstream of ERK1/2 activation, p53 may be necessary for calcimycin-induced apoptosis. To further test the role of p53 in calcimycin-induced apoptosis, we have either overexpressed p53 through the expression construct, pCMV-p53 or inhibited p53 expression using an antisense construct, pCMV-antisense-p53 (Figure 3B). The cells overexpressing p53 were more sensitive to calcimycin-induced apoptosis (Table 1, row 9). In contrast, cells with p53 expression repressed are much more resistant to calcimycin-induced apoptosis (Table 1, row 10). Thus, the presence or absence of p53 is directly linked to the level of calcimycin-induced apoptosis.
Figure 3.
Analysis of the apoptotic pathway downstream of ERK1/2 activation. (A) Western blot analysis to show that calcimycin induces up-regulation of p53 (left) and that such up-regulation is abrogated when ERK1/2 activation is inhibited (right). N/N1003A cells were grown to 100% confluence and then either pretreated with 50 μM UO126 (right) or 0.01% DMSO (left) for 6 h followed by treatment with 5 μM calcimycin for 0, 1, 5, or 10 h in MEM. Then, total proteins were isolated, resolved in 10% SDS-PAGE and analyzed with antibodies against p53 and β-actin as described previously (Li et al., 1998). Note that the UO126 pretreatment enhanced the background p53 expression but abrogated its up-regulation by calcimycin. (B) Western blot analysis to show the different p53 levels at pCMV vector-transfected N/N1003A cells, pCMV-p53 transfected N/N1003A cells, and pCMV-antisense-p53-transfected N/N1003A cells. The three types of cells were grown to 100% confluence, and then total proteins were isolated, resolved in 10% SDS-PAGE, and analyzed with antibodies against p53 and β-actin as described in Figure 3A. (C) Western blot analysis of p53 and Bax levels in the vector-transfected cells (1), p53-transfected cells (2), and antisense-p53-transfected cells (3) under treatment of 0.01% DMSO (-5 μM calcimycin) or 5 μM calcimycin (+5 μM calcimycin). Western blot was conducted with antibodies against p53, Bax, and βactin as described previously (Li et al., 1998). Both exogenous and endogenous p53 has the same molecular weight. (D) p53 positively regulates Bax in RLECs. Columns 1-4, the results from the reporter gene with wild-type p53 binding site (pBax-CAT), and columns 5-8, the results from the reporter gene with mutant p53 binding sites [pBax(m)-CAT]. Columns 1 and 5, parental N/N1003A cells; columns 2 and 6, vector-transfected cells (pCMV-N/N1003A), columns 3 and 7, p53-transfected cells (pCMV-p53-N/N1003A), and columns 4 and 8, antisense-p53-transfected cells (pCMV-antisense-p53-N/N1003A). pBax-CAT or pBax(m)-CAT and pRSV-β-Gal were cotransfected into the four types of cells. Twenty-four hours after transfection, the four types of cells were treated with either 0.01% dimethyl sulfoxide or 5 μM calcimycin for 6 h. Thereafter, the different cell samples were harvested for assay of both β-Gal and CAT activity as described previously (Xiang et al., 2002; Feng et al., 2004). As controls, the Bax-CAT with p53 site mutated was also cotransfected with pRSV-β-Gal. The results of CAT activity generated from this mutant construct was close to background and displayed little difference in different cell lines with or without calcimycin treatment (columns 5-8). (E) UO126 prevents release of cytochrome c. N/N1003A cells were grown to 100% confluence and then pretreated with either 50 μM UO126 (right) or 0.01% DMSO (left) for 6 h followed by treatment with 5 μM calcimycin (calcimycin) or 0.01% DMSO (DMSO) for 12 h in MEM. Then, the treated cells were collected for isolation of mitochondria (M) and soluble fraction (S). Total proteins in both mitochondria and soluble fraction were resolved in 12% SDS-PAGE and analyzed with an antibody against cytochrome c as described previously (Mao et al., 2004). (F) UO126 prevents activation of caspase-3. N/N1003A grown to 100% confluence were pretreated with either 50 μM UO126 or 0.01% DMSO for 6 h followed by treatment with 5 μM calcimycin (calcimycin) or 0.01% DMSO (DMSO) for 12 h in MEM. At the end of the treatment, each sample was collected for caspase-3 activity assays as described previously (Li et al., 2001; Mao et al., 2001, 2004).
The Tumor Suppressor p53 positively Regulates Expression of Bax to Deliver the Calcimycin-induced Death Signal
To explore how p53 may regulate calcimycin-induced apoptosis, we next examined the expression of Bax, a downstream target gene of p53 (Miyashita and Reed, 1995). As shown in Figure 3C, Bax expression in N/N1003A cells is directly associated with p53 level. Calcimycin treatment of N/N1003A cells induced up-regulation of both p53 and Bax (Figure 3C, lane 1). Overexpression of p53 enhanced Bax expression in the absence and presence of calcimycin treatment (Figure 3C, lane 2). On the other hand, inhibition of p53 expression greatly attenuated Bax expression without or with calcimycin treatment (Figure 3C, lane 3). To confirm that p53 directly regulates Bax in N/N1003A cells, we next introduced a CAT reporter gene driven by a Bax promoter which contains one perfect p53 binding site and three imperfect p53 binding sites or by the same expression construct with the p53 binding site mutated (Miyashita and Reed, 1995) into N/N1003 Cells. After transfection, the transfected cells were grown in normal culture medium for 24 h followed by treatment with 0.01% DMSO or 5 μM calcimycin for 6 h. At the end of treatment, both 0.01%dimethyl sulfoxide and 5 μM calcimycin-treated Bax-CAT-transfected cells were harvested for examination of reporter gene activity. As shown in column 1 of Figure 3D, compared with DMSO, calcimycin induced more than threefold increase in CAT activity from Bax-CAT-transfected cells. In contrast, such induction was not observed in the cells transfected by the same construct with the p53 binding sites mutated (Figure 3D, column 5). These results indicate that p53 can directly regulate Bax in N/N1003A cells. Overexpression of p53 induced a six- and ninefold CAT activity in the absence or presence of calcimycin treatment (Figure 3D, column 3). In contrast, inhibition of p53 expression blocked the calcimycin-induced CAT activity (Figure 3D, column 4). The CAT activity generated from the reporter construct with p53 binding site mutated was close to background and displayed little difference in different cell lines with or without calcimycin treatment (Figure 3D, columns 5-8). To determine that Bax up-regulation played an important role in calcimycin-induced apoptosis, we next overexpressed mouse Bax in N/N1003A cells. As shown in the row 11 of Table 1, cells overexpressing Bax, as those overexpressing p53, were more sensitive to calcimycin-induced apoptosis than the vector-transfected cells. Inhibition of Bax expression with antisense-bax RNA also attenuated calcimycin-induced apoptosis (Table 1, row 12). Together, these results demonstrate that Bax is an important downstream target of p53 during calcimycin-induced apoptosis.
Calcimycin-induced Release of Cytochrome c and Activation of Caspase-3 Can Be Substantially Blocked by the ERK Inhibitor UO126
Because Bax promotes apoptosis through interactions with antiapoptotic regulators of the Bcl-2 family in mitochondria (Oltvai et al., 1993; Gross et al., 1999) and induces release of cytochrome c (Eskes et al., 1998; Jürgensmeier et al., 1998; Pastorino et al., 1999), we predicted that p53-regulated Bax expression would alter the permeability of mitochondria in the calcimycin-treated cells and thus allow release of cytochrome c. To test this possibility, we isolated cytosol and mitochondria from both DMSO and calcimycin-treated cells without or with UO126 pretreatment. As shown in Figure 3E, Western blot analysis revealed that calcimycin treatment indeed led to leakage of mitochondria because a substantial amount of cytochrome c was released from the mitochondria to cytoplasm in calcimycin-treated cells but not in DMSO-treated cells (Figure 3E, left). Pretreatment with UO126 greatly attenuated this release of cytochrome c (Figure 3E, right). As a result of differential release of cytochrome c in RLECs without or with pretreatment of UO126, differential caspase-3 activation was observed. As shown in Figure 3F, caspase-3 activity was up-regulated approximately ninefold by calcimycin in N/N1003A cells. In contrast, a <3-fold up-regulation of caspase-3 activity was observed in UO126-pretreated N/N1003A cells after calcimycin treatment. Thus, UO126 prevention of ERK1/2 activation induced by calcimycin also repressed the downstream apoptotic events.
