Abstract
Myoblast C2C12 cells cultured in the presence of FGF2 actively proliferate and showed a differentiation-defective phenotype compared with cells cultured in low serum or in the presence of insulin. These FGF2 effects are associated with sustained activation of p44/p42-MAPK and lack of activation of AKT. Here we demonstrate that Sprouty-2, a protein involved in the negative feedback of receptor tyrosine kinase signaling, when stably overexpressed in C2C12 cells and in the presence of FGF2 produces growth arrest (precluding the expression of PCNA and the phosphorylation of retinoblastoma and inducing the expression of p21CIP) and myogenesis (multinucleated myotubes formation, induction of creatine kinase and expression of myosin heavy chain protein). These events were accompanied by repression of p44/p42-MAPK and activation of AKT. When C2C12 cells were stably transfected with a Sprouty-2 (Y55F) mutant defective in inhibiting p44/p42-MAPK activation by FGF, myoblasts in the presence of FGF continue to grow and completely fail to form myotubes. This work is the first evidence of the contribution of sprouty genes to myogenic differentiation in the presence of FGF2.
INTRODUCTION
The development of skeletal muscle is a multistep process in which pluripotent mesodermal cells give rise to myoblasts that subsequently withdraw from the cell cycle, acquire an apoptosis-resistant phenotype, and differentiate to myotubes (Blau et al., 1985; Wang and Walsh, 1996). Most rat and mouse skeletal muscle cell lines proliferate in high serum conditions, and postconfluent cells spontaneously differentiate after several days in low serum due in part to the autocrine expression of insulin-like growth factor (IGF) II acting through the IGF-I receptor (Ewton et al., 1994; Bach et al., 1995). Moreover, myogenic differentiation can be accelerated by including insulin, IGF-I or IGF-II in the culture medium (Coolican et al., 1997). Studies on signaling through the insulin/IGF-I receptors, members of the receptor tyrosine kinase (RTK) family, have revealed two main pathways by which these signals might be transmitted; the phosphatidylinositol 3 kinase (PI3K) cascade and the Ras/Raf/p44.p42 mitogen-activated protein kinase (MAPK) cascade (White, 2003). PI3K is one of the primary signaling pathways leading to skeletal muscle differentiation, as demonstrated by pharmacological and genetic approaches (Kaliman et al., 1996, 1998). Downstream of PI3K several Ser/Thr kinases, including AKT, p70S6K, and p38-MAPK, are involved in the myogenic differentiation program (Calera and Pilch, 1998; Cuenda and Cohen, 1999; Baeza-Raja and Munoz-Canoves, 2004). On the other hand, activation of Ras/Raf/p44.p42-MAPK cascade seems to be detrimental to the differentiation process (Coolican et al., 1997). We have previously delineated the signaling pathways accompanying the formation of myotubes in C2C12 cells after treatment with insulin; sequential activation of PI3K, AKT, p70S6K, and p38-MAPK in parallel to the induction of muscle specific proteins, with a concomitant inhibition of p44/p42-MAPK and growth arrest (Conejo and Lorenzo, 2001; Conejo et al., 2001). In this regard, it has been shown that AKT activation inhibited Raf/MAPK signaling pathway in differentiated myotubes, but not in myoblasts (Rommel et al., 1999).
Members of the fibroblast growth factor (FGF) family are also critical participants in the genesis of skeletal muscle and bone formation. Skeletal myoblasts normally expressed FGFs and their receptors that stimulate DNA synthesis through autocrine and paracrine mechanisms (Hannon et al., 1996). However, during myocyte differentiation both factors and receptors are down-regulated (Scata et al., 1999). Accordingly, FGFs are mitogenic for skeletal myocytes and prevent myocyte terminal differentiation (Hannon et al., 1996; Kudla et al., 1998). Although FGF signals through RTK, using intracellular pathways similar to that described for insulin/IGFs, FGF-mediated repression of myogenic differentiation requires the action of pertussis-toxin-sensitive heterotrimeric G proteins as well as the activation of p44/p42-MAPK (Fedorov et al., 1998; Kudla et al., 1998).
Sprouty (Spry) proteins have been identified as repressors of RTK signaling in vertebrates and invertebrates, as recently reviewed (Kim and Bar-Sagi, 2004). The mouse and human genomes each contain four sprouty genes encoding proteins of 32-34 kDa, with a conserved cysteine-rich domain present in the carboxy terminus necessary for interaction with the plasma membrane in response to growth factor stimulation. In the amino-terminal domain, Spry proteins contain a conserved tyrosine residue (Tyr 55) that undergoes phosphorylation in response to FGF or epidermal growth factor (EGF). Interestingly, forced expression of Spry2 inhibited FGF-, but not EGF-induced p44/p42-MAPK activation. Furthermore, mutagenesis of the tyrosine 55 of Spry2 conferred dominant-negative activities with respect to inhibition of FGF signaling while sparing EGF signaling (Sasaki et al., 2001; Hanafusa et al., 2002; Mason et al., 2004). Several biological actions of Spry have been related to inhibition of FGF- and vascular EGF-induced proliferation, differentiation, and migration in cultured endothelial cells (Impagnatiello et al., 2001). Furthermore, overexpression of Spry in mouse embryos inhibits angiogenesis, a process regulated by FGF (Lee et al., 2001; Sasaki et al., 2003). Moreover, because Spry proteins have the capacity to repress p44/p42-MAPK activation by certain growth factors, attenuation of fibroblasts proliferation and PC12 differentiation has been reported after ectopic expression of this protein (de Maximy et al., 1999; Tefft et al., 1999; Hanafusa et al., 2002). So far, the contribution of Spry to skeletal muscle differentiation has not been explored yet, although Spry proteins are expressed in embryonic muscle (Tefft et al., 1999), and vertebrate spry genes can cause chondrodysplasia when overexpressed because of an inhibition of chondrocyte differentiation (Minowada et al., 1999). Accordingly, we undertook the present study to investigate the impact of Spry2 overexpression (wild-type and a dominant-negative mutant) in muscle differentiation by generation of stably transfected C2C12 myoblasts. Our data identify Spry2 as a repressor of p44/p42-MAPK activation by FGF that confers myogenic differentiation properties in the presence of this factor.
