Abstract
Bardet-Biedl syndrome (BBS) is a multisystemic disorder characterized by postaxial polydactyly, progressive retinal dystrophy, obesity, hypogonadism, renal dysfunction, and learning difficulty. Other manifestations include diabetes mellitus, heart disease, hepatic fibrosis, and neurological features. The condition is genetically heterogeneous, and eight genes (BBS1–BBS8) have been identified to date. A mutation of the BBS1 gene on chromosome 11q13 is observed in 30%–40% of BBS cases. In addition, a complex triallelic inheritance has been established in this disorder—that is, in some families, three mutations at two BBS loci are necessary for the disease to be expressed. The clinical features of BBS that can be observed at birth are polydactyly, kidney anomaly, hepatic fibrosis, and genital and heart malformations. Interestingly, polydactyly, cystic kidneys, and liver anomalies (hepatic fibrosis with bile-duct proliferation) are also observed in Meckel syndrome, along with occipital encephalocele. Therefore, we decided to sequence the eight BBS genes in a series of 13 antenatal cases presenting with cystic kidneys and polydactyly and/or hepatic fibrosis but no encephalocele. These fetuses were mostly diagnosed as having Meckel or “Meckel-like” syndrome. In six cases, we identified a recessive mutation in a BBS gene (three in BBS2, two in BBS4, and one in BBS6). We found a heterozygous BBS6 mutation in three additional cases. No BBS1, BBS3, BBS5, BBS7, or BBS8 mutations were identified in our series. These results suggest that the antenatal presentation of BBS may mimic Meckel syndrome.
Introduction
Bardet-Biedl syndrome (BBS [MIM 209900]) is a multisystemic genetic disorder characterized by postaxial polydactyly, progressive retinal dystrophy, obesity, hypogonadism, learning difficulty, and renal dysfunction. Other manifestations include diabetes mellitus, neurological impairments (mainly ataxia), heart disease, dental malformations, and hepatic fibrosis. This condition is genetically heterogeneous, and six genes (BBS1–BBS6) were identified by genetic linkage studies (Katsanis et al. 2000; Slavotinek et al. 2000; Mykytyn et al. 2001, 2002; Nishimura et al. 2001; Chiang et al. 2004; Fan et al. 2004; Li et al. 2004). Two more genes (BBS7 [Badano et al. 2003a] and BBS8 [Ansley et al. 2003]) have been identified on the basis of their homology to previously identified BBS genes. The major locus, BBS1, is on chromosome 11q13. It is responsible for 30%–40% of BBS cases (Beales et al. 2001). In addition to genetic heterogeneity, a complex mode of inheritance called “triallelism” has been established for this disorder, since, at least in some families, three mutations at two BBS loci are necessary for the condition to be expressed (Katsanis et al. 2001, 2002).
Because of the late onset of symptoms, the diagnosis of BBS is usually made during childhood. For example, obesity appears around age 2–3 years, and retinal degeneration becomes clinically apparent only at age 8 years (Beales et al. 1999). The only features that may be present at birth are polydactyly, kidney anomaly, hepatic fibrosis, and genital or heart malformations. Interestingly, polydactyly and cystic kidneys are two malformations—along with occipital encephalocele—that characterize Meckel syndrome (MKS) (Mecke and Passarge 1971), a fetal-lethal autosomic recessive condition. Liver anomalies (hepatic fibrosis and bile-duct proliferation) are constant in MKS (Salonen 1984). On the basis of this phenotypic overlap between the two syndromes and the absence of major signs of BBS in the perinatal period, we hypothesized that fetuses presenting with cystic kidneys, polydactyly, and/or hepatic fibrosis but without encephalocele could be either misdiagnosed as MKS or referred to as “Meckel-like.” Therefore, we decided to sequence the eight known BBS genes (BBS1–BBS8) in a series of 13 antenatal cases presenting with kidney anomaly, polydactyly, and/or hepatic fibrosis but not encephalocele. We identified a recessive mutation in a BBS gene in six cases and observed a heterozygous mutation in BBS6 in three additional cases (fig. 1). In the present study, we describe the antenatal phenotype of patients with BBS and discuss the overlap with the clinical spectrum of MKS.
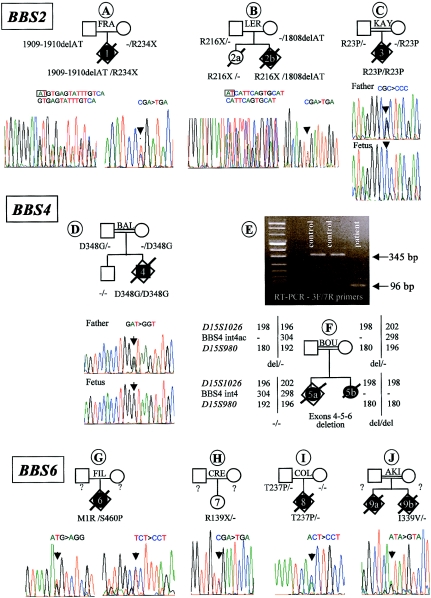
Figure 1.
Results of the BBS2, BBS4, and BBS6 mutation screening. The pedigrees and mutations are indicated. The black symbols indicate the affected cases. Below each pedigree, sequence chromatographs are shown. In family BOU, the results of the RT-PCR study confirming the deletion of three BBS4 exons in proband 5b (E) and the results of haplotype analysis at the BBS4 locus (F) are shown.
Material and Methods
Patients
A total of 13 patients, presenting with the association of kidney anomaly, polydactyly, and/or hepatic fibrosis, diagnosed prenatally, were included in the study. In 11 cases, pregnancy was terminated because of either severe renal dysfunction (oligohydramnios) or brain anomaly (corpus callosum agenesis/hypoplasia or Dandy-Walker malformation [DWM]), in accordance with French legislation. In the two postnatal cases (1 and 13b), the parents declined pregnancy termination, after genetic counseling. Chromosome analyses and clinicopathological examinations were performed in 11 cases after parental consent was obtained. Clinical and histological features are summarized in table 1.
Table 1.
