Abstract
Activating mutations in the genes for fibroblast growth factor receptors 1–3 (FGFR1–3) are responsible for a diverse group of skeletal disorders. In general, mutations in FGFR1 and FGFR2 cause the majority of syndromes involving craniosynostosis, whereas the dwarfing syndromes are largely associated with FGFR3 mutations. Osteoglophonic dysplasia (OD) is a “crossover” disorder that has skeletal phenotypes associated with FGFR1, FGFR2, and FGFR3 mutations. Indeed, patients with OD present with craniosynostosis, prominent supraorbital ridge, and depressed nasal bridge, as well as the rhizomelic dwarfism and nonossifying bone lesions that are characteristic of the disorder. We demonstrate here that OD is caused by missense mutations in highly conserved residues comprising the ligand-binding and transmembrane domains of FGFR1, thus defining novel roles for this receptor as a negative regulator of long-bone growth.
The fibroblast growth factors (FGFs) and their receptors (FGFR1–4) play key roles in skeletal development. The FGFRs are part of the tyrosine kinase receptor family and comprise an extracellular ligand-binding domain, a single transmembrane domain, and an intracellular tyrosine kinase region (Ruta et al. 1989; Ornitz and Itoh 2001). Activating mutations in FGFR1–3 result in skeletal disorders. FGFR1 and FGFR2 mutations cause syndromes involving craniosynostosis, such as Pfieffer, Crouzon, and Apert syndromes, whereas the dwarfing syndromes, such as achondroplastic and hypochondroplastic dwarfism, are associated with FGFR3 mutations (Muenke et al. 1994; Reardon et al. 1994; Shiang et al. 1994). Osteoglophonic dysplasia (OD [MIM 166250]) is an autosomal dominant disorder that shares characteristics with both the craniosynostosis syndromes and the dwarfing syndromes. OD is characterized by craniosynostosis, prominent supraorbital ridge, and depressed nasal bridge, as well as by rhizomelic dwarfism and nonossifying bone lesions. However, the genetic basis for the unique skeletal phenotype associated with OD is unknown.
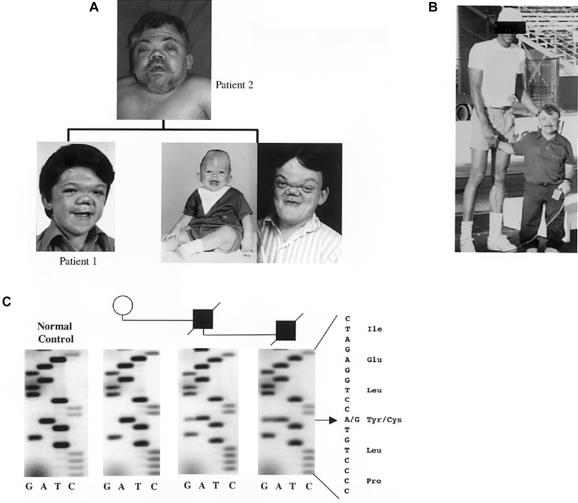
A kindred with OD that consisted of a father and two sons was recognized because of skeletal complications and progressive weakness in the family members. The proband (patient 1) and the affected father (patient 2) were evaluated (fig. 1a). Patient 1 possessed a distinct OD facial phenotype characterized by craniosynostosis, severe nasal maxillary hypoplasia, telechanthus, and prominent supraorbital ridge (fig. 1a). Patient 1 also had growth retardation, with a peak stature of 40 in (101.6 cm) and a weight of 100 lb (45.36 kg). The proband’s father and brother (fig. 1a) had the same skeletal syndrome (the father’s peak stature was 48 in [121.92 cm]; see fig. 1b). They also had shortened necks, broad and shortened thumbs, brachydactyly, and generalized osteopenia. In addition, patients 1 and 2 never had tooth eruption, as documented by radiographs.
Figure 1.
Kindred including patients 1 and 2 with OD. a, The proband (patient 1, shown here after multiple corrective surgeries). He had severe craniofacial abnormalities, including craniosynostosis, telechanthus, and facial hypoplasia, as did his father (patient 2) and brother (infant and adult photographs of the brother are shown). b, Patient 2; note reduced stature. c, The heterozygous 1115G→A (Y372C) missense mutation in patients 1 and 2, as detected by DNA sequencing. This change was not found in normal controls or in the proband’s grandmother.
