Abstract
Migraine is the most common type of chronic episodic headache. Several population-based family studies have suggested a strong genetic predisposition to migraine, especially migraine with aura (MA). Although several susceptibility loci have been identified, none of the numerous studies performed to date have led to the identification of a gene responsible for the more common forms of migraine. GABA-A receptors and their modulator sites seem to be involved in the pathophysiological events that underlie migraine. We report on clinical and molecular data from a total of 10 families with MA, in which MA segregates as an autosomal dominant trait and presents with homogeneous clinical features. After excluding linkage with the known candidate loci, we used a functional candidate approach and genotyped these families with markers from the 15q11-q13 genomic region, which contains the genes encoding GABA-A receptor subunits. Evidence of linkage was obtained with a parametric two-point linkage analysis (maximum LOD score of 5.56 at a recombination fraction of 0.001 for marker GABRB3) and was supported by multipoint analysis (maximum LOD score of 6.54 between markers D15S113 and D15S1019). The critical region spanned 3.6 Mb. These results provide the basis for further investigation of the hypothesized relationship between a GABA-A receptor dysfunction and migraine.
Migraine is the most common type of chronic episodic headache, affecting 11% of the general population of Western Europe and the United States (Goadsby et al. 2002). Up to 31% of migraine patients have an aura consisting of transient (usually <60 min in duration) neurological symptoms, whereas 5% have an aura without headache (Goadsby et al. 2002). On the basis of clinical manifestations, two types of migraine can be distinguished: migraine with aura (MA) and migraine without aura (Rasmussen and Olesen 1992).
Several population-based family studies have suggested a strong genetic predisposition to migraine, especially to MA (Russel and Olesen 1995; Ziegler et al. 1998), although the mode of inheritance remains unclear. Most likely, the two types of migraine are inherited as multifactorial traits, with the exception of a few families that display an autosomal dominant pattern of inheritance (Russel 2001). In any case, the genetic factors are likely to act by determining a constitutional migraine threshold modulated by external and internal triggers (Pietrobon and Striessnig 2003). Although several susceptibility loci have been identified—on chromosomes 1q (MGR6 [MIM 607516]) (Ducros et al. 1997; Gardner et al. 1997), 4q24-q28 (MGR1 [MIM 157300]) (Wessman et al. 2002), Xq24-q28 (MGR2 [MIM 300125]) (Nyholt et al. 2000), 19p13 (MGR5 [MIM 607508]) (Jones et al. 2001), and 14q21-q22 (MGR4 [MIM 607501]) (Soragna et al. 2003)—none of the numerous studies performed to date have led to the identification of a gene responsible for the most common forms of migraine. The only exception is familial hemiplegic migraine (MIM 141500), a rare autosomal dominant subtype of migraine (Joutel et al. 1993) that is caused by mutations in a gene on chromosome 19 encoding a calcium-channel alpha subunit (CACNA1A [MIM 601011]) (Ophoff et al. 1996).
Little progress has been made toward understanding the neurobiology of migraine. Activation of the trigeminovascular system is thought to be responsible for the pain, whereas cortical spreading depression (CSD) seems to underlie the aura symptoms (Pietrobon and Striessnig 2003). In a recent study, Bolay et al. (2002) showed that CSD activates trigeminal afferents that cause inflammation of the pain-sensitive meninges, generating a headache. These data point to CSD as a critical event in the mechanisms of MA and support the emerging notion that CSD is a valid therapeutic target (Iadecola 2002).
An increase in excitability could explain the predisposition of the brain to episodes of CSD (Parsons 1998). The mechanisms that underlie the cortical excitability and its periodicity remain unclear. A possible cause is an abnormal release of excitatory neurotransmitters or, alternatively, a reduced intracortical inhibition (Pietrobon and Striessnig 2003). With regard to the latter alternative, several studies demonstrate a very likely involvement of GABAergic circuits through deficient intracortical inhibitory processes, especially in patients with MA (Aurora et al. 1998; Palmer et al. 2000).
Moreover, it is currently thought that the neuronal mechanisms underlying headache may work similarly in healthy individuals and individuals with migraines, but, because of central neuronal hyperexcitability in patients with migraine, headache is more easily triggered in these individuals. In recent years, the concept of migraine as a result of CNS hyperexcitability has led to the use of GABAergic anticonvulsivant medications as the first line of therapy for prevention of migraine (Silberstein 2004). In particular, GABA-A receptors and their modulator sites seem to be involved in the pathophysiological events that underlie migraine, mainly at the trigeminovascular-complex level (Cutrer and Moskowitz 1996; Storer et al. 2001, 2004).