Clinical and Pathological Findings Observed in Patients
|
Finding in Patient (Family)a |
|||||||||||||||||
| Trait | 1 (FRA) | 2a (LER) | 2b (LER) | 3 (KAY) | 4 (BAL) | 5a (BOU) | 5b (BOU) | 6 (FIL) | 7 (CRE) | 8 (COL) | 9a (AKI) | 9b (AKI) | 10 (STA) | 11 (AND) | 12 (KAL) | 13a (MOU) | 13b (MOU) |
| Consanguinity | − | − | − | + | + | + | + | − | − | − | + | + | − | − | + | − | − |
| National origin | France | France | France | Turkey | Turkey | Tunisia | Tunisia | France | France | France | Turkey | Turkey | France | France | Algeria | France | France |
| Ageb | 28 wk | 2 d | 22 wk | 26 wk | 26 wk | 26 wk | 12 d | 24 wk | 12 years | 32 wk | 32 wk | ? | 27 wk | 37 wk | 25 wk | 29 wk | 18 wk |
| Brain: | |||||||||||||||||
| Anomalyc | − | − | + | − | + | + | ? | − | − | − | + | ? | − | + | + | + | + |
| Supratentorial | VD | CCA | CCH, Arh | ||||||||||||||
| Infratentorial | Nect | DWM | ME | DWM | DWM | ME, DWM | OD,DWM | ||||||||||
| Hand polydactyly | −/− | −/− | +/− | −/− | +/+ | −/− | −/− | +/+ | +/+ | −/− | −/− | −/− | +/+ | −/− | +/+ | +/+ | −/− |
| Foot polydactyly | +/− | −/− | +/+ | +/+ | +/+ | −/− | +/− | +/+ | +/− | +/+ | −/− | −/− | +/− | −/− | +/+ | −/− | −/− |
| Kidney anomalyc | + | Enlargedd | + | + | + | + | + | + | + | Enlarged | + | + | + | + | + | + | + |
| Pathology | MC | NA | MKL | MKL | MC | MKL | NA | MKS | Failure | Normal | MC | NA | MC | MC | MKL | MC | MC |
| Portal fibrosis | − | NA | + | + | − | + | NA | − | NA | − | + | NA | − | + | − | − | − |
| BDP | − | NA | − | − | − | + | NA | − | NA | − | + | NA | − | − | − | − | +/− |
| Heart defect | − | − | − | − | − | − | + | − | − | − | + | NA | − | − | |||
| Other defects | − | − | + | − | − | + | NA | − | + | + | − | NA | − | − | |||
| Initial diagnosis | BBS | ? | MKS | MKL | MKL | MKS | MKS | MKL | BBS | BBS | MKS var | MKS var | BBS | Ago/Gold | MKS | Ago,MKS var | Ago,MKS var |
| BBS mutation(s) | 2 Htz BBS2 +1 BBS4 | 1 HtzBBS2 | 2 HtzBBS2 | 1 HmzBBS2 | 1 HmzBBS4 | NoneBBS4 | 1 HmzBBS4 | 2 HtzBBS6 | 1 HtzBBS6 | 1 HtzBBS6 | NoneBBS6 | 1 HtzBBS6 | |||||
+ = present; − = absent; ? = unknown; NA = not available; Ago = Agostino syndrome; Arh = arhinencephaly; BDP = bile-duct proliferation; CCA = corpus callosum agenesis; CCH = corpus callosum hypoplasia; Gold = Goldston syndrome; Htz = heterozygous mutation; Hmz = homozygous mutation; ME = meningocele; MC = medullary cysts; MKL = Meckel-like; Nect = neuronal ectopias; OD = occipital defect; var = variant; VD = ventricular dilatation.
Wk indicates weeks of gestation.
Detected on ultrasound examination.
Enlarged left kidney.
Mutation Screening of BBS Genes
Genomic DNA was extracted from frozen fetal tissue in 11 cases and from peripheral blood samples in 2 postnatal cases, by use of standard procedures. Primers were designed using introns flanking the coding exons of the eight BBS genes and are available on request. Direct sequencing of both strands was performed using the Big Dye Terminator Cycle Sequencing kit (Applied Biosystems) and was analyzed on an ABI 3100 automated sequencer (Applied Biosystems).
Results
BBS2
Mutations were identified in three fetuses. Case 1 (in family FRA) was a 28-wk-old fetus presenting with enlarged hyperechogenic kidneys and unilateral foot polydactyly. After pregnancy termination, neuropathological study disclosed moderate cerebral ventricular dilatation with neuronal ectopias. Histological study of the kidneys showed preserved corticomedullar differentiation but the presence of multiple medullary cysts (fig. 2E and 2F). The liver was normal (fig. 2G and 2H). We identified two heterozygous truncating BBS2 mutations: a 2-bp deletion in exon 15 (1909–1910delAT), resulting in a frameshift mutation (M637fsX648), which was inherited from the father, and a C→T transversion in exon 6, resulting in a nonsense mutation at codon 234 (R234X) (fig. 1A), which was inherited from the mother. In this fetus, a heterozygous 4-bp deletion was also detected in BBS4 intron 7, potentially removing the lariat branch site. This mutation was inherited from the asymptomatic father. RNA was extracted from a blood sample, but RT-PCR failed to find an abnormal supernumerary transcript. This case is the only one of our series in which three BBS mutated alleles have been identified.
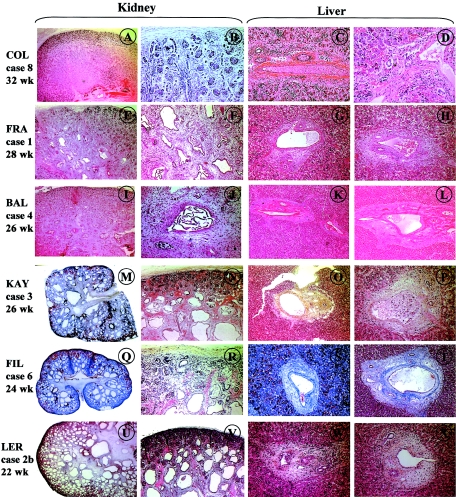
Figure 2.
Histological sections (hematoxylin/eosin) of kidneys and livers of fetuses carrying BBS mutations. Case 8 shows normal kidney histology. Cases 1 and 4 show medullary cysts, whereas cases 3, 6, and 2b show kidneys “Meckel-like” lesions. The liver shows moderate portal fibrosis in cases 3 and 2b.
Case 2b (in family LER) was a BBS2 compound heterozygote, 22-wk-old fetus (fig. 1B). He carried a paternally inherited nonsense mutation in exon 6 (R216X) and a 2-bp deletion in exon 15 (1808delAT), resulting in a frameshift mutation (Y603fsX612), which he inherited from his mother (fig. 1B). The pregnancy was terminated because of the presence, on ultrasound examination, of cystic kidneys associated with unilateral upper-limb and bilateral feet postaxial polydactyly. This case was first diagnosed as MKS, on the basis of kidney macroscopic and histological features showing severe disorganization of the renal parenchymal architecture, involving cortical and medullary layers (fig. 2U and 2V). However, the liver showed mild portal fibrosis but no bile-duct proliferation (fig. 2W and 2X). In this family, a previous child (case 2a) had died with hydrops at age 2 d (fig. 1B), and no autopsy was performed. She did not have polydactyly or brain anomaly, and the left kidney was slightly larger than normal on ultrasound examination. DNA analysis showed that she was heterozygous for the paternal R216X mutation.