Calcimycin-induced Apoptosis in N/N1003A Cells Is Greatly Attenuated in Cells Expressing the Exogenous Mouse αB-Crystallin
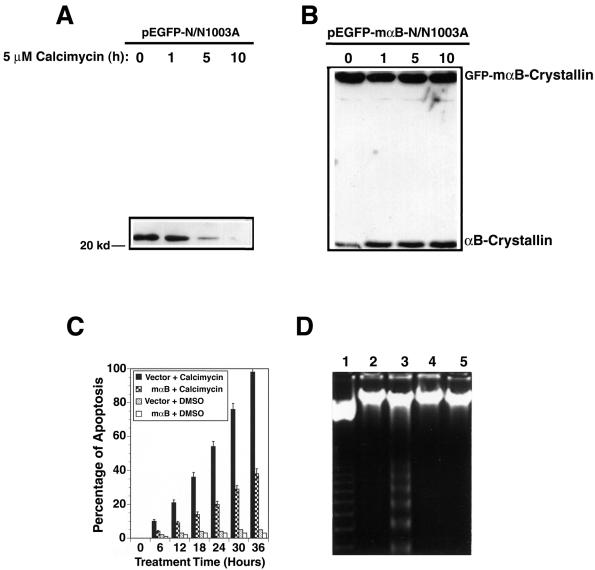
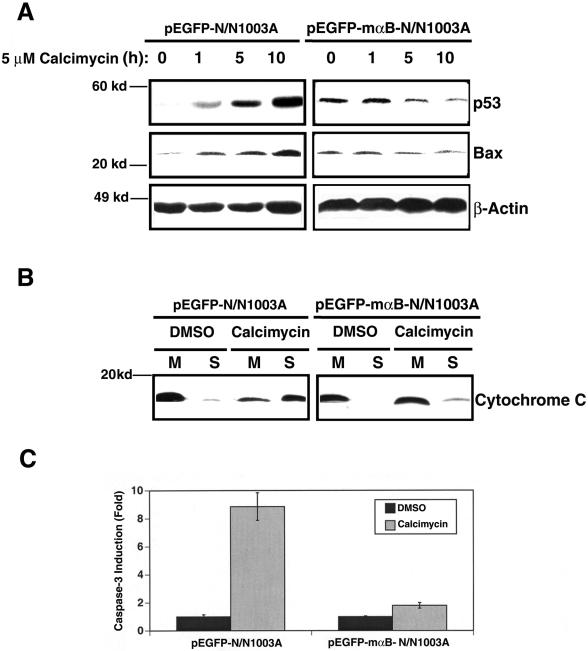
During calcimycin-induced apoptosis, we observed distinct down-regulation of αB-crystallin (Figure 4A). Because αB-crystallin is an antiapoptotic regulator (Li et al., 2001; Mao et al. 2001, 2004), we predicted that overexpression of αB-crystallin may prevent calcimycin-induced apoptosis. As shown in Figure 4B, overexpression of the fusion protein of GFP and mouse αB-crystallin in N/N1003A cells provided resistance to calcimycin-induced degradation of both the endogenous αB-crystallin, and the fusion protein of GFP and mαB-crystallin (Figure 4B). When the viability of the vector-transfected cells (pEGFP-N/N1003A) and the mouse αB-crystallin-transfected N/N1003A cells (pEGFP-mαB-N/N1003A) were compared, it was found that >90% of the vector-transfected cells were dead or undergoing apoptosis after 36-h treatment by 5 μM calcimycin. In contrast, <40% of the mouse αB-crystallin expression cells were apoptotic after the same length of treatment by 5 μM calcimycin (Figure 4C). The apoptotic nature of these cells was verified by DNA fragmentation. As shown in the lane 3 of Figure 4D, an 18-h treatment caused distinct DNA fragmentation laddering in the vector-transfected N/N1003A cells. However, DNA fragmentation ladder was hardly detectable at this stage from the αB-crystallin expression cells (Figure 4D, lane 5 from the left). Hoechst staining further confirmed the above-mentioned results (our unpublished data). Treatment of either vector-transfected cells or mouse αB-transfected cells with 0.01% DMSO induced very few apoptotic cells (our unpublished data).
Figure 4.
αB-crystallin prevents calcimycin-induced apoptosis. (A) Calcimycin-induced degradation of the endogenous αB-crystallin. The vector-transfected cells (pEGFP-N/N1003A) were grown to 100% confluence, and subsequently treated with 5 μM calcimycin for 0, 1, 5, or 10 h in MEM. Then, total proteins were isolated, resolved in 10% SDS-PAGE, and analyzed with antibody against αB-crystallin as described previously (Mao et al., 2001, 2004). Note that the endogenous αB-crystallin became gradually degraded after treatment by 5 μM calcimycin. (B) Overexpression of the fusion protein of GFP and mouse αB-crystallin not only provided resistance to the exogenous fusion protein but also stabilized the endogenous αB-crystallin. The GFP-αB-crystallin-transfected N/N1003A cells were grown to 100% confluence, and subsequently treated with 5 μM calcimycin for 0, 1, 5, or 10 h in MEM. Western blot analysis was conducted as described in Figure 4A. (C) Apoptosis assays. Vector-transfected cells (vector), and mouse αB-crystallin-transfected cells (mαB) were grown to 100% confluence in MEM with 400 μg/ml G418. Then, they were treated with 5 μM calcimycin (calcimycin) or 0.01% DMSO (DMSO) for 0-36 h. The percentage of cell death was determined as described previously (Li et al., 1998). (D) DNA fragmentation assay. The vector-transfected cells (lanes 2 and 3), and mouse αB-crystallin-transfected cells (lanes 4 and 5) were treated with 0.01% DMSO (lanes 2 and 4) or 5 μM calcimycin (lanes 3 and 5) for 18 h. These cells were collected for extraction of genomic DNAs which were processed as described in Figure 1B. The 123 base pairs DNA marker (Invitrogen) is shown in lane 1.
αB-Crystallin Suppresses Calcimycin-induced Activation of ERK1/2
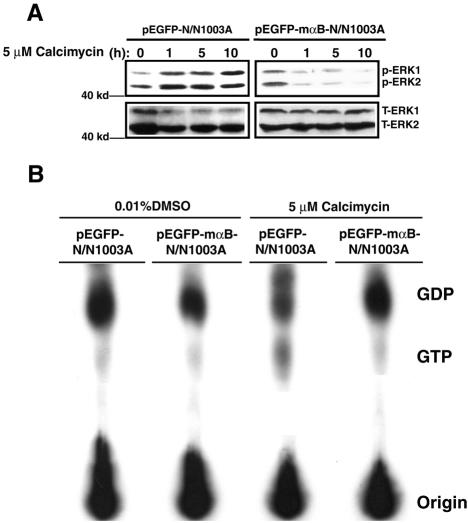
Next, we investigated the possible mechanism by which αB-crystallin prevents calcimycin-induced apoptosis. Knowing that inhibition of ERK1/2 leads to blockage of calcimycin-induced apoptosis, we first tested whether αB-crystallin could abrogate calcimycin-induced activation of ERK1/2. As shown in Figure 5A, left, Western blot analysis revealed that although calcimycin also induced steady activation of ERK1/2 in vector-transfected cells as in the parent N/N1003A cells, calcimycin-induced ERK1/2 activation was not observed in pEGFP-mαB-N/N1003A cell (Figure 5A, right two panels). These results indicate that αB-crystallin prevent calcimycin-induced apoptosis through suppression of calcimycin-induced activation of ERK1/2.
Figure 5.
αB-crystallin blocks calcimycin-induced RAS activation to suppress activation of ERK1/2. (A) Western blot analysis of ERK1/2 activation in vector-transfected cells (pEGFP-N/N1003A) and mouse αB-crystallin-transfected cells (pEGFP-mαB-N/N1003A). Both types of cells were grown to 100% confluence and then treated with 5 μM calcimycin for 0, 1, 5, and 10 h in MEM. After treatment, total proteins were isolated and processed for Western blot analysis as described in Figure 1F. Note that ERK1/2 were steady activated by calcimycin in vector-transfected cells (left). In mouse αB-crystallin-transfected cells, activation of ERK1/2 by calcimycin was suppressed (right). (B). GTP loading assays to show that αB-crystallin blocks calcimycin-induced RAS activation. GTP loading assays were conducted as described in Materials and Methods. Note that after calcimycin stimulation, the amount of GTP released from RAS-GTP in vector-transfected cells was ∼10-fold more than that in αB-crystallin-transfected cells from the intensity analysis using an automatic digit system from the Silk Scientific (Orem, UT) (Li et al., 2003).
αB-Crystallin Blocks RAS-GRF2-mediated RAS Activation to Suppress ERK Activation
The fact that in αB-crystallin expression cells, calcimycin-induced activation of ERK1/2 (Figure 5A), p38 and JNK2 (our unpublished data) was all inhibited indicates that αB-crystallin may acts on the initial steps of RAS/RAF/MEK/ERK signaling pathway. For this reason, we investigated the effect of αB-crystallin on RAS activation. Both pEGFP-N/N1003A and pEGFP-mαB-N/N1003A cells were label with [32P]orthophosphate and then treated with DMSO and 5 μM calcimycin. Cell extracts were prepared from labeled and treated cells, and the RAS protein in each sample was immunoprecipitated. After that, the levels of GTP-bound RAS and GDP-bound RAS were determined with TLC. As shown in Figure 5B, DMSO treatment of both vector and αB-crystallin expression cells only induced background levels of RAS activation as reflected by the faint spots of labeled GTP released from RAS-GTP. Treatment of the vector-transfected cells with 5 μM calcimycin induced substantially activation of RAS. However, such activation of RAS was not observed in the αB-crystallin expression cells. Thus, αB-crystallin blocks RAS activation to suppress activation of the downstream MAP kinases after treatment by calcimycin.