MATERIALS AND METHODS
Materials
Serum and culture media were from Life Technologies, (Paisley, United Kingdom). Geneticin was purchased from Invitrogen (Carlsbad, CA). Insulin, FGF2, and anti-α-actin antibody were from Sigma Chemical Co. (St. Louis, MO). Antibodies against phospho-tyrosine and Spry2 were purchased from Upstate Biotechnology (Lake Placid, NY); against myogenin, MyoD, MHC and p21CIP from Santa Cruz (Palo Alto, CA); against Rb from PharMingen (Heidelberg, Germany); against caveolin-3 from Transduction Laboratories (Lexington, Y); against PCNA and protein A-agarose from Roche Molecular Biochemicals (Mannheim, Germany); against AU5 from Berkeley Antibody Company (Berkeley, CA); against phospho- and total (p44/p42-MAPK, p38-MAPK, AKT, and p70S6K) from Cell Signaling (Beverly, MA).
DNA Constructs
Full-length cDNA of human Spry2 was obtained by RT-PCR from total RNA of Molt 4 cells using primers designed for the published sequence and providing BglII and NotI sites at the 5′ and 3′ ends, respectively. The amplified product was then subcloned into BglII and NotI sites of pCEFL-KZ-AU5. The mutant hSpry2 Y55F was generated from the above pCEFL-KZ-AU5-Spry2 wild-type (wt) by site-directed PCR-mutagenesis, using specific primers in which tyrosine was substituted for phenylalanine and subcloned into BglII and NotI sites of pCEFL-KZ-AU5.
Cell Line Generation
Mouse C2C12 myoblasts (American Type Culture Collection; Blau et al., 1985) were maintained in DMEM supplemented with 10% fetal serum (FS) and antibiotics, at 37°C and 5% CO2. C2C12 cells were stably transfected with 4 μg of hSpry2 wt or the single mutant (Y55F) to generate the cell lines C2C12-Spry wt and C2C12-Spry Y55F. C2C12-AU5 cell line was the negative control from cells transfected with the empty vector (pCEFL-KZ-AU5). After transfection according to the calcium phosphate-mediated protocol (Stratagene, La Jolla, CA), cell lines were selected by geneticin (0.5 mg/ml) for 3 wk. Several clones were obtained for this procedure, and then clones were analyzed for Spry2 wt or mutant overexpression. Two clones were finally selected without significant phenotypic differences between them. The same clone was used for all experiments, and each time that a frozen vial was resuscitated cells were keep cells for 10 d in geneticin, and ectopic expression of Spry2 was determined. Cells showed a Spry2 stable expression after several passages. All cell lines were grown to confluence in DMEM supplemented with 10% FS before the differentiation protocol that consisted in culturing cells in low serum medium, DMEM supplemented with 2% horse serum (HS), or in the presence of insulin 50 nM or 1 nM FGF2 for up to 4 d. In some experiments overnight serum-starved myoblasts were acute stimulated with insulin or FGF for up to 2 h. In some experiments NIH 3T3 fibroblasts were used for transient transfections with the Spry2 constructs.
Creatine Kinase Activity
Creatine kinase activity was determined in cell lysates using the NADPH-coupled assay following the protocol supplied by the manufacturer (Biomerieux SA, Lyon, France), as previously described (Conejo et al., 2002). Results are expressed as Units (μmoles per minute) of NADPH formed per mg of DNA, and are means ± SEM from duplicates from three independent experiments.
Immunoprecipitations
Cells were lysed as previously described (Conejo et al., 2002), and equal amounts of protein (1 mg) were immunoprecipitated at 4°C with monoclonal antibodies anti-AU5 or with anti-Spry. The immune complexes were collected on protein A-agarose beads and analyzed by SDS-PAGE. PI3-kinase activity was measured in anti-phospho-tyrosine immunoprecipitates by in vitro phosphorylation of phosphatidylinositol as previously described (Conejo and Lorenzo, 2001).
Western Blotting
Cellular proteins (30 μg) were submitted to SDS-PAGE, transferred to Immobilon membranes, and immunodetected as previously described (Conejo et al., 2002). Immunoreactive bands were visualized using the enhanced chemiluminescence (ECL-Plus) Western blotting protocol from Amersham Biosciences (Little Chalfont, United Kingdom). In experiments using x-ray films, different exposure times were used to ensure that bands were not saturated.
RESULTS
Characterization of C2C12 Stably Transfected Spry2 wt and Y55F Mutant Cell Lines
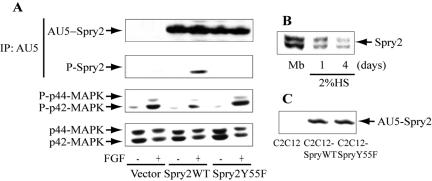
Spry genes negatively modulate intracellular signaling from RTK, being necessary for phosphorylation of the tyrosine residue 55 after stimulation with growth factors (Kim and Bar-Sagi, 2004). We used a Spry2 wild-type form (wt) and the single mutant (Y55F), where the tyrosine 55 was replaced with phenylalanine, cloned in pCEFL-KZ-AU5 harboring the AU5 tag. This mutation compromises the ability of Spry to interfere with p42/p44-MAPK, but it has no effect on the subcellular localization of Spry2 (Sasaki et al., 2001; Hanafusa et al., 2002; Mason et al., 2004). Before generating stable muscle cell lines, we checked the properties of these constructs by transient transfection in NIH 3T3 fibroblasts (Figure 1A). In control cells stimulation for 15 min with 1 nM FGF2 produces a high phosphorylation of p44/p42-MAPK. Cells transfected with Spry2 wt construct showed tyrosine phosphorylation of this protein after FGF2 stimulation, and the activation of p44/p42-MAPK was very poor compared with the control. The transfectant bearing the (Y55F) mutant construct was unable to phosphorylate Spry2 in tyrosine and maintained the activation of p44/p42-MAPK by FGF at a similar level as the control cells. Both Spry2 wt and mutant were expressed at similar levels as assessed by immunodetection with the anti-AU5 antibody (Figure 1A). Endogenous expression of Spry2 was detected in the mouse fetal skeletal muscle cell line C2C12 before differentiation by immunodetection with anti-Spry2 antibody, this expression decreases after a protocol of differentiation in low serum (2% HS) for 1-4 d (Figure 1B). Next, Spry2 wt and mutant constructs were used for the generation of C2C12 stable cell lines. From the different cell lines generated, two representative clones, named C2C12-Spry wt and C2C12-Spry Y55F were selected because they expressed similar level of the AU5-Spry2 protein (Figure 1C).
