Abstract
The Phox2b gene is necessary for autonomic nervous-system development. Phox2b−/− mice die in utero with absent autonomic nervous system circuits, since autonomic nervous system neurons either fail to form or degenerate. We first identified the Phox2b human ortholog, PHOX2B, as the gene underlying congenital central hypoventilation syndrome (CCHS, or Ondine curse), with an autosomal dominant mode of inheritance and de novo mutation at the first generation. We have subsequently shown that heterozygous mutations of PHOX2B may account for several combined or isolated disorders of autonomic nervous-system development—namely, tumors of the sympathetic nervous system (TSNS), such as neuroblastoma and late-onset central hypoventilation syndrome. Here, we report the clinical and molecular assessments of a cohort of 188 probands with CCHS, either isolated or associated with Hirschsprung disease and/or TSNS. The mutation-detection rate was 92.6% (174/188) in our series, and the most prevalent mutation was an in-frame duplication leading to an expansion of +5 to +13 alanines in the 20-alanine stretch at the carboxy terminal of the protein. Such findings suggest PHOX2B mutation screening as a simple and reliable tool for the diagnosis of CCHS, independent of the clinically variable phenotype. In addition, somatic mosaicism was detected in 4.5% of parents. Most interestingly, analysis of genotype-phenotype interactions strongly supports the contention that patients with CCHS who develop malignant TSNS will harbor either a missense or a frameshift heterozygous mutation of the PHOX2B gene. These data further highlight the link between congenital malformations and tumor predisposition when a master gene in development is mutated.
Congenital central hypoventilation syndrome (CCHS, or Ondine curse [MIM 209880]) is a life-threatening disorder primarily manifested as sleep-associated respiratory insufficiency and markedly impaired ventilatory responses to hypercarbia and hypoxaemia (Gozal 1998). Progress in the recognition and clinical management of patients with CCHS has revealed the presence of broader structural and functional impairments of the autonomic nervous system (ANS) (Vanderlaan et al. 2004). In particular, Hirschsprung disease (HSCR [MIM 142623]) (Croaker et al. 1998) and tumors of autonomic neural crest derivatives, such as neuroblastoma, ganglioneuroblastoma, and ganglioneuroma (Rohrer et al. 2002), are noted in 20% and 5%–10% of patients with CCHS, respectively. The association of CCHS and HSCR is known as “Haddad syndrome” (MIM 209880). Finally, a heterogeneous group of patients with late-onset central hypoventilation syndrome (LO-CHS) have also been recently described (Katz et al. 2000), although whether CCHS and LO-CHS should be regarded as the same entity remains controversial.
In mice, the development of all ANS reflex circuits is critically dependent on the paired-like homeobox gene Phox2b (Pattyn et al. 1999; Brunet and Pattyn 2002). The human PHOX2B gene (MIM 603851) maps on chromosome 4p12 and encodes a highly conserved 314–amino-acid homeobox transcription factor with two short and stable polyalanine repeats of 9 and 20 residues. (Yokoyama et al. 1999).
On the basis of our initial report indicating a relatively high frequency of mutations in the human PHOX2B gene among patients with CCHS, we sought to examine genotype-phenotype relationships in a large group of these patients and their families, with particular attention to the tumoral phenotype. We report on a correlation between the nature of the PHOX2B mutation and a tumor risk significant enough to allow a focused follow-up among patients with CCHS.
Patients
Clinical information and blood samples were obtained with informed consent of the probands or the parents from a series of 188 patients with CCHS (91 females and 97 males) and their parents, when possible. Samples were primarily from the United States, Great Britain, France, Germany, and Sweden. Twenty-nine patients have been reported elsewhere (Amiel et al. 2003). Full detailed clinical data were not available for 10 patients (5.3%).