The direct sequencing of all exons in FGFR1–4, as well as the novel decoy receptor gene FGFR5, by use of DNA samples from this family, revealed a heterozygous 1115G→A missense mutation within exon 10 of FGFR1 (fig. 1c). This change replaces a tyrosine with a cysteine residue at amino acid position 372 (Y372C), which maps to the extracellular juxtamembrane region of FGFR1. Unaffected family members were negative for the mutation, and no other nucleotide substitutions in FGFR1 were found. In addition, this change disrupts an RsaI restriction site and was not found in RFLPs from 880 control alleles (not shown). Tyrosine at position 372 is conserved among all FGFRs. The analogous mutations that result in unpaired cysteine residues in FGFR2 (Y375C) and FGFR3 (Y373C) cause Beare-Stevenson cutis gyrata syndrome (characterized by furrowed skin, acanthosis nigricans, craniosynostosis, digital anomalies, and early death [Hall et al. 1992; Przylepa et al. 1996]) and thanatophoric dwarfism type I (characterized by craniosynostosis, short ribs and bones of the extremities, reduced vertebral bodies, and neonatal lethality [Rousseau et al. 1996]), respectively. It is interesting that, although these mutations are analogous to FGFR2 mutations that cause the skin disorders mentioned above, these patients do not have dermatologic findings. Follow-up analysis of this kindred was not possible because the patients are deceased. Patient 1 died at age 28 years, presumably from a pulmonary embolism due to extended immobilization; patient 2 died at age 59 years, from respiratory distress under similar immobilizing conditions; and the brother of patient 1 died at age 24 years, from pneumonia.
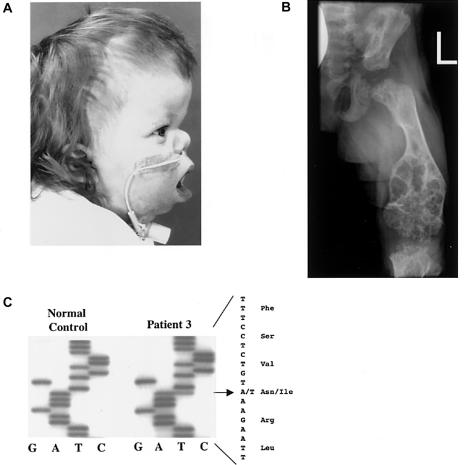
An unrelated patient with OD (patient 3), was found to have severe craniosynostosis, midface hypoplasia (fig. 2a), right choanal atresia, and mild rhizomelic shortening of the limbs, which became more pronounced with further growth. In radiological analysis, severe femur metaphyseal lesions were present (fig. 2b), and, similar to the findings for patient 1, marked demineralization of the long bones (fig. 2b), the mandible, and the maxilla was observed. Patient 3 developed swelling that arose from the lower gums, with a histologic appearance resembling giant-cell granulomata. The swelling did not respond to vinblastine or methotrexate, and, ultimately, the growth of the lesions was suppressed by intravenous bisphosphonate treatment.
Figure 2.
Patient 3. a, Midface hypoplasia with low-set ears and telechanthus. b, Radiograph of the lower leg, revealing severe lesions at the metaphases, as well as shortened bone length and decreased mineral density. c, The 929T→A (N330I) missense mutation of patient 3.
The karyotype of patient 3 was normal, and DNA analysis was negative for Pfeiffer syndrome mutations and Muenke and Saethre-Chotzen syndromes mutations—FGFR1 P252R and FGFR3 P250R, respectively (Muenke et al. 1997; Paznekas et al. 1998). We then identified a heterozygous T→A mutation (929T→A) in exon 9 of FGFR1 (fig. 2c), which resulted in an asparagine-to-isoleucine (N330I) mutation. This change creates a Tsp509I restriction site, which was not found in 200 control individuals by RFLP analysis (not shown). Furthermore, the parents of patient 3 were negative for the mutation, which indicates that it was a de novo DNA mutation. N330 is a predicted glycosylation site, and homologous mutations in FGFR2 (N331I) and FGFR3 (N328I) cause Crouzon syndrome (Steinberger et al. 1996) and hypochondroplasia (Winterpacht et al. 2000), respectively. Of note, exon 9 includes the sequence encoding the C-terminal portion of D3, a domain that plays a role in FGF binding and activation (Ornitz et al. 1996). Exon 9 is present only in the “c” isoform of FGFR1, indicating that specific changes in FGFR1c will lead to OD.