Figure 1.
Characterization of Spry2 wt and Y55F mutant and generation of C2C12 stably transfected cell lines. (A) NIH 3T3 fibroblasts were transiently transfected with 2 μg of Spry2, corresponding to the wild-type form (wt) or the single mutant (Y55F) in pCEFL-KZ-AU5. As negative control cells were transfected with the vector (pCEFL-KZ-AU5). The transfected cells were serum-starved for 18 h and then treated without or with 1 nM FGF for 15 min, and the cell extracts were incubated with anti-AU5 antibody. The proteins immunoprecipitated were analyzed by immunoblotting with anti-phospho-tyrosine and after stripping with anti-AU5. Furthermore, they were determined the levels of phosphorylation of p44/p42-MAPK using specific anti-phospho- and total antibodies. Results show a representative blot out of three. (B) Endogenous expression level of Spry2 were detected in C2C12 undifferentiated myoblasts growing with 10% FS (Mb) and after culture for 1-4 d in 2% HS to induce differentiation. (C) C2C12-Spry wt, C2C12-Spry Y55F, and C2C12-AU5 cell lines were generated by stably transfection of C2C12 cells with 4 μg of Spry2 constructs (wt or Y55F mutant) or vector, after selection for several weeks with geneticin. The blot shows the expression level of AU5-Spry2 from the C2C12 transfectants generated.
Short FGF2 Exposure Induces a Transient Activation of p44/p42-MAPK in C2C12-Spry2 wt Cells and a Sustained Activation in C2C12-Spry2 Y55F Cells
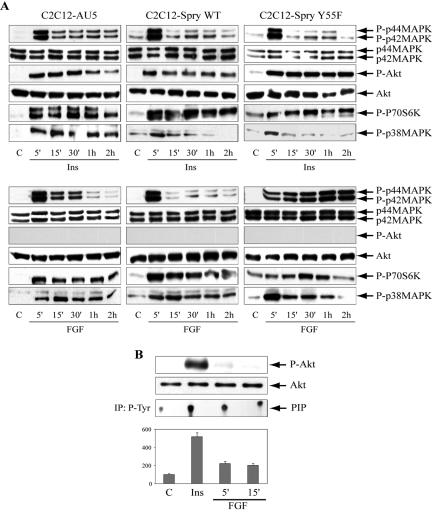
Mouse C2C12 cells can be induced to differentiate in low serum or in the presence of insulin or IGF-I, whereas treatment with growth factors such as EGF or FGF inhibits differentiation (Fedorov et al., 1998; Kudla et al., 1998). We evaluated the main signaling pathways induced by stimulation with these factors in the Spry wt and mutant cell lines (Figure 2). Treatment with 50 nM insulin caused a maximal p44/p42-MAPK phosphorylation at 5 min (5-fold), with lower effect (2-fold) maintained for 1 h; a sustained AKT and p70S6K phosphorylation (4-fold) for 2 h; and a transient phosphorylation of p38-MAPK regardless the expression of Spry wt or Y55F mutant (Figure 2A). These data indicated that neither wt or mutant Spry proteins greatly affected acute insulin signaling. A sharply different behavior was detected in response to FGF2. In control cells, C2C12-AU5, stimulation with 1 nM FGF2 induced a maximal p44/p42-MAPK phosphorylation at 5 min (5-fold), with lower phosphorylation (3-fold) maintained for up to 30 min. FGF2 induced a transient p44/p42-MAPK phosphorylation at 5 min that disappeared after longer incubation in the C2C12-Spry wt cell line. However, FGF induced a sustained p44/p42-MAPK phosphorylation for the 2 h of treatment in C2C12-Spry Y55F cell line. Surprisingly, AKT phosphorylation was not detected under FGF2 stimulation in either cell line (Figure 2A). This behavior not can be attributed to different levels of expression of p44/p42-MAPK or AKT in the cell lines, as demonstrated by the immunodetection with the anti-total p44/p42-MAPK and AKT antibodies. However, FGF2 either at 5 and 15 min stimulated PI3K in C2C12-AU5 cells but to a lower extent than insulin (Figure 2B). Although FGF fails to activate AKT in C2C12 cells acutely, a sustained phosphorylation of p70S6K and p38-MAPK was produced by this factor in the three cell lines (Figure 2A). The lack of activation of AKT by FGF has been described in osteoblastic cells, although in this system FGF also failed to activate p70S6K, but not PI3K (Debiais et al., 2004). Activation of p38-MAPK by FGF has been reported in chondrocytes (Raucci et al., 2004) and in NIH 3T3 fibroblasts (unpublished data). All these data clearly indicate that Spry2 wt is cutting down the length of time of p44/p42-MAPK activation by FGF, whereas the Spry2 Y55F mutant prolonged this MAPK phosphorylation.
Figure 2.
Insulin and FGF acute signaling in Spry2 wt and Y55F mutant stably transfected C2C12 cell lines. Myoblasts from the Spry2 wild-type form (C2C12-Spry wt) or the single mutant (C2C12-Spry Y55F) or the negative control (C2C12-AU5) were serum-starved overnight and then incubated in the absence (C) or in the presence of 50 nM insulin (Ins) or 1 nM FGF for the indicated times. (A) Cells were lysed and total protein (50 μg) was submitted to SDS-PAGE and immunodetected with anti-phospho-Akt, P70S6K, -p44/p42MAPK, or -p38MAPK antibodies and with total anti-Akt or -p44/p42MAPK) antibodies. (B) C2C12-AU5 cells were cultured as indicated above, and lysates were immunoprecipitated with anti-phospho-tyrosine antibodies and assayed for PI3-kinase activity. Histograms from densitometric analysis of PI3K activity expressed in arbitrary units are means ± SEM. Representative experiments out of three are shown.