Case 3 (in family KAY) was a 26-wk-old fetus of Turkish consanguineous descent. The pregnancy was terminated because the fetus presented with enlarged cystic kidneys, oligoamnios, and bilateral foot polydactyly. Histological examination of the liver showed mild portal fibrosis with no bile-duct proliferation (fig. 2O and 2P). The corticomedullary architecture of the enlarged kidneys was severely affected by numerous irregular cysts, lined with a single cell layer. These cysts involved the entire renal parenchyma, with volume enlargement toward the medulla. Only one to two ranges of immature glomeruli were observed in the cortical nephrogenic zone (fig. 2M and 2N). There were no other visceral malformations, and examination of the CNS was unremarkable. In this case, a homozygous G→C transversion in exon 1 of the BBS2 gene, leading to the substitution of an arginine with a proline at codon 23 (R23P), was identified. Both parents were heterozygous for this mutation (fig. 1C). This missense mutation concerned a conserved amino acid and was not detected in 100 control chromosomes.
BBS4
Mutations were identified in two fetuses. Case 4 (in family BAL) was a 26-wk-old fetus presenting with quadrilateral postaxial polydactyly and bilateral enlarged kidneys with cysts located in the deep cortex and the renal medulla (fig. 2I and 2J). The liver was normal (fig. 2K and 2L). Corpus callosum agenesis was observed on ultrasound examination and was confirmed at autopsy. No other malformation was observed. Sequence analysis of BBS4 revealed a homozygous A→G transition in exon 13, resulting in a missense mutation (D348G) (fig. 1D). This mutation was inherited from consanguineous Turkish parents who were heterozygous for this mutation. A healthy brother did not carry this mutation and had inherited both wild-type alleles. This missense mutation involved a conserved amino acid and was not detected in 100 control chromosomes.
Case 5b (the proband in family BOU) was a girl who died at age 12 d. She had unilateral foot polydactyly, cystic kidneys, and endocardial cushion defects. No brain anomaly was apparent. Autopsy was refused. The absence of amplification of BBS4 exons 4, 5, and 6 led to the suspicion of a homozygous deletion of these exons. The deletion was confirmed by RT-PCR analysis of RNA extracted from lymphoblastoid cells, by use of primers located in exons 3 and 7. The expected wild-type fragment was 345 bp in length, whereas the 96-bp amplification product (fig. 1E) observed in proband 5b corresponded to a lack of three exons, as confirmed by sequencing (data not shown). Haplotyping was performed using two flanking markers and one intragenic marker located in intron 4 of the BBS4 gene. The absence of amplification of the intragenic marker in proband 5b and the hemizygosity observed in the parents are in accordance with both of the consanguineous parents being heterozygous for this deletion (fig. 1F). Interestingly, in this family, an earlier fetus (5a) presented with occipital meningocele, cystic kidneys, hepatic portal fibrosis, and bile-duct proliferation, a presentation considered characteristic of MKS. DNA was extracted from paraffin blocks, and haplotyping at the BBS4 locus showed that sib 5a had a different haplotype from the proband and did not carry the BBS4 deletion (fig. 1F).
BBS6
Mutations were identified in four fetuses. Case 6 (in family FIL) was a 24-wk-old fetus. The pregnancy was terminated after detection, on ultrasound examination, of enlarged and cystic kidneys, anamnios, and quadrilateral postaxial hexadactyly. Autopsy confirmed the absence of other malformations. Microscopic examination of the liver was normal (fig. 2S and 2T), but the kidneys showed histopathological changes reminiscent of MKS, with both cortical and medullar cystic formations. These cysts were larger in the medulla than in the cortex and were lined with a thin cuboidal epithelium. A thin cortical glomerular layer was present (fig. 2Q and 2R). Sequencing of the BBS genes revealed that this fetus was a BBS6 compound heterozygote—the first missense mutation resulted in the substitution of the methionine initiator codon with an arginine (M1R), and the second change was a missense mutation in exon 6, resulting in the substitution of a serine with a proline at codon 460 (S460P) (fig. 1G). Unfortunately, DNA of the parents was not available to establish the inheritance of these mutations.
Case 7 (in family CRE) was a 12-year-old girl presenting with BBS. Enlarged kidneys, bilateral hand polydactyly, and left-foot polydactyly were detected antenatally. After a genetic-counseling discussion about the risk of MKS, the parents declined pregnancy termination. After birth, the size of the kidneys decreased to normal, whereas progressive renal failure appeared. Obesity started at age 3 years, and an electroretinogram examination established the diagnosis of BBS. At age 12 years, vision was normal. We found one heterozygous BBS6 C→G transversion in exon 3 of the BBS6 gene, resulting in a nonsense mutation (R139X) (fig. 1H). No other BBS mutations were identified in this patient.
In case 8 (in family COL), pregnancy was terminated at 32 wk of gestation because of enlarged kidneys and bilateral foot polydactyly detected on ultrasound examination. Autopsy and histological examination showed no other malformations. The CNS, liver, and kidneys were unremarkable (fig. 2A–2D). The diagnosis of BBS was suggested. We identified one heterozygous missense mutation in exon 3 of BBS6, resulting in the substitution of a threonine with a proline at codon 237 (T237P). This mutation was inherited from the father (fig. 1I) and was not observed in >100 control chromosomes. We failed to find any other change in the coding sequence of BBS6 or the other BBS genes in this fetus.
In family 9 (AKI), a fetus (case 9a) presented with cystic kidneys and heart defect. Corpus callosum hypoplasia was detected on ultrasound examination, and the parents elected to terminate the pregnancy at 32 wk of gestation. Brain examination showed absence of olfactory bulbs. Microscopical examination of the liver showed portal fibrosis and focal bile-duct proliferation and dilatation in some large portal areas. In the kidneys, medullary microcysts were noted. During the pregnancy that followed (proband 9b), abnormal kidneys were detected on ultrasound examination, and the pregnancy was terminated. The parents declined autopsy but agreed to molecular analysis of a blood sample. A heterozygous BBS6 A→G transition was identified, resulting in the substitution of an isoleucine with a valine at codon 339 (I339V) (fig. 1J). This change was not observed in 100 control chromosomes and involved a conserved amino acid. No other BBS6 coding-sequence mutations could be found in the fetus. Paraffin blocks were obtained from sib 9a, and DNA was extracted for molecular analysis, but we failed to find the same mutation.