Therefore, we hypothesized that genes encoding GABA-A receptor subunits should be good candidate genes and performed linkage analysis in 10 families with MA by use of markers from the 15q11-q13 genomic region, which harbors three genes encoding three GABA-A receptor subunits. The genotyping of 10 families with MA by use of microsatellite markers from candidate regions identified elsewhere (19p13, 1q21-q23, 1q31, 4q24, and 14q24) showed several recombinations in affected individuals. Multipoint analysis did not support linkage with these loci, clearly showing negative LOD score values (data not shown).
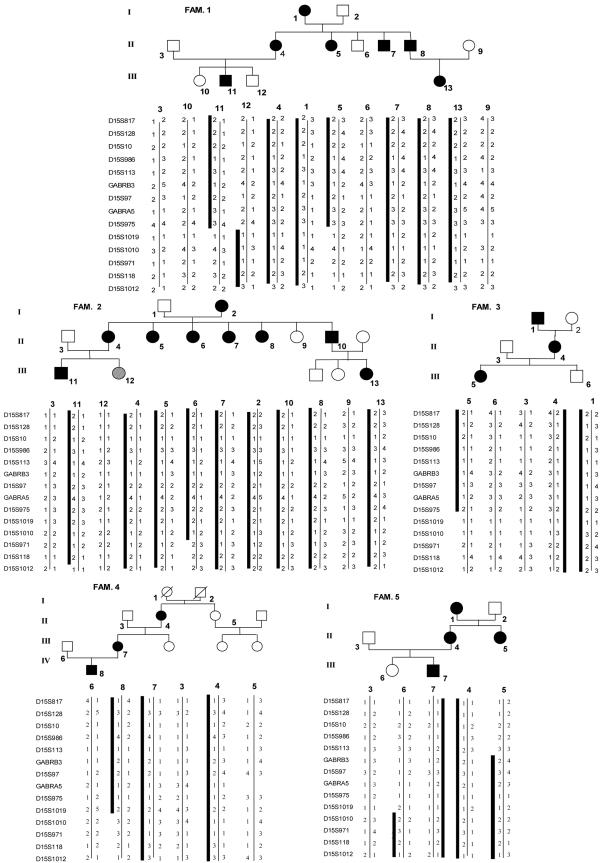
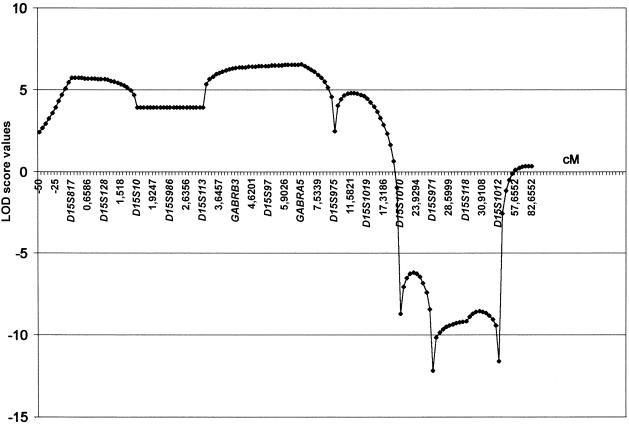
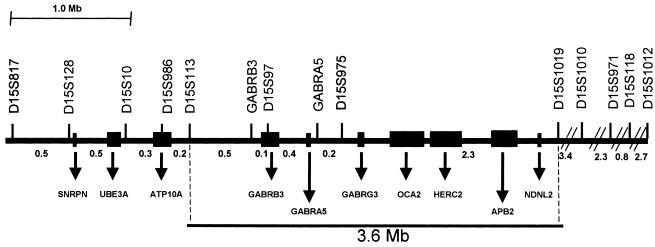
On the contrary, microsatellite analysis with markers from the 15q11-q13 region showed no recombinants in affected individuals from five families with MA. In the other five families with MA, we did not observe cosegregation of haplotypes with the disease. Haplotypes and pedigrees of the five linked families are shown in figure 1. Clinical data from 25 affected members of these families are reported in table 1. In a linkage analysis (by use of LINKAGE 5.1 software [Lathrop et al. 1984]), the highest two-point values were obtained with markers GABRB3 (Zmax = 5.56 at θmax = 0.001) and D15S97 (Zmax = 5.31 at θmax = 0.001), under the assumption of 100% penetrance (table 2). The two-point score values, under the assumption of 80% penetrance, are shown in table 3. The highest Zmax values for the same two markers were 5 and 4.75, respectively, at θmax = 0.001. Multipoint linkage analysis (by use of SIMWALK 2.89 software [Sobel and Lange 1996]) provided strong evidence of linkage between markers D15S113 and D15S1019, with a maximum LOD score of 6.54 for marker GABRA5 (fig. 2), confirming the results of the two-point analysis. The genomic region within proximal and distal recombinants spanned 3.6 Mb (fig. 3).