The clinical presentations of the 178 remaining patients are summarized in table 1. With regard to the ventilatory phenotype, the patients fulfilled the inclusion criteria established in 1999 (Weese-Mayer et al. 1999)—namely, (1) persistent central alveolar hypoventilation (PaCO2 >60 mmHg) during sleep, detected by polysomnography while the patient spontaneously breathes room air; (2) lack of ventilatory responses to inhaled CO2; and (3) absence of primary lung, neuromuscular, or cardiac disease. HSCR and tumors of the sympathetic nervous system (TSNS) were observed in 45 (24%) and 10 (5.3%) of the cases, respectively. As opposed to the distribution of isolated HSCR—in which the sex ratio is skewed in favor of females, with the short-segment form of the disease in 80% of the cases (Croaker et al. 1998)—males and females were equally affected, and the long-segment form of the disease was most frequent among cases for which the size of the aganglionic tract was known (22 cases with long-segment form, 12 cases with short-segment form, and 11 cases with unknown length of the aganglionic tract). Cases of TSNS had various degrees of differentiation and were classified as neuroblastoma (MIM 256700), ganglioneuroblastoma, or ganglioneuroma in seven, one, and two cases, respectively; these were either isolated or multifocal (table 1). Finally, our series included three familial cases: two cases with vertical transmission of the disease from an affected parent (the father in one case and the mother in the other case) to his or her child and one case with recurrence in siblings.
Table 1.
Clinical Findings in a Series of 188 Patients with CCHS
|
No. of Patients with |
|||||||
| Syndromic CCHS |
|||||||
| Patients | Isolated CCHS | HSCR | HSCR+TSNS | TSNS | Unknown | LO-CHS | Total No. of Patients |
| Males | 58 | 21 | 4 | 2 | 6 | 6 | 97 |
| Females | 62 | 18 | 2 | 2 | 4 | 3 | 91 |
| All | 120 | 39 | 6 | 4 | 10 | 9 | 188 |
Methods
DNA was extracted in accordance with standard protocols. We screened the coding sequence of the PHOX2B gene by denaturing high-pressure liquid chromatography and/or direct DNA sequencing, as described elsewhere (Amiel et al. 2003). The screening of exon 3 of the PHOX2B gene was improved by using the protocol described by Matera et al. (2004). When no mutation was detected in the PHOX2B coding sequence, we studied the 533 bp of the promoter region that is known to be extremely conserved among species and includes pbx-Hox– and Prep/Meis–binding sites in the mouse (Samad et al. 2004) (primers available on request). Direct DNA sequencing was performed by use of the fluorometric method (Big Dye Terminator Cycle Sequencing kit [Applied Biosystems]).
Whenever the parental DNA of a CCHS index case with an identified PHOX2B mutation was available for study, it was screened by direct DNA sequencing (105 pairs of parents and 19 single parents).
Results
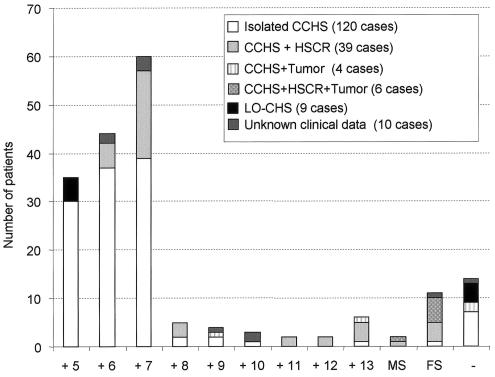
A heterozygous mutation of the PHOX2B gene was identified in 174 (92.6%) of 188 cases (table 2 and fig. 1). In 161 (92.5%) of the 174 cases with an identifiable mutation, the mutation consisted of an in-frame duplication of 15–39 nucleotides, leading to an expansion of +5 to +13 alanines within the 20-alanine tract of the carboxy terminal of the homeodomain of the protein (fig. 1). In 10 cases, we identified a small nucleotide insertion or deletion located 3′ to the homeobox. Such frameshift mutations presumably resulted in either a shortened or an elongated protein that may have retained or lost the conserved 20-alanine tract (table 2). Futhermore, in three of the cases, a missense mutation in the homeodomain (428A→G [Q143R] or 422G→A [R141Q]) or in the stop codon (945A→C [X315C]) was identified. The clinical features presented by these patients are summarized in table 2. Most of them presented with HSCR and TSNS (11 and 6 cases, respectively). Finally, direct sequencing of the PHOX2B coding sequence, the intron-exon boundaries, and 530 bp of the promoter region failed to detect a nucleotide variation in 14 cases (4 cases of LO-CHS, 7 cases of isolated CCHS, 2 cases of CCHS + tumor, and 1 case of unknown presentation). Of the nine previously reported patients with CCHS in whom no PHOX2B mutation was identified, seven were found to harbor an in-frame duplication leading to a polyalanine expansion after PCR amplification, by use of the protocol described by Matera et al. (2004).
