Abstract
This study investigates the potential of Trichoderma harzianum to mitigate the effects of Beet curly top Iran virus (BCTIV) on tomato plants. Tomato seedlings at the four-leaf stage were treated with a T. harzianum suspension and subsequently agroinoculated with a BCTIV infectious clone. The experiment included four treatments: mock plants (C), BCTIV-inoculated plants (V), Trichoderma-treated plants (T), and plants both infected with BCTIV and treated with Trichoderma (TV). Three weeks post-inoculation, symptom development and virus accumulation were assessed. At 45 days post-inoculation, root colonization by T. harzianum was confirmed. The disease severity index indicated a significant reduction in TV plants compared to V plants. Virus accumulation was also significantly lower in TV plants. Real-time PCR analysis showed increased expression of defense-related genes (HSP90, AGO2a, PR1) in TV plants, suggesting enhanced plant defense responses. Additionally, TV plants exhibited the highest fresh and dry weight among all groups. The presence of T. harzianum spores in the roots of TV plants confirmed successful colonization. These findings demonstrate that T. harzianum enhances tomato resistance to BCTIV by activating plant defense mechanisms, reducing disease severity and viral replication, promoting healthier growth and greater biomass in the treated tomato plants.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-025-96068-6.
Keywords: Biocontrol, Gene expression, Signaling pathways, Viral accumulation
Subject terms: Plant biotechnology, Biochemistry, Biological techniques, Biotechnology, Molecular biology, Plant sciences
Introduction
The Trichoderma genus has gained significant attention as a valuable biological agent in diverse crop systems, making it an attractive choice for integrated disease management strategies1. Numerous species of Trichoderma have been known for their exceptional ability to enhance biofertilization properties, induce resistance, and serve as effective commercial biopesticides in controlling a wide range of plant pathogens2–4. Trichoderma enhances nutrient uptake, modifies the rhizosphere, and bolsters tolerance to environmental and biological stresses5. The interactions between Trichoderma and plants are characterized by intricate signaling pathways, hormone modulation, nutrient enhancement, and activation of the defense system, collectively contributing to the observed benefits on plant growth, seed germination, and induced resistance6. Trichoderma fungi utilize various biocontrol techniques, including resource competition, antibiosis, and parasitism, achieved through the synthesis of hydrolytic enzymes and metabolites7. These fungi demonstrate high adaptability in the rhizosphere and can effectively induce systemic defense responses in host plants by activating diverse signaling pathways, such as JA/ET-dependent induced systemic resistance in cucumbers8–12. Phytohormone-mediated mechanisms are pivotal in enhancing plant defense responses against pathogens, including systemic acquired resistance (SAR) and induced systemic resistance. Studies reveal that Trichoderma strains can activate both SAR and induced systemic resistance pathways in plants, with interactions influenced by phytohormones and detoxifying enzymes produced by Trichoderma10,13,14. Specifically, Trichoderma interactions involve a complex interplay of phytohormones such as abscisic acid (ABA), ethylene, jasmonic acid (JA), and auxin, modulating defense pathways including SA-independent ISR and JA/ET responses15–23.
Trichoderma synthesizes diverse proteins and metabolites that finely tune plant defense responses and developmental processes24. Notably, T. hamatum strain Th23 shows promising efficacy in suppressing Tomato mosaic virus (TMV) infection in tomato plants by inducing the overexpression of pathogenesis-related genes PR-1 and PR-7, thereby reducing TMV accumulation by 84.69% and promoting plant growth25. Similarly, a specific strain of T. harzianum has been shown to induce resistance against Cucumber mosaic virus (CMV) in tomato plants through biochemical and molecular pathways, while also alleviating various stresses when applied to tomato seeds26,27. Key transcription factors like WRKYs, MYBs, and MYCs serve as regulatory hubs in the transcriptional network of systemic defense following stress perception, while Trichoderma spp. may contribute to long-term priming in plants through epigenetic regulation involving histone modifications, DNA methylation, RNA-directed DNA methylation (RdDM) and regulation of MYB, PR-1, WRKY, AZI1 and EDS1 genes10. Additionally, substantial expression of plant defense-related PR genes is corroborated by the distinct expression patterns of hormone-responsive marker genes, as demonstrated in a study where Arabidopsis-T. virens interaction led to increased accumulation of salicylic acid (SA) and expression of SA-responsive marker genes such as PR-128. Heat shock proteins (Hsps) play a crucial role in protein folding or re-folding, particularly during stress conditions, and their expression is dynamic, occurring in response to stress as well as during basal metabolism. Research on Trichoderma Hsps primarily focuses on their overexpression in plants or Trichoderma itself, leading to enhanced stress tolerance29.