Figure 1.
Haplotypes and pedigrees of the five families with MA in linkage with the 15q11-q13 genomic region. The gray shading of the symbol representing individual III-12 of family 2 indicates an MA-like phenotype (see text for discussion).
Table 1.
Clinical Features of Patients
|
Age(years) |
Presence of Featurea |
||||||||||
| Family andPatient | Sex | AtStudy | AtOnset | VisualDisturbance | LanguageDisturbance | Sensory/MotorDisturbance | Confusion | Ataxia | Dizziness | Headache Sideb | Highest Attack Frequency |
| 1: | |||||||||||
| III-11 | M | 17 | 13 | + | + | + | L | 10/mo | |||
| III-13 | F | 20 | 12 | + | R-L alternating | 2–3/mo | |||||
| II-4 | F | 43 | 10 | + | + | + | R | 3–4/mo | |||
| II-5 | F | 45 | 8 | + | R-L alternating | 1–2/mo | |||||
| II-7 | M | 48 | 9 | + | + | R-L alternating | 1–2/mo | ||||
| II-8 | M | 50 | 8 | + | R-L alternating | 2/mo | |||||
| I-1 | F | 80 | <18 | + | + | + | + | R-L alternating | 2/mo | ||
| 2: | |||||||||||
| III-11 | M | 13 | 13 | + | L | 2/year | |||||
| II-4 | F | 46 | 8 | + | + | + | L | 2–3/mo | |||
| II-5 | F | 48 | 7 | + | R-L alternating | 2/mo | |||||
| II-6 | F | 50 | 8 | + | R-L alternating | 1–2/mo | |||||
| II-7 | F | 53 | 9 | + | R-L alternating | 2/mo | |||||
| II-10 | M | 59 | 10 | + | + | R-L alternating | 1–2/mo | ||||
| II-8 | F | 55 | 7 | + | R-L alternating | 2/mo | |||||
| 3: | |||||||||||
| III-5 | F | 16 | 10 | + | Bilateral | Daily | |||||
| I-1 | M | 75 | <18 | + | Bilateral | 4/mo | |||||
| II-4 | F | 55 | <18 | + | Bilateral | 3–4/year | |||||
| 4: | |||||||||||
| IV-8 | M | 15 | 14 | + | + | + | Bilateral | 5–6/year | |||
| III-7 | F | 35 | 12 | + | R-L alternating | 2–3/mo | |||||
| II-4 | F | 38 | 10 | + | + | R-L alternating | 2/mo | ||||
| 5: | |||||||||||
| III-7 | M | 10 | 6 | + | + | + | Bilateral | 2–3/year | |||
| II-4 | F | 28 | 8 | + | R-L alternating | 2/mo | |||||
| II-5 | F | 35 | 7 | + | R-L alternating | 2–3/mo | |||||
| I-1 | F | 50 | 12 | + | R-L alternating | 1/mo | |||||
A plus sign (+) indicates presence of clinical feature.
R= right; L = left.
Table 2.
