Abstract
Recently, a quantitative-trait locus (QTL) for whole blood serotonin level was identified in a genomewide linkage and association study in a founder population. Because serotonin level is a sexually dimorphic trait, in the present study, we evaluated the sex-specific genetic architecture of whole blood serotonin level in the same population. Here, we use an extended homozygosity-by-descent linkage method that is suitable for large complex pedigrees. Although both males and females have high broad heritability (H2=0.99), females have a higher additive component (h2=0.63 in females; h2=0.27 in males). Furthermore, the serotonin QTL on 17q that was identified previously in this population, integrin β3 (ITGB3), and a novel locus on 2q influence serotonin levels only in males, whereas linkage to a region on chromosome 6q is specific to females. Both sexes contribute to linkage signals on 12q and 16p. There were, overall, more associations meeting criteria for suggestive significance in males than in females, including those of ITGB3 and the serotonin transporter gene (5HTT). This analysis is consistent with heritable sexual dimorphism in whole blood serotonin levels resulting from the effects of a combination of sex-specific and sex-independent loci.
Introduction
Genetically complex traits, which likely determine most of the heritable variation in humans, pose challenges to the gene-mapping community. In particular, gene-environment and gene-gene interactions, genetic heterogeneity, and incomplete penetrance make thorough genetic dissection of complex traits difficult, if not impossible. Gene-environment interactions are most often ignored by geneticists, simply because we know little about the environmental factors that influence the expression of most traits or about how to incorporate known environmental factors into our genetic models. Sex could be considered an environmental factor that can modify both the penetrance and expressivity of a wide variety of traits. Sex is easily determined and has measurable effects on recognizable morphology, neurobiological circuits, susceptibility to autoimmune disease, and quantitative traits like hypertension, obesity, and lipid levels, among others. In fact, sex-specific genetic influence has been found even for traits with no prior evidence of sexual dimorphism. For example, although phenotypic differences between the sexes in fasting glucose level are not evident, men show higher heritability for this trait than do women (Schousboe et al. 2003). Thus, it is possible that autosomal loci have sex-specific effects on a wide range of human traits, as has been observed for heritable quantitative traits in model organisms (Mackay 2001).
The serotonin system contributes to complex traits, including cognition, affect, endocrine regulation, neurotrophic effects, pain, appetite, emesis, sex, sleep, aggression, perception, sensory-motor function, and vascular and gastrointestinal regulation (Heninger 1997), and the role of serotonin in some of these traits is sex limited. For example, serotonin dysfunction has been observed in women with premenstrual dysphoric disorder (Steiner et al. 1999), and serotonin is thoughtto contribute to aggression in males by interacting with testosterone (Clark and Henderson 2003). In several population studies, females had increased average whole blood serotonin, as compared with males (Ashcroft et al. 1964; Wirz-Justice et al. 1977; Gonzales 1980; Ortiz et al. 1988). Although the genetic basis underlying variation in serotonin levels is not well understood, heritability estimates (narrow heritability [h2] 0.52; broad heritability [H2] 0.99) suggest that it is strongly genetically regulated (Abney et al. 2001). Therefore, an examination of gene-sex interactions with regard to serotonin level could identify sex differences in the genetic architecture of this trait and could further elucidate its underlying etiology.
Recently, genomewide linkage and association mapping was performed for whole blood serotonin levels in the Hutterites, a founder population of European descent (Weiss et al. 2004). In that study, we identified a novel QTL, integrin β3 (ITGB3), on chromosome 17q. One additional linkage result on chromosome 16p met criteria for suggestive significance. The goal of the present study was to examine whether there are different male and female genetic contributions to interindividual variation in serotonin levels and different contributions to the evidence for linkage and association for this trait in the Hutterites.
Material and Methods
Subjects
The Hutterites are a young founder population who practice a communal agricultural lifestyle. Details of the population, the sampling strategy, and the utility of this population for mapping complex traits have been described elsewhere (Abney et al. 2000; Ober et al. 2001; Weiss et al. 2004). The 806 Hutterites in our studies are related to each other through multiple lines of descent in a known pedigree. The mean inbreeding coefficient of the individuals in this sample is 0.034 (SD 0.015), slightly greater than that of 1.5 cousins. A complete genealogy of 806 individuals was constructed from a >12,000-member Hutterite pedigree. This yielded a 1,623-person pedigree that included all known ancestors of the 806 individuals (Abney et al. 2000). Whole blood serotonin was measured in 567 of these Hutterites (300 females; 267 males) after informed consent was obtained, as described elsewhere (Ober et al. 2001; Weiss et al. 2004). Whole blood serotonin measurements were ln transformed to establish a normal distribution. Distributions of the male-only sample, the female-only sample, and the whole sample were normal by the Kolmogorov-Smirnov test (P>.9). The mean age of females was 27.1 years (SD 15.0; range 5–89); the mean age of males was 27.0 years (SD 15.1; range 6–79). This study was approved by the institutional review boards of The University of Chicago and the University of South Dakota.