Table 2.
Patients with CCHS in Whom Heterozygous Missense or Frameshift Mutations of the PHOX2B Gene Were Identified
|
Clinical Findingsa |
||||||
| Patient | CCHS | HSCR | NB | NucleotideVariation | Putative PHOX2B(aa) | Alanine Stretchb |
| O.61 | + | + (SS) | + (NB) | 422G→A | 314 (R141Q) | P |
| O.82 | + | + (LS) | − | 428A→G | 314 (Q143R) | P |
| O.179 | + | + (LS) | − | 606–607insA | 358 | A |
| S.253 | + | + | + (x NB) | 618–619insC | 358 | A |
| O.188 | ? | ? | ? | 692delG | 307 | A |
| O.110 | + | + (TCA) | + (x NB) | 693–700del8 | 355 | A |
| O.185 | + | − | ? | 689–696dup8 | 310 | A |
| O.203 | + | + (TCA) | − | 722del35 | 346 | A |
| S.247 | + | + | + (NB) | 722del38 | 345 | A |
| O.123 | + | + (LS) | − | 722del38 | 345 | A |
| O.43 | + | + | + (x NB) | 931del5 | 356 | P |
| O.22 | + | + (TCA) | + (GGB) | 936insT | 358 | P |
| O.150 | + | + (SS) | ? | 945A→C | 355 | P |
+ = presence; − = absence; ? = unknown; SS = short-segment HSCR; LS = long-segment HSCR; TCA = total colonic aganglionosis; NB = neuroblastoma; x = multiple; GGB = ganglioneuroblastoma.
20-alanine stretch in putative protein. P= present; A = absent.
Figure 1.
PHOX2B mutations among subgroups of patients with CCHS. The mutation types are reported on the X-axis. Alanine expansions are symbolized by a plus sign (+) followed by the number of extra alanines. MS = missense mutations; FS = frameshift mutations. The minus sign (−) represents the group of patients (n=14) with no PHOX2B mutation identified. Clinical subgroups are listed in the graph key.
Regarding familial cases: the vertical transmission of an alanine expansion (+5 and +7 alanines) from an affected parent to his or her affected child was demonstrated in two cases. In the third familial case, with recurrence in sibs, we were able to detect paternal somatic mosaicism for the mutation that was identified in the index case (+7 alanines). A somatic mosaicism was detected in 10 cases (6 fathers and 4 mothers). In all cases, the nucleotide in-frame duplication leading to alanine expansion remained unchanged in transmission, suggesting that such polyalanine expansions are both meiotically and mitotically stable. These data argue for unequal allelic homologous recombination as the mutation-causing mechanism, as proposed by Warren (1997) for alanine expansion in HOXD13.
In the group of patients with LO-CHS (nine cases), a PHOX2B mutation was found in five cases (fig. 1) and always consisted of an expansion of +5 alanines. Neither alanine triplet expansions nor frameshift mutations were found in 250 control chromosomes from various ethnic backgrounds. Interestingly, 2 of 250 control alleles exhibited polyalanine contractions—one of five and the other of seven triplets (not shown).
Discussion
The PHOX2B gene has recently been identified as the primary gene underlying CCHS, with an autosomal dominant mode of inheritance and de novo mutation at the first generation (Amiel et al. 2003). The PHOX2B mutation-detection rate is high in CCHS and was initially underestimated because of major-allele drop-out (Amiel et al. 2003; Sasaki et al. 2003; Matera et al. 2004). Moreover, the vast majority of the mutations (161 [92.5%] of 174 cases) are located within the cryptic triplet region encoding the carboxy terminal stretch of 20 alanines. These observations indicate that PHOX2B molecular screening may provide a fast and reliable diagnostic test in neonates presenting with clinical manifestations suggestive of CCHS. The group of patients with no detectable PHOX2B mutation (14/188) is clinically heterogeneous, although nine patients are not clinically distinguishable from patients with CCHS who harbor a PHOX2B mutation (fig. 1). Such a small percentage of failure to detect the molecular event is not, per se, a strong argument for genetic heterogeneity.