Two-Point LOD Scores under the Assumption of 100% Penetrance[Note]
| LOD at θ = |
|||||||||
| Marker | .001 | .01 | .05 | .1 | .2 | .3 | .4 | θmax | Zmax |
| D15S817 | −1.95 | −.96 | −.30 | −.06 | .11 | .17 | .14 | .3 | .17 |
| D15S128 | 1.73 | 2.65 | 3.02 | 2.88 | 2.27 | 1.47 | .59 | .05 | 3.02 |
| D15S10 | .78 | 1.57 | 1.98 | 1.93 | 1.50 | .93 | .35 | .05 | 1.98 |
| D15S986 | −.41 | 1.36 | 2.40 | 2.56 | 2.23 | 1.58 | .78 | .1 | 2.56 |
| D15S113 | −.05 | 1.72 | 2.76 | 2.92 | 2.56 | 1.84 | .91 | .1 | 2.92 |
| GABRB3 | 5.56 | 5.47 | 5.04 | 4.48 | 3.32 | 2.10 | .87 | .001 | 5.56 |
| D15S97 | 5.31 | 5.22 | 4.80 | 4.26 | 3.12 | 1.94 | .78 | .001 | 5.31 |
| GABRA5 | 3.16 | 4.07 | 4.34 | 4.08 | 3.20 | 2.10 | .92 | .05 | 4.34 |
| D15S975 | .74 | 1.67 | 2.02 | 1.88 | 1.31 | .80 | .36 | .05 | 2.02 |
| D15S1019 | 4.48 | 4.41 | 4.07 | 3.63 | 2.71 | 1.71 | .69 | .001 | 4.48 |
| D15S1010 | −7.44 | −3.59 | −.88 | .13 | .75 | .71 | .35 | .2 | .75 |
| D15S971 | −13.62 | −7.64 | −3.59 | −1.99 | −.69 | −.22 | −.06 | .4 | −.06 |
| D15S118 | −11.80 | −5.88 | −2.02 | −.65 | .26 | .37 | .16 | .3 | .37 |
| D15S1012 | −14.11 | −8.14 | −4.08 | −2.46 | −1.05 | −.43 | −.14 | .4 | −.14 |
Note.— The highest values for each marker are shown in bold.
Table 3.
Two-Point LOD Scores under the Assumption of 80% Penetrance[Note]
| LOD at θ = |
|||||||||
| Marker | .001 | .01 | .05 | .1 | .2 | .3 | .4 | θmax | Zmax |
| D15S817 | −1.62 | −.87 | .27 | −.05 | .11 | .16 | .13 | .3 | .16 |
| D15S128 | 1.55 | 2.35 | 2.72 | 2.62 | 2.07 | 1.34 | .54 | .05 | 2.72 |
| D15S10 | .35 | 1.13 | 1.57 | 1.55 | 1.21 | .74 | .27 | .05 | 1.57 |
| D15S986 | −.64 | .92 | 1.96 | 2.16 | 1.92 | 1.37 | .68 | .1 | 2.16 |
| D15S113 | −.28 | 1.36 | 2.40 | 2.58 | 2.28 | 1.64 | .80 | .1 | 2.58 |
| GABRB3 | 5.00 | 4.91 | 4.52 | 4.01 | 2.95 | 1.85 | .76 | .001 | 5.00 |
| D15S97 | 4.75 | 4.67 | 4.28 | 3.78 | 2.75 | 1.69 | .67 | .001 | 4.75 |
| GABRA5 | 2.83 | 3.61 | 3.89 | 3.67 | 2.87 | 1.88 | .81 | .05 | 3.89 |
| D15S975 | 2.57 | 2.51 | 2.25 | 1.90 | 1.19 | .65 | .27 | .05 | 2.57 |
| D15S1019 | 4.14 | 4.07 | 3.75 | 3.34 | 2.48 | 1.56 | .62 | .001 | 4.14 |
| D15S1010 | −6.76 | −3.69 | −1.11 | −.11 | .55 | .56 | .27 | .2 | .56 |
| D15S971 | −8.62 | −5.40 | −2.68 | −1.53 | −.56 | −.19 | −.05 | .4 | −.05 |
| D15S118 | −9.44 | −4.97 | −1.78 | −.61 | .17 | .27 | .11 | .3 | .27 |
| D15S1012 | −10.41 | −7.01 | −3.71 | −2.31 | −1.05 | −.47 | −.16 | .4 | −.16 |
Note.— The highest values for each marker are shown in bold.
Figure 2.
Graphic representation of the results of multipoint linkage analysis
Figure 3.
Map of the critical region for MA. The microsatellite markers used for genotyping are indicated at top. Distances between markers are as given in the UCSC database. Known genes are indicated at bottom. The critical region between markers D15S113 and D15S1019 is boxed.
According to the University of California–Santa Cruz (UCSC) database, this critical region harbors the following genes: GABRB3 (MIM 137192; GenBank accession number NM_021912), GABRA5 (MIM 137142), GABRG3 (MIM 600233), OCA2 (MIM 203200), HERC2 (MIM 605837), APB2, and NDNL2 (MIM 608243).
