Primary aldosteronism (PA), recognized as the leading cause of secondary hypertension, has long been linked to an increased risk of cardiovascular diseases such as coronary artery disease, atrial fibrillation, congestive heart failure, and stroke. 1 , 2 However, establishing a causal relationship between PA and cardiovascular outcome has been challenging. Traditionally, 2 effective approaches to achieving this goal are the thorough analysis of large observational cohorts and conducting controlled trials that involve interventions aimed at altering the suspected risk factor. Nonetheless, observational cohort studies may have unrecognized confounders, and randomized control trials are impractical in this association. Mendelian randomization is a promising and powerful tool that uses genetic variants as instrumental variables to assess causal relationships using observational data. This approach helps to overcome some limitations of traditional observational studies, such as confounding and reverse causation, by using the random assortment of genes from parents to offspring as a natural experiment.
In this issue of the Journal of the American Heart Association (JAHA), Inoue and colleagues performed an updated cross‐ancestry meta‐analysis of genome‐wide association studies, encompassing 1560 PA cases and 742 139 controls of East Asian and European descent. 3 Based on previously published genome‐wide association studies, 4 , 5 this meta‐analysis of the cross‐ancestry analysis identified 7 genetic loci significantly associated with PA, which include genes such as CASZ1, WNT2B, HOTTIP, LSP1, TBX3, RXFP2, and NDP. These findings not only reinforce previous genetic discoveries but also expand our understanding of the genetic underpinnings of PA (Figure). The identified loci highlight potential pathways involved in aldosterone regulation and PA pathophysiology, offering promising targets for future research and therapeutic interventions. 6 , 7 , 8 In the 2‐sample Mendelian randomization analysis, by using these 7 genetic variants as instrumental variables, the study demonstrated that PA significantly increases the risk of coronary artery disease, congestive heart failure, and stroke in both East Asian and European populations in a causal relationship. The consistent findings across different populations underscore the robustness of the results and suggest a universal cardiovascular burden associated with PA. This study did not investigate the relationship between atrial fibrillation and PA, likely due to limitations in the available data. Atrial fibrillation is associated with stroke and heart failure, which are important comorbidities in patients with PA. Additionally, genetic variants on the X chromosome were excluded due to a lack of available data in the European ancestry, which might also impact the results.
Figure 1. Causal relationship of cardiovascular disease in primary aldosteronism.
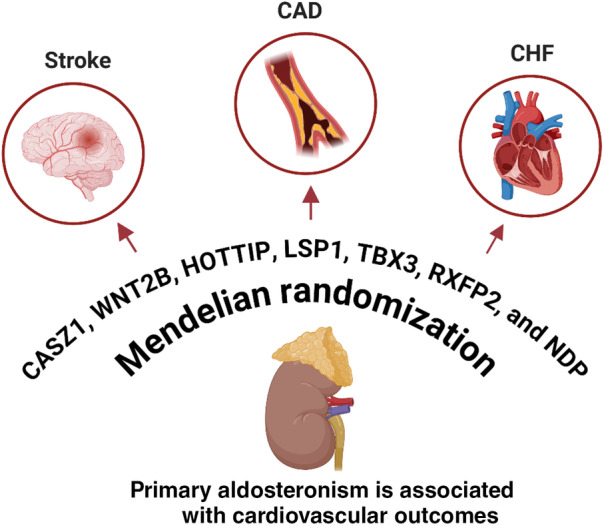
Illustration of potential genetic risks associated with cardiovascular diseases in patients with primary aldosteronism, as identified through genome‐wide association and Mendelian randomization studies. CAD indicates coronary artery disease; and CHF, congestive heart failure.
The study's findings have significant clinical implications. First, they underscore the necessity of early and systematic screening for PA in patients with hypertension, especially those with resistant hypertension. The strong causal link between PA and adverse cardiovascular outcomes emphasizes the need for clinicians to be vigilant in diagnosing and managing PA to prevent future cardiovascular events. Moreover, the study elucidates the multifaceted role of aldosterone in promoting cardiovascular damage through mechanisms such as inflammation, endothelial dysfunction, and oxidative stress. These mechanisms are mainly triggered by aldosterone‐activated mineralocorticoid receptors and subsequent genomic and nongenomic pathways. 9 The results advocate for effective mineralocorticoid receptor blockade in patients with PA, beyond mere blood pressure control.
The genetic insights gained from this study pave the way for personalized medicine approaches in the management of PA. Although the current study did not provide treatment outcomes analysis, more intensive PA treatment such as surgical adrenalectomy or aggressive mineralocorticoid receptor antagonist use for those carrying genetic risk might potentially benefit these patients. 10 Understanding individual genetic predispositions to PA can help tailor interventions more precisely, improving outcomes and reducing the cardiovascular burden. However, this study did not delve into PA subtyping and the association of these genetic variants with PA subtypes or somatic mutations. Future research should focus on the heterogeneity of PA, exploring variations in cardiovascular risk based on different PA subtypes and somatic mutations.