Somatic mosaicism in one of the parents of a patient with CCHS was detected in 10 cases by studying DNA extracted from peripheral leukocytes. This ratio is unexpectedly high and has direct and important consequences for genetic counseling. Indeed, although a recurrence-risk figure cannot be accurately predicted—since the percentage of germline mosaicism is unknown—prenatal diagnosis for a future pregnancy should be proposed, since the recurrence risk may be as high as 50%. Conversely, it will be of interest to confirm that somatic mosaicism for a PHOX2B mutation may be detectable in one of the parents of all cases in which recurrence in siblings has occurred (Haddad et al. 1978; Hamilton and Bodurtha 1989; Kerbl et al. 1996).
Heterozygous PHOX2B mutations were identified in five of the nine patients with LO-CHS in our series. Some of these patients are therefore at risk to transmit either CCHS or LO-CHS to their progeny. It is thus important to study the PHOX2B gene in all patients with LO-CHS, so as to provide accurate genetic counseling for these families, even though whether these patients harbor a germline mutation or a somatic mosaicism remains a question. It is interesting to note that, in contrast to the manifestations of CCHS in humans, the ventilatory phenotype observed in Phox2b+/− mice (i.e., impaired ventilatory response to inhaled CO2) is transitory and also less severe than in patients with CCHS, with the mice showing normal spontaneous breathing (Dauger et al. 2003). Such discrepant observations between human and mice argue against haploinsufficiency as the disease-causing mechanism responsible for the alanine-expansion mutations observed in humans. Indeed, there is growing evidence for a common disease-causing mechanism resulting from heterozygous polyalanine expansions—namely, a dominant negative effect due to cytoplasmic aggregation of both mutant and wild-type proteins (Albrecht et al. 2004; Amiel et al. 2004).
Small alanine expansions (range of +5 to +7 alanines) are, by far, the most frequent mutations identified in our series (fig. 1) and in other series reported to date (Sasaki et al. 2003; Weese-Mayer et al. 2003; Matera et al. 2004). Interestingly, the distribution of alanine expansions differs greatly among the patient subgroups (fig. 1). Indeed, the smallest alanine expansion was the only one identified in patients with LO-CHS (5 cases), whereas it was never found in patients with CCHS + HSCR (45 patients). Although patients with CCHS + HSCR tend to harbor a longer alanine expansion, in comparison with that of patients with isolated CCHS (fig. 1), one cannot predict the accurate phenotype from the genotype, since the +7-alanine mutation is the most frequent expansion in both the group of patients with isolated CCHS and the group with CCHS + HSCR.
A high predisposition to TSNS has been long recognized in patients with CCHS (Rohrer et al. 2002). The incidence of TSNS is indeed 500-fold greater in these patients than in the general population (5%–10% vs. 1/10,000 in the general population [Rohrer et al. 2002]). Moreover, when present, the tumors tend to be multifocal in patients with CCHS. We propose that such tumor predisposition may be a direct consequence of the PHOX2B germline heterozygous mutation. Indeed, such mutations have been identified in both familial and sporadic cases of neuroblastoma with no CCHS phenotype (Mosse et al. 2004; Trochet et al. 2004). Interestingly, frameshift and missense PHOX2B mutations may predispose to neuroblastoma, as opposed to mutations leading to polyalanine expansions (table 2). Indeed, an invasive tumor (neuroblastoma) was diagnosed in 6 (46%) of 13 patients harboring either a missense or a frameshift mutation, whereas a differentiated tumor (ganglioneuroma) was diagnosed in only 2 (1.24%) of 161 patients harboring an in-frame duplication leading to an expansion of +9 alanines in one and +13 in the other. Interestingly, the latter two patients did not have HSCR. Similar findings in 67 patients were reported by Weese-Mayer et al. (2003), whereby a PHOX2B frameshift mutation (K155X) was found once and in the only patient with CCHS with neuroblastoma. Matera et al. (2004) also reported three frameshift mutations in a series of 27 cases, although the presence of TSNS is not mentioned in this series. Finally, in the series of 10 patients with CCHS reported by Sasaki et al. (2003), a PHOX2B frameshift mutation was found once, in a patient with Haddad syndrome. In this case, there was no mention of TSNS up to the age of 4 mo (Sasaki et al. 2003). Thus, PHOX2B molecular testing points to a subset of patients with CCHS who are at very high risk for developing tumors and therefore justify a very careful clinical follow-up, at least in the first 2 years of life. Finally, no loss of heterozygosity at the PHOX2B locus was observed in the tumors of patients with a PHOX2B gene mutation, whenever it could be tested (Mosse et al. 2004; Trochet et al. 2004). Although we showed that the mutant 931del5 allele was transcriptionally stable, it remains an open question as to whether frameshift mutations result in stable and active proteins (Trochet et al. 2004).