We focused on the three genes encoding GABA-A receptor subunits and sequenced the coding region in affected and unaffected individuals from the five linked families. We did not detect any significant mutation; rather, we detected a polymorphism, C75T, in the first exon of the GABRB3 gene in all affected members of family 1.
We detected a significant linkage between the MA phenotype and markers within the 15q11-q13 genomic region, thus providing strong evidence of the existence of an MA susceptibility locus on the proximal chromosome 15q. Evidence of linkage was obtained by parametric two-point linkage analysis and was supported by multipoint analysis (Lander and Kruglyak 1995). We could use this type of analysis because, in the families we studied, MA segregates as an autosomal dominant trait and presents with homogeneous clinical features. The only exception was individual III-12 in family 2 (see fig. 1), who suffered from a single hemiplegic attack, unprecedented in her family, resembling a form of sporadic hemiplegic migraine (SHM), which is considered to be a separate diagnostic entity from typical MA (Headache Classification Subcommittee of the International Headache Society 2004). This patient did not share the affected haplotype, which confirms that she is affected by a different condition—that is, SHM. Alternatively, her condition can be interpreted as a phenocopy. The presence of phenocopies is not an uncommon phenomenon in families with MA (Soragna et al. 2003).
As illustrated in figure 3, the MA critical region spans 3.6 Mb and is defined by the proximal marker D15S113 and the distal marker D15S1019. A number of genes map to this interval. Among these, GABRB3, GABRG3, and GABRA5—encoding β3, γ3, and α5 GABA-A receptor subunits, respectively—can be considered the best candidates. Two imprinted genes, maternally expressed, are located in the proximity of the centromeric boundary of the critical region: UBE3A (MIM 601623) (Matsuura et al. 1997) and the more closely located ATP10A (MIM 605855) (Meguro et al. 2001). Since we observe an excess of maternal transmission of the disease in these families, we cannot rule out the existence of an imprinting effect on the MA phenotype, even though the sequencing of the ATP10A gene did not detect any mutation (data not shown). In addition, we performed a mutational analysis of the coding sequences of three GABA-A receptor genes in at least one affected member from each family, but we did not detect any significant sequence variation. Only a single polymorphism (C75T) in the first exon of GABRB3 was found in family 1. Even though we failed to detect significant mutations, we cannot rule out the possibility that dysfunctional activity of these GABA receptor subunits is due either to polymorphisms, such as C75T, that have functional effect or to mutations in noncoding regulatory sequences.
Given the ubiquitous expression and the vital function of the GABA-A receptors, which ligate a neurotransmitter widely distributed throughout the CNS, we expected to find not clear pathogenetic mutations in patients with MA but, rather, variants with a mild effect on protein function. However, we cannot exclude the possibility that mutations in regulatory sequences located outside the coding region might have strong biochemical effects, similar to what would be expected for an autosomal dominant condition. In addition, there are several lines of evidence suggesting that GABAergic drugs modulate biochemical and physiological events involved in the pathophysiology of migraine, particularly via GABA-A receptor–mediated mechanisms.
It has been proposed elsewhere that an increase in inhibitor GABAergic neurotransmission may suppress the abnormal cortical events underlying the migraine aura (Cutrer et al. 1997). The GABAergic migraine prophylactic drugs may restore a normal cortical inhibitory potential by elevating cortical threshold for spreading depression propagation in patients with migraines (Palmer et al. 2000). Valproic acid blocks neurogenic inflammation within the meninges via GABA-A receptor–mediated mechanisms (Cutrer et al. 1997). Other studies underline the specific importance of GABA-A receptors in modulating trigeminovascular nociceptive neurotransmission (Cutrer et al. 1995, 1997; Storer et al. 2001, 2004). It is also very interesting that an anaesthetic agent, propofol, acting in a well-documented manner at GABA-A receptor subtypes, may represent a rapid and highly effective form of abortive headache treatment (Krusz et al. 2000).
Our study also confirmed the role of GABAergic medications. During the period of clinical observation, individuals II-7 and II-8 of family 1 needed prophylactic therapy for the worsening of migraine. Within 2 wk of administration, sodium valproate completely abolished their headaches.