Genotyping
A genome screen using 658 autosomal microsatellite markers (Marshfield screening sets 9 and 51) was completed by the Mammalian Genotyping Service of the National Heart, Lung, and Blood Institute, yielding an ∼5-cM map (see Marshfield Web site). In addition, 226 microsatellite markers and 239 intragenic SNPs or in/dels related to asthma and cardiovascular diseases (e.g., see Newman et al. [2004]) were genotyped in this sample. None of the markers were selected because they were functional or positional “candidates” for serotonin levels. Genotyping was performed blind to all phenotypic information. Distances for framework markers were based on the Marshfield map (see Marshfield Web site); all other markers were placed by use of the physical map (see UCSC Web site) and estimations of recombination within the Hutterite pedigree as calcuated by CRI-MAP (see CRI-MAP Web site). The final map had an average intermarker distance of 3.2 cM.
Statistical Analysis
Estimation of heritability
The methods used to estimate narrow and broad heritability were described in detail elsewhere (Abney et al. 2001) but will be briefly reviewed here. We analyzed serotonin by use of a variance-components maximum-likelihood method (Abney et al. 2000). This method estimates additive, dominance, and environmental variance by using information about the kinship coefficient and the probability that a given pair of individuals share two alleles identical by descent (IBD) without either individual being autozygous. Accurate estimation of the dominance variance, as opposed to just a sibship correlation, is possible, because essentially every pair of Hutterites has a nonzero probability of sharing two alleles IBD. We considered models that had—in addition to an environmental variance component—only additive variance, only dominance variance, and both additive and dominance variance components. To assess the best-fitting model, we used the Bayesian information criterion (Schwarz 1978) to compare themodels and used the likelihood-ratio χ2 test to determine which components (including sex) were significant.
Mapping
Genome scans were performed using homozygosity-by-descent (HBD) linkage and allele-specific-HBD (ASHBD) association methods, and significance was assessed using a permutation-based method. The linkage method tests for correlations between regions inherited HBD and trait value, whereas ASHBD tests for correlations between each allele at a marker inherited HBD and trait value. For both methods, we assess empirical locus-specific and genomewide significance, using a Monte Carlo permutation test. This test keeps the genotypes fixed while permuting trait values. This has the advantage of assessing significance conditional on characteristics of the genotype data (e.g., informativeness, heterozygosity, and linkage disequilibrium) while preserving the covariance structure in the phenotype data. The methods used in the present study are identical to those described elsewhere (Abney et al. 2002), except that we have now extended the original HBD computations to include genotype information from related individuals (see appendix). Because of these changes, the results of the genomewide screens vary slightly from our previous report (Weiss et al. 2004).
All analyses were run first on the entire sample and then separately by sex. In the sex-specific analyses, the phenotype values for individuals of the opposite sex were entered as missing data. Genomewide suggestive significance was met if the P value at a locus was less than the minimum P value expected under the null hypothesis, which was estimated by finding the minimum locus-specific P value across the genome for each of 1,000 permutations and averaging these values.
Locus-specific P values, on the basis of Gaussian theory, were Bonferroni corrected, such that the number of alleles at a marker locus equaled the number of tests for ASHBD. The Bonferroni-corrected P values were very close to the locus-specific permutation-based P values (Abney et al. 2002). Although likelihood ratios were not calculated, we can assign an equivalent 1-df LOD score to each of our P values by using the formula LOD=0.217F-1(1-P), where F−1 is the inverse cumulative distribution function of a χ2 random variable with 1 df.
Results
Serotonin Levels
The mean whole blood serotonin level in the entire sample was 191 ng/ml (SD 79). The mean for females (N=300) was 206 ng/ml (SD 79) and the mean for males (N=267) was 174 ng/ml (SD 76). Sex was a significant predictor in the best-fitting model for age-adjusted, ln-transformed serotonin values (P=2.4×10-9).