In summary, our findings in this extensive series of patients with CCHS and their families support a major role for PHOX2B in the generation of normal respiratory patterning and in the normal development of the ANS in humans. Although modifier genes in both LO-CHS and Haddad syndrome are likely, PHOX2B is the major disease-causing gene in CCHS and is a predisposing gene to neuroblastoma whenever the mutation does not result in a polyalanine expansion.
Acknowledgments
We are thankful to the patients and families who participated in the study; to the French, U.S., and German parent support groups of CCHS, for their support; to I. Ceccherini, for sharing her PCR protocol of the PHOX2B gene before publication; to the ARC; to INSERM; and to the Children’s Foundation Research Endowment and Kosair Charities.
Electronic-Database Information
The URL for data presented herein is as follows:
- Online Mendelian Inheritance in Man (OMIM), http://www.ncbi.nlm.nih.gov/Omim/ (for CCHS, Ondine curse, Hirschsprung disease, Haddad syndrome, PHOX2B, and neuroblastoma)
References
- Albrecht AN, Kornak U, Boddrich A, Suring K, Robinson PN, Stiege AC, Lurz R, Stricker S, Wanker EE, Mundlos S (2004) A molecular pathogenesis for transcription factor associated polyalanine tract expansions. Hum Mol Genet 13:2351–2359 10.1093/hmg/ddh277 [DOI] [PubMed] [Google Scholar]
- Amiel J, Laudier B, Attie-Bitach T, Trang H, de Pontual L, Gener B, Trochet D, Etchevers H, Ray P, Simonneau M, Vekemans M, Munnich A, Gaultier C, Lyonnet S (2003) Polyalanine expansion and frameshift mutations of the paired-like homeobox gene PHOX2B in congenital central hypoventilation syndrome. Nat Genet 33:459–461 10.1038/ng1130 [DOI] [PubMed] [Google Scholar]
- Amiel J, Trochet D, Clement-Ziza M, Munnich A, Lyonnet S (2004) Polyalanine expansions in human. Hum Mol Genet 13:R235–R243 10.1093/hmg/ddh251 [DOI] [PubMed] [Google Scholar]
- Brunet JF, Pattyn A (2002) Phox2 genes—from patterning to connectivity. Curr Opin Genet Dev 12:435–440 10.1016/S0959-437X(02)00322-2 [DOI] [PubMed] [Google Scholar]
- Croaker GD, Shi E, Simpson E, Cartmill T, Cass DT (1998) Congenital central hypoventilation syndrome and Hirschsprung’s disease. Arch Dis Child 78:316–322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dauger S, Pattyn A, Lofaso F, Gaultier C, Goridis C, Gallego J, Brunet JF (2003) Phox2b controls the development of peripheral chemoreceptors and afferent visceral pathways. Development 130:6635–6642 10.1242/dev.00866 [DOI] [PubMed] [Google Scholar]
- Gozal D (1998) Congenital central hypoventilation syndrome: an update. Pediatr Pulmonol 26:273–282 [DOI] [PubMed] [Google Scholar]
- Haddad GG, Mazza NM, Defendini R, Blanc WA, Driscoll JM, Epstein MA, Epstein RA, Mellins RB (1978) Congenital failure of automatic control of ventilation, gastrointestinal motility and heart rate. Medicine (Baltimore) 57:517–526 [DOI] [PubMed] [Google Scholar]
- Hamilton J, Bodurtha JN (1989) Congenital central hypoventilation syndrome and Hirschsprung’s disease in half sibs. J Med Genet 26:272–274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz ES, McGrath S, Marcus CL (2000) Late-onset central hypoventilation with hypothalamic dysfunction: a distinct clinical syndrome. Pediatr Pulmonol 29:62–68 [DOI] [PubMed] [Google Scholar]
- Kerbl R, Litscher H, Grubbauer HM, Reiterer F, Zobel G, Trop M, Urlesberger B, Eber E, Kurz R (1996) Congenital central hypoventilation syndrome (Ondine’s curse syndrome) in two siblings: delayed diagnosis and successful noninvasive treatment. Eur J Pediatr 155:977–980 [DOI] [PubMed] [Google Scholar]