No mutation was identified in the coding sequences of the BBS1, BBS3, BBS5, BBS7, and BBS8 genes. Polymorphic changes observed in the BBS genes are summarized in table 2.
Table 2.
Polymorphisms and Variants Observed in BBS Genes
| Gene andNucleotide Variation | Protein Change |
| BBS1: | |
| G379A | L126L |
| IVS6+55 C→T | |
| C684T | L228L |
| IVS8−8 G→C | |
| C1413T | L471L |
| IVS17+7 G→A | |
| BBS2: | |
| A367G | I123V |
| IVS5−54 G→C | |
| IVS6−34 C→T | |
| A1413C | V471V |
| BBS3: | |
| IVS4−18 T→C | |
| IVS5−49 A→G | |
| IVS8+75 A→G | |
| IVS8+80 A→G | |
| IVS8+82–86 del5 | |
| BBS4: | |
| IVS1−17 C→T | |
| IVS1−38 C→A | |
| IVS2+19 G→T | |
| IVS2−6 A→G | |
| A137G | K46R |
| A180G | Q60Q |
| IVS6+7 C→T | |
| IVS7+23 G→C | |
| IVS10−17 G→C | |
| C1061T | T354I |
| BBS5: | |
| IVS1−40 G→C | |
| BBS6: | |
| C117T | P39P |
| C534T | I178I |
| IVS3+17 A→C | |
| IVS3+34 C→G | |
| G1595T | G532V |
| C1549T | R517C |
| BBS7: | |
| −133 C→G | |
| IVS3−45 C→T | |
| IVS9+32 A→G | |
| IVS9+32–34del4 | |
| IVS14+24 C→A | |
| IVS17+16 G→A | |
| IVS17−12 C→A | |
| BBS8: | |
| IVS3+18 A→G | |
| IVS3+48 T→C | |
| IVS6+67 A→G | |
| IVS14−12 C→G |
Discussion
We sequenced the eight known BBS genes in 13 patients presenting prenatally with a kidney anomaly associated with polydactyly and/or hepatic fibrosis but with no encephalocele. Most of these cases were considered to be MKS or “Meckel-like” syndromes, on the basis of the presence of a CNS anomaly (cases 2b, 4, 9, 11, 12, and 13), kidney histology (cases 3 and 6), or MKS in the same family (case 5). We identified recessive mutations in BBS genes in six cases—BBS2 in cases 1, 2b, and 3; BBS4 in cases 4 and 5b; and BBS6 in case 6—and identified a BBS6 heterozygous mutation in cases 7, 8, and 9b. Since mutations in one of the eight known BBS genes are found in only 40% of BBS cases (Katsanis 2004), the diagnosis of BBS is not excluded in the remaining cases of our series.
Two mutations identified in our series have been reported elsewhere in patients with BBS. As in case 9b, the I339V BBS6 mutation was reported in the heterozygous state in a patient with BBS in whom no other BBS gene mutations were identified (Slavotinek et al. 2002). Although this change may be a rare variant, neither Slavotinek et al. (2002) nor we found it in 100 controls, and it is not listed as a polymorphism in human SNP databases (see dbSNP and Ensembl Web sites). The R216X BBS2 mutation identified in case 2b was reported in a BBS case carrying, in addition, a BBS2 frameshift mutation and a BBS6 missense mutation (Katsanis et al. 2001). This case presented a typical BBS phenotype with renal involvement.
No mutation in the BBS1 gene, the major gene responsible for 30%–40% of postnatal BBS cases, was identified in our series. However, a single M390R mutation with a founder effect from Northern Europe accounts for 80% of cases with BBS1 mutations (Beales et al. 2003; Mykytyn et al. 2003), and none of our cases was of North European extraction. In agreement with previous studies, a high rate of heterozygous BBS6 mutations was observed (cases 7, 8, and 9b), and we failed to find any other BBS gene mutations in the three cases with a heterozygous BBS6 mutation. These alleles might correspond to a “third allele,” and further molecular analysis will be necessary to establish whether these cases carry a recessive mutation at another as-yet-unidentified BBS gene. In case 1, two BBS2 mutations were identified, and, in addition, a 4-bp deletion was identified in BBS4 intron 7, potentially located in the lariat branch site. As mentioned above, this is the only case in our series in which three BBS mutations were identified.
In the present study, polydactyly and cystic kidneys were the only features observed in seven fetuses on prenatal ultrasound examination (cases 1, 3, 5b, 6, 7, 8, and 10). In one of them (case 3), mild liver portal fibrosis without bile-duct proliferation was found on histological examination. A BBS mutation was identified in 6/7 of these cases. In two of them (cases 3 and 6), the kidney histopathological changes were reminiscent of MKS, and, interestingly, the occurrence of such severe cystic kidneys in a sib with BBS has been reported elsewhere (Gershoni-Baruch et al. 1992). The association of polydactyly and cystic kidneys is not reported as a single entity in OMIM but is observed in numerous syndromes, such as BBS, MKS, and Pallister-Hall syndrome (table 3). In addition, these features have been reported in patients with Joubert, Jeune, Smith-Lemli-Opitz, oro-facio-digital I (OFDI), and Simpson-Golabi syndromes. However, in all these syndromes, other clinical features can be detected antenatally. Cassart et al. (2004) already suggested that BBS was a possible diagnosis for cases in which polydactyly and enlarged kidneys were observed antenatally. We demonstrate that 6/7 of cases presenting this association, with or without liver portal fibrosis but with no other findings, are cases of BBS. In the absence of polydactyly, other congenital hepatorenal fibrocystic syndromes can be discussed (Johnson et al. 2003).
Table 3.