Heritability
In an earlier study of whole blood serotonin levels in the Hutterites, the best-fitting heritability model included both additive and dominance genetic effects (Abney et al. 2001). In that study, the narrow heritability (h2) was estimated to be 0.52, and the broad heritability (H2), to be 0.99, indicating that loci with both additive and nonadditive effects influence serotonin levels (Abney et al. 2001). In the sex-specific analyses, these estimates were h2=0.63 and H2=0.99 in females and h2=0.27 and H2=0.99 in males. Thus, whereas the broad heritabilities in males and females were similar to each other (and to the combined sample), the additive genetic component was greater in females (0.63 ± 0.20), as compared with that in males (0.27 ± 0.21). Although, in the combined sample, the model that included both additive and dominance components was a significantly better fit, in the sex-specific analyses, the models including a dominance component were not significantly better than purely additive models, in which h2=0.79±0.13 (likelihood-ratio χ2, P=.11) in females and h2=0.59±0.15 (likelihood-ratio χ2, P=.06) in males. Therefore, we used both models (one with additive and dominance components together and another with just an additive component) for our mapping studies in males and females. However, because the results were very similar (data not shown), we present here only results for analyses using the model that includes both additive and dominance components.
Linkage Results
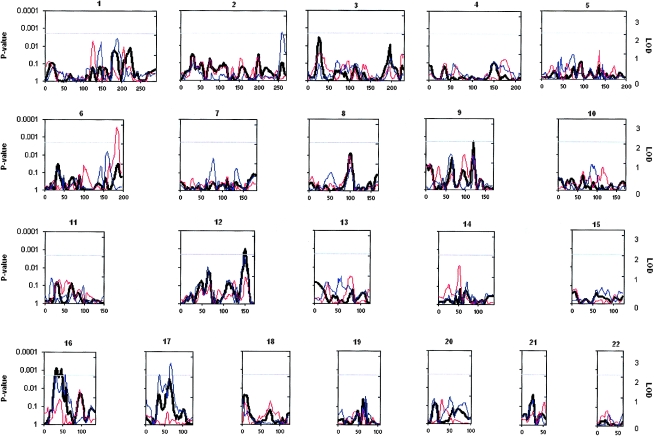
In our previous genomewide linkage analysis of the whole sample, one region on chromosome 16p at 35 cM met the criteria for suggestive significance (Weiss et al. 2004). By use of the modified method for estimating HBD, in the present study, the region on 16p at 35 cM remained the most significant, but linkage signals at two additional locations also met the criteria for suggestive significance (P<.0020): one on 16p at 46 cM and one on 12q at 150 cM (fig. 1).
Figure 1.
HBD linkage results with serotonin level. Locus-specific P values are shown on the left axis, with LOD equivalents on the right axis (see “Material and Methods” section). The gray line denotes the threshold for suggestive significance. The thick black line shows results for the full sample, the blue line shows results for males, and the pink line shows results for females.
In the male-only analysis, regions on 2q (260 cM), 16p (56 cM), 17p (36 cM), and 17q (66 cM) reached criteria for suggestive significance (P<.0020) (fig. 1). The region on 17q at 66 cM, with the most significant linkage in males, contains ITGB3, the previously reported QTL in this population.
In contrast, a region on 6q at 183 cM had the strongest linkage in the female-only analysis, meeting the criteria for suggestive significance (P<.0022) (fig. 1). Whereas there was a small signal on 12q at 150 cM (LOD=1.0; P=.03) and on 16p at 34 cM and 46 cM (LOD=0.7 and 0.9; P=.06 and .05, respectively), the regions on 2q, 17p, 17q, and 16p at 56 cM that were suggestive for linkage in males showed no evidence for linkage in females (P>.1). Similarly, the region on 6q that was most significant in females had no evidence for linkage in males (P>.1).
Association Results
The results of the genomewide association analysis of the entire sample were similar to what was reported elsewhere (Weiss et al. 2004): only one association met the criteria for suggestive significance (P<.00045), in a region corresponding to the linkage peak on chromosome 17 at 66 cM (ITGB3 L33P [dbSNP accession number rs5918]) (table 1). However, many more associations met this criteria in the sex-specific analyses. Although, in females, only one association (on chromosome 10) met the criteria for suggestive significance (P<.00065), suggestive associations were observed at seven loci in males (P<.00058), including ITGB3 and 5HTT on chromosome 17 (table 1). The 5HTT locus (also known as “SLC6A4” or “SERT”) encodes the serotonin transporter, at which variation has been associated with whole blood serotonin level and with autism (Cook et al. 1997; Klauck et al. 1997; Hanna et al. 1998; Tordjman et al. 2001; Yirmiya et al. 2001; Kim et al. 2002; Conroy et al. 2004; McCauley et al. 2004). Furthermore, evidence for linkage in families with autism to the region on 17q that includes 5HTT and marker D17S1294 has been reported (International Molecular Genetic Study of Autism Consortium 2001; Yonan et al. 2003). It is perhaps relevant, in light of our results, that autism is significantly more prevalent in males (Fombonne 2003).