C2C12-AU5 Myoblasts Failed to Differentiate in the Presence of FGF by Their Inability to Produce Growth Arrest
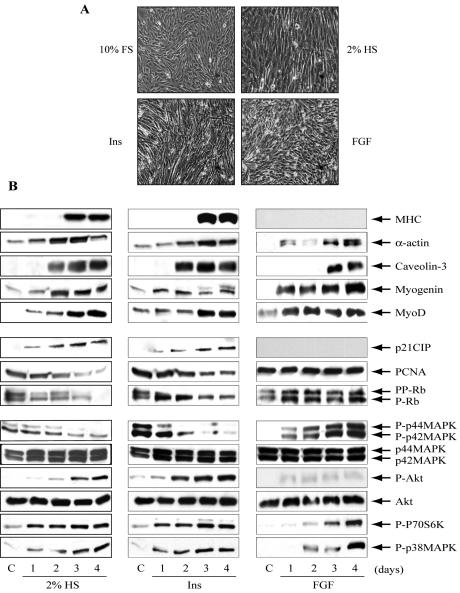
C2C12-AU5 myoblasts were cultured for up to 4 d in a serum-free medium in the presence of 50 nM insulin or 1 nM FGF2 or low serum (2% HS). The myogenic differentiation of cells cultured with insulin or HS was detected morphologically by the alignment, elongation, and fusion of mono-nucleated myoblasts into multinucleated myotubes, as shown in Figure 3A. However, cells treated with FGF2 failed to form myotubes. Differentiation of skeletal muscle cells involves the expression of specific transcription factors such as MyoD, and also early and terminal differentiation markers such as myogenin and myosin heavy chain (MHC), respectively (Weintraub, 1993). The expression of these proteins was detected in differentiated cells after 3 d in the presence of insulin or HS (Figure 3B). Intriguingly, the expression of myogenin and MyoD started before in cells treated with FGF2 than with insulin or HS. However, cells treated with FGF defective in terminal differentiation did not express MHC protein. Other muscle-specific proteins, such as caveolin-3 and α-actin, were also detected in cell treated with FGF2, although their expression was delayed 1 d compared with cells treated with insulin or HS. Myoblast differentiation requires growth arrest, so next we checked the expression of several genes controlling cell cycle such as p21CIP, a cell cycle inhibitor, PCNA, an essential marker for DNA replication, and retinoblastoma (Rb), a tumor suppressor gene controlling the entrance on the cell cycle by its phosphorylation level (Figure 3B). Proliferating myoblasts expressed PCNA protein and Rb in the hyperphosphorylated state. Treatment for 4 d with insulin or with HS produced sequential growth arrest as demonstrated by the down-regulation of PCNA protein, the up-regulation of the cell cycle inhibitor p21CIP, and maintenance of Rb hypophosphorylation (Figure 3B). However, myoblasts treated with FGF2 failed to produce growth arrest because they did not express p21CIP and maintained a high expression of PCNA and hyperphosphorylated Rb (Figure 3B).
Figure 3.
FGF fails to differentiate C2C12-AU5 myoblasts by its inability to produce growth arrest. C2C12 myoblasts carrying the empty vector pCEFL-KZ-AU5 (C2C12-AU5) were grown in medium DMEM supplemented with 10% FS until confluence (C) and then cells were further cultured up to 4 d in DMEM with 2% HS or with 50 nM insulin or with 1 nM FGF. (A) Phase-contrast images of the cells were taken after these treatments for detection of multinucleated myotubes. Magnification, ×10. Representative experiments out of three are shown. (B) At different days of culture (1-4) cells were lysed and total protein (30 μg) was submitted to SDS-PAGE and immunodetected with anti-MHC, -α-actin, -Caveolin-3, -Myogenin, -MyoD, -p21, -PCNA or -Rb antibodies, with anti-phospho-Akt, P70S6K, -p44/p42MAPK, or -p38MAPK) antibodies and with total anti-Akt or -p44/p42MAPK antibodies. Results show representative blots out of three.
Specific intracellular signaling pathways control the differentiation process. Accordingly, we studied the main signaling cascades in these cells. Phosphorylation of p44/p42-MAPK decreases while phosphorylation of AKT, p70S6K and p38-MAPK increases during differentiation with either insulin or HS, in agreement with our previous observations (Conejo et al., 2001). In an opposite manner, a high phosphorylation of p44/p42-MAPK was detected in undifferentiated cells under FGF2 treatment, and AKT phosphorylation was hardly detectable. However, FGF2 caused activation of p70S6K and p38MAPK delayed 1 d compared with insulin or HS. The changes observed in the amount of phospho- (AKT and p44/p42-MAPK) under insulin and FGF treatment reflected changes in the kinase activity, because the protein levels were similar in the experimental conditions. Neither changes in total p70S6K nor total p38-MAPK protein content were detected (unpublished data).
Spry2 wt Overexpression in C2C12 Cells Confers Myogenic Differentiation Properties in the Presence of FGF2, Allowing Growth Arrest and Formation of Myotubes
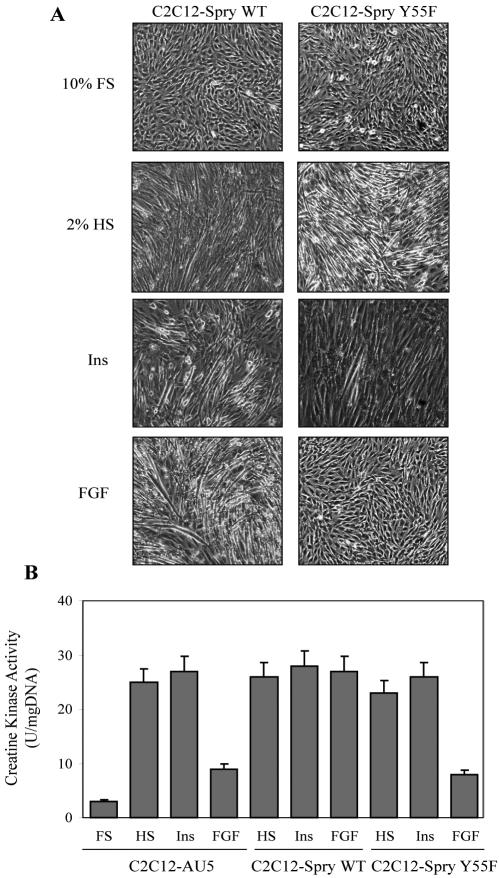
C2C12-Spry wt myoblasts were cultured for up to 4 d with FGF and compared with cells treated with insulin or 2% HS as positive differentiation controls (Figure 4). The expression of Spry2 wt allows the myogenic differentiation of cells in the presence of FGF2, as detected morphologically under the microscope by the formation of multinucleated myotubes (Figure 4A). Indeed, to quantify the differentiation level we determined the activity of creatine kinase as a metabolic marker characteristic of terminally differentiated myotubes (Figure 4B). Undifferentiated C2C12 cells showed an almost undetectable creatine kinase activity, that increased by 10-fold under the differentiation protocol with insulin or HS either in control or in Spry2 wt cells. C2C12-AU5 myoblasts cultured with FGF2 failed to induce creatine kinase activity. However, C2C12-Spry2 wt cells showed activation of creatine kinase after 4 d in the presence of FGF2 (Figure 4B). Moreover, the expression of specific muscle proteins such as myogenin, MyoD, MHC, caveolin-3, and α-actin in differentiated C2C12-Spry wt cells in the presence of FGF seems to be higher than that detected in cells differentiated with insulin (Figure 5). Furthermore, a sequential growth arrest was observed in C2C12-Spry wt cells in the presence of FGF2 in a manner similar to that detected with insulin, with down-regulation of the expression of PCNA protein, up-regulation of the expression of p21CIP, and maintenance of hypophosphorylated Rb (Figure 5). We further explored how Spry2 wt affected intracellular signaling in the presence of either FGF2 or insulin. We observed that p44/p42-MAPK was phosphorylated after 1 and 2 d of treatment with FGF. However, this phosphorylation was completely abolished at day 3 and 4, allowing growth arrest. In an opposite manner, a robust AKT activation was detected upon 3 and 4 d in the presence of FGF2 in C2C12-Spry wt cells, in parallel to the differentiation process. Activation of p70S6K and p38MAPK was also detected, but the maximal effect was produced 1 d before that the activation of AKT. The insulin signaling profile detected in C2C12-Spry2 wt cells (Figure 5) did not differ essentially from that detected in control cells (Figure 3).