Syndromes Associated with or Occasionally Reported with Polydactyly, Cystic Kidney Dysplasia, and/or Brain Anomalies
|
Clinical Findingsa |
|||||||||||
| Brain |
|||||||||||
| Syndrome | MIM | PD | CKD | DWM/VA | CCA/CCH | Other | Liver | Heart | Genital | Cleft | Other |
| MKS | 249000 | + | + | DWM | + | Occipital encephalocele | HF+BDP | + | + | + | Pancreas cysts |
| BBS | 209900 | + | + | DWM | Normal | HF+BDP | + | + | Obesity, diabetes, retinal dysplasia | ||
| Pallister-Hall | 146510 | + | + | − | + | Hamartoma | + | + | Imperforate anus, short limbs | ||
| Joubert | 213300 | + | + | DWM/VA | Occipital meningocele | HF | Coloboma,b retinal dystrophy, abnormal eye movements, tachypnea | ||||
| Jeune | 208500 | + | + | − | − | − | HF+BDP | Short stature and ribs, retinal degeneration, pancreas | |||
| SLO | 270400 | + | + | DWM | − | Hydrocephaly, heterotopia | + | + | + | IUGR, microcephaly | |
| CVAc | 213010 | + | + | VA | − | Occipital encephalocele | HF+BDP | Coloboma | |||
| OFDI | 311200 | + | + | − | + | Hamartoma, hydrocephaly, porencephaly | + | Syn-clino-brachy-dactyly, tongue anomalies, alopecia | |||
| Simpson-Golabi | 312870 | + | + | VA | + | Hydrocephaly | + | + | + | Pancreas and somatic overgrowth, macrocephaly, macroglossia | |
| Miller-Diecker | 247200 | + | + | − | + | Lissencephaly, microcephaly | + | + | IUGR | ||
| DWM | 220200 | − | − | DWM | − | − | |||||
| DWM with PD | 220220 | + | − | DWM | − | ||||||
| Goldston (1963)d | − | + | DWM | − | − | ||||||
| Goldston | 267010 | − | + | DWM | + | Pancreas | |||||
| Scalp defects and PD | 181250 | + | +/− | DWM | Occiptial defect | Autosomic dominant | |||||
| Mohr/OFDII | 252100 | + | +/− | DWM | Cerebellar defect | + | Lingual malformation, supernumerary sutures in skull, hearing loss, tachypnea | ||||
| EVC | 225500 | + | +/− | DWM | Normal | + | Short limbs, ribs, nails, teeth | ||||
| CDG | 212065 | − | + | DWM | Cerebellar defect | + | − | ||||
| 3C | 220210 | − | DWM | + | + | Coloboma | |||||
| COACH | 216360 | − | + | VA | Occipital encephalocele | Coloboma, congenital ataxia | |||||
| Hydrolethalus | 236680 | + | − | DWM | + | Hydrocephaly | − | + | + | − | |
+ = present; +/− = occasionally present; − = absent; PD = polydactyly; CKD = cystic kidney dysplasia; VA = vermis agenesis; CCA/CCH = corpus callosum agenesis or hypoplasia; HF= hepatic fibrosis; BDP= bile-duct proliferation.
See MIM 243910.
Cerebellar vermis aplasia with associated features suggesting SLO and MKS.
To our knowledge, corpus callosum agenesis has never been reported in patients with BBS. In the present study, a homozygous BBS4 mutation was found in one patient (case 4) with corpus callosum agenesis associated with polydactyly and cystic kidneys. These data suggest that corpus callosum agenesis might be associated with the antenatal presentation of BBS. Interestingly, hypoplasia of the corpus callosum was also present in patient 9a, in addition to cystic kidneys and a heart defect. Portal fibrosis and focal bile-duct proliferation and dilatation in some large portal areas were noted on histological examination. The pregnancy that followed (case 9b) was terminated for cystic kidneys, and the fetus was found to carry a heterozygous I339V BBS6 mutation, previously identified in a patient with BBS (Slavotinek et al. 2002). However, analysis of DNA from paraffin blocks of fetus 9a failed to find the same BBS6 mutation. Either this change is a rare variant or this “third” BBS mutated allele not shared by the sibs—who may still share another homozygous BBS gene mutation—acts as a modifier and modulates the phenotype, as reported elsewhere in some families with BBS and a third mutation present in the more severely affected sib but not the other (Badano et al. 2003b).
The association of DWM with either polydactyly (Hart et al. 1972; Tal et al. 1980) or cystic kidney dysplasia (D’Agostino et al. 1963; Goldston et al. 1963) has been reported. In addition, DWM, cystic kidneys, and hepatic fibrosis have been documented in several cases (Kudo et al. 1985; Gloeb et al. 1989; Pierquin et al. 1989; Hunter et al. 1991; Walpole et al. 1991; Gulcan et al. 2001) and have been recorded as Goldston syndrome. Despite the lack of bile-duct proliferation, Goldston syndrome has been suggested to be a variant of MKS (Walpole et al. 1991; Gulcan et al. 2001). Furthermore, the association of DWM, cystic kidneys, and hepatic fibrosis with polydactyly (as observed in case 2b) has been reported several times; most authors considered these patients as having MKS (Summers and Donnenfeld 1995; Cincinnati et al. 2000), suggesting that DWM belongs to the spectrum of MKS brain malformations. By other authors, these cases were classified as “Meckel-like,” in the context of the cerebro-reno-digital syndrome (Lurie et al. 1991; Genuardi et al. 1993). In these reports, however, hepatic fibrosis but no bile-duct proliferation was present (Genuardi et al. 1993; Summers and Donnenfeld 1995; Cincinnati et al. 2000). Although a molecular study is necessary to establish whether these patients had BBS, these findings suggest that they did not have MKS. Finally, the present study shows that infratentorial malformations should be added to the spectrum of malformations observed in BBS. Along this line, vermis agenesis and mega cisterna magna have been reported once in BBS (Baskin et al. 2002).
Other syndromes constantly or occasionally associating DWM with cystic kidneys and/or polydactyly—namely, MKS, Goldston, Joubert, hydrolethalus (Morava et al. 1996), Ellis-Van Creveld (EVC), SLO, congenital disorder of glycosylation (CDG), OFDII, and scalp defects with polydactyly—are summarized in table 3. In all these syndromes (except Joubert and CDG), other clinical signs, such as intrauterine growth retardation (IUGR) (in SLO), short ribs (in EVC), and tongue anomalies (in OFDII), are observed antenatally. In the present report, three cases presented with this association, but no BBS mutation was detected. Although other BBS genes could be mutated, some cases could also correspond to prenatal cases of Joubert syndrome.
Several patients with Goldston syndrome, MKS, or Joubert syndrome have been reported to have both DWM and an occipital meningocele (Miranda et al. 1972; Malpuech et al. 1979; Walpole et al. 1991; Moerman et al. 1993; Piantanida et al. 1993; Al-Gazali et al. 1996; Yapar et al. 1996). This raised the possibility of a common mechanism for both malformations, even though discordant sibs with either encephalocele or DWM have been reported (Blankenberg et al. 1987; Moerman et al. 1993). In most cases, however, the so-called DWM was diagnosed on the basis of brainimaging criteria alone, and one can postulate that the occipital meningo encephalocele may interfere with brainstem and cerebellum development, leading to an infratentorial dysplasia mimicking DWM. Only a neuropathological examination could help distinguish these two entities.