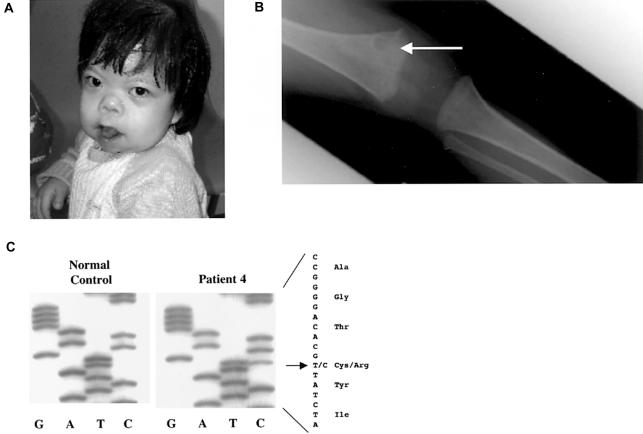
Similar to patients 1–3, patient 4 had clinical features at age 2.5 years that were consistent with OD and included prominent eyes, a short nose, palpable cranial sutures, pansutural craniosynostosis (confirmed by radiograph and CT scans; not shown), pectus excavatum, and delayed tooth eruption (fig. 3a). Her head circumference was in the 3rd percentile, and she had growth retardation (percentile <0.4) with bilateral flexion contractures of her fingers. Furthermore, a skeletal survey of patient 4 showed multiple lesions in the metaphyseal regions of the long bones (fig. 3b), although they were not as severe as those of patient 2.
Figure 3.
Patient 4. a, Craniosynostosis, midface hypoplasia, and telechanthus. b, Radiograph of the femur, showing lesions at metaphysis (arrow). c, The 1135T→C (C379R) mutation of patient 4.
In sequence analysis of FGFR1 in patient 4, a heterozygous T→C mutation (1135T→C; C379R) was identified in exon 10 (fig. 3c), which resulted in a cysteineto-arginine change. This substitution interrupts an HpyCH4V restriction site, and the change was not found in 100 control individuals by RFLP analysis. Furthermore, both parents were negative for the mutation, which indicates that it was a de novo substitution (not shown). Cysteine at position 379 is predicted to reside within the FGFR1 transmembrane domain, and mutations in FGFR3 at the position homologous to C379 (G380R) are responsible for >90% of achondroplastic dwarfism cases (Rousseau et al. 1994; Shiang et al. 1994; Ikegawa et al. 1995).
In examination of the biochemistries of the patients with OD, parallel observations were made in several cases. Patient 1 and his father, patient 2, were hypophosphatemic (1.0 mg/dl; normal, 2.7–4.5 mg/dl) secondary to renal phosphate wasting (tubular maximum phosphate transport/glomerular filtration rates, 0.9 mg/dl for patient 1 and 0.3 mg/dl for patient 2; normal, 2.5–4.5 mg/dl). This family also had 1,25-dihydroxyvitamin D concentrations that were inappropriately low, given the degree of hypophosphatemia (10 pg/ml; normal, 25–45 pg/ml). In a similar manner, patient 3 became hypophosphatemic (3.2 mg/dl; normal for children, 4.5–5.5 mg/dl) at age 3 years as a result of isolated renal phosphate wasting, with normal serum calcium, 25-hydroxyvitamin D, and alkaline phosphatase concentrations. In this patient, parathyroid hormone (PTH) levels fluctuated above the normal range, with unknown etiology, early in life. Although PTH was suppressed during the period of bisphosphonate treatment for lesions (aged 3–3.5 years), patient 3 remained hypophosphatemic. It is interesting that isolated renal phosphate wasting has not been a reported finding in OD. We previously determined that a known phosphaturic factor, FGF23, is produced by the nonossifying lesions in some patients with fibrous dysplasia of bone and that elevated circulating concentrations of FGF23 are positively correlated with phosphate wasting in these patients (Riminucci et al. 2003). Similarly, the hypophosphatemia and disturbed vitamin D metabolism observed in OD may be caused by increased FGF23 production within the characteristic radiolucent OD lesions (Beighton et al. 1980). We therefore tested serum levels of FGF23 in patient 3, which were indeed elevated (101 pg/ml; control mean ± SD, 30 ± 20 pg/ml). Of note, patient 4 had normal plasma phosphate concentrations and a normal serum FGF23 concentration (32.6 pg/ml). Indeed, the lesional burden is far greater in patient 3 than in patient 4 (figs. 2b and 3b), which is inversely correlated with the serum phosphate concentrations in these patients (not shown). Patients 1 and 2 are deceased; therefore, we were unable to determine their serum FGF23 values. However, because this family, parallel to patient 3, had the characteristic OD skeletal phenotype and renal phosphate wasting, we would hypothesize that serum FGF23 was elevated.