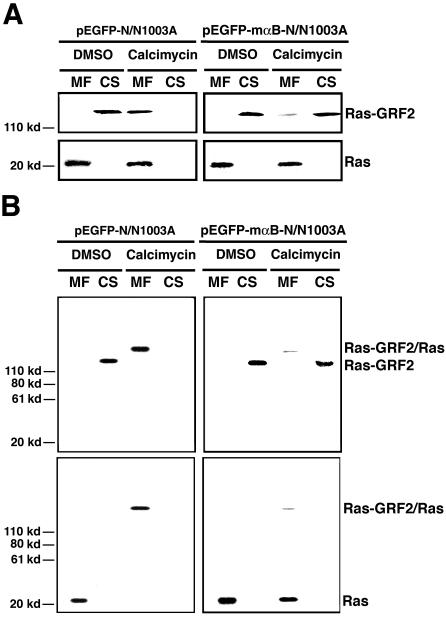
To explore how αB-crystallin may block the activation of RAS, we examined the upstream regulators of RAS. The RAS small GTPases are normally activated by the quanine nucleotide exchange factors (GEFs), which induce the dissociation of GDP to allow association of GTP (Cullen and Lockyer, 2002). On response to growth factor stimulation, two common RAS GEFs, son-of-sevenless 1 (Sos 1) and 2 (Sos 2), are activated to transfer from cytosol to the plasma membrane, and there they activate RAS (Campbell et al., 1998). However, under calcium treatment, RAS is activated by other factors, the RAS-guanine-nucleotide-releasing factor 1 (RAS-GRF1) and 2 (RAS-GRF2) (Cullen and Lockyer, 2002). Because RAS-GRF1 is mainly expressed in the nerve system and RAS-GRF2 is widely expressed in many tissues (Farnsworth et al., 1995; Fam et al., 1997), we therefore examined the activation of RAS by RAS-GRF2 after calcimycin treatment. As shown in Figure 6A, left, after calcimycin treatment, the 135-kDa RAS-GRF2 was transferred from cytosol (CS) to plasma membrane (MF). In the αB-crystallin expression cells, however, the translocation of the RAS-GRF2 from cytosol to plasma membrane was largely inhibited (Figure 6A, right). To further confirm that the RAS-GRF2 translocated to plasma membrane activated RAS, we performed in vivo cross-linking after calcimycin treatment as described in the Materials and Methods. If the RAS-GRF2 indeed activated RAS after calcimycin treatment, it would bind to RAS and thus could be cross-linked together to form a complex. As showed in Figure 6B, left, calcimycin treatment induced formation of the RAS-GRF2 and RAS complex in the vector-transfected N/N1003A cells as demonstrated by the Western blot analysis. In contrast, in the αB-crystallin expression cells, RAS-GRF2 activation of RAS was largely inhibited as reflected by the fact that majority of RAS and RAS-GRF2 were not coupled together (Figure 6B, right). These results demonstrated that in N/N1003A cells, calcimycin activated RAS mainly through RAS-GRF2, and αB-crystallin negatively regulates activation of RAS through suppression of RAS-GRF2 translocation.
Figure 6.
Calcimycin induces RAS-GRF2 to activate RAS, which could be inhibited by αB-crystallin. (A) Western blot analysis of RAS-GRF2 and RAS in vector-transfected cells (pEGFP-N/N1003A) and mouse αB-crystallin-transfected cells (pEGFP-mαB-N/N1003A) treated by DMSO (mock) and calcimycin (experiment). Both membrane (MF) and cytosol (CS) proteins were isolated according to Brennan and Lin (1996) from two types of cells after treatment with 0.01% DMSO (DMSO) or 5 μM calcimycin (calcimycin) for 1 h in MEM. These proteins were processed for Western blot analysis as described in Figure 1F. Note that RAS-GRF2 was transferred from cytosol into membrane fraction after calcimycin treatment (left top). In mouse αB-crystallin-transfected cells, RAS-GRF2 translocation induced by calcimycin was largely suppressed (right top). RAS was always associated with membrane fraction. (B) Cross-linking to show that RAS-GRF2 directly binds to and activates RAS. In vivo cross-linking was conducted as described in Materials and Methods. Note that after calcimycin stimulation, RAS-GRF2 and RAS form a complex that can be cross-linked and detected by anti-RAS-GRF2 antibody (Santa Cruz Biotechnology, Santa Cruz, CA) and anti-RAS antibody (Oncogene Science, Cambridge, MA) (B, left). In αB-crystallin-transfected cells, the RAS-GRF2 was largely retained in the cytosol and only a very small fraction was bound to RAS, indicating that αB-crystallin prevents RAS activation by calcimycin through regulation of RAS-GRF2 translocation.
αΒ-crystallin Prevents Calcimycin-induced Up-Regulation of p53 and Bax, Release of cytochrome c, and Activation of Caspase-3
As described above, inhibition of ERK1/2 activation by UO126 prevents p53 up-regulation (Figure 4A). In αB-crystallin-transfected N/N1003A cells, calcimycin-induced p53 up-regulation was completely abrogated (Figure 7A, right and top). Moreover, calcimycin-induced Bax up-regulation was also suppressed (Figure 7A, right and middle). Thus, by repressing activation of ERK1/2, αB-crystallin also suppressed calcimycin-induced up-regulation of p53 and Bax.
Figure 7.
αB-crystallin abrogates downstream apoptotic events. (A) αB-crystallin abrogates calcimycin-induced up-regulation of p53 and Bax. Both vector-transfected cells (pEGFP-N/N1003A) and mouse αB-crystallin-transfected cells (pEGFP-mαB-N/N1003A) were grown to 100% confluence, then treated with 5 μM calcimycin for 0, 1, 5, or 10 h in MEM. After treatment, Western blot analysis was conducted as described in Figure 3C. (B) αB-crystallin prevents release of cytochrome c from mitochondria. Analysis of cytochrome c was conducted as described in Figure 3E. (C) αB-crystallin prevents activation of caspase-3. Analysis of caspase-3 activity was conducted as described in Figure 3F.
Because αB-crystallin suppressed calcimycin-induced upregulation of p53 and Bax, we predicted that αB-crystallin might preserve the integrity of mitochondria and thus prevent release of cytochrome c. To test this possibility, again, we isolated cytosol and mitochondria from both DMSO and calcimycin-treated vector- and αB-crystallin-transfected cells. As shown in Figure 7B, Western blot analysis of the proteins from these different samples revealed that calcimycin treatment led to malfunction of mitochondria in vector-transfected cells and a substantial amount of cytochrome c was released from the mitochondria to cytoplasm in calcimycin-treated cells but only barely detectable in DMSO-treated cells (Figure 7B, left). Expression of αB-crystallin greatly attenuated this release of cytochrome c (Figure 7B, right). As a result of differential release of cytochrome c in vector- and αB-crystallin-transfected cells, differential caspase-3 activation was observed. As shown in Figure 7C, caspase-3 activity was up-regulated >8.5-fold in the vector-transfected N/N1003A cells. In contrast, <2-fold up-regulation of caspase-3 activity was observed in mouse αB-crystallin-transfected N/N1003A cells after calcimycin treatment (Figure 7C). Together, through repression of ERK1/2 activation induced by calcimycin, αB-crystallin prevents activation of the downstream apoptotic events.
Overexpression of p53 Attenuates the Protective Function of αB-Crystallin
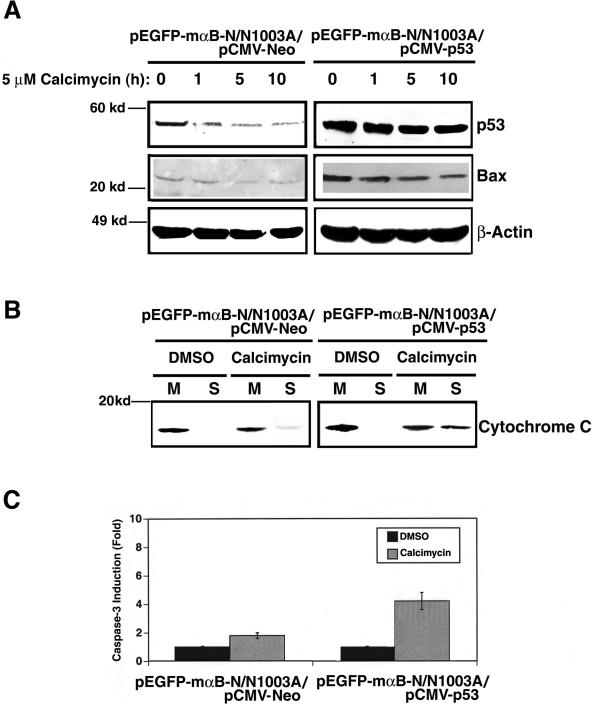
To further confirm that p53 is a key component downstream of ERK1/2 activation during calcimycin-induced apoptosis, we overexpressed p53 in αB-crystallin-transfected N/N1003A cells. Overexpression of p53 in αB-crystallin-transfected N/N1003A cells attenuated the αB-crystallin functions in suppressing expression of Bax (Figure 8A, right), preventing release of cytochrome c (Figure 8B, right), and inhibiting of caspase-3 activation (Figure 8C). As a result, overexpression of p53 in αB-crystallin-transfected N/N1003A cells enhanced apoptosis (Table 1, row 16).
Figure 8.
Overexpression of p53 attenuates the antiapoptotic function of αB-crystallin. (A) Overexpression of p53 attenuates αB-crystallin-mediated down-regulation of p53 and Bax. Treatment of the two types (shown in the figure) of cells was conducted as described in Figure 7A. Western blot analysis was performed as described in Figure 3C. (B) Overexpression of p53 counteracts αB-crystallin-inhibited release of cytochrome c. Analysis of cytochrome c was conducted as described in Figure 3E. (C) Overexpression of p53 attenuates αB-crystallin-repressed caspase-3 activation. Analysis of caspase-3 activity was conducted as described in Figure 3F.
DISCUSSION
Signaling Pathways Mediating Calcium-induced Apoptosis
Calcimycin is one of the earliest known factors shown to induce apoptosis of cells first in the immune system and later in others (McConkey et al., 1989a,b, 1992; Asai et al., 1999; Orrenius et al., 2003). After calcimycin treatment, an increase in the intracellular calcium is a necessary event in the induction of apoptosis (McConkey et al., 1992; Orrenius et al., 2003). After calcium increase, several downstream signaling pathways are apparently activated in different types of cells. One pathway activated by calcium to mediate apoptosis is through activation of the protein phosphatase-2B (PP-2B) (Asai et al., 1999; Wang et al., 1999). Activated PP-2B has been reported to dephosphorylate Bad (Wang et al., 1999), an important proapoptotic member of the Bcl-2 family (Yang et al., 1995; Gross et al., 1999). Another pathway seems to be mediated by the p38 kinase. In mouse myeloid leukemic cells, Lotem et al. (1999) have demonstrated that activation of p38 kinase is necessary for calcimycin-induced apoptosis. How activation of p38 kinase signals the downstream apoptotic events is not defined. In the present study, we demonstrate that calcimycin induces activation of ERK1/2 (Figure 1F), p38 kinase, and also JNK2 (Figure 2, C and D). Activation of p38 kinase and JNK2 is not necessary for calcimycin-induced apoptosis in RLECs because inhibition of p38 kinase activation by PD169316 and JNK2 activation by SP600125 does not interfere with calcimycin-induced apoptosis (Table 1), which is apparently different from that observed in mouse myeloid leukemic cells (Lotem et al., 1999). Furthermore, inhibition of PP-2B activation by cyclosporine A does not block calcimycin-induced apoptosis (our unpublished data), suggesting that an alternative pathway is activated. Indeed, when activation of ERK1/2 is blocked by UO126 or a dominant negative mutant ERK, calcimycin-induced apoptosis is mostly abrogated. Moreover, upstream of ERK activation, the ERK activation kinases, MEK1/2 and MEK1/2 activation kinase, RAF1 also were activated after calcimycin treatment. Activation of both MEK1/2 and RAF1 is necessary for calcium-induced apoptosis of lens epithelial cells based on the inhibition studies with dominant negative mutant RAF1. RAF1 is activated by RAS because a dominant negative mutant RAS interfered with RAF1 activation. Thus, our data show that the RAS-activated RAF/MEK/ERK pathway alone mediates calcium-induced apoptosis.