Figure 4.
Spry2 overexpression in C2C12 cells, but not the mutant Spry2 Y55F, restores myoblasts differentiation by FGF. Myoblasts from the Spry2 wild-type form (C2C12-Spry wt) or the single mutant (C2C12-Spry Y55F) or the negative control (C2C12-AU5) were cultured as in Figure 3. (A) At the end of the culture time phase-contrast images of the cells were taken for detection of multinucleated myotubes. Magnification, ×10. Representative experiments out of three are shown. (B) Cells were collected and processed for determination of creatine kinase activity. Results are expressed as Units (μmoles per minute) of NADPH formed per mg of DNA and are means ± SEM from duplicates from three independent experiments.
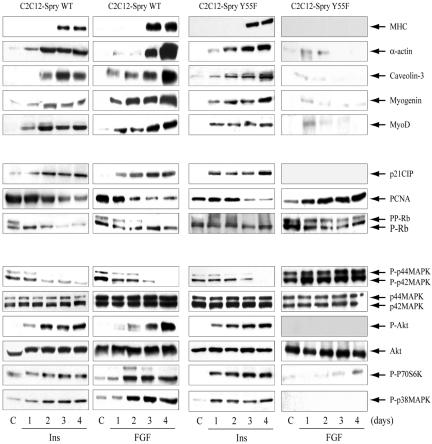
Figure 5.
Spry2 overexpression, but not Spry2 Y55F, represses p42/p44-MAPK and activates AKT, allowing growth arrest and the expression of terminal differentiation markers by FGF. Myoblasts from the Spry2 wild-type form (C2C12-Spry wt) or the single mutant (C2C12-Spry Y55F) were cultured up to 4 d in DMEM with 50 nM insulin or with 1 nM FGF. Western blot analysis of proteins was performed as in Figure 3.
To confirm that the differentiated phenotype observed under FGF treatment was due to Spry2 wt overexpression, we performed the opposite experiment, the study of myogenic differentiation in the presence of FGF in a stably transfected cell line, C2C12-Spry2 Y55F, harboring an inactive Spry2 protein as described above. Cells in the presence of FGF2 failed to differentiate either phenotypically by the formation of myotubes or metabolically by the induction of creatine kinase (Figure 4, A and B). Cells continued growing as indicated by the cell cycle markers previously described (Figure 5). In addition, a high phosphorylation of p44/p42-MAPK prolonged for 4 d of differentiation was detected, whereas AKT phosphorylation was undetectable. Furthermore, in these differentiation-defective cells p70S6K and p38-MAPK phosphorylation was also undetectable, and expression of skeletal-muscle proteins was barely detectable, although the total amount of p70S6K and p38-MAPK remained unaltered (unpublished data). However, insulin effects in C2C12-Spry2 Y55F on cell morphology, activation of creatine kinase, induction of differentiation markers, inhibition of cell cycle markers, and signaling profile were not different from those detected in C2C12-Spry2 wt or control cells, indicating that Spry2 is not involved in the insulin signaling cascade (Figures 4 and 5). Furthermore, neither Spry2 wt nor mutant overexpression modifies the differentiation profile observed in cells under low serum (Supplementary Figure S1), which is essentially like that observed in the presence of insulin (Figure 5). Data from Figure 5 clearly indicate that Spry2 wt repressed p44/p42-MAPK phosphorylation by FGF2, whereas the Spry2 Y55F mutant prolonged MAPK activation for 4 d of treatment.
DISCUSSION
FGFs provide the signals that induce limb formation in vertebrates and maintain initial outgrowth of the limb bud. Vertebrate Spry genes are expressed near FGF signaling centers at stages when organogenesis is commencing in the mouse embryo (E8.5-E9.5; Minowada et al., 1999). FGF signaling regulates expression of Spry and in turn, Spry gene expression antagonizes FGF signaling (Minowada et al., 1999). Then, a balanced expression of FGF and Spry determines the length of the skeletal elements. In this regard, overexpression of Spry genes in the chick limb leads to inhibition of chondrocyte differentiation that results in a chondrodysplasia, resembling that observed in activating mutations in Fgfr3 (Minowada et al., 1999).
In this work we investigated the contribution of Spry to muscle development. Our data provide the first time evidence of a key role of Spry proteins in skeletal muscle lineage, modulating FGF signaling and allowing terminal myogenic differentiation. C2C12 myoblasts in the presence of FGF2 actively proliferate and fail to differentiate due to failure to repress p44/p42-MAPK and to activate AKT pathway (Figure 3). Activation of p44/p42-MAPK leads to proliferation, and myogenic differentiation requires activation of AKT (Conejo and Lorenzo, 2001; Sumitani et al., 2002). We found that FGF2 prevents myocyte terminal differentiation in C2C12 cells, in a manner similar to that described for mouse MM14 and Sol 8 myocytes (Hannon et al., 1996; Scata et al., 1999). However, the expression of early muscle proteins, such as MyoD, myogenin, caveolin-3, or α-actin was not affected by FGF2. This could be due to the activation of p38-MAPK produced by FGF2, because this kinase has been proposed as an upstream regulator of the expression of caveolin-3 (Galbiati et al., 1999), and the kinetics of p38-MAPK versus caveolin-3 expression is consistent with this idea (Figure 3B).