Table 1.
Results of ASHBD Association Test with Serotonin Level[Note]
|
P Valuea for |
||||||
| Chromosomeand Locus | Distance from p-ter(cM) | AssociatedAllele | Frequency | All Samples | Males | Females |
| 1: | ||||||
| D1S1589 | 192 | 205 | .48 | .0232 | .0006 | >.1 |
| 2: | ||||||
| D2S125 | 261 | 94 | .20 | .0028 | .0003 | >.1 |
| 10: | ||||||
| D10S1677 | 100 | 174 | .32 | .0504 | >.1 | .0005 |
| 17: | ||||||
| ATA58E08 | 47 | 111 | .23 | .0289 | .0004 | >.1 |
| D17S1294 | 51 | 260 | .22 | .0106 | .0002 | >.1 |
| 5HTT_SNP09b | 51 | A | .57 | .0191 | .0003 | >.1 |
| D17S1299 | 62 | 196 | .55 | .0005 | .0001 | >.1 |
| ITGB3_33 | 66 | L | .80 | .0002 | .0003 | .0751 |
Note.— All ASHBD results with P values reaching the threshold for suggestive significance (see “Material and Methods” section) in one of the three samples are shown. All loci included here showed association with decreased serotonin levels.
P values are Bonferroni corrected (see “Material and Methods” section for details). Results in bold italics met criteria for suggestive significance.
dbSNP accession number rs2066713.
Covariate Analysis by Locus-Specific HBD
Elsewhere, we showed that HBD for the Leu33 allele at the ITGB3 locus accounted for the linkage signal on chromosome 17q in the combined sample (Weiss et al. 2004). Here, we repeated the linkage analysis including the probability of HBD for the Leu33 allele as a covariate in males and females separately.
As in our previous study, evidence for linkage on chromosome 17 was nearly eliminated in the covariate analysis of the combined sample (P=.79). Thus, HBD for the Leu33 allele—or for other variants that are in nearly complete linkage disequilibrium with it—explained almost all the evidence for linkage on 17q. In the male-only covariate analysis, the linkage peak in the ITGB3 region on 17q was also nearly completely eliminated (P=.28), confirming that it is a likely QTL for whole blood serotonin level in males. Notably, the peak on 17p, which is 30 cM from ITGB3, was relatively unchanged in the covariate analysis, indicating that these regions are independent.
Discussion
Serotonin levels have long been reported to be sexually dimorphic (Ashcroft et al. 1964; Wirz-Justice et al. 1977; Gonzales 1980; Ortiz et al. 1988). Further, the serotonin system has been implicated in differing male and female susceptibility to phenotypes influenced by the peripheral and central serotonergic systems, such as the increased prevalence among females of mortality after ischemic coronary events (Loop et al. 1983; Lerner and Kannel 1986; Weaver et al. 1996; Vaccarino et al. 1999) and of major depression (Weissman and Olfson 1995; Gater et al. 1998). In addition, selective serotonin-reuptake inhibitors (SSRIs) are more effective in treating depression in women (Kornstein et al. 2000), suggesting sex differences in metabolism and action of SSRIs.
In the present study, we not only show that whole blood serotonin levels differ between male and female Hutterites, but we also report, for the first time, that the underlying genetic architecture differs between the sexes. Although broad heritability was high in both sexes (H2=0.99), female heritability had a larger additive component (h2=0.63 in females; h2=0.27 in males). Sex-specific heritabilities in humans have been reported elsewhere for behavioral traits like aggression (van Beijsterveldt et al. 2003) and for quantitative traits like fasting insulin level (Schousboe et al. 2003). In model organisms, sex-specific differences in heritability are common, such as for lifespan in Drosophila (Leips and Mackay 2000) and for morphological traits in birds (Jensen et al. 2003). Our mapping studies of serotonin levels in the combined Hutterite sample, in males, and in females revealed that different loci influence serotonin levels in males and females. QTLs on chromosomes 17q (ITGB3) and 2q are specific to males, whereas a locus on chromosome 6q is specific to females (fig. 1). When ITGB3 L33 was included as a covariate, the signal on 17q in males was eliminated, indicating that this is a likely QTL. In humans, although many candidate genes are tested for sex-specific effects, only a few other traits have been found to have sex-specific susceptibility loci in genomewide screens. Psychiatric traits—such as neuroticism (Fullerton et al. 2003) and mood disorders (Zubenko et al. 2003)—as well as immune-mediated disorders—such as inflammatory bowel disease (Fisher et al. 2002) and osteoarthritis (Loughlin et al. 2000)—have shown sex-specific linkages.