- Matera I, Bachetti T, Puppo F, Di Duca M, Morandi F, Casiraghi GM, Cilio MR, Hennekam R, Hofstra R, Schober JG, Ravazzolo R, Ottonello G, Ceccherini I (2004) PHOX2B mutations and polyalanine expansions correlate with the severity of the respiratory phenotype and associated symptoms in both congenital and late onset central hypoventilation syndrome. J Med Genet 41:373–380 10.1136/jmg.2003.015412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosse YP, Laudenslager M, Khazi D, Carlisle AJ, Winter CL, Rappaport E, Maris JM (2004) Germline PHOX2B mutation in hereditary neuroblastoma. Am J Hum Genet 75:727–730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pattyn A, Morin X, Cremer H, Goridis C, Brunet JF (1999) The homeobox gene Phox2b is essential for the development of autonomic neural crest derivatives. Nature 399:366–370 10.1038/20700 [DOI] [PubMed] [Google Scholar]
- Rohrer T, Trachsel D, Engelcke G, Hammer J (2002) Congenital central hypoventilation syndrome associated with Hirschsprung’s disease and neuroblastoma: case of multiple neurocristopathies. Pediatr Pulmonol 33:71–76 10.1002/ppul.10031 [DOI] [PubMed] [Google Scholar]
- Samad OA, Geisen MJ, Caronia G, Varlet I, Zappavigna V, Ericson J, Goridis C, Rijli FM (2004) Integration of anteroposterior and dorsoventral regulation of Phox2b transcription in cranial motoneuron progenitors by homeodomain proteins. Development 131:4071–4083 10.1242/dev.01282 [DOI] [PubMed] [Google Scholar]
- Sasaki A, Kanai M, Kijima K, Akaba K, Hashimoto M, Hasegawa H, Otaki S, Koizumi T, Kusuda S, Ogawa Y, Tuchiya K, Yamamoto W, Nakamura T, Hayasaka K (2003) Molecular analysis of congenital central hypoventilation syndrome. Hum Genet 114:22–26 10.1007/s00439-003-1036-z [DOI] [PubMed] [Google Scholar]
- Trochet D, Bourdeaut F, Janoueix-Lerosey I, Deville A, de Pontual L, Schleiermacher G, Coze C, Philip N, Frebourg T, Munnich A, Lyonnet S, Delattre O, Amiel J (2004) Germline mutations of the paired-like homeobox 2B (PHOX2B) gene in neuroblastoma. Am J Hum Genet 74:761–764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderlaan M, Holbrook CR, Wang M, Tuell A, Gozal D (2004) Epidemiologic survey of 196 patients with congenital central hypoventilation syndrome. Pediatr Pulmonol 37:217–229 10.1002/ppul.10438 [DOI] [PubMed] [Google Scholar]
- Warren ST (1997) Polyalanine expansion in synpolydactyly might result from unequal crossing-over of HOXD13. Science 275:408–409 10.1126/science.275.5298.408 [DOI] [PubMed] [Google Scholar]
- Weese-Mayer DE, Berry-Kravis EM, Zhou L, Maher BS, Silvestri JM, Curran ME, Marazita ML (2003) Idiopathic congenital central hypoventilation syndrome: analysis of genes pertinent to early autonomic nervous system embryologic development and identification of mutations in PHOX2b. Am J Med Genet 123:267–278 10.1002/ajmg.a.20527 [DOI] [PubMed] [Google Scholar]
- Weese-Mayer DE, Shannon DC, Keens TG, Silvestri JM (1999) Idiopathic congenital central hypoventilation syndrome: diagnosis and management. Am J Respir Crit Care Med 160:368–373 [DOI] [PubMed] [Google Scholar]
- Yokoyama M, Watanabe H, Nakamura M (1999) Genomic structure and functional characterization of NBPhox (PMX2B), a homeodomain protein specific to catecholaminergic cells that is involved in second messenger-mediated transcriptional activation. Genomics 59:40–50 10.1006/geno.1999.5845 [DOI] [PubMed] [Google Scholar]