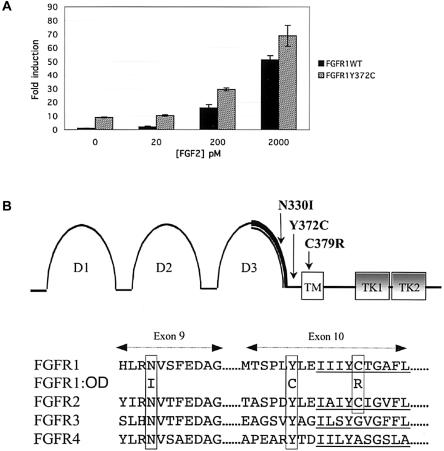
To determine whether the OD mutations were activating mutations, wild-type FGFR1c and Y372C FGFR1c were assayed for ligand-independent and ligand-dependent activity. The FGFR1 Y372C missense mutation was introduced into the FGFR1 cDNA by use of a nested-PCR site-directed mutagenesis approach (Yang et al. 1994). Wild-type and mutant receptors were cotransfected with an osteocalcin FGF response element promoter-luciferase reporter into the osteogenic MC3T3 cell line (Newberry et al. 1996). In the absence of exogenous FGF, the basal activity of Y372C FGFR1 was elevated 9-fold, as compared with that of wild-type FGFR1 (P<.01), which indicates that the mutation causes ligand-independent activation of FGFR1 (fig. 4a). Both wild-type and Y372C FGFR1 responded to increasing concentrations of FGF2, a known ligand for FGFR1c (Ornitz et al. 1996). At all concentrations tested, FGF2-mediated activity was significantly higher for the Y372C FGFR1 mutant compared with the wild type (fig. 4a).
Figure 4.
Characteristics of OD mutations. a, Activation of FGFR1 and Y372C FGFR1 tested using cotransfection of wild-type FGFR1 and Y372C mutant FGFR1 with an FGFR1-responsive osteocalcin promoter-luciferase construct. Y372C FGFR1 had a 9-fold greater basal activity, compared with that of wild-type FGFR1 (P<.01), and showed increased activity with FGF2 as ligand (20 pM, P<.01; 200 pM, P<.02; 2,000 pM, P<.03). b, Structure of FGFR1, with the relevant domains labeled. D1–3 = extracellular immunoglobulin-like domains that comprise the FGF-binding domain; TM = transmembrane region; TK = tyrosine kinase domains 1–2. The thick bold line within D3 represents the known IIIb(exon 8)/IIIc(exon 9) splicing region. The positions of the OD mutations within FGFR1 exons 9 and 10 are indicated. In the partial protein-sequence alignment of FGFR1-4 (bottom), the location of conserved residues with OD FGFR1 mutations are boxed, and the transmembrane domains encoded by sequences within exon 10 are underlined.