Sex differences could result from parent-of-origin effects, linkage to or interaction with sex chromosomes, or differences arising from sex-specific hormonal environments. Our results did not separate individual genotypes on the basis of parent of origin, so imprinting, maternal effects, or mitochondrial inheritance would not explain the differences in male and female linkage signals observed in our study. Although we did not test for linkage or association on the sex chromosomes, X- or Y-linked loci that interact with autosomal loci could theoretically explain our results. It should be noted that the serotonin-metabolizing monoamine oxidases (MAO-A and MAO-B) are encoded by genes on the X chromosome and are good functional candidates to influence serotonin level. However, a model of interaction with sex-linked loci that would explain a female-specific locus on 6q and male-specific loci on 2q and 17q would require multiple sex-linked genes. Therefore, a more biologically plausible mechanism may be that hormonal interaction at the transcriptional or posttranslational level underlies the sex-specific linkages and associations observed in the study. In support of the latter mechanism, ITGB3 mRNA levels were suppressed by estrogen both in intercaruncular bovine stromal cells (Kimmins et al. 2003) and in human osteoclasts (Saintier et al. 2004). Similarly, 5HTT mRNA expression in serotonergic neurons was reduced by estrogen in Rhesus macaques (Pecins-Thompson et al. 1998). If the trans effect of estrogen on these loci in females is epistatic to effects of interindividual cis variation, linkage and association may be more difficult to detect in females, in whom higher circulating levels of estrogen could mask the effects of genetic variation at these loci. Consistent with this hypothesis, the linkage and association in the regions containing ITGB3 and 5HTT on chromosome 17 in the Hutterites was present only in males. Regardless of the mechanism for the sex-specific linkage and association observed in our study, these data clearly show that the loci influencing whole blood serotonin levels differ between males and females.
Lastly, if the sex-specific genetic effects observed in the present study are a more general phenomenon, then these results would have broad implications for mapping complex trait genes. Failure to model for sex-specific architecture may significantly hamper the ability to detect signals of susceptibility loci in genomewide screens. In our study, only the two regions (16p and 12q) that show some evidence for linkage in both sexes met criteria for suggestive significance in the combined sample: the relatively larger effects of 17q and 2q in males and 6q in females did not meet these criteria. Thus, although combining data to increase sample size or performing meta-analysis on different samples are tempting approaches to increase power, caution should be taken, since our results suggest that appropriately choosing and subdividing populations or groups of patients may be critical for detection of linkage in complex traits.
Acknowledgments
The authors acknowledge Dr. Rodney Parry, for help in planning and conducting field trips; Harvey Dytch and Elle Profits, for computational assistance; and Suzanne Cheng, at Roche Molecular Systems, for providing genotyping reagents. This study was supported in part by grants HL56399 and HL66533 (to C.O.), DK55889 and HG02899 (to M.A.), and HD35482 (to E.H.C. Jr.); by a grant from the National Heart, Lung, and Blood Institute Mammalian Genotyping Service (to C.O.); and by a grant from Hoffmann-LaRoche (to C.O.). L.A.W. is supported by a National Science Foundation graduate research fellowship.
Appendix
Here, we briefly describe the extensions made to the original HBD computation, to include genotype information from related individuals. The earlier version of the HBD-linkage-mapping method computed the probability of an individual being HBD at a locus, conditional on only the genotypes of that individual (Abney et al. 2002). Ignoring the genotypes of other individuals allowed rapid estimation of HBD and proved to be a good approximation, with sufficiently dense and informative markers. However, in areas of the chromosome in which information content is low, inclusion of genotype data from other individuals improves the accuracy of the estimation. The computation of the probability of being HBD at a locus is based on a hidden Markov model (HMM). The HMM relies on three basic quantities: the inbreeding coefficient, the transition probabilities between HBD and non-HBD states, and the single-locus probabilities of observing the marker-genotype data, given the HBD state. Computation of the last of these quantities was previously done solely on the basis of the frequency of the observed marker alleles but is now done conditional on all known ancestral genotypes at that marker, for the individual in question. More precisely, we compute the probability of HBD (or non-HBD), given the genotype of the individual at the marker and given the genotypes of all the individual’s ancestors at that marker. The algorithm for accomplishing this uses the same basic recursion strategy proposed by Davis et al. (1996), with two important differences. The first difference is in the estimation of parent-offspring transmission when parental genotype data is missing. For instance, consider the case of a child having genotype (1,2) at a marker, with the father's genotype (1,2) and the mother's genotype unknown (0,0). Rather than assigning the probability of one-half to the child’s 1 allele being inherited from the father and one-half to it being inherited from the mother, we recursively look up the maternal lineage and check genotypes. So, if the maternal grandparents had genotypes (2,3) and (2,3), for example, then we would assign a probability equal to zero to the child’s 1 allele being inherited from the mother and a probability equal to one to it being inherited from the father. If the mother is a founder, then we assign the probability that each of the mother’s alleles are of type 1 equal to the frequency of the allele. The transmission probability, then, becomes weighted by the allele frequency. If allele 1 were very rare, for instance, then the probability that it was inherited from the father is increased, whereas the probability that it was inherited from the mother is decreased.