Accordingly, Spry2 wt overexpressed in C2C12 cells repressed chronic activation of p44/p42-MAPK by FGF2, causing cell cycle arrest (Figure 5). As in smooth muscle (Zhang et al., 2005), Spry2 promotes differentiation, acting as much as a tumor suppressor gene does in lowering the threshold for myogenic differentiation. The capacity of Spry2 wt to repress p44/p42-MAPK activation by FGF (under both acute and chronic treatment, as shown in Figures 2A and 5) in skeletal muscle cells was not unexpected because similar repression has been described in other cellular systems such a fibroblasts, neuronal, or endothelial cells, associated with attenuation of proliferation, differentiation, or migration, respectively (Tefft et al., 1999; Lee et al., 2001; Sasaki et al., 2001; Hanafusa et al., 2002; Sasaki et al., 2003). The molecular mechanism by which Spry2 inhibits FGF-induced MAPK signaling has not yet been clarified. Some reports indicate that Spry2 prevents the formation or maintenance of activated GTP-Ras (Gross et al., 2001); meanwhile, others suggest that it impairs the recruitment of the Grb2-Sos complex to FGFR (Hanafusa et al., 2002). Unlike these models, other authors consider that Spry2 inhibits Raf activation rather than Ras (Sasaki et al., 2003). Furthermore, other data show that Spry2 sequesters active c-Cbl molecules impeding RTK ubiquitination, down-regulation, and degradation (Hall et al., 2003).
The inability of the dominant-negative Spry2 Y55F protein to allow myogenic differentiation in the presence of FGF2 was due to the prolonged p44/p42-MAPK phosphorylation and high expression of proliferation mediators (Figures 4 and 5). This effect is consistent with the published behavior of this mutant in other biological systems; thus, the expression of this mutant in PC12 cells resulted in the activation of p44/p42-MAPK, in that model necessary for growth factor-induced neuronal differentiation (Hanafusa et al., 2002). In this regard, a prolonged activation of p44/p42-MAPK by oncogenic forms of N-ras and H-ras has been reported to prevent skeletal myoblast differentiation (Mitin et al., 2001; Conejo et al., 2002). Furthermore, activation of K-ras or N-ras genes by point mutations is frequently found in human embryonic and cardiac rhabdomyosarcomas (Stratton et al., 1989; Garcia et al., 2000).
In addition to repression of p44/p42-MAPK as an absolute requirement for growth arrest (Conejo and Lorenzo, 2001), myogenic terminal differentiation is dependent on AKT activation (Tureckova et al., 2001). In previous work we found that to restore differentiation of Ras-transformed myoblasts besides pharmacological repression of p44/p42-MAPK, it was necessary to activate AKT either with insulin or by transfection of a constitutively active AKT (Conejo et al., 2002). In this study, Spry2-mediated repression of p44/p42-MAPK allows the phosphorylation of AKT in the presence of FGF2. This AKT activation was not produced in Spry2 Y55F cells (Figure 5). Because FGF2 failed to stimulate AKT at short times regardless of the presence of Spry2 wt, the activation of AKT detected at the end of the differentiation (days 3 and 4) could be attributed to the endogenous expression of IGF-II (Ewton et al., 1994;Bach et al., 1995). In this regard, IGF-II transcription during skeletal myogenesis is produced by activation of mTOR, an upstream regulator of p70S6K (Erbay et al., 2003). However, FGF2 in the absence of Spry2 activated p70S6K but not AKT, and p44/p42-MAPK seemed to be exerting an inhibitory effect precluding AKT activation. This suggests a negative cross-talk between p44/p42-MAPK and AKT under FGF2 treatment. This cross-talk is opposite that the observed in this work under insulin-induced differentiation, where AKT activation inhibited Raf/MAPK signaling, as has been described (Rommel et al., 1999). Somehow, Spry2 allows AKT activation in the presence of FGF2 and this event seems to be essential for expression of muscle creatine kinase and MHC, terminal markers of differentiation (Sumitani et al., 2002; Hribal et al., 2003) and for the formation of multinucleated myotubes (Figures 4 and 5).
In conclusion, our work identifies Spry2 for the first time as a repressor of p44/p42-MAPK activation by FGF2 in skeletal muscle cells that confers myogenic differentiation properties in the presence of FGF2. Furthermore, the Spry2 biological effects are opposite to those generated by FGF: Where FGF induces cell differentiation Spry2 inhibits these processes; where FGF blocks differentiation (such as the skeletal muscle cells) Spry2 counteracts these effects. Whether this model is applicable in other lineages is currently being studied, as is the identification of the proteins that interact with Spry2 upon FGF stimulation.
Supplementary Material
Acknowledgments
C.dA. was fellowship recipient from the Ministerio de Educacion y Ciencia (MEC), Spain. This work was supported by Grants BMC2002-01322 and SAF2003-02604 from Ministerio de Ciencia y Tecnologia (Spain); 08.6/0002.1/2003 and GR/SAL/0291/2004 from Comunidad de Madrid (Spain). We also thank the support of COST B17 Action from the European Commission, and Red degrupos de Diabetes Mellitus G03/212 from Instituto de Salud Carlos III, Ministerio de Sanidad y Consumo.
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E05-05-0419) on July 6, 2005.
Abbreviations used: FGF, fibroblast growth factor; FS, fetal serum; HS, horse serum; IGF, insulin-like growth factor; MAPK, mitogen-activated protein kinase; MHC, myosin heavy chain; PI3K, phosphatidylinositol 3 kinase; Rb, retinoblastoma; RTK, receptor tyrosine kinase; Spry, Sprouty.
The online version of this article contains supplemental material at MBC Online (http://www.molbiolcell.org).