In summary, OD is caused by activating mutations in highly conserved residues of FGFR1, within a limited region comprising the D3 domain, a linker region, and the initial transmembrane domain. Previously characterized mutations in FGFR1–3-associated skeletal dysplasias lead to inappropriate receptor signaling through covalent and noncovalent receptor dimerization and activation (Neilson and Friesel 1996; Li et al. 1997), increased ligand-binding affinity (Anderson et al. 1998; Ibrahimi et al. 2001), altered specificity for FGF binding (Yu et al. 2000), and direct activation of the FGFR tyrosine kinase domains (Neilson and Friesel 1996). In contrast, inactivating mutations in FGFR1 are responsible for autosomal dominant Kallmann syndrome, characterized by hypogonadism and anosmia (Dod et al. 2003); thus, it is highly likely that the OD N330I and C379R mutations, in addition to the Y372C substitution, are gain-of-function mutations. It is thought that activation of FGFR3 is responsible for negative regulation of bone elongation, because Fgfr3 null mice have increased long-bone length (Deng et al. 1996) and because of the relatively common occurrence of activating mutations in FGFR3-related achondroplasia (Naski et al. 1996). The most common FGFR1-activating mutation (P252R) causes the craniosynostosis disorder Pfeiffer syndrome (Muenke et al. 1994); thus, FGFR1 has been associated primarily with flat bone growth and skull formation. Because the Fgfr1 null mouse is neonatal lethal (Deng et al. 1994) and because of the rarity of OD, the role of FGFR1 in long-bone growth has not been fully recognized. In long bones, FGFR1 expression is spatially separated from that of FGFR3, as the epiphyseal growth plate is formed. Prehypertrophic and hypertrophic chondrocytes, osteoblasts, and perichondrium express FGFR1, whereas FGFR3 is found in proliferating chondrocytes and adult osteoblasts (Peters et al. 1992; Deng et al. 1996; Delezoide et al. 1998; Xiao et al. 2004). Together with the expression profile of FGFR1 in bone, the exon 9 mutation (N330I) demonstrates that the activation of the FGFR1c isoform has profound effects on long bones, possibly by regulation of skeletal formation through suppression of chondrocyte or osteoblast function. It is interesting that patients 1 and 2 had respiratory difficulties, which is not a typical characteristic of other FGFR syndromes. The patients did not have rib deformities; thus, there are two things that most likely affected respiratory function. The hypophosphatemia and marked reduction in calcitriol concentration may have led to weakness, which resulted in poor inspiratory effort with atelectasis, thereby predisposing the patients to pneumonia. In addition, it is likely that immobilization led to a pulmonary embolus in patient 1 and, possibly, in his father (patient 2) and/or brother.
In summary, OD is a disorder that shares skeletal characteristics with both the craniosynostoses and the dwarfing syndromes. OD is caused by activating mutations in FGFR1, which thus reveals novel critical functions of the FGFR1 receptor in the modulation of bone elongation.
Acknowledgments
We are very grateful to the kindreds for their participation and for permission to present family photographs. We also thank David Weaver, M.D., for assistance in characterizing the craniofacial features of OD; Mack Harrell, M.D., and Dawn Vickers, R.N., for taking many of the original patient photographs and obtaining blood samples; and Jean Kirk, M.D., for facilitating the sample processing. The authors also greatly appreciate the scientific advice from Moosa Mohammadi, Ph.D., and from Omar Ibrahimi, given during the course of these studies. This work was supported by National Institutes of Health grant DK063934 (to K.E.W.), training grant T32 AR007033 (to K.Y.), grant HD39952 (to D.M.O.), and grants AR42228, AG18397, AR02095, and AR47866 (to M.J.E.). The authors would also like to acknowledge the support of the Indiana Genomics Initiative, supported in part by Lilly Endowment.