The Becurtovirus genus, including Beet curly top Iran virus (BCTIV), is a significant threat to tomato plants globally, with symptoms such as leaf curling and thickening caused by its single-stranded DNA circular genome. BCTIV is transmitted by the leafhopper vector Circulifer hematoceps, posing challenges for control due to its complex epidemiology and broad host range. Utilizing resistant tomato cultivars and inducing plant defenses through chemical means offer promising strategies for managing BCTIV and other viral plant diseases30–32. This study endeavors to explore the groundbreaking impact of T. harzianum on the genetic defense mechanism of tomatoes infected with BCTIV, aiming to unravel the intricate dynamics of gene expression under the influence of this beneficial fungus. By meticulously examining the effects of T. harzianum colonization on BCTIV replication, symptom manifestation, and the modulation of key defense genes, we delve into a novel realm of understanding the interplay between fungal colonization and viral infection in tomato plants.
Material and methods
Trichoderma and BCTIV sources
Seeds of the tomato cultivar “Super Urbana” were acquired for this experiment. This particular cultivar has been previously identified as susceptible to BCTIV infection, as documented by Khoshnazar and Eini30. Consequently, it was selected as an appropriate candidate to examine the potential impact of T. harzianum on BCTIV infection. The infectious clone of BCTIV in Agrobacterium tumefaciens was obtained from the virology collection at the Department of Plant Protection, University of Zanjan33 (Fig. 1a). Meanwhile, T. harzianum (strain T12-N) was provided by Ferdowsi University of Mashhad (Mashhad, Iran). That deposited in the Ferdowsi University collection by 89124FM number.
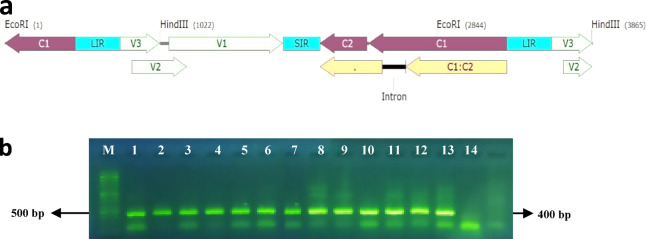
Fig. 1.
(a) The schematic diagram of BCTIV Infectious clone with ORFs, (b) Electrophoresis of PCR product for Agroinuclated plants at 21 dpi by C4F/C4R Primers, (Lane 1–3: Inoculated by BCTIV and treated by 108 Trichoderma spore ml−1 (T3V), Lane 4–6: Inoculated by BCTIV and treated by 106 Trichoderma spore ml−1 (T2V), Lane 7–9: Inoculated by BCTIV and treated by 103 Trichoderma spore ml−1 (T1V), Lane 10–12: Inoculated by BCTIV and not treated by Trichoderma (CV), Lane 13: Positive Control, Lane 14: Healthy Plant, M: DNA ladder 100 bp (SMOBIO, Taiwan).
Experimental design
To ensure seed sanitation, tomato seeds were subjected to a standard sterilization protocol. Initially, the seeds were treated with a 1% sodium hypochlorite solution, followed by thorough rinsing with sterile distilled water. Subsequently, the sterilized seeds were left to air dry at room temperature. For optimal germination conditions, germination trays composed of sand, peat moss, and perlite were used to plant the treated seeds. All material that are used for cultivation were sterilized before use. After 2 weeks, the emerging seedlings were carefully transplanted into 2 L pots. These pots were filled with a sterile and free of pathogen and Trichoderma mixture of perlite, field soil, and sand in a proportionate ratio of 1:1:1. This clean substrate composition was chosen to provide an ideal growth environment for the tomato seedlings. Experiments were performed using a completely randomized design (CRD) with four treatments and four replicates in two times that each pots including three plants (Each treatment 18 plants including nine BCTIV-infected and nine plant non-infected). Treatment groups involving different concentrations of T. harzianum spores in inoculated and non-inoculated plants with BCTIV. The treatments included: (1) control plants without inoculation with BCTIV or treatment by Trichoderma (C), (2) control inoculated plants with BCTIV but without treatment by Trichoderma (CV), (3) plants treated with 103, 106, and 108 spores ml−1 of Trichoderma and not inoculated with BCTIV (T1, T2, and T3, respectively), and (4) plants treated with 103, 106, and 108 spores ml−1 of Trichoderma and inoculated with BCTIV (T1V, T2V, and T3V, respectively). Under controlled greenhouse conditions, the temperature was maintained at 26 °C for 16 h and 20 °C for 8 h during the dark period while ensuring a relative humidity of 70%.