How could RAS be activated by calcium? In PC 12 cells, it has been shown that membrane depolarization and calcium influx leads to activation of RAS (Rosen et al., 1994). Elevation of intracellular calcium through various stimuli also induces activation of the proline-rich tyrosine kinase 2 (Pyk2) (Lev et al., 1995; Dikic et al., 1996). In neuronal and hematopoietic cells, activated Pyk2 binds to Src, which allows the formed complex to interact with Sos, and thus induces the calcium-dependent activation of RAS (Avraham et al., 2000). RAS also can be activated by the RAS-guanine-nucleotide-releasing factor 1 (RAS-GRF1) (Martegani et al., 1992; Shou et al., 1992; Farnsworth et al., 1995) and 2 (RAS-GRF2) (Fam et al., 1997). Although RAS-GRF1 is mainly expressed in the nerve system, our data suggests that in N/N1003A cells, calcimycin treatment leads to RAS activation mainly through RAS-GRF2. Calcimycin not only induces translocation of the RAS-GRF2 from cytosol to plasma membrane but also causes the direct binding of RAS-GRF2 to RAS (Figure 6). Moreover, αB-crystallin that substantially blocks calcimycin-induced activation of RAS also suppresses the translocation of RAS-GRF2. Thus, through activation of the calcium-triggered RAS-GRF2, calcium can indirectly activated RAS in rabbit lens epithelial cells.
Once ERK1 and ERK2 are activated, the key downstream event is up-regulation and activation of p53 as evidenced from several observations. First, calcimycin induces tremendous up-regulation of the endogenous p53 protein in N/N1003A cells. Regulation of p53 expression by RAS-activated RAF/MEK/ERK pathway has recently been observed in human fibrosarcoma cell line HT1080 (Agarwal et al., 2001). The importance of p53 in mediating calcimycin-induced apoptosis is further demonstrated by the fact that overexpression of human p53 in N/N1003A cells enhanced the sensitivity of calcimycin-induced apoptosis. In contrast, down-regulation of p53 through antisense technology attenuated calcimycin-induced apoptosis.
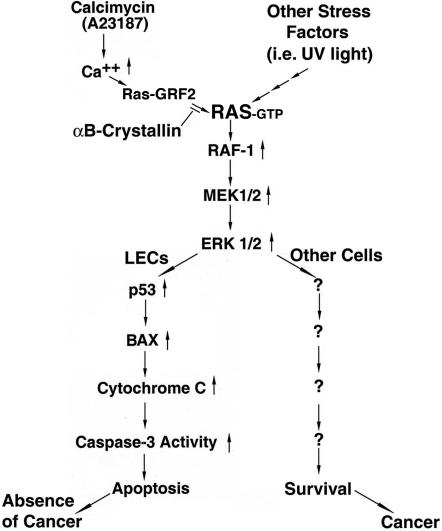
Downstream of p53, Bax, a direct target of p53, plays important role in calcimycin-induced apoptosis. This is demonstrated by several lines of experimental evidence. First, calcimycin-induced up-regulation of Bax parallels with the up-regulation of p53 (Figure 3C). Furthermore, p53 over-expression positively regulates bax promoter-driven reporter gene activity in N/N1003A cells (Figure 3D). Finally, overexpression of either p53 or Bax induces enhanced sensitivity of the N/N1003A cells to calcimycin-induced apoptosis (Table 1). Bax promotes apoptosis through its interaction with antiapoptotic members such as Bcl-2 and Bcl-XL in the mitochondria (Gross et al., 1999). Such interactions have been shown to change the permeability of mitochondria, allowing release of cytochrome c followed by activation of caspase-3 (Eskes et al., 1998; Jürgensmeier et al., 1998; Pastorino et al., 1999). Indeed, in our study, we found that Bax up-regulation changed the permeability of mitochondria as reflected by the fact that a substantial amount of cytochrome c was released after calcimycin treatment. As a result of cytochrome c release, caspase-3, the apoptosis executioner was also activated by calcimycin. Together, our results provide evidence for a novel signaling pathway mediating calcium-induced apoptosis (Figure 9).
Figure 9.
Diagram to show a novel pathway for calcimycin-induced apoptosis in N/N1003A cells, also a novel protective mechanism of αB-crystallin against calcimycin-induced apoptosis and the partial explanation why the ocular lens lacks spontaneous tumor. Calcimycin induces an increase of the intracellular calcium, which induces RAS-GRF2 translocation from cytosol to plasma membrane to activate RAS. RAS then activates RAF1, leading to activation of the downstream MEK1/2, which activates ERK1/2. Activation of this pathway triggers p53 up-regulation and activation, which through regulation of Bax, signals the mitochondrial death pathway as reflected by the release of cytochrome c followed by activation of caspase-3. In lens epithelial cells (LECs), stress-activated RAS/RAF/MEK/ERK signaling pathway mediates apoptosis, leading to elimination of cells with genome lesion. In contrast, in most other cells, the same pathway activated by stress promotes survival, allowing accumulation of cells with damaged DNA, which likely develops into cancer. This partially explains why the ocular lens lacks spontaneous tumor. αB-crystallin suppresses calcimycin-induced RAS-GRF2 translocation to block RAS activation and thus suppress the activation of ERK signaling pathway. Through repression of ERK1/2 activation, it prevents downstream apoptotic events including calcimycin-induced up-regulation of p53 and Bax, release of cytochrome c and activation of caspase-3. Up-arrow stands for the increase of intracellular calcium (Ca2+), or up-regulation of enzyme activity (RAS, RAF1, MEK1/2, ERK1/2, and capase-3), or up-regulation of protein (p53, Bax, and cytochrome c).
Stress-activated RAF/MEK/ERK Signaling Pathway Mediates Apoptosis, which may partially Contribute to Absence of Spontaneous Tumor in the Ocular Lens
Xia et al. (1995) first described the differential functions of MAPKs in regulating apoptosis/survival. These authors show that activation of the JNKs and p38 kinase signaling pathways tends to promote apoptosis, whereas the activation of ERK signaling pathway tends to be antiapoptotic. After this pioneering observation, subsequent studies from several aspects support that the RAF/MEK/ERK pathway is predominantly involved in promotion of survival. First, RAS, the most upstream kinase, is an important signaling molecule activating multiple signaling pathways (Hingorani and Tuveson, 2003). Constitutive activity of RAS (through expression of Harvey RAS) results in the transformation of cells such as NIH 3T3 cells (Cuadrado et al., 1993). During the transformation, RAS-activated RAF/MEK/ERK pathway plays an important role at least in rodent (Cowley et al., 1994). Although recent studies suggest that the RAS-activated RAF/MEK/ERK pathway may not be necessary for transformation of human cells, this pathway is still necessary for the transformed cells to develop tumors in nude mice (Hamad et al., 2002). Second, at the RAF kinase level, numerous studies have shown that RAF kinases are needed for growth factors-mediated cell proliferation (Muszynski et al., 1995; Kerkhoff and Rapp, 1997). Knockout of either B-RAF or RAF1 kinase results in embryonic death (Wojnowski et al., 1997, 1998), and mice without A-RAF also die shortly after birth (Pritchard et al., 1996). On the other hand, over-expression of different RAF kinases (RAF1, A-RAF, and B-RAF) is capable of preventing apoptosis as demonstrated in hematopoietic cells, FDC-P1 and TF1 lines (Hoyle et al., 2000). The major signaling step for RAF kinases to promote survival is through activation of the downstream MEK kinases because inhibition of MEK activity blocks the RAF overexpression-promoted survival (Hoyle et al., 2000). Besides, RAF-1 is associated with mitochondria (Wang et al., 1996) and has been shown to phosphorylate the proapoptotic protein, Bad, to promote survival (Wang and Reed, 1998). Third, at the MEK kinase level, knockout of MEK1 also leads to embryonic death (Giroux et al., 1999), and overexpression of MEK kinase promotes cell survival such as TF1 cells (Blalock et al., 2000). Finally, although the ERK1(-/-) mice are able to survive, the thymocytes from these mice display reduced proliferation in response to ligation of T-cell receptor (Pages et al., 1999). After activation, ERK likely activates p90Rsk to prevent apoptosis through multiple mechanisms. The kinase p90Rsk is either to phosphorylate the transcriptional factor CREB to promote survival (Bos, 1989; Jean et al., 1998) or phosphorylate Bad to prevent apoptosis (Lizcano et al., 2000; Tan et al., 1999).