Electronic-Database Information
The URL for data presented herein is as follows:
- Online Mendelian Inheritance in Man (OMIM), http://www.ncbi.nlm.nih.gov/Omim/ (for OD) [PubMed]
References
- Anderson J, Burns HD, Enriquez-Harris P, Wilkie AO, Heath JK (1998) Apert syndrome mutations in fibroblast growth factor receptor 2 exhibit increased affinity for FGF ligand. Hum Mol Genet 7:1475–1483 [DOI] [PubMed] [Google Scholar]
- Beighton P, Cremin BJ, Kozlowski K (1980) Osteoglophonic dwarfism. Pediatr Radiol 10:46–50 [DOI] [PubMed] [Google Scholar]
- Delezoide AL, Benoist-Lasselin C, Legeai-Mallet L, Le Merrer M, Munnich A, Vekemans M, Bonaventure J (1998) Spatio-temporal expression of FGFR 1, 2 and 3 genes during human embryo-fetal ossification. Mech Dev 77:19–30 [DOI] [PubMed] [Google Scholar]
- Deng C, Wynshaw-Boris A, Zhou F, Kuo A, Leder P (1996) Fibroblast growth factor receptor 3 is a negative regulator of bone growth. Cell 84:911–921 [DOI] [PubMed] [Google Scholar]
- Deng CX, Wynshaw-Boris A, Shen MM, Daugherty C, Ornitz DM, Leder P (1994) Murine FGFR-1 is required for early postimplantation growth and axial organization. Genes Dev 8:3045–3057 [DOI] [PubMed] [Google Scholar]
- Dode AC, Levilliers J, Dupont JM, De Paepe A, Le Dû N, Soussi-Yanicostas N, Coimbra RS, et al (2003) Loss-of-function mutations in FGFR1 cause autosomal dominant Kallmann syndrome. Nat Genet 33:463–465 [DOI] [PubMed] [Google Scholar]
- Hall BD, Cadle RG, Golabi M, Morris CA, Cohen MM Jr (1992) Beare-Stevenson cutis gyrata syndrome. Am J Med Genet 44:82–89 [DOI] [PubMed] [Google Scholar]
- Ibrahimi OA, Eliseenkova AV, Plotnikov AN, Yu K, Ornitz DM, Mohammadi M (2001) Structural basis for fibroblast growth factor receptor 2 activation in Apert syndrome. Proc Natl Acad Sci USA 98:7182–7187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikegawa S, Fudushima Y, Isomura M, Takada F, Nakamura Y (1995) Mutations of the fibroblast growth factor receptor-3 gene in one familial and six sporadic cases of achondroplasia in Japanese patients. Hum Genet 96:309–311 [DOI] [PubMed] [Google Scholar]
- Li Y, Mangasarian K, Mansukhani A, Basilico C (1997) Activation of FGF receptors by mutations in the transmembrane domain. Oncogene 14:1397–1406 [DOI] [PubMed] [Google Scholar]
- Muenke M, Gripp KW, McDonald-McGinn DM, Gaudenz K, Whitaker LA, Bartlett SP, Markowitz RI, et al (1997) A unique point mutation in the fibroblast growth factor receptor 3 gene (FGFR3) defines a new craniosynostosis syndrome. Am J Hum Genet 60:555–564 [PMC free article] [PubMed] [Google Scholar]
- Muenke M, Schell U, Hehr A, Robin N, Losken HW, Schinzel A, Pulleyn LJ, Rutland P, Reardon W, Malcolm S, Winter RM (1994) A common mutation in the fibroblast growth factor receptor 1 gene in Pfeiffer syndrome. Nat Genet 8:269–274 [DOI] [PubMed] [Google Scholar]
- Naski MC, Wang Q, Xu J, Ornitz DM (1996) Graded activation of fibroblast growth factor receptor 3 by mutations causing achondroplasia and thanatophoric dysplasia. Nat Genet 13:233–237 [DOI] [PubMed] [Google Scholar]
- Neilson KM, Friesel R (1996) Ligand-independent activation of fibroblast growth factor receptors by point mutations in the extracellular, transmembrane, and kinase domains. J Biol Chem 271:25049–25057 [DOI] [PubMed] [Google Scholar]
- Newberry EP, Boudreaux JM, Towler DA (1996) The rat osteocalcin fibroblast growth factor (FGF)-responsive element: an okadaic acid-sensitive, FGF-selective transcriptional response motif. Mol Endocrinol 10:1029–1040 [DOI] [PubMed] [Google Scholar]