References
- Bach, L. A., Salemi, R., and Leeding, K. S. (1995). Roles of insulin-like growth factor (IGF) receptors and IGF-binding proteins in IGF-II-induced proliferation and differentiation of L6A1 rat myoblasts. Endocrinology 136, 5061-5069. [DOI] [PubMed] [Google Scholar]
- Baeza-Raja, B., and Munoz-Canoves, P. (2004). p38 MAPK-induced nuclear factor-kappaB activity is required for skeletal muscle differentiation: role of interleukin-6. Mol. Biol. Cell 15, 2013-2026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blau, H. M., Pavlath, G. K., Hardeman, E. C., Chiu, C. P., Silberstein, L., Webster, S. G., Miller, S. C., and Webster, C. (1985). Plasticity of the differentiated state. Science 230, 758-766. [DOI] [PubMed] [Google Scholar]
- Calera, M. R., and Pilch, P. F. (1998). Induction of Akt-2 correlates with differentiation in Sol8 muscle cells. Biochem. Biophys. Res. Commun. 251, 835-841. [DOI] [PubMed] [Google Scholar]
- Conejo, R., de Alvaro, C., Benito, M., Cuadrado, A., and Lorenzo, M. (2002). Insulin restores differentiation of Ras-transformed C2C12 myoblasts by inducing NF-kappaB through an AKT/P70S6K/p38-MAPK pathway. Oncogene 21, 3739-3753. [DOI] [PubMed] [Google Scholar]
- Conejo, R., and Lorenzo, M. (2001). Insulin signaling leading to proliferation, survival, and membrane ruffling in C2C12 myoblasts. J. Cell. Physiol. 187, 96-108. [DOI] [PubMed] [Google Scholar]
- Conejo, R., Valverde, A. M., Benito, M., and Lorenzo, M. (2001). Insulin produces myogenesis in C2C12 myoblasts by induction of NF-kappaB and downregulation of AP-1 activities. J. Cell. Physiol. 186, 82-94. [DOI] [PubMed] [Google Scholar]
- Coolican, S. A., Samuel, D. S., Ewton, D. Z., McWade, F. J., and Florini, J. R. (1997). The mitogenic and myogenic actions of insulin-like growth factors utilize distinct signaling pathways. J. Biol. Chem. 272, 6653-6662. [DOI] [PubMed] [Google Scholar]
- Cuenda, A., and Cohen, P. (1999). Stress-activated protein kinase-2/p38 and a rapamycin-sensitive pathway are required for C2C12 myogenesis. J. Biol. Chem. 274, 4341-4346. [DOI] [PubMed] [Google Scholar]
- de Maximy, A. A., Nakatake, Y., Moncada, S., Itoh, N., Thiery, J. P., and Bellusci, S. (1999). Cloning and expression pattern of a mouse homologue of Drosophila sprouty in the mouse embryo. Mech. Dev. 81, 213-216. [DOI] [PubMed] [Google Scholar]
- Debiais, F., Lefevre, G., Lemonnier, J., Le Mee, S., Lasmoles, F., Mascarelli, F., and Marie, P. J. (2004). Fibroblast growth factor-2 induces osteoblast survival through a phosphatidylinositol 3-kinase-dependent, -beta-catenin-independent signaling pathway. Exp. Cell Res. 297, 235-246. [DOI] [PubMed] [Google Scholar]
- Erbay, E., Park, I. H., Nuzzi, P. D., Schoenherr, C. J., and Chen, J. (2003). IGF-II transcription in skeletal myogenesis is controlled by mTOR and nutrients. J. Cell Biol. 163, 931-936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ewton, D. Z., Roof, S. L., Magri, K. A., McWade, F. J., and Florini, J. R. (1994). IGF-II is more active than IGF-I in stimulating L6A1 myogenesis: greater mitogenic actions of IGF-I delay differentiation. J. Cell Physiol. 161, 277-284. [DOI] [PubMed] [Google Scholar]
- Fedorov, Y. V., Jones, N. C., and Olwin, B. B. (1998). Regulation of myogenesis by fibroblast growth factors requires beta-gamma subunits of pertussis toxin-sensitive G proteins. Mol. Cell Biol. 18, 5780-5787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galbiati, F., Volonte, D., Engelman, J. A., Scherer, P. E., and Lisanti, M. P. (1999). Targeted down-regulation of caveolin-3 is sufficient to inhibit myotube formation in differentiating C2C12 myoblasts. Transient activation of p38 mitogen-activated protein kinase is required for induction of caveolin-3 expression and subsequent myotube formation. J. Biol. Chem. 274, 30315-30321. [DOI] [PubMed] [Google Scholar]
- Garcia, J. M., Gonzalez, R., Silva, J. M., Dominguez, G., Vegazo, I. S., Gamallo, C., Provencio, M., Espana, P., and Bonilla, F. (2000). Mutational status of K-ras and TP53 genes in primary sarcomas of the heart. Br. J. Cancer 82, 1183-1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross, I., Bassit, B., Benezra, M., and Licht, J. D. (2001). Mammalian sprouty proteins inhibit cell growth and differentiation by preventing ras activation. J. Biol. Chem. 276, 46460-46468. [DOI] [PubMed] [Google Scholar]
- Hall, A. B., Jura, N., DaSilva, J., Jang, Y. J., Gong, D., and Bar-Sagi, D. (2003). hSpry2 is targeted to the ubiquitin-dependent proteasome pathway by c-Cbl. Curr. Biol. 13, 308-314. [DOI] [PubMed] [Google Scholar]
- Hanafusa, H., Torii, S., Yasunaga, T., and Nishida, E. (2002). Sprouty1 and Sprouty2 provide a control mechanism for the Ras/MAPK signalling pathway. Nat. Cell Biol. 4, 850-858. [DOI] [PubMed] [Google Scholar]
- Hannon, K., Kudla, A. J., McAvoy, M. J., Clase, K. L., and Olwin, B. B. (1996). Differentially expressed fibroblast growth factors regulate skeletal muscle development through autocrine and paracrine mechanisms. J. Cell Biol. 132, 1151-1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hribal, M. L., Nakae, J., Kitamura, T., Shutter, J. R., and Accili, D. (2003). Regulation of insulin-like growth factor-dependent myoblast differentiation by Foxo forkhead transcription factors. J. Cell Biol. 162, 535-541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Impagnatiello, M. A., Weitzer, S., Gannon, G., Compagni, A., Cotten, M., and Christofori, G. (2001). Mammalian sprouty-1 and -2 are membrane-anchored phosphoprotein inhibitors of growth factor signaling in endothelial cells. J. Cell Biol. 152, 1087-1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaliman, P., Canicio, J., Shepherd, P. R., Beeton, C. A., Testar, X., Palacin, M., and Zorzano, A. (1998). Insulin-like growth factors require phosphatidylinositol 3-kinase to signal myogenesis: dominant negative p85 expression blocks differentiation of L6E9 muscle cells. Mol. Endocrinol. 12, 66-77. [DOI] [PubMed] [Google Scholar]