This result further supports the link between the patients’ conditions, drug response, and genetic findings in the families we studied. In conclusion, our results may provide the basis for further investigation of the hypothesized relationship between a possible GABA-A receptor dysfunction and migraine (Cutrer and Moskowitz 1996).
Electronic-Database Information
Accession numbers and URLs for data presented herein are as follows:
- GenBank, http://www.ncbi.nih.gov/Genbank/ (for GABRB3 [accession number NM_021912])
- Online Mendelian Inheritance in Man (OMIM), http://www.ncbi.nlm.nih.gov/Omim/ (for MGR6, MGR1, MGR2, MGR5, MGR4, familial hemiplegic migraine, CACNA1A, GABRB3, GABRA5, OCA2, HERC2, NDNL2, UBE3A, and ATP10A)
- UCSC, http://www.genome.ucsc.edu
References
- Aurora SK, Ahmad BK, Welch KM, Bhardhwaj P, Ramadan HM (1998) Transcranial magnetic stimulation confirms hyperexcitability of occipital cortex in migraine. Neurology 50:1111–1114 [DOI] [PubMed] [Google Scholar]
- Bolay H, Reuter U, Dunn AK, Huang Z, Boas DA, Moskowitz MA (2002) Intrinsic brain activity triggers trigeminal meningeal afferents in a migraine model. Nat Med 8:136–142 [DOI] [PubMed] [Google Scholar]
- Cutrer FM, Limmroth V, Ayata G, Moskowitz MA (1995) Attenuation by valproate of c-fos immunoreactivity in trigeminal nucleus caudalis induced by intracisternal capsaicin. Br J Pharmacol 116:3199–3204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cutrer FM, Limmroth V, Moskowitz MA (1997) Possible mechanisms of valproate in migraine prophylaxis. Cephalalgia 17:93–100 [DOI] [PubMed] [Google Scholar]
- Cutrer FM, Moskowitz MA (1996) Wolff Award 1996. The actions of valproate and neurosteroids in a model of trigeminal pain. Headache 36:579–585 [DOI] [PubMed] [Google Scholar]
- Ducros A, Joutel A, Vahedi K, Cecillon M, Ferreira A, Bernard E, Verier A, Echenne B, Lopez de Munain A, Bousser MG, Tournier-Lasserve E (1997) Mapping of a second locus for familial hemiplegic migraine to 1q21-q23 and evidence of further heterogeneity. Ann Neurol 42:885–890 [DOI] [PubMed] [Google Scholar]
- Gardner K, Barmada M, Ptacek L, Hoffman E (1997) A new locus for hemiplegic migraine maps to chromosome 1q31. Neurology 49:1231–1238 [DOI] [PubMed] [Google Scholar]
- Goadsby P, Lipton R, Ferrari M (2002) Migraine—current understanding and treatment. N Engl J Med 346:257–270 [DOI] [PubMed] [Google Scholar]
- Headache Classification Subcommittee of the International Headache Society (2004) The international classification of headache disorder. 2nd ed. Cephalalgia Suppl 24:9–160 [DOI] [PubMed] [Google Scholar]
- Iadecola C (2002) From CSD to headache: a long and winding road. Nat Med 8:110–112 [DOI] [PubMed] [Google Scholar]
- Jones K, Ehm M, Pericak-Vance M, Haines J, Boyd P, Peroutka S (2001) Migraine with aura susceptibility locus on chromosome 19p13 is distinct from the familial hemiplegic migraine locus. Genomics 78:150–154 [DOI] [PubMed] [Google Scholar]
- Joutel A, Bousser MG, Biousse V, Labauge P, Chabriat H, Nibbio A, Maciazek J, Meyer B, Bach MA, Weissenbach J (1993) A gene for familial hemiplegic migraine maps to chromosome 19. Nat Genet 5:40–45 [DOI] [PubMed] [Google Scholar]
- Krusz JC, Scott V, Belanger J (2000) Intravenous propofol: unique effectiveness in treating intractable migraine. Headache 40:224–230 [DOI] [PubMed] [Google Scholar]
- Lander ES, Kruglyak L (1995) Genetic dissection of complex traits: guidelines for interpreting and reparting linkage results. Nat Genet 11:241–247 [DOI] [PubMed] [Google Scholar]