The other significant change in the algorithm is the assumption of unrelated founders. Normally, in considering the IBD status of two alleles, the recursive strategy will trace the possible paths of the alleles up the pedigree until either the paths join—in which case the alleles are IBD—or the paths end in different founders—in which case the alleles are not IBD. In a very deep pedigree, such as the Hutterites, in which there are many generations with untyped individuals, this approach gives poor results. Instead, we apply the recursion only up to the oldest typed individuals, a group we refer to as “quasi-founders.” In this case, in which possible allele paths end in separate quasi-founders, instead of assigning a zero probability of the alleles being IBD, we compute the probability of the alleles being IBD given the genotypes of the two quasi-founders and their previously computed identity coefficients (Abney et al. 2002). The net result is that we are able to rapidly compute multilocus HBD probabilities across the genome conditional on the individual’s genotypes and all the individual's ancestors’ genotypes in the entire intact Hutterite pedigree, for each of our study individuals.
Electronic-Database Information
Accession numbers and URLs for data presented herein are as follows:
- CRI-MAP, http://biobase.dk/Embnetut/Crimap/
- dbSNP, http://www.ncbi.nlm.nih.gov/SNP/ (for ITGB3 L33P [accession number rs5918] and 5HTT_SNP09 [accession number rs2066713])
- Marshfield, http://research.marshfieldclinic.org/genetics/
- UCSC, http://genome.ucsc.edu/
References
- Abney M, McPeek M, Ober C (2000) Estimation of variance components of quantitative traits in inbred populations. Am J Hum Genet 66:629–650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- ——— (2001) Broad and narrow heritabilities of quantitative traits in a founder population. Am J Hum Genet 68:1302–1307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abney M, Ober C, McPeek MS (2002) Quantitative-trait homozygosity and association mapping and empirical genomewide significance in large complex pedigrees: fasting serum-insulin levels in the Hutterites. Am J Hum Genet 70:920–934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashcroft GW, Crawford TB, Binns JK, Macdougall EJ (1964) Estimation of 5-hydroxytryptamine in human blood. Clin Chim Acta 45:364–369 [DOI] [PubMed] [Google Scholar]
- Clark AS, Henderson LP (2003) Behavioral and physiological responses to anabolic-androgenic steroids. Neurosci Biobehav Rev 27:413–436 [DOI] [PubMed] [Google Scholar]
- Conroy J, Meally E, Kearney G, Fitzgerald M, Gill M, Gallagher L (2004) Serotonin transporter gene and autism: a haplotype analysis in an Irish autistic population. Mol Psychiatry 9:587–593 [DOI] [PubMed] [Google Scholar]
- Cook EH Jr, Courchesne R, Lord C, Cox NJ, Yan S, Lincoln A, Haas R, Courchesne E, Leventhal BL (1997) Evidence of linkage between the serotonin transporter and autistic disorder. Mol Psychiatry 2:247–250 [DOI] [PubMed] [Google Scholar]
- Davis S, Schroeder M, Goldin LR, Weeks DE (1996) Nonparametric simulation-based statistics for detecting linkage in general pedigrees. Am J Hum Genet 58:867–880 [PMC free article] [PubMed] [Google Scholar]
- Fisher SA, Hampe J, Macpherson AJ, Forbes A, Lennard-Jones JE, Schreiber S, Curran ME, Mathew CG, Lewis CM (2002) Sex stratification of an inflammatory bowel disease genome search shows male-specific linkage to the HLA region of chromosome 6. Eur J Hum Genet 10:259–265 [DOI] [PubMed] [Google Scholar]
- Fombonne E (2003) The prevalence of autism. JAMA 289:87–89 [DOI] [PubMed] [Google Scholar]