Inoue et al's study represents a significant milestone in cardiovascular research, providing evidence of the causal relationship between PA and cardiovascular diseases. These findings highlight the critical importance of early detection and targeted treatment of PA. As we continue to unravel the genetic and molecular mechanisms underlying PA, we move closer to more effective and personalized strategies for managing this common, yet often underdiagnosed, condition. This study not only advances our scientific knowledge but also holds the promise of improving clinical practice and patient outcomes in the fight against hypertension and cardiovascular disease.
Disclosures
None.
The opinions expressed in this article are not necessarily those of the editors or of the American Heart Association.
This article was sent to Bruce Ovbiagele, MD, MSc, MAS, MBA, MLS, Editor‐in‐Chief, for editorial decision and final disposition.
See Article by Inoue et al.
For Disclosures, see page 2.
References
- 1. Monticone S, D'Ascenzo F, Moretti C, Williams TA, Veglio F, Gaita F, Mulatero P. Cardiovascular events and target organ damage in primary aldosteronism compared with essential hypertension: a systematic review and meta‐analysis. Lancet Diabetes Endocrinol. 2018;6:41–50. doi: 10.1016/S2213-8587(17)30319-4 [DOI] [PubMed] [Google Scholar]
- 2. Tsai CH, Pan CT, Chang YY, Chen ZW, Wu VC, Hung CS, Lin YH. Left ventricular remodeling and dysfunction in primary aldosteronism. J Hum Hypertens. 2021;35:131–147. doi: 10.1038/s41371-020-00426-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Inoue K, Naito T, Fuji R, Sonehara K, Yamamoto K, Baba R, Kodama T, Otagaki Y, Okada A, Itcho K. Primary aldosteronism and risk of cardiovascular outcomes: genome‐wide association and Mendelian randomization study. J Am Heart Assoc. 2024. doi: 10.1161/JAHA.123.034180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Naito T, Inoue K, Sonehara K, Baba R, Kodama T, Otagaki Y, Okada A, Itcho K, Kobuke K, Kishimoto S, et al. Genetic risk of primary aldosteronism and its contribution to hypertension: a cross‐ancestry meta‐analysis of genome‐wide association studies. Circulation. 2023;147:1097–1109. doi: 10.1161/CIRCULATIONAHA.122.062349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Le Floch E, Cosentino T, Larsen CK, Beuschlein F, Reincke M, Amar L, Rossi GP, de Sousa K, Baron S, Chantalat S, et al. Identification of risk loci for primary aldosteronism in genome‐wide association studies. Nat Commun. 2022;13:5198. doi: 10.1038/s41467-022-32896-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Drelon C, Berthon A, Sahut‐Barnola I, Mathieu M, Dumontet T, Rodriguez S, Batisse‐Lignier M, Tabbal H, Tauveron I, Lefrancois‐Martinez AM, et al. PKA inhibits WNT signalling in adrenal cortex zonation and prevents malignant tumour development. Nat Commun. 2016;7:12751. doi: 10.1038/ncomms12751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Peng KY, Chang HM, Lin YF, Chan CK, Chang CH, Chueh SJ, Yang SY, Huang KH, Lin YH, Wu VC, et al. miRNA‐203 modulates aldosterone levels and cell proliferation by targeting Wnt5a in aldosterone‐producing adenomas. J Clin Endocrinol Metab. 2018;103:3737–3747. doi: 10.1210/jc.2018-00746 [DOI] [PubMed] [Google Scholar]
- 8. Yokota K, Shibata H, Kurihara I, Itoh H, Sone M. CASZ1: a promising factor modulating aldosterone biosynthesis and mineralocorticoid receptor activity. Hypertens Res. 2023;46:417–420. doi: 10.1038/s41440-022-01131-8 [DOI] [PubMed] [Google Scholar]
- 9. Buffolo F, Tetti M, Mulatero P, Monticone S. Aldosterone as a mediator of cardiovascular damage. Hypertension. 2022;79:1899–1911. doi: 10.1161/HYPERTENSIONAHA.122.17964 [DOI] [PubMed] [Google Scholar]
- 10. Hundemer GL, Curhan GC, Yozamp N, Wang M, Vaidya A. Cardiometabolic outcomes and mortality in medically treated primary aldosteronism: a retrospective cohort study. Lancet Diabetes Endocrinol. 2018;6:51–59. doi: 10.1016/S2213-8587(17)30367-4 [DOI] [PMC free article] [PubMed] [Google Scholar]