In contrast to these studies, here we have presented evidence to show that calcium-activated RAS/RAF/MEK/ERK pathway alone mediate calcimycin-induced apoptosis. Interruption of RAF, MEK or ERK kinase activation with either pharmacological inhibitor or dominant negative mutants substantially abrogates the calcimycin-induced apoptosis. On the other hand, blockage of activation of either p38 kinase or JNK2 does not interfere with apoptosis triggered by calcimycin. Similar results were obtained with UVA-induced apoptosis (Liu et al., 2004). Our demonstration that RAF/MEK/ERK signaling pathway instead of JNKs-p38 kinase signaling pathways mediates stress-induced apoptosis reveals some unique features of the lens epithelial cells. In this regard, it is worth to note that the ocular lens is the only organ that does not develop spontaneous tumor (Veromann, 1994). Considering that the ERK pathway is normally involved in promotion of surviving in most other tissues and that oncogenic RAS persistently activates the ERK1 and ERK2 pathways, which contributes to the increased proliferative rate of tumor cells (Johnson and Lapadat, 2002), lack of natural tumor in the ocular lens may partially be due to the fact that in the lens epithelial cells (the only cells that have complete sets of cellular organelles including nuclei in the lens organ), the RAF/MEK/ERK pathway mediates stress-induced apoptosis. On DNA damages induced by various stress factors, activation of the RAF/MEK/ERK pathway, a major pathway for multiple functions in the ocular lens (Gong et al., 2001; Lovicu and McAvoy, 2001; Zatechka and Lou, 2002a,b; Li et al., 2003; Wang et al., 2005), instead of promoting survival of the cells with genetic lesions, signals apoptosis of the damaged cells. In this way, the cells bearing the genetic damages are removed in a timely manner and thus tumor development is avoided (Figure 9).
Mechanism Mediating αB-Crystallin Prevention of Calcimycin-induced Apoptosis
αB-crystallin is initially known as a major lens structural protein that plays an essential role in maintaining the transparency of the ocular lens (Bloemendal, 1981). Later, αΒ-crystallin is found to show chaperone-like activity (Horwitz, 1992; Rao et al., 1993) and also displays autokinase activity (Kantorow and Piatigorsky, 1994). αB-crystallin is a member of the small heat-shock protein family (Ingolia and Craig, 1982). It shares close homology with another small heat shock protein, HSP 27 (Ingolia and Craig, 1982; Xanthoudakis and Nicholson, 2000). Both of them play distinct role in preventing cells from stress-induced apoptosis. These factors have been shown to prevent apoptosis of murine fibrosarcoma cells induced by tumor necrosis factor (TNF) and staurosporin (Mehlen et al., 1996a,b), murine cardiomyocytes induced by ischemia and reperfusion (Ray et al., 2001) and lens epithelial cell triggered by TNF and staurosporin (Andley et al., 2000; Mao et al., 2004), UVA irradiation (Andley et al., 2000; Liu et al., 2004), the phosphatase inhibitor okadaic acid (Li et al., 1998, 2001), and oxidative stress (Mao et al., 2001). Although the two small heat-shock proteins can prevent cells from apoptosis induced by similar or even the same stress conditions, their antiapoptotic mechanisms differ to a large degree. They can act on the same target but display difference in the molecular mechanisms or work on different targets of the apoptotic signaling pathways. Numerous studies have shown that HSP27 prevents apoptosis through 1) binding to caspase-3 to modulate caspase-3 activity (Pandey et al., 2000b), 2) interacting with cytochrome c to prevent activation of procaspase-9 (Garrido et al., 1999; Bruey et al., 2000), and 3) regulating Bid intracellular distribution and protecting F-actin integrity (Paul et al., 2002). In contrast, in our recent study of αB-crystallin protection on hydrogen peroxide-induced apoptosis, we have demonstrated that αB-crystallin interacts with both procaspase-3 and partially processed procaspase-3 to repress caspase-3 activation (Mao et al., 2001). Similar interaction was reported in nonlens system (Kamradt et al., 2001). Second, instead of regulating Bid distribution, more recently in our comparison of the antiapoptotic abilities of the two closely related family members, αA- and αB-crystallins, we have shown that both crystallins can interact with Bax and Bcl-XS to prevent their translocation from cytoplasm into mitochondria, thus abrogating release of cytochrome c, activation of caspase-3 and eventual activation of apoptosis induced by staurosporine (Mao et al., 2004). In this communication, we have presented evidence to show that αB-crystallin is able to abrogate calcimycin-induced apoptosis. The mechanism for such abrogation is through repression of calcimycin-induced activation of ERK1/2. By repressing ERK 1/2 activation, αB-crystallin greatly attenuates up-regulation of p53 and Bax, prevents release of cytochrome c, and finally represses caspase-3 activation. In our recent study of the UVA-induced apoptosis, we found that αB-crystallin is also able to repress UVA-induced activation of the ERK signaling pathway. In contrast, the highly related family member, αA-crystallin, does not seem to have such ability. Instead, it activates the AKT surviving pathway to prevent UVA-induced apoptosis (Liu et al., 2004).
How could αB-crystallin suppress the ERK signaling pathway? Our results presented here (Figure 5B) demonstrate that αB-crystallin is able to block calcimycin-induced activation of the small GTP binding protein RAS, which is the most upstream kinases in the MAP kinase activation hierarchy (Kyriakis and Avruch; 2001; Pearson et al., 2001; Weston and Davis, 2002). Under calcimycin treatment, calcium induces RAS-GRF2 translocation from cytosol to the plasma membrane and thus activates RAS. αB-crystallin prevents RAS activation through suppression of RAS-GRF2 translocation. Whether αB-crystallin regulates RAS-GRF2-translocation by controlling endogenous calcium level or through other strategies is currently under investigation but beyond the scope of the present study. Taken together, our results that αB-crystallin blocks calcimycin-induced RAS activation to suppress RAF/MEK/ERK signaling pathway and related downstream apoptotic events provide a novel mechanism to explain the antiapoptotic ability of this small heat-shock protein (Figure 9).
Acknowledgments
We thank Dr. John Reddan for the N/N1003A cells, Dr. Stanley Korsmeyer for the mouse Bax cDNA, and Patricia C. Schmid for cell flow cytometry analysis. This study was supported in part by the Hormel Foundation, the National Institutes of Health/National Eye Institute Grant EY 15765 (to D.W.L.) and National Institutes of Health grants to Z. D., R. B., and J. R.
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E05-01-0010) on July 6, 2005.
References
- Agarwal, M. L., Ramana, C. V., Hamilton, M., Taylor, W. R., DePrimo, S. E., Bean, L. J., Agarwal, A., Agarwal, M. K., Wolfman, A., and Stark, G. R. (2001). Regulation of p53 expression by the RAS-MAP kinase pathway. Oncogene 20, 2527-2536. [DOI] [PubMed] [Google Scholar]
- Andley, U. P., Song, Z., Wawrousek, E. F., and Fleming, T. P. (2000). Differential protective activity of αA- and αB-crystallin in lens epithelial cells. J. Biol. Chem. 275, 36823-36831. [DOI] [PubMed] [Google Scholar]
- Asai, A., Qiu, J., Narita, Y., Chi, S., Saito, N., Shinoura, N., Hamada, H., Kuchino, Y., and Kirino, T. (1999). High level calcineurin activity predisposes neuronal cells to apoptosis. J. Biol. Chem. 274, 34450-34458. [DOI] [PubMed] [Google Scholar]
- Ausubel, F. M., Brent, R., Kingston, R. E., Moore, D. D., Seidman, J. E., Smith, J. A., and Struhl, K. (2002). Current Protocols in Molecular Biology, New York: John Wiley & Sons.
- Avraham, H., Park, S. Y., Schinkmann, K., and Avraham, S. (2000). RAFTK/Pyk2-mediated cellular signalling. Cell Signal. 12, 123-133. [DOI] [PubMed] [Google Scholar]
- Ballif, B. A., and Blenis, J. (2001). Molecular mechanisms mediating mammalian mitogen-activated protein kinase (MAPK) kinase (MEK)-MAPK cell survival signals. Cell Growth Differ. 12, 397-408. [PubMed] [Google Scholar]
- Blalock, W. L., Pearce, M., Steelman, L. S., Franklin, R. A., McCarthy, S. A., Cherwinski, H., McMahon, M., and McCubrey, J. A. (2000). A conditionally-active form of MEK1 results in autocrine transformation of human and mouse hematopoietic cells. Oncogene 19, 526-536. [DOI] [PubMed] [Google Scholar]
- Beere, H. M., Wolf, B. B., Cain, K., Mosser, D. D., Mahboubi, A., Kuwana, T., Tailor, P., Morimoto, R. I., Cohen, G. M., and Green, D. R. (2000). Heat-shock protein 70 inhibits apoptosis by preventing recruitment of procaspase-9 to the Apaf-1 apoptosome. Nat. Cell. Biol. 2, 469-475. [DOI] [PubMed] [Google Scholar]
- Behrens, A., Sibilia, M., and Wagner, E. F. (1999). Amino-terminal phosphorylation of c-Jun regulates stress-induced apoptosis and cellular proliferation. Nat. Genet. 21, 326-329. [DOI] [PubMed] [Google Scholar]
- Bloemendal, H. (1981). Molecular Biology of the Eye Lens, New York: John Wiley & Sons.
- Bonni, A., Brunet, A., West, A. E., Datta, S. R., Takasu, M. A., and Greenberg, M. E. (1999). Cell survival promoted by the RAS-MAPK signaling pathway by transcription-dependent and -independent mechanisms. Science 286, 1358-1362. [DOI] [PubMed] [Google Scholar]
- Bos, J. L. (1989). RAS oncogenes in human cancer: a review. Cancer Res. 49, 4682-4689. [PubMed] [Google Scholar]
- Brennan, W. A., Jr., and Lin, S.-W. (1996). Solubilization and purification of the rat liver insulin receptor. In: Strategies for Protein Purification and Characterization. A Laboratory Course Manual, ed. D. R. Marshak, J. T. Kadonoga, R. R. Burgess, M. W. Knuth, W. A. Brennan, Jr., and S. H. Lin, Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press, 275-343.