In view of the results of the present study, the question of whether MKS and BBS are allelic disorders arose. First, MKS is a genetically heterogeneous condition. Three loci have been mapped on 17q23 (MKS1 [Paavola et al. 1995]), 11q14 (MKS2 [Roume et al. 1998]), and 8q24 (MKS3 [Morgan et al. 2002]), but no gene has been identified yet. One locus is common to both MKS and BBS, since both BBS1 and MKS2 map on chromosome 11q13-q14. Although the BBS1 gene is located almost 10 cM centromeric to the MKS2 locus, we sequenced the BBS1 gene in 17 MKS cases, including the familial cases linked to 11q13 (Roume et al. 1998), and identified a heterozygous BBS1 mutation in two cases: the recurrent M390R mutation and a new G559D mutation. No other BBS gene mutation could be identified in these two cases. These results may suggest genetic interactions between BBS and MKS. However, although renal histological features in some cases are very similar to those observed in MKS (fig. 2M, 2N, and 2Q), the typical liver ductal plate anomaly, considered a constant in MKS, was absent from cases in the present study. Finally, other malformations frequently found in MKS, such as cleft lip/palate and pancreatic and epidydymal cysts, are not observed in patients with BBS (Fraser and Lytwyn 1981). These observations argue against the hypothesis that the two disorders are allelic. Also, in the family in which a homozygous BBS4 deletion was found in one sib presenting with severe BBS (case 5b), the sib born earlier (case 5a) with an MKS phenotype did not carry this deletion. Therefore, it is likely that, in this consanguineous family, two different recessive disorders—both characterized by the association of polydactyly and cystic kidneys—were segregating. Indeed, both BBS and MKS are frequently found in consanguineous populations (Teebi 1994; Zlotogora 1997). Here, also, one can hypothesize that an as-yet-unidentified MKS allele may be shared between sibs and may add to the severity of the BBS phenotype in case 5b. Interestingly, family 5 illustrates how the clinical spectrum of a genetic disorder might be extended wrongly when two different disorders segregate in consanguineous families.
In conclusion, our study shows that the association of DWM, cystic kidneys, and hepatic fibrosis without bile-duct proliferation, reported as Goldston syndrome or as “Meckel-like,” belongs to the clinical spectrum of BBS. Although BBS and MKS kidney histopathological findings may be similar, the present study suggests that, although genetic interaction may exist between BBS and MKS genes, the two disorders are not caused by the same gene mutations. This hypothesis can be definitively established when MKS genes are identified. The recent demonstration of the role of BBS proteins in ciliary function and the clinical overlap between BBS and MKS will hopefully open the way to discovery of the MKS disease-causing genes.
Acknowledgments
We are thankful to the Société Française de Foetopathologie and to all the clinicians, for sending us patient data and material, in particular Sophie Chemouni, Albert David, Gérard Dray, Yvette Hillion, Nathalie Leporrier, Françoise Menez, Marie-France Nombalais, Joelle Roume, Jaqueline Vigneron, and Dominique Zachar. We thank Corinne Stoetzel for technical assistance. H.K.-B. was granted a fellowship from the Fondation pour la Recherche Médicale.
Electronic-Database Information
The URLs for data presented herein are as follows:
- dbSNP, http://www.ncbi.nlm.nih.gov/SNP/
- Ensembl, http://www.ensembl.org/
- Online Mendelian Inheritance in Man (OMIM), http://www.ncbi.nlm.nih.gov/Omim/
References
- Al-Gazali LI, Abdel Raziq A, Al-Shather W, Shahzadi R, Azhar N (1996) Meckel syndrome and Dandy Walker malformation. Clin Dysmorphol 5:73–76 [DOI] [PubMed] [Google Scholar]
- Ansley SJ, Badano JL, Blacque OE, Hill J, Hoskins BE, Leitch CC, Kim JC, Ross AJ, Eichers ER, Teslovich TM, Mah AK, Johnsen RC, Cavender JC, Lewis RA, Leroux MR, Beales PL, Katsanis N (2003) Basal body dysfunction is a likely cause of pleiotropic Bardet-Biedl syndrome. Nature 425:628–633 10.1038/nature02030 [DOI] [PubMed] [Google Scholar]
- Badano JL, Ansley SJ, Leitch CC, Lewis RA, Lupski JR, Katsanis N (2003a) Identification of a novel Bardet-Biedl syndrome protein, BBS7, that shares structural features with BBS1 and BBS2. Am J Hum Genet 72:650–658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badano JL, Kim JC, Hoskins BE, Lewis RA, Ansley SJ, Cutler DJ, Castellan C, Beales PL, Leroux MR, Katsanis N (2003b) Heterozygous mutations in BBS1, BBS2 and BBS6 have a potential epistatic effect on Bardet-Biedl patients with two mutations at a second BBS locus. Hum Mol Genet 12:1651–1659 10.1093/hmg/ddg188 [DOI] [PubMed] [Google Scholar]
- Baskin E, Kayiran SM, Oto S, Alehan F, Agildere AM, Saatci U (2002) Cerebellar vermis hypoplasia in a patient with Bardet-Biedl syndrome. J Child Neurol 17:385–387 [DOI] [PubMed] [Google Scholar]
- Beales PL, Badano JL, Ross AJ, Ansley SJ, Hoskins BE, Kirsten B, Mein CA, Froguel P, Scambler PJ, Lewis RA, Lupski JR, Katsanis N (2003) Genetic interaction of BBS1 mutations with alleles at other BBS loci can result in non-Mendelian Bardet-Biedl syndrome. Am J Hum Genet 72:1187–1199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beales PL, Elcioglu N, Woolf AS, Parker D, Flinter FA (1999) New criteria for improved diagnosis of Bardet-Biedl syndrome: results of a population survey. J Med Genet 36:437–446 [PMC free article] [PubMed] [Google Scholar]