Treatment with T. harzianum and agroinoculation of BCTIV
After transferring the tomato seedlings to the pots, a suspension of T. harzianum spores was prepared in three different concentrations (103, 106, and 108 spores ml−1) and added to the crown of the plans in each pot. Trichoderma inoculum prepared by serial dilution was used at a concentration of 50 ml kg−1 in each pot. The application was repeated twice, with a 1-week interval between applications. Plants were not irrigated for 3 days after each treatment to allow the fungus to establish in the crown area of the plant. The verified infectious BCTIV clone was injected into V and TV plants by Agrobacterium cells 1 week following the last T. harzianum treatment30. Plants were observed for the emergence of symptoms, and samples were taken 21-, 32-, and 45 days post-virus inoculation (dpi).
Disease severity evaluation
Disease severity was evaluated in all treated and untreated plants 21-, 32-, and 45 days post-inoculation (dpi). Disease severity responses were assessed using a numerical scale grade (0–4) according to Friedmann et al.34, with some modifications; where, 0 = symptomless; 1 = mild leaf thickening; 2 = mild leaf thickening and curling; 3 = leaf thickening, leaf curling, swollen veins, and stunting; 4 = leaf thickening, curling, swollen veins, epinasty and stunting of the whole plant. Each plant was ranked separately, and the disease severity was estimated using the following formulae35.
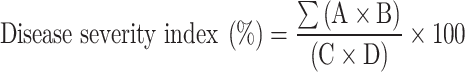 |
where A: disease rating, B: number of plants in each rating grade, C: total number of observed plants, D: maximum disease rating.
Analysis of variance was used for the calculated disease severity data to statistically differentiate (ANOVA-Bonferroni post hoc-test, P < 0.05) the response of each treatment against virus infection.
Virus accumulation
Leaves from all treatments were collected 21 days after BCTIV inoculation and total DNA was extracted using the Gem-CTAB technique. A NanoDrop 2000 spectrophotometer (Thermo Scientific, USA) was used to measure the DNA content, and PCR was used to detect BCTIV infection by amplifying a segment corresponding to the C4 gene using the specific primers (BCC4-F/BCC4-R) (Table 1). Real-time PCR was used to measure the amount of virus in each treatment. The qPCR mix included 100 ng of DNA, 1 µl of each specific primer (10 pmol) (BCC4-F and BCC4-R), and SYBER Green (SMOBiO, Taiwan). As a reference gene, the actin gene was utilized. The optimized qPCR protocol consisted of 95 °C/3 min one cycle, 35 cycles of 95 °C/20 s, 50 °C/30 s, 72 °C/30 s, and a 72 °C/3 min final extension. The approach was outlined by calculating the relative amount of virus in each sample (log2)36.
Table 1.
The primer sequences utilized in this study.
| Primers | Sequences (5′ to 3′) | Reference |
|---|---|---|
|
BC C4 F BC C4 R |
CAACACCAAGGAGGAGTTC TTACGAAATATATATTTTG |
Ebrahimi et al.50 |
|
ACT F ACT R |
GCCCCTCGTCTGTGACAA CCTTGGCCGACCCACAATA |
Tokhmechi et al.51 |
|
PR1 F PR1 R |
ATGCTCCATTTAAAGGAC AGGACATGTTGACGCAAC |
Tokhmechi et al.51 |
|
HSP90 F HSP90 R |
GCACAGGCACTTAGGGACTC CTGAGGTGAGAAGGGCAGTC |
Tokhmechi et al.51 |
|
AGO2a F AGO2a R |
CTCCTTCACCATTCCCACAC CCCCCAAAGCAGATAAAACA |
Tokhmechi et al.51 |
Gene expression analysis
Using the GeneAll RiboEx kit (GenAll, South Korea), total RNA was isolated from tomato leaves 21 days post inoculation. The NanoDrop 2000 spectrophotometer (Thermo Scientific, USA) measured the amount of RNAs present. We created cDNA from 1000 ng of RNA using the Easy™ cDNA Synthesis Kit (Partous, Itan). The transcript levels of three genes, including pathogenesis-related protein 1 (PR-1), heat shock protein 90 (HSP90), and argonaute-2a (AGO2a) were measured by Real-Time PCR (Rotor-Gene Q, QIAGEN). ACT was also employed as a housekeeping gene. 3 µl of SYBR Green (SMOBiO, Taiwan), 0.3 µl of forwards and reverse primers (10 pmol), 2 µl of cDNA (100 ng/µl), and 9.4 µl of ddH2O were used in the reactions. The qPCR condition consisted of 95 °C for 3 min, 35 cycles of 95 °C for 20 s, 50 °C for 30 s, and 72 °C for 30 s, followed by 72 °C for 1 min. The relative expression of genes was calculated using the 2−ΔΔCt method. The actin gene was used as a reference gene by using ACTF/ACTR primer pairs (Table 1). Each sample’s relative BCTIV copy number was determined when compared to the actin gene. The approach was outlined by calculating the relative amount of virus in each sample (log2)36. Three biological replicates were performed for each treatment. The mean of biological replicates was statistically analyzed using one-way ANOVA and Bonferroni post-hoc test, comparing virus accumulation between treatments.