- Kaliman, P., Vinals, F., Testar, X., Palacin, M., and Zorzano, A. (1996). Phosphatidylinositol 3-kinase inhibitors block differentiation of skeletal muscle cells. J. Biol. Chem. 271, 19146-19151. [DOI] [PubMed] [Google Scholar]
- Kim, H. J., and Bar-Sagi, D. (2004). Modulation of signalling by Sprouty: a developing story. Nat. Rev. Mol. Cell Biol. 5, 441-450. [DOI] [PubMed] [Google Scholar]
- Kudla, A. J., Jones, N. C., Rosenthal, R. S., Arthur, K., Clase, K. L., and Olwin, B. B. (1998). The FGF receptor-1 tyrosine kinase domain regulates myogenesis but is not sufficient to stimulate proliferation. J. Cell Biol. 142, 241-250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, S. H., Schloss, D. J., Jarvis, L., Krasnow, M. A., and Swain, J. L. (2001). Inhibition of angiogenesis by a mouse sprouty protein. J. Biol. Chem. 276, 4128-4133. [DOI] [PubMed] [Google Scholar]
- Mason, J. M., Morrison, D. J., Bassit, B., Dimri, M., Band, H., Licht, J. D., and Gross, I. (2004). Tyrosine phosphorylation of Sprouty proteins regulates their ability to inhibit growth factor signaling: a dual feedback loop. Mol. Biol. Cell 15, 2176-2188. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Minowada, G., Jarvis, L. A., Chi, C. L., Neubuser, A., Sun, X., Hacohen, N., Krasnow, M. A., and Martin, G. R. (1999). Vertebrate Sprouty genes are induced by FGF signaling and can cause chondrodysplasia when overexpressed. Development 126, 4465-4475. [DOI] [PubMed] [Google Scholar]
- Mitin, N., Kudla, A. J., Konieczny, S. F., and Taparowsky, E. J. (2001). Differential effects of Ras signaling through NFkappaB on skeletal myogenesis. Oncogene 20, 1276-1286. [DOI] [PubMed] [Google Scholar]
- Raucci, A., Laplantine, E., Mansukhani, A., and Basilico, C. (2004). Activation of the ERK1/2 and p38 mitogen-activated protein kinase pathways mediates fibroblast growth factor-induced growth arrest of chondrocytes. J. Biol. Chem. 279, 1747-1756. [DOI] [PubMed] [Google Scholar]
- Rommel, C., Clarke, B. A., Zimmermann, S., Nunez, L., Rossman, R., Reid, K., Moelling, K., Yancopoulos, G. D., and Glass, D. J. (1999). Differentiation stage-specific inhibition of the Raf-MEK-ERK pathway by Akt. Science 286, 1738-1741. [DOI] [PubMed] [Google Scholar]
- Sasaki, A., Taketomi, T., Kato, R., Saeki, K., Nonami, A., Sasaki, M., Kuriyama, M., Saito, N., Shibuya, M., and Yoshimura, A. (2003). Mammalian Sprouty4 suppresses Ras-independent ERK activation by binding to Raf1. Nat. Cell Biol. 5, 427-432. [DOI] [PubMed] [Google Scholar]
- Sasaki, A., Taketomi, T., Wakioka, T., Kato, R., and Yoshimura, A. (2001). Identification of a dominant negative mutant of Sprouty that potentiates fibroblast growth factor-but not EGF-induced ERK activation. J. Biol. Chem. 276, 36804-36808. [DOI] [PubMed] [Google Scholar]
- Scata, K. A., Bernard, D. W., Fox, J., and Swain, J. L. (1999). FGF receptor availability regulates skeletal myogenesis. Exp. Cell Res. 250, 10-21. [DOI] [PubMed] [Google Scholar]
- Stratton, M. R., Fisher, C., Gusterson, B. A., and Cooper, C. S. (1989). Detection of point mutations in N-ras and K-ras genes of human embryonal rhabdomyosarcomas using oligonucleotide probes and the polymerase chain reaction. Cancer Res. 49, 6324-6327. [PubMed] [Google Scholar]
- Sumitani, S., Goya, K., Testa, J. R., Kouhara, H., and Kasayama, S. (2002). Akt1 and Akt2 differently regulate muscle creatine kinase and myogenin gene transcription in insulin-induced differentiation of C2C12 myoblasts. Endocrinology 143, 820-828. [DOI] [PubMed] [Google Scholar]
- Tefft, J. D., Lee, M., Smith, S., Leinwand, M., Zhao, J., Bringas, P., Jr., Crowe, D. L., and Warburton, D. (1999). Conserved function of mSpry-2, a murine homolog of Drosophila sprouty, which negatively modulates respiratory organogenesis. Curr. Biol. 9, 219-222. [DOI] [PubMed] [Google Scholar]
- Tureckova, J., Wilson, E. M., Cappalonga, J. L., and Rotwein, P. (2001). Insulin-like growth factor-mediated muscle differentiation: collaboration between phosphatidylinositol 3-kinase-Akt-signaling pathways and myogenin. J. Biol. Chem. 276, 39264-39270. [DOI] [PubMed] [Google Scholar]
- Wang, J., and Walsh, K. (1996). Resistance to apoptosis conferred by Cdk inhibitors during myocyte differentiation. Science 273, 359-361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weintraub, H. (1993). The MyoD family and myogenesis: redundancy, networks, and thresholds. Cell 75, 1241-1244. [DOI] [PubMed] [Google Scholar]
- White, M. F. (2003). Insulin signaling in health and disease. Science 302, 1710-1711. [DOI] [PubMed] [Google Scholar]
- Zhang, C., Chaturvedi, D., Jaggar, L., Magnuson, D., Lee, J. M., and Patel, T. B. (2005). Regulation of vascular smooth muscle cell proliferation and migration by human sprouty 2. Arterioscler. Thromb. Vasc. Biol. 25, 533-538. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.