- Lathrop GM, Lalouel JM, Julier C, Ott J (1984) Strategies for multilocus linkage analysis in humans. Proc Natl Acad Sci USA 81:3443–3446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuura T, Sutcliffe JS, Fang P, Galjaard RJ, Jiang YH, Benton CS, Rommens JM, Beaudet AL (1997) De novo truncating mutations in E6-AP ubiquitin-protein ligase gene (UBE3A) in Angelman syndrome. Nat Genet 15:74–77 [DOI] [PubMed] [Google Scholar]
- Meguro M, Kashiwagi A, Mitsuya K, Nakao M, Kondo I, Saitoh S, Oshimura M (2001) A novel maternally expressed gene, ATP10C, encodes a putative aminophospholipid translocase associated with Angelman syndrome. Nat Genet 28:19–20 [DOI] [PubMed] [Google Scholar]
- Nyholt D, Curtain R, Griffiths L (2000) Familial typical migraine: significant linkage and localization of a gene to Xq24-28. Hum Genet 107:18–23 [DOI] [PubMed] [Google Scholar]
- Ophoff RA, Terwindt GM, Vergouwe MN, van Eijk R, Oefner PJ, Hoffman SM, Lamerdin JE, Mohrenweiser HW, Bulman DE, Ferrari M, Haan J, Lindhout D, van Ommen GJ, Hofker MH, Ferrari MD, Frants RR (1996) Familial hemiplegic migraine and episodic ataxia type-2 are caused by mutations in the Ca2+ channel gene CACNL1A4. Cell 87:543–552 [DOI] [PubMed] [Google Scholar]
- Palmer JE, Chronicle EP, Rolan P, Mulleners WM (2000) Cortical hyperexcitability is cortical under-inhibition: evidence from a novel functional test of migraine patients. Cephalalgia 20:525–532 [DOI] [PubMed] [Google Scholar]
- Parsons AA (1998) Recent advances in mechanism of spreading depression. Curr Opin Neurol 11:227–231 [DOI] [PubMed] [Google Scholar]
- Pietrobon D, Striessnig J (2003) Neurobiology of migraine. Nat Rev Neurosci 4:386–398 [DOI] [PubMed] [Google Scholar]
- Rasmussen BK, Olesen J (1992) Migraine with aura and migraine without aura: an epidemiological study. Cephalalgia 12:221–228 [DOI] [PubMed] [Google Scholar]
- Russel MB (2001) Genetics of migraine without aura, migraine with aura, migrainous disorder, head trauma migraine without aura and tension-type headache. Cephalalgia 21:778–780 [DOI] [PubMed] [Google Scholar]
- Russel MB, Olesen J (1995) Increased familial risk and evidence of genetic factor in migraine. BMJ 311:541–544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silberstein (2004) Topiramate in migraine prevention: evidence-based medicine from clinical trials. Neurol Sci (Suppl 3) 25:S244–S245 [DOI] [PubMed] [Google Scholar]
- Sobel E, Lange K (1996) Descent graphs in pedigree analysis: applications to haplotyping, location scores, and marker sharing statistics. Am J Hum Genet 58:1323–1337 [PMC free article] [PubMed] [Google Scholar]
- Soragna D, Vettori A, Carraro G, Marchioni E, Vazza G, Tupler R, Savoldi F, Mostacciolo M (2003) A locus for migraine without aura maps on chromosome 14q21.2-q22.3. Am J Hum Genet 72:161–167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storer RJ, Akerman S, Goadsby PJ (2001) GABA receptors modulate trigeminovascular nociceptive neurotransmission in the trigeminocervical complex. Br J Pharmacol 134:896–904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storer RJ, Akerman S, Shields KG, Goadsby PJ (2004) GABAA receptor modulation of trigeminovascular nociceptive neurotransmission by midazolam is antagonized by flumazenil. Brain Res 1013:188–193 [DOI] [PubMed] [Google Scholar]
- Wessman M, Kallela M, Kaunisto M, Marttila P, Sobel E, Sartiala J, Oswell G, Leal S, Papp J, Hamalainen E, Broas P, Joslyn G, Hovatta I, Hiekkalinna T, Kaprio J, Ott J, Cantor R, Zwart JA, Ilmavirta M, Havanka H, Farkkila M, Peltonen L, Palotie A (2002) A susceptibility locus for migraine with aura, on chromosome 4q24. Am J Hum Genet 70:652–662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler DK, Hur YM, Bouchard TJ Jr, Hassaneim RS, Barter R (1998) Migraine in twins raised together and apart. Headache 38:417–422 [DOI] [PubMed] [Google Scholar]