- Fullerton J, Cubin M, Tiwari H, Wang C, Bomhra A, Davidson S, Miller S, Fairburn C, Goodwin G, Neale MC, Fiddy S, Mott R, Allison DB, Flint J (2003) Linkage analysis of extremely discordant and concordant sibling pairs identifies quantitative-trait loci that influence variation in the human personality trait neuroticism. Am J Hum Genet 72:879–890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gater R, Tansella M, Korten A, Tiemens BG, Mavreas VG, Olatawura MO (1998) Sex differences in the prevalence and detection of depressive and anxiety disorders in general health care settings: report from the World Health Organization Collaborative Study on Psychological Problems in General Health Care. Arch Gen Psychiatry 55:405–413 [DOI] [PubMed] [Google Scholar]
- Gonzales GF (1980) Blood levels of 5-hydroxytryptamine in human beings under several physiological situations. Life Sci 27:647–650 [DOI] [PubMed] [Google Scholar]
- Hanna GL, Himle JA, Curtis GC, Koram DQ, Weele JVV, Leventhal BL, Cook EH Jr (1998) Serotonin transporter and seasonal variation in blood serotonin in families with obsessive-compulsive disorder. Neuropsychopharmacology 18:102–111 [DOI] [PubMed] [Google Scholar]
- Heninger GR (1997) Serotonin, sex, and psychiatric illness. Proc Natl Acad Sci USA 94:4823–4824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- International Molecular Genetic Study of Autism Consortium (2001) A genomewide screen for autism: strong evidence for linkage to chromosomes 2q, 7q, and 16p. Am J Hum Genet 69:570–581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen H, Saether BE, Ringsby TH, Tufto J, Griffith SC, Ellegren H (2003) Sexual variation in heritability and genetic correlations of morphological traits in house sparrow (Passer domesticus). J Evol Biol 16:1296–1307 [DOI] [PubMed] [Google Scholar]
- Kim S-J, Cox N, Courchesne R, Lord C, Corsello C, Akshoomoff N, Guter S, Leventhal B, Courchesne E, Cook E (2002) Transmission disequilibrium mapping in the serotonin transporter gene (SLC6A4) region in autistic disorder. Mol Psychiatry 7:278–288 [DOI] [PubMed] [Google Scholar]
- Kimmins S, Lim HC, Parent J, Fortier MA, MacLaren LA (2003) The effects of estrogen and progesterone on prostaglandins and integrin beta 3 (β3) subunit expression in primary cultures of bovine endometrial cells. Domest Anim Endocrinol 25:141–154 [DOI] [PubMed] [Google Scholar]
- Klauck SM, Poustka F, Benner A, Lesch K-P, Poustka A (1997) Serotonin transporter (5-HTT) gene variants associated with autism? Hum Molec Genet 6:2233–2238 [DOI] [PubMed] [Google Scholar]
- Kornstein SG, Schatzberg AF, Thase ME, Yonkers KA, McCullough JP, Keitner GI, Gelenberg AJ, Davis SM, Harrison WM, Keller MB (2000) Gender differences in treatment response to sertraline versus imipramine in chronic depression. Am J Psychiatry 157:1445–1452 [DOI] [PubMed] [Google Scholar]
- Leips J, Mackay TF (2000) Quantitative trait loci for life span in Drosophila melanogaster: interactions with genetic background and larval density. Genetics 155:1773–1788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerner DJ, Kannel WB (1986) Patterns of coronary heart disease morbidity and mortality in the sexes: a 26-year follow-up of the Framingham population. Am Heart J 111:383–390 [DOI] [PubMed] [Google Scholar]
- Loop FD, Golding LR, MacMillan JP, Cosgrove DM, Lytle BW, Sheldon WC (1983) Coronary artery surgery in women compared with men: analyses of risks and long-term results. J Am Coll Cardiol 1:383–390 [DOI] [PubMed] [Google Scholar]
- Loughlin J, Mustafa Z, Smith A, Irven C, Carr AJ, Clipsham K, Chitnavis J, Bloomfield VA, McCartney M, Cox O, Sinsheimer JS, Sykes B, Chapman KE (2000) Linkage analysis of chromosome 2q in osteoarthritis. Rheumatology (Oxford) 39:377–381 [DOI] [PubMed] [Google Scholar]
- Mackay TF (2001) The genetic architecture of quantitative traits. Annu Rev Genet 35:303–339 [DOI] [PubMed] [Google Scholar]
- McCauley JL, Olson LM, Dowd M, Amin T, Steele A, Blakely RD, Folstein SE, Haines JL, Sutcliffe JS (2004) Linkage and association analysis at the serotonin transporter (SLC6A4) locus in a rigid-compulsive subset of autism. Am J Med Genet 127B:104–112 [DOI] [PubMed] [Google Scholar]
- Newman DL, Hoffjan S, Bourgain C, Abney M, Nicolae RI, Profits ET, Grow MA, Walker K, Steiner L, Parry R, Reynolds R, McPeek MS, Cheng S, Ober C (2004) Are common disease susceptibility alleles the same in outbred and founder populations? Eur J Hum Genet 12:584–590 [DOI] [PubMed] [Google Scholar]