- Beales PL, Katsanis N, Lewis RA, Ansley SJ, Elcioglu N, Raza J, Woods MO, Green JS, Parfrey PS, Davidson WS, Lupski JR (2001) Genetic and mutational analyses of a large multiethnic Bardet-Biedl cohort reveal a minor involvement of BBS6 and delineate the critical intervals of other loci. Am J Hum Genet 68:606–616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blankenberg TA, Ruebner BH, Ellis WG, Bernstein J, Dimmick JE (1987) Pathology of renal and hepatic anomalies in Meckel syndrome. Am J Med Genet Suppl 3:395–410 [DOI] [PubMed] [Google Scholar]
- Cassart M, Eurin D, Didier F, Guibaud L, Avni EF (2004) Antenatal renal sonographic anomalies and postnatal follow-up of renal involvement in Bardet-Biedl syndrome. Ultrasound Obstet Gynecol 24:51–54 10.1002/uog.1086 [DOI] [PubMed] [Google Scholar]
- Chiang AP, Nishimura D, Searby C, Elbedour K, Carmi R, Ferguson AL, Secrist J, Braun T, Casavant T, Stone EM, Sheffield VC (2004) Comparative genomic analysis identifies an ADP-ribosylation factor–like gene as the cause of Bardet-Biedl syndrome (BBS3). Am J Hum Genet 75:475–484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cincinnati P, Neri ME, Valentini A (2000) Dandy-Walker anomaly in Meckel-Gruber syndrome. Clin Dysmorphol 9:35–38 [DOI] [PubMed] [Google Scholar]
- D’Agostino AN, Kernohan JW, Brown JR (1963) The Dandy-Walker syndrome. J Neuropathol Exp Neurol 22:450–470 [DOI] [PubMed] [Google Scholar]
- Fan Y, Esmail MA, Ansley SJ, Blacque OE, Boroevich K, Ross AJ, Moore SJ, Badano JL, May-Simera H, Compton DS, Green JS, Lewis RA, Van Haelst MM, Parfrey PS, Baillie DL, Beales PL, Katsanis N, Davidson WS, Leroux MR (2004) Mutations in a member of the Ras superfamily of small GTP-binding proteins causes Bardet-Biedl syndrome. Nat Genet 36:989–993 10.1038/ng1414 [DOI] [PubMed] [Google Scholar]
- Fraser FC, Lytwyn A (1981) Spectrum of anomalies in the Meckel syndrome, or: “maybe there is a malformation syndrome with at least one constant anomaly.” Am J Med Genet 9:67–73 [DOI] [PubMed] [Google Scholar]
- Genuardi M, Dionisi-Vici C, Sabetta G, Mignozzi M, Rizzoni G, Cotugno G, Martini Neri ME (1993) Cerebro-reno-digital (Meckel-like) syndrome with Dandy-Walker malformation, cystic kidneys, hepatic fibrosis, and polydactyly. Am J Med Genet 47:50–53 [DOI] [PubMed] [Google Scholar]
- Gershoni-Baruch R, Nachlieli T, Leibo R, Degani S, Weissman I (1992) Cystic kidney dysplasia and polydactyly in 3 sibs with Bardet-Biedl syndrome. Am J Med Genet 44:269–273 [DOI] [PubMed] [Google Scholar]
- Gloeb DJ, Valdes-Dapena M, Salman F, O’Sullivan MJ, Quetel TA (1989) The Goldston syndrome: report of a case. Pediatr Pathol 9:337–343 [DOI] [PubMed] [Google Scholar]
- Goldston AS, Burke EC, D’Agostino A, McCaughey WT, Maccaughey WT (1963) Neonatal polycystic kidney with brain defect. Am J Dis Child 106:484–488 [DOI] [PubMed] [Google Scholar]
- Gulcan YH, Duman N, Kumral A, Sagol, Lebe B, Kavukcu S, Ercal D, Celiloglu M, Ozkan H (2001) Goldston syndrome: report of a case. Genet Couns 12:263–267 [PubMed] [Google Scholar]
- Hart MN, Malamud N, Ellis WG (1972) The Dandy-Walker syndrome: a clinicopathological study based on 28 cases. Neurology 22:771–780 [DOI] [PubMed] [Google Scholar]
- Hunter AG, Jimenez C, Tawagi FG (1991) Familial renalhepatic-pancreatic dysplasia and Dandy-Walker cyst: a distinct syndrome? Am J Med Genet 41:201–207 [DOI] [PubMed] [Google Scholar]
- Johnson CA, Gissen P, Sergi C (2003) Molecular pathology and genetics of congenital hepatorenal fibrocystic syndromes. J Med Genet 40:311–319 10.1136/jmg.40.5.311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsanis N (2004) The oligogenic properties of Bardet-Biedl syndrome. Hum Mol Genet Suppl 13:R65–R71 10.1093/hmg/ddh092 [DOI] [PubMed] [Google Scholar]
- Katsanis N, Ansley SJ, Badano JL, Eichers ER, Lewis RA, Hoskins BE, Scambler PJ, Davidson WS, Beales PL, Lupski JR (2001) Triallelic inheritance in Bardet-Biedl syndrome, a Mendelian recessive disorder. Science 293:2256–2259 10.1126/science.1063525 [DOI] [PubMed] [Google Scholar]
- Katsanis N, Beales PL, Woods MO, Lewis RA, Green JS, Parfrey PS, Ansley SJ, Davidson WS, Lupski JR (2000) Mutations in MKKS cause obesity, retinal dystrophy and renal malformations associated with Bardet-Biedl syndrome. Nat Genet 26:67–70 10.1038/79201 [DOI] [PubMed] [Google Scholar]
- Katsanis N, Eichers ER, Ansley SJ, Lewis RA, Kayserili H, Hoskins BE, Scambler PJ, Beales PL, Lupski JR (2002) BBS4 is a minor contributor to Bardet-Biedl syndrome and may also participate in triallelic inheritance. Am J Hum Genet 71:22–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudo M, Tamura K, Fuse Y (1985) Cystic dysplastic kidneys associated with Dandy-Walker malformation and congenital hepatic fibrosis: report of two cases. Am J Clin Pathol 84:459–463 [DOI] [PubMed] [Google Scholar]
- Li JB, Gerdes JM, Haycraft CJ, Fan Y, Teslovich TM, May-Simera H, Li H, Blacque OE, Li L, Leitch CC, Lewis RA, Green JS, Parfrey PS, Leroux MR, Davidson WS, Beales PL, Guay-Woodford LM, Yoder BK, Stormo GD, Katsanis N, Dutcher SK (2004) Comparative genomics identifies a flagellar and basal body proteome that includes the BBS5 human disease gene. Cell 117:541–552 10.1016/S0092-8674(04)00450-7 [DOI] [PubMed] [Google Scholar]
- Lurie IW, Lazjuk GI, Korotkova IA, Cherstvoy ED (1991) The cerebro-reno-digital syndromes: a new community. Clin Genet 39:104–113 [DOI] [PubMed] [Google Scholar]