- Bruey, J. M., et al. (2000). Hsp27 negatively regulates cell death by interacting with cytochrome c. Nat. Cell Biol. 2, 645-652. [DOI] [PubMed] [Google Scholar]
- Campbell, S. L., Khosravi-Far, R., Rossman, K. L., Clark, G. J., and Der, C. J. (1998). Increasing complexity of calcium signaling. Oncogene 17, 1395-1413. [DOI] [PubMed] [Google Scholar]
- Carson, D. A., and Ribeiro, J. M. (1993). Apoptosis and disease. Lancet 341, 1251-1254. [DOI] [PubMed] [Google Scholar]
- Cowan, W. M., Fawcett, J. W., O'Leary, D.D.M., and Stanfield, B. B. (1984). Regressive events in neurogenesis. Science 225, 1258-1265. [DOI] [PubMed] [Google Scholar]
- Cowley, S., Paterson, H., Kemp, P., and Marshall, C. J. (1994). Activation of MAP kinase kinase is necessary and sufficient for PC12 differentiation and for transformation of NIH 3T3 cells. Cell 77, 841-852. [DOI] [PubMed] [Google Scholar]
- Cuadrado, A., Bruder, J. T., Heidaran, M. A., App, H., Rapp, U. R., and Aaronson, S. A. (1993). H-RAS and raf-1 cooperate in transformation of NIH3T3 fibroblasts. Oncogene 8, 2443-2448. [PubMed] [Google Scholar]
- Cullen, P. J., and Lockyer, P. J. (2002). Integration of calcium and RAS signalling. Nat. Rev. Mol. Cell. Biol. 3, 339-348. [DOI] [PubMed] [Google Scholar]
- Dickens, M., Rogers, J. S., Cavanagh, J., Raitano, A., Xia, Z., Halpern, J. R., Greenberg, M. E., Sawyers, C. L., and Davis, R. J. (1997). A cytoplasmic inhibitor of the JNK signal transduction pathway. Science 277, 693-696. [DOI] [PubMed] [Google Scholar]
- Dikic, I., Tokiwa, G., Lev, S., Courtneidge, S. A., and Schlessinger, J. (1996). A role for Pyk2 and Src in linking G-protein-coupled receptors with MAP kinase activation. Nature 383, 547-550. [DOI] [PubMed] [Google Scholar]
- Eskes, R., Antonsson, B., Osen-Sand, A., Montessuit, S., Richter, C., Sadoul, R., Mazzei, G., Nichols, A., and Martinou, J. C. (1998). Bax-induced cytochrome C release from mitochondria is independent of the permeability transition pore but highly dependent on Mg 2+ ions. J. Cell Biol. 143, 217-224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eskes, R., Desagher, S., Antonsson, B., and Martinou, J. C. (2000). Bid induces the oligomerization and insertion of Bax into the outer mitochondrial membrane. Mol. Cell. Biol. 20, 929-935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fam, N. P., Fan, W. T., Wang, Z. X., Zhang, L. J., Chen, H., and Moran, M. F. (1997). Cloning and characterization of RAS-GRF2, a novel guanine nucleotide exchange factor for RAS. Mol. Cell. Biol. 17, 1396-1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farnsworth, C. L., Freshney, N. W., Rosen, L. B., Ghosh, A., Greenberg, M. E., and Feig, L. A. (1995). Calcium activation of RAS mediated by neuronal exchange factor RAS-GRF. Nature 376, 524-527. [DOI] [PubMed] [Google Scholar]
- Feng, H., et al. (2004). Human Bcl-2 activates ERK signaling pathway to regulate activating protein-1, lens epithelium-derived growth factor and downstream genes. Oncogene 23, 7310-7321. [DOI] [PubMed] [Google Scholar]
- Franklin, R. A., and McCubrey, J. A. (2000). Kinases: positive and negative regulators of apoptosis. Leukemia 14, 2019-2034. [DOI] [PubMed] [Google Scholar]
- Garrido, C., Bruey, J. M., Fromentin, A., Hammann, A., Arrigo, A. P., and Solary, E. (1999). HSP27 inhibits cytochrome c-dependent activation of procaspase-9. FASEB J. 13, 2061-2070. [DOI] [PubMed] [Google Scholar]
- Giroux, S., et al. (1999). Embryonic death of Mek1-deficient mice reveals a role for this kinase in angiogenesis in the labyrinthine region of the placenta. Curr. Biol. 9, 369-372. [DOI] [PubMed] [Google Scholar]
- Gong, X., Wang, X., Han, J., Niesman, I., Huang, Q., and Horwitz, J. (2001). Development of cataractous macrophthalmia in mice expressing an active MEK1 in the lens. Invest. Ophthalmol. Vis. Sci. 42, 539-548. [PubMed] [Google Scholar]
- Gross, A., McDonnell, J. M., and Korsmeyer, S. J. (1999). BCL-2 family members and the mitochondria in apoptosis. Genes Dev. 13, 1899-1911. [DOI] [PubMed] [Google Scholar]
- Hamad, N. M., Elconin, J. H., Karnoub, A. E., Bai, W., Rich, J. N., Abraham, R. T., Der, C. J., and Counter, C. M. (2002). Distinct requirements for RAS oncogenesis in human versus mouse cells. Genes Dev. 16, 2045-2057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayakawa, J., Ohmichi, M., Kurachi, H., Ikegami, H., Kimura, A., Matsuoka, T., Jikihara, H., Mercola, D., and Murata, Y. (1999). Inhibition of extracellular signal-regulated protein kinase or c-Jun N-terminal protein kinase cascade, differentially activated by cisplatin, sensitizes human ovarian cancer cell line. J. Biol. Chem. 274, 31648-31654. [DOI] [PubMed] [Google Scholar]
- Hingorani, S. R., and Tuveson, D. A. (2003). RAS redux: rethinking how and where RAS acts. Curr. Opin. Genet. Dev. 13, 6-13. [DOI] [PubMed] [Google Scholar]
- Horwitz, J. (1992). Alpha-crystallin can function as a molecular chaperone. Proc. Natl. Acad. Sci. USA 89, 10449-10453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoyle, P. E., Moye, P. W., Steelman, L. S., Blalock, W. L., Franklin, R. A., Pearce, M., Cherwinski, H., Bosch, E., McMahon, M., and McCubrey, J. A. (2000). Differential abilities of the Raf family of protein kinases to abrogate cytokine dependency and prevent apoptosis in murine hematopoietic cells by a MEK1-dependent mechanism. Leukemia 14, 642-656. [DOI] [PubMed] [Google Scholar]
- Huang, C., Ma, W. Y., and Dong, Z. (1999). The extracellular-signal-regulated protein kinases (Erks) are required for UV-induced AP-1 activation in JB6 cells. Oncogene 18, 2828-2835. [DOI] [PubMed] [Google Scholar]
- Huang, X.-Q., et al. (2005). hTERT extends proliferative lifespan and prevents oxidative stress-induced apoptosis in human lens epithelial cells. Invest. Ophthalmol. Vis. Sci. 46, 2503-2513. [DOI] [PubMed] [Google Scholar]
- Ingolia, T. D., and Craig, E. A. (1982). Four small Drosophila heat shock proteins are related to each other and to mammalian α-crystallin. Proc. Natl. Acad. Sci. USA 79, 2360-2364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaattela, M., Wissing, D., Kokholm, K., Kallunki, T., and Egeblad, M. (1998). Hsp70 exerts its anti-apoptotic function downstream of caspase-3-like proteases. EMBO J. 17, 6124-6134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jean, D., Harbison, M., McConkey, D. J., Ronai, Z., and Bar-Eli, M. (1998). CREB and its associated proteins act as survival factors for human melanoma cells. J. Biol. Chem. 273, 24884-24890. [DOI] [PubMed] [Google Scholar]
- Johnson, G. L., and Lapadat, R. (2002). Mitogen-activated protein kinase pathways mediated by ERK, JNK, and p38 protein kinases. Science 298, 1911-1912. [DOI] [PubMed] [Google Scholar]
- Jürgensmeier, J. M., Xie, Z., Deveraux, Q., Ellerby, L., Bredesen, D., and Reed, J. C. (1998). Bax directly induces release of cytochrome C from isolated mitochondria. Proc. Natl. Acad. Sci. USA 95, 4997-5002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamradt, M. C., Chen, F., and Cryns, V. L. (2001). The small heat shock protein αB-crystallin negatively regulates cytochrome c- and caspase-8-dependent activation of caspase-3 by inhibiting its autoproteolytic maturation. J. Biol. Chem. 276, 16059-16063. [DOI] [PubMed] [Google Scholar]
- Kantorow, M., and Piatigorsky, J. (1994). α-Crystallin/small heat shock protein has autokinase activity. Proc. Natl. Acad. Sci. USA 91, 3112-3116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerkhoff, E., and Rapp, U. R. (1997). Induction of cell proliferation in quiescent NIH 3T3 cells by oncogenic c-Raf-1. Mol. Cell. Biol. 17, 2576-2586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr, J.F.R., Wyllie, A. H., and Currrie, A. R. (1972). Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br. J. Cancer 26, 239-257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr, J.F.R., Harmon, B., and Searle, J. (1974). An electron-microscope study of cell deletion in the anuran tadpole tail during spontaneous metamorphosis with special reference to apoptosis of striated muscle fibers. J. Cell Sci. 14, 571-585. [DOI] [PubMed] [Google Scholar]
- Kyriakis, J. M., and Avruch, J. (2001). Mammalian mitogen-activated protein kinase signal transduction pathways activated by stress and inflammation. Physiol. Rev. 81, 807-869. [DOI] [PubMed] [Google Scholar]
- Lev, S., Moreno, H., Martinez, R., Canoll, P., Peles, E., Musacchio, J. M., Plowman, G. D., Rudy, B., and Schlessinger, J. (1995). Protein tyrosine kinase PYK2 involved in Ca(2+)-induced regulation of ion channel and MAP kinase functions. Nature 376, 737-745. [DOI] [PubMed] [Google Scholar]
- Li, D.W.-C. (1997). The lens epithelium, apoptosis and cataract formation. In: Eye Lens Epithelium: Damaging Mechanisms and Lens Transparency, Chapter 8, ed. G. Glaesser, O. Hockwin, and G.F.J.M. Vrensen, Halle: Nova Acta Leopoldina NF 75, 81-108.