- Ornitz DM, Itoh N (2001) Fibroblast growth factors [review]. Genome Biol 2:3005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ornitz DM, Xu J, Colvin JS, McEwen DG, MacArthur CA, Coulier F, Gao G, Goldfarb M (1996) Receptor specificity of the fibroblast growth factor family. J Biol Chem 271:15292–15297 [DOI] [PubMed] [Google Scholar]
- Paznekas WA, Cunningham ML, Howard TD, Korf BR, Lipson MH, Grix AW, Feingold M, Goldberg R, Borochowitz Z, Aleck K, Mulliken J, Yin M, Jabs EW (1998) Genetic heterogeneity of Saethre-Chotzen syndrome due to Twist and FGFR mutations. Am J Hum Genet 62:1370–1380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters KG, Werner S, Chen G, Williams LT (1992) Two FGF receptor genes are differentially expressed in epithelial and mesenchymal tissues during limb formation and organogenesis in the mouse. Development 114:233–243 [DOI] [PubMed] [Google Scholar]
- Przylepa KA, Paznekas W, Zhang M, Golabi M, Bias W, Bamshad MJ, Carey JC, Hall BD, Stevenson R, Orlow S, Cohen MM Jr, Jabs EW (1996) Fibroblast growth factor receptor 2 mutations in Beare-Stevenson cutis gyrata syndrome. Nat Genet 13:492–494 [DOI] [PubMed] [Google Scholar]
- Reardon W, Winter RM, Rutland P, Pulleyn LJ, Jones BM, Malcolm S (1994) Mutations in the fibroblast growth factor receptor 2 gene cause Crouzon syndrome. Nat Genet 8:98–103 [DOI] [PubMed] [Google Scholar]
- Riminucci M, Collins MT, Fedarko NS, Cherman N, Corsi A, White KE, Waguespack S, Gupta A, Hannon T, Econs MJ, Bianco P, Gehron Robey P (2003) FGF-23 in fibrous dysplasia of bone and its relationship to renal phosphate wasting. J Clin Invest 112:683–692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rousseau F, Bonaventure J, Legeal-Mallet L, Pelet A, Michel-Rozet J, Maroteaux P, Merrer ML, Munnich A (1994) Mutations in the gene encoding fibroblast growth factor receptor-3 in achondroplasia. Nature 371:252–254 [DOI] [PubMed] [Google Scholar]
- Rousseau F, el Ghouzzi V, Delezoide AL, Legeai-Mallet L, Le Merrer M, Munnich A, Bonaventure J (1996) Missense FGFR3 mutations create cysteine residues in thanatophoric dwarfism type I (TD1). Hum Mol Genet 5:509–512 [DOI] [PubMed] [Google Scholar]
- Ruta M, Burgess W, Givol D, Epstein J, Neiger N, Kaplow J, Crumley G, Dionne C, Jaye M, Schlessinger J (1989) Receptor for acidic fibroblast growth factor is related to the tyrosine kinase encoded by the fms-like gene (FLG). Proc Natl Acad Sci USA 86:8722–8726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiang R, Thompson LM, Zhu YZ, Church DM, Fielder TJ, Bocian M, Winokur ST, Wasmuth JJ (1994) Mutations in the transmembrane domain of FGFR3 cause the most common genetic form of dwarfism, achondroplasia. Cell 78:335–342 [DOI] [PubMed] [Google Scholar]
- Steinberger D, Mulliken JB, Muller U (1996) Crouzon syndrome: previously unrecognized deletion, duplication, and point mutation within FGFR2 gene. Hum Mutat 8:386–390 [DOI] [PubMed] [Google Scholar]
- Winterpacht A, Hilbert K, Stelzer C, Schweikardt T, Decker H, Segerer H, Spranger J, Zabel B (2000) A novel mutation in FGFR-3 disrupts a putative N-glycosylation site and results in hypochondroplasia. Physiol Genomics 2:9–12 [DOI] [PubMed] [Google Scholar]
- Xiao L, Naganawa T, Obugunde E, Gronowicz G, Ornitz DM, Coffin JD, Hurley MM (2004) Stat1 controls postnatal bone formation by regulating fibroblast growth factor signaling in osteoblasts. J Biol Chem 279:27743–27752 [DOI] [PubMed] [Google Scholar]
- Yang X-F, Fournier H, Dion N, Crine P, Boileau G (1994) Site-directed mutagenesis and transfection methods in the study of prohormone processing. Neuroprotocols 5:157–168 [Google Scholar]
- Yu K, Herr AB, Waksman G, Ornitz DM (2000) Loss of fibroblast growth factor receptor 2 ligand-binding specificity in Apert syndrome. Proc Natl Acad Sci USA 97:14536–14541 [DOI] [PMC free article] [PubMed] [Google Scholar]