- Ober C, Abney M, McPeek MS (2001) The genetic dissection of complex traits in a founder population. Am J Hum Genet 69:1068–1079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortiz J, Artigas F, Gelpi E (1988) Serotonergic status in human blood. Life Sci 43:983–990 [DOI] [PubMed] [Google Scholar]
- Pecins-Thompson M, Brown NA, Bethea CL (1998) Regulation of serotonin re-uptake transporter mRNA expression by ovarian steroids in Rhesus macaques. Brain Res Mol Brain Res 53:120–129 [DOI] [PubMed] [Google Scholar]
- Saintier D, Burde MA, Rey JM, Maudelonde T, de Vernejoul MC, Cohen-Solal ME (2004) 17β-estradiol downregulates β3-integrin expression in differentiating and mature human osteoclasts. J Cell Physiol 198:269–276 [DOI] [PubMed] [Google Scholar]
- Schousboe K, Visscher PM, Henriksen JE, Hopper JL, Sorensen TI, Kyvik KO (2003) Twin study of genetic and environmental influences on glucose tolerance and indices of insulin sensitivity and secretion. Diabetologia 46:1276–1283 [DOI] [PubMed] [Google Scholar]
- Schwarz G (1978) Estimating the dimension of a model. Ann Stat 6:461–464 [Google Scholar]
- Steiner M, Yatham LN, Coote M, Wilkins A, Lepage P (1999) Serotonergic dysfunction in women with pure premenstrual dysphoric disorder: is the fenfluramine challenge test still relevant? Psychiatry Res 87:107–115 [DOI] [PubMed] [Google Scholar]
- Tordjman S, Gutneckt L, Carlier M, Spitz E, Antoine C, Slama F, Cohen D, Ferrari P, Roubertoux P, Anderson G (2001) Role of the serotonin transporter in the behavioral expression of autism. Mol Psychiatry 6:434–439 [DOI] [PubMed] [Google Scholar]
- Vaccarino V, Parsons L, Every NR, Barron HV, Krumholz HM, for the National Registry of Myocardial Infarction 2 Participants (1999) Sex-based differences in early mortality after myocardial infarction. N Engl J Med 341:217–225 [DOI] [PubMed] [Google Scholar]
- van Beijsterveldt CE, Bartels M, Hudziak JJ, Boomsma DI (2003) Causes of stability of aggression from early childhood to adolescence: a longitudinal genetic analysis in Dutch twins. Behav Genet 33:591–605 [DOI] [PubMed] [Google Scholar]
- Weaver WD, White HD, Wilcox RG, Aylward PE, Morris D, Guerci A, Ohman EM, Barbash GI, Betriu A, Sadowski Z, Topol EJ, Califf RM (1996) Comparisons of characteristics and outcomes among women and men with acute myocardial infarction treated with thrombolytic therapy. GUSTO-I investigators. JAMA 275:777–782 [PubMed] [Google Scholar]
- Weiss LA, Veenstra-VanderWeele J, Newman DL, Kim SJ, Dytch H, McPeek MS, Cheng S, Ober C, Cook EH, Abney M (2004) Genome-wide association study identifies ITGB3 as a QTL for whole blood serotonin. Eur J Hum Genet 12:949–954 [DOI] [PubMed] [Google Scholar]
- Weissman MM, Olfson M (1995) Depression in women: implications for health care research. Science 269:799–801 [DOI] [PubMed] [Google Scholar]
- Wirz-Justice A, Lichtsteiner M, Feer H (1977) Diurnal and seasonal variations in human platelet serotonin in man. J Neural Transm 41:7–15 [DOI] [PubMed] [Google Scholar]
- Yirmiya N, Pilowsky T, Nemanov L, Arbelle S, Feinsilver T, Fried I, Ebstein R (2001) Evidence for an association with the serotonin transporter promoter region polymorphism and autism. Am J Med Genet 105:381–386 [DOI] [PubMed] [Google Scholar]
- Yonan AL, Alarcon M, Cheng R, Magnusson PK, Spence SJ, Palmer AA, Grunn A, Juo SH, Terwilliger JD, Liu J, Cantor RM, Geschwind DH, Gilliam TC (2003) A genomewide screen of 345 families for autism-susceptibility loci. Am J Hum Genet 73:886–897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zubenko GS, Maher B, Hughes HB III, Zubenko WN, Stiffler JS, Kaplan BB, Marazita ML (2003) Genome-wide linkage survey for genetic loci that influence the development of depressive disorders in families with recurrent, early-onset, major depression. Am J Med Genet 123B:1–18 [DOI] [PubMed] [Google Scholar]