- Li, D.W.-C., Fass, U., Huizar, I., and Spector, A. (1998). Okadaic acid-induced lens epithelial cell apoptosis requires inhibition of phosphatase-1 and is associated with induction of gene expression including p53 and bax. Eur. J. Biochem. 257, 351-361. [DOI] [PubMed] [Google Scholar]
- Li, D.W.-C., Liu, J. P., Mao, Y. W., Wang, J., and Hou, L. H. (2003). Expression and Activity of the Signaling Molecules for Mitogen-Activated Protein Kinase Pathways in Human, Bovine and Rat Lenses. Investig. Ophthalmol. Vis. Sci. 44, 5277-5286. [DOI] [PubMed] [Google Scholar]
- Li, D.W.-C., Xiang, H., Mao, Y. W., Wang, J., Fass, U., Zhang, X. Y., and Xu, C. (2001). Caspase-3 is actively involved in okadaic acid-induced lens epithelial cell apoptosis. Exp. Cell. Res. 266, 279-291. [DOI] [PubMed] [Google Scholar]
- Li, W. C., et al. (1995a). Lens epithelial cell apoptosis appears to be a common cellular basis for non-congenital cataract development in humans and animals. J. Cell Biol. 130, 169-181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, W. C., Kuszak, J. R., Wang, G. M., Wu, Z. Q., and Spector, A. (1995b). Calcimycin-induced lens epithelial cell apoptosis contributes to cataract formation. Exp. Eye Res. 61, 91-98. [DOI] [PubMed] [Google Scholar]
- Li, W. C., and Spector, A. (1996). Lens epithelial cell apoptosis is an early event in the development of UVB-induced cataract. Free Radic. Biol. Med. 20, 301-311. [DOI] [PubMed] [Google Scholar]
- Liu, J.-P., Schlosser, R., Ma, W.-Y., Dong, Z., Feng, H., Liu, L., Huang, X.-Q., Liu, Y., and Li, D.W.-C. (2004). Human αA- and αB-crystallins prevent UVA-induced apoptosis through regulation of protein kinase C (PKC)α, RAF/MEK/ERK and AKT signaling pathways. Exp. Eye Res. 79, 393-403. [DOI] [PubMed] [Google Scholar]
- Lizcano, J. M., Morrice, N., and Cohen, P. (2000). Regulation of BAD by cAMP-dependent protein kinase is mediated via phosphorylation of a novel site, Ser155. Biochem. J. 349, 547-557. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Lotem, J., Kama, R., and Sachs, L. (1999). Suppression or induction of apoptosis by opposing pathways downstream from calcium-activated calcineurin. Proc. Natl. Acad. Sci. USA 96, 12016-12020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovicu, F. J., and McAvoy, J. W. (2001). FGF-induced lens cell proliferation and differentiation is dependent on MAPK (ERK1/2) signaling. Development 128, 5075-5084. [DOI] [PubMed] [Google Scholar]
- Mao, Y. W., Liu J.-P, Xiang, H., and Li, D. W. (2004). Human αA- and αB-crystallins bind to Bax and Bcl-XS to sequester their translocation during staurosporine-induced apoptosis. Cell Death Differ. 11, 512-526. [DOI] [PubMed] [Google Scholar]
- Mao, Y. W., Xiang, H., Wang, J., Korsmeyer, S., Reddan, J., and Li, D. W. (2001). Human bcl-2 gene attenuates the ability of RLECs against H2O2-induced apoptosis through down-regulation of the αB-crystallin gene. J. Biol. Chem. 276, 43435-43445. [DOI] [PubMed] [Google Scholar]
- Martegani, E., Vanoni, M., Zippel, R., Coccetti, P., Brambilla, R., Ferrari, C., Sturani, E., and Alberghina, L. (1992). Cloning by functional complementation of a mouse cDNA encoding a homologue of CDC25, a Saccharomyces cerevisiae RAS activator. EMBO J. 11, 2151-2157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAvoy, J. W. (1980). Induction of the eye lens. Differentiation 17, 137-149. [DOI] [PubMed] [Google Scholar]
- McConkey, D. J., Hartzell, P., Jondal, M., and Orrenius, S. (1989a). Inhibition of DNA fragmentation in thymocytes and isolated thymocyte nuclei by agents that stimulate PKC. J. Biol. Chem. 264, 13399-13402. [PubMed] [Google Scholar]
- McConkey, D. J., Hartzell, P., Nicotera, P., and Orrenius, S. (1989b). Calcium-activated DNA fragmentation kills immature thymocytes. FASEB J. 3, 1843-1849. [DOI] [PubMed] [Google Scholar]
- McConkey, D. J., Jondal, M., and Orrenius, S. (1992). Cellular signaling in thymocyte apoptosis. Semin. Immunol. 4, 371-377. [PubMed] [Google Scholar]
- Mehlen, P., Kretz-Remy, C., Preville, X., and Arrigo, A. P. (1996a). Human Hsp27, Drosophila Hsp27 and human αB-crystallin expression-mediated increase in glutathione is essential for the protective activity of these proteins against TNFα-induced cell death. EMBO J. 15, 2695-2706. [PMC free article] [PubMed] [Google Scholar]
- Mehlen, P., Schulze-Osthoff, K., and Arrigo, A. P. (1996b). Small stress proteins as novel regulators of apoptosis. Heat shock protein 27 blocks Fas/APO-1- and staurosporine-induced cell death. J. Biol. Chem. 271, 16510-16514. [DOI] [PubMed] [Google Scholar]
- Meyaard, L., Otto, S. A., Jonker, R. R., Janneke, M. M., Keet, R.P.M., and Miederma, F. (1992). Programmed death of T cells in HIV-1 infection. Science 257, 217-220. [DOI] [PubMed] [Google Scholar]
- Mischak, H., Seitz, T., Janosch, P., Eulitz, M., Steen, H., Schellerer, M., Philipp, A., and Kolch, W. (1996). Negative regulation of Raf-1 by phosphorylation of serine 621. Mol. Cell. Biol. 16, 5409-5418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyashita, T., and Reed, J. C. (1995). Tumor suppressor p53 is a direct transcriptional activator of the human bax gene. Cell 80, 293-299. [DOI] [PubMed] [Google Scholar]
- Morgenbesser, S. D., Williams, B. O., Jacks, T., and DePinho, R. A. (1994). p53-dependent apoptosis produced by Rb-deficiency in the developing mouse lens. Nature 371, 72-74. [DOI] [PubMed] [Google Scholar]
- Muszynski, K. W., Ruscetti, F. W., Heidecker, G., Rapp, U., Troppmair, J., Gooya, J. M., and Keller, J. R. (1995). Raf-1 protein is required for growth factor-induced proliferation of hematopoietic cells. J. Exp. Med. 181, 2189-2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemoto, S., Xiang, J., Huang, S., and Lin, A. (1998). Induction of apoptosis by SB202190 through inhibition of p38β mitogen-activated protein kinase. J. Biol. Chem. 273, 16415-16420. [DOI] [PubMed] [Google Scholar]
- Orrenius, S., Zhivotovsky, B., and Nicotera, P. (2003). Regulation of cell death: the calcium apoptosis link. Nat. Rev. Mol. Cell. Biol. 4, 552-565. [DOI] [PubMed] [Google Scholar]
- Ormerod, M. G. (1994). Further applications to cell biology. In: Flow Cytometry: A Practical Approach, 2nd ed., ed. M. G. Ormerod, Oxford: IRL Press, 119-135.