- Malpuech G, Palcoux JB, Desbordes AM, Dalens B (1979) Meckel’s syndrome: an unusual pedigree. J Genet Hum 27:167–174 [PubMed] [Google Scholar]
- Mecke S, Passarge E (1971) Encephalocele, polycystic kidneys, and polydactyly as an autosomal recessive trait simulating certain other disorders: the Meckel syndrome. Ann Genet 14:97–103 [PubMed] [Google Scholar]
- Miranda D, Schinella RA, Finegold MJ (1972) Familial renal dysplasia: microdissection studies in siblings with associated central nervous system and hepatic malformations. Arch Pathol 93:483–491 [PubMed] [Google Scholar]
- Moerman P, Pauwels P, Vandenberghe K, Lauweryns JM, Fryns JP (1993) Goldston syndrome reconsidered. Genet Couns 4:97–102 [PubMed] [Google Scholar]
- Morava E, Adamovich K, Czeizel AE (1996) Dandy-Walker malformation and polydactyly: a possible expression of hydrolethalus syndrome. Clin Genet 49:211–215 [DOI] [PubMed] [Google Scholar]
- Morgan NV, Gissen P, Sharif SM, Baumber L, Sutherland J, Kelly DA, Aminu K, Bennett CP, Woods CG, Mueller RF, Trembath RC, Maher ER, Johnson CA (2002) A novel locus for Meckel-Gruber syndrome, MKS3, maps to chromosome 8q24. Hum Genet 111:456–461 10.1007/s00439-002-0817-0 [DOI] [PubMed] [Google Scholar]
- Mykytyn K, Braun T, Carmi R, Haider NB, Searby CC, Shastri M, Beck G, Wright AF, Iannaccone A, Elbedour K, Riise R, Baldi A, Raas-Rothschild A, Gorman SW, Duhl DM, Jacobson SG, Casavant T, Stone EM, Sheffield VC (2001) Identification of the gene that, when mutated, causes the human obesity syndrome BBS4. Nat Genet 28:188–191 10.1038/88925 [DOI] [PubMed] [Google Scholar]
- Mykytyn K, Nishimura DY, Searby CC, Beck G, Bugge K, Haines HL, Cornier AS, Cox GF, Fulton AB, Carmi R, Iannaccone A, Jacobson SG, Weleber RG, Wright AF, Riise R, Hennekam RCM, Lüleci G, Berker-Karauzum S, Biesecker LG, Stone EM, Sheffield VC (2003) Evaluation of complex inheritance involving the most common Bardet-Biedl syndrome locus (BBS1). Am J Hum Genet 72:429–437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mykytyn K, Nishimura DY, Searby CC, Shastri M, Yen HJ, Beck JS, Braun T, Streb LM, Cornier AS, Cox GF, Fulton AB, Carmi R, Luleci G, Chandrasekharappa SC, Collins FS, Jacobson SG, Heckenlively JR, Weleber RG, Stone EM, Sheffield VC (2002) Identification of the gene (BBS1) most commonly involved in Bardet-Biedl syndrome, a complex human obesity syndrome. Nat Genet 31:435–438 [DOI] [PubMed] [Google Scholar]
- Nishimura DY, Searby CC, Carmi R, Elbedour K, Van Maldergem L, Fulton AB, Lam BL, Powell BR, Swiderski RE, Bugge KE, Haider NB, Kwitek-Black AE, Ying L, Duhl DM, Gorman SW, Heon E, Iannaccone A, Bonneau D, Biesecker LG, Jacobson SG, Stone EM, Sheffield VC (2001) Positional cloning of a novel gene on chromosome 16q causing Bardet-Biedl syndrome (BBS2). Hum Mol Genet 10:865–874 10.1093/hmg/10.8.865 [DOI] [PubMed] [Google Scholar]
- Paavola P, Salonen R, Weissenbach J, Peltonen L (1995) The locus for Meckel syndrome with multiple congenital anomalies maps to chromosome 17q21-q24. Nat Genet 11:213–215 10.1038/ng1095-213 [DOI] [PubMed] [Google Scholar]
- Piantanida M, Tiberti A, Plebani A, Martelli P, Danesino C (1993) Cerebro-reno-digital syndrome in two sibs. Am J Med Genet 47:420–422 [DOI] [PubMed] [Google Scholar]
- Pierquin G, Deroover J, Levi S, Masson T, Hayez-Delatte F, Van Regemorter N (1989) Dandy-Walker malformation with postaxial polydactyly: a new syndrome? Am J Med Genet 33:483–484 [DOI] [PubMed] [Google Scholar]
- Roume J, Genin E, Cormier-Daire V, Ma HW, Mehaye B, Attie T, Razavi-Encha F, Fallet-Bianco C, Buenerd A, Clerget-Darpoux F, Munnich A, Le Merrer M (1998) A gene for Meckel syndrome maps to chromosome 11q13. Am J Hum Genet 63:1095–1101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salonen R (1984) The Meckel syndrome: clinicopathological findings in 67 patients. Am J Med Genet 18:671–689 [DOI] [PubMed] [Google Scholar]
- Slavotinek AM, Searby C, Al-Gazali L, Hennekam RC, Schrander-Stumpel C, Orcana-Losa M, Pardo-Reoyo S, Cantani A, Kumar D, Capellini Q, Neri G, Zackai E, Biesecker LG (2002) Mutation analysis of the MKKS gene in McKusick-Kaufman syndrome and selected Bardet-Biedl syndrome patients. Hum Genet 110:561–567 10.1007/s00439-002-0733-3 [DOI] [PubMed] [Google Scholar]
- Slavotinek AM, Stone EM, Mykytyn K, Heckenlively JR, Green JS, Heon E, Musarella MA, Parfrey PS, Sheffield VC, Biesecker LG (2000) Mutations in MKKS cause Bardet-Biedl syndrome. Nat Genet 26:15–16 10.1038/79116 [DOI] [PubMed] [Google Scholar]
- Summers MC, Donnenfeld AE (1995) Dandy-Walker malformation in the Meckel syndrome. Am J Med Genet 55:57–61 [DOI] [PubMed] [Google Scholar]
- Tal Y, Freigang B, Dunn HG, Durity FA, Moyes PD (1980) Dandy-Walker syndrome: analysis of 21 cases. Dev Med Child Neurol 22:189–201 [DOI] [PubMed] [Google Scholar]
- Teebi AS (1994) Autosomal recessive disorders among Arabs: an overview from Kuwait. J Med Genet 31:224–233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walpole IR, Goldblatt J, Hockey A, Knowles S (1991) Dandy-Walker malformation (variant), cystic dysplastic kidneys, and hepatic fibrosis: a distinct entity or Meckel syndrome? Am J Med Genet 39:294–298 [DOI] [PubMed] [Google Scholar]
- Yapar EG, Ekici E, Dogan M, Gokmen O (1996) Meckel-Gruber syndrome concomitant with Dandy-Walker malformation: prenatal sonographic diagnosis in two cases. Clin Dysmorphol 5:357–362 [PubMed] [Google Scholar]
- Zlotogora J (1997) Autosomal recessive diseases among Palestinian Arabs. J Med Genet 34:765–766 [DOI] [PMC free article] [PubMed] [Google Scholar]