- Oltvai, Z. N., Milliman, C. L., and Korsmeyer, S. J. (1993). Bcl-2 heterodimerizes in vivo with a conserved homolog, Bax, that accelerates programmed cell death. Cell 74, 609-619. [DOI] [PubMed] [Google Scholar]
- Pages, G., Guerin, S., Grall, D., Bonino, F., Smith, A., Anjuere, F., Auberger, P., and Pouyssegur, J. (1999). Defective thymocyte maturation in p44 MAP kinase (Erk 1) knockout mice. Science 286, 1374-1377. [DOI] [PubMed] [Google Scholar]
- Pandey, P., et al. (2000a). Negative regulation of cytochrome c-mediated oligomerization of Apaf-1 and activation of procaspase-9 by heat shock protein 90. EMBO J. 19, 4310-4322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey, P., Farber, R., Nakazawa, A., Kumar, S., Bharti, A., Nalin, C., Weichselbaum, R., Kufe, D., and Kharbanda, S. (2000b). Hsp27 functions as a negative regulator of cytochrome c-dependent activation of procaspase-3. Oncogene 19, 1975-1981. [DOI] [PubMed] [Google Scholar]
- Pastorino, J. G., Tafani, M., Rothman, R. J., Marcinkeviciute, A., Hoek, J. B., Farber, J. L., and Marcineviciute, A. (1999). Functional consequences of the sustained or transient activation by Bax of the mitochondrial permeability transition pore. J. Biol. Chem. 274, 31734-31739. [DOI] [PubMed] [Google Scholar]
- Paul, C., Manero, F., Gonin, S., Kretz-Remy, C., Virot, S., and Arrigo, A. P. (2002). Hsp27 as a negative regulator of cytochrome C release. Mol. Cell. Biol. 22, 816-834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson, G., Robinson, F., Beers Gibson, T., Xu, B. E., Karandikar, M., Berman, K., and Cobb, M. H. (2001). Mitogen-activated protein (MAP) kinase pathways: regulation and physiological functions. Endocr. Rev. 22, 153-183. [DOI] [PubMed] [Google Scholar]
- Persons, D. L., Yazlovitskaya, E. M., Cui, W., and Pelling, J. C. (1999). Cisplatin-induced activation of mitogen-activated protein kinases in ovarian carcinoma cells: inhibition of extracellular signal-regulated kinase activity increases sensitivity to cisplatin. Clin. Cancer Res. 5, 1007-1014. [PubMed] [Google Scholar]
- Piatigorsky, J. (1981). Lens differentiation in vertebrates. A review of cellular and molecular features. Differentiation 19, 134-153. [DOI] [PubMed] [Google Scholar]
- Pritchard, C. A., Bolin, L., Slattery, R., Murray, R., and McMahon, M. (1996). Post-natal lethality and neurological and gastrointestinal defects in mice with targeted disruption of the A-Raf protein kinase gene. Curr. Biol. 6, 614-617. [DOI] [PubMed] [Google Scholar]
- Raff, M. C. (1992). Social controls on cell survival and cell death. Nature 356, 397-400. [DOI] [PubMed] [Google Scholar]
- Rao, P. V., Horwitz, J., and Zigler, J. S., Jr. (1993). α-Crystallin, a molecular chaperone, forms a stable complex with carbonic anhydrase upon heat denaturation. Biochem. Biophys. Res. Commun. 190, 786-793. [DOI] [PubMed] [Google Scholar]
- Ray, P. S., Martin, J. L., Swanson, E. A., Otani, H., Dillmann, W. H., and Das, D. K. (2001). Transgene over-expression of αB crystallin confers simultaneous protection against cardiomyocyte apoptosis and necrosis during myocardial ischemia and reperfusion. FASEB J. 15, 393-402. [DOI] [PubMed] [Google Scholar]
- Rosen, L. B., Ginty, D. D., Weber, M. J., and Greenberg, M. E. (1994). Membrane depolarization and calcium influx stimulate MEK and MAP kinase via activation of RAS. Neuron 12, 1207-1221. [DOI] [PubMed] [Google Scholar]
- Shou, C., Farnsworth, C. L., Nell, B. G., and Feig, L. A. (1992). Molecular cloning of cDNAs encoding a guanine-nucleotide-releasing factor for RAS p21. Nature 358, 351-354. [DOI] [PubMed] [Google Scholar]
- Spector, A. (1995). (1995). Oxidative stress-induced cataract: mechanism of action. FASEB J. 9, 1173-1182. [PubMed] [Google Scholar]
- Strasser, A., Harris, A. W., and Cory, S. (1991). bcl-2 transgene inhibits T cell death and perturbs thymic self-censorship. Cell 67, 889-899. [DOI] [PubMed] [Google Scholar]
- Takayama, S., Bimston, D. N., Matsuzawa, S., Freeman, B. C., Aime-Sempe, C., Xie, Z., Morimoto, R. I., and Reed, J. C. (1997). BAG-1 modulates the chaperone activity of Hsp70/Hsc70. EMBO J. 16, 4887-4896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan, Y., Ruan, H., Demeter, M. R., and Comb, M. J. (1999). p90(RSK) blocks bad-mediated cell death via a PKC-dependent pathway. J. Biol. Chem. 274, 34859-34867. [DOI] [PubMed] [Google Scholar]
- Thompson, C. B. (1995). Apoptosis in the pathogenesis and treatment of disease. Science 267, 1456-1462. [DOI] [PubMed] [Google Scholar]
- Tournier, C., Hess, P., Yang, D. D., Xu, J., Turner, T. K., Nimnual, A., Bar-Sagi, D., Jones, S. N., Flavell, R. A., and Davis, R. J. (2000). Requirement of JNK for stress-induced activation of the cytochrome c-mediated death pathway. Science 288, 870-874. [DOI] [PubMed] [Google Scholar]
- Veromann, S. (1994). Eye lens tumors: an experimental-histological and theoretical approach. Tumour Biol. 15, 135-140. [DOI] [PubMed] [Google Scholar]
- Wang, H. G., Rapp, U. R., and Reed, J. C. (1996). Bcl-2 targets the protein kinase Raf-1 to mitochondria. Cell 87, 629-638. [DOI] [PubMed] [Google Scholar]
- Wang, H. G., Pathan, N., Ethell, I. M., Krajewski, S., Yamaguchi, Y., Shibasaki, F., McKeon, F., Bobo, T., Franke, T. F., and Reed, J. C. (1999). Ca2+-induced apoptosis through calcineurin dephosphorylation of BAD. Science 284, 339-343. [DOI] [PubMed] [Google Scholar]
- Wang, H. G., and Reed, J. C. (1998). Bc1-2, Raf-1 and mitochondrial regulation of apoptosis. Biofactors 8, 13-16. [DOI] [PubMed] [Google Scholar]
- Wang J, et al. (2005). Human telomerase reverse transcriptase immortalizes bovine lens epithelial cells and suppresses differentiation through regulation of the ERK signaling pathway. J. Biol. Chem. 280, 22776-22787. [DOI] [PubMed] [Google Scholar]
- Wang, Y., Huang, S., Sah, V. P., Ross, Jr., J., Brown, J. H., Han, J., and Chien, K. R. (1998). Cardiac muscle cell hypertrophy and apoptosis induced by distinct members of the p38 mitogen-activated protein kinase family. J. Biol. Chem. 273, 2161-2168. [DOI] [PubMed] [Google Scholar]
- Wang, X. (2001). The expanding role of mitochondria in apoptosis. Genes Dev. 15, 2922-2933. [PubMed] [Google Scholar]
- Weston, C. R., and Davis, R. J. (2002). The JNK signal transduction pathway. Curr. Opin. Genet. Dev. 12, 14-21. [DOI] [PubMed] [Google Scholar]
- Williams, G. T., Smith, C. A., Spooncer, E., Dexter, T. M., and Taylor, D. R. (1990). Haemopoietic colony stimulating factors promote cell survival by suppressing apoptosis. Nature 343, 76-79. [DOI] [PubMed] [Google Scholar]
- Wojnowski, L., Stancato, L. F., Zimmer, A. M., Hahn, H., Beck, T. W., Larner, A. C., Rapp, U. R., and Zimmer, A. (1998). Craf-1 protein kinase is essential for mouse development. Mech. Dev. 76, 141-149. [DOI] [PubMed] [Google Scholar]
- Wojnowski, L., Zimmer, A. M., Beck, T. W., Hahn, H., Bernal, R., Rapp, U. R., and Zimmer, A. (1997). Endothelial apoptosis in Braf-deficient mice. Nat. Genet. 16, 293-297. [DOI] [PubMed] [Google Scholar]
- Wyllie, A. H. (1980). Glucocorticoid-induced thymocyte apoptosis is associated with endogenous endonuclease activation. Nature 284, 555-556. [DOI] [PubMed] [Google Scholar]
- Xanthoudakis, S., and Nicholson, D. W. (2000). Heat-shock proteins as death determinants. Nat. Cell. Biol. 2, E163-E165. [DOI] [PubMed] [Google Scholar]
- Xia, Z., Dickens, M., Raingeaud, J., Davis, R. J., and Greenberg, M. E. (1995). Opposing effects of ERK and JNK-p38 MAP kinases on apoptosis. Science 270, 1326-1331. [DOI] [PubMed] [Google Scholar]
- Xiang, H., Wang, J., Mao, Y., Liu, M., Reddy, V. N., and Li, D. W. (2002). Human telomerase accelerates growth of lens epithelial cells through regulation of the genes mediating RB/E2F pathway. Oncogene 21, 3784-3791. [DOI] [PubMed] [Google Scholar]
- Yang, D. D., Kuan, C. Y., Whitmarsh, A. J., Rincon, M., Zheng, T. S., Davis, R. J., Rakic, P., and Flavell, R. A. (1997). Absence of excitotoxicity-induced apoptosis in the hippocampus of mice lacking the Jnk3 gene. Nature 389, 865-870. [DOI] [PubMed] [Google Scholar]
- Yang, E., Zha, J., Jockel, J., Boise, L. H., Thompson, C. B., and Korsmeyer, S. J. (1995). Bad, a heterodimeric partner for Bcl-XL and Bcl-2, displaces Bax and promotes cell death. Cell 80, 285-291. [DOI] [PubMed] [Google Scholar]
- Yuan, J. (1997). Transducing signals of life and death. Curr. Opin. Cell Biol. 9, 247-251. [DOI] [PubMed] [Google Scholar]
- Zatechka, S. D., Jr., and Lou, M. F. (2002a). Studies of the mitogen-activated protein kinases and phosphatidylinositol-3 kinase in the lens. 1. The mitogenic and stress responses. Exp. Eye Res. 74, 703-717. [DOI] [PubMed] [Google Scholar]
- Zatechka, S. D., Jr., and Lou, M. F. (2002b). Studies of the mitogen-activated protein kinases and phosphatidylinositol-3 kinase in the lens. 2. The inter-communications. Exp. Eye Res. 75, 177-192. [DOI] [PubMed] [Google Scholar]
- Zechner, D., Craig, R., Hanford, D. S., McDonough, P. M., Sabbadini, R. A., and Glembotski, C. C. (1998). MKK6 activates myocardial cell NF-κB and inhibits apoptosis in a p38 mitogen-activated protein kinase-dependent manner. J. Biol. Chem. 273, 8232-8239. [DOI] [PubMed] [Google Scholar]
- Zychlinsky, A., Prevost, M. C., and Sansonett, P. J. (1992). Shigella flexneri induces apoptosis in infected macrophages. Nature 358, 167-182. [DOI] [PubMed] [Google Scholar]