Enzymes activity assay
Catalase and peroxidase enzyme activity were evaluated37. Catalase activity was determined by measuring the rate of hydrogen peroxide disappearance at 240 nm using a spectrophotometer, with the rate of decomposition calculated using an extinction coefficient of 39.4 mM−1 cm−1. Ascorbate peroxidase activity was assessed by monitoring the conversion of ascorbate to monohydrate ascorbates in the presence of hydrogen peroxide, with absorbance changes measured at 290 nm for 30 s and the rate of oxidized ascorbate calculated using an extinction coefficient of 6.22 mM−1 cm−1. Peroxidase enzyme activity was quantified based on the consumption of μmol of NADH per minute per mg of protein.
GC–MS analysis
To use GC–MS to analyze various components, such as enzymes and phytochemicals, homogenized solution containing 3 ml of 70% methanol and 1000 mg of leaf tissue from each treatment was centrifuged at 3000g for 5 min. After mixing the supernatant with 5 ml of 70% methanol and centrifuging, 3 ml of 100% methanol was added and combined with the supernatant. The integrated solution was filtered using filter paper and then dried. The dried mixture was then dissolved in 1 ml of methanol (80%) for the GC–MS analysis. The GC–MS analysis was carried out using an Agilent 6890 series mass spectrometer and an Agilent 5973 (SpectraLab Scientific, Canada) GC with a Varian column, VF-1 ms column length of 30 m, inner diameter of 0.25 mm, and film thickness of 0.5 µm. The sample splitting ratio was set at 1:100, and the injector temperature was maintained at 280 °C. A high-purity helium gas (99.999%) was utilized as the carrier gas, with an injection volume of 1 µl and a constant flow rate of 1 ml per min. The oven temperature program consisted of a total running period of 42 min, starting at an initial temperature of 80 °C for 2 min, then gradually increasing to 280 °C at a rate of 10 °C per min for 20 min. For the mass spectrometry (MS) analysis, the quadrupole analyzer was operated in electron-influenced ionization mode (EI) with a source temperature of 230 °C. The electron energy was set at 70 eV, and a solvent delay of 3 min was employed. Data was analyzed using the Wiley mass spectrum library and the NIST MS spectral software.
Statistical analysis
Analysis of variance (ANOVA-one-way) was carried out statistically using either GenStat (Version 14) or GraphPad Prism Software (version 10), using a completely randomized design. Comparison among means was determined by using Fisher’s protected least significant difference (LSD) test (P ≤ 0.05). Each experiment was run in four replicates, and all the experiment was repeated two time individually, and the significant differences in each treatment group were assessed at P ≤ 0.05. The data obtained from two individual experiments were combined and analyzed together. At least three plants were selected at random from the 16 plants within each condition across both repeated experiments.
Root colonization
Finally, the colonization of Trichoderma isolates in the plant root was assessed at 45 days post-inoculation (dpi). The roots were collected, washed with water to remove adhering soils, and divided into upper, middle, and lower parts. Each part was then divided into small sections and surface-disinfected by immersing in 1% sodium hypochlorite solution for 30 s to survey the endosymbiosis of Trichoderma with tomato root. The disinfected sections were cultured on petri dishes containing PDA medium, and the growth rate of Trichoderma isolates was observed at 28 °C after 10 days38. Additionally, soil samples from the Trichoderma-treated plots were collected weekly to evaluate the Trichoderma population.
Results
Phenotypic responses
The result showed that agroinoculated plants were infected by BCTIV (Fig. 1b). Following, phenotypic responses were observed in plants infected with BCTIV, treated with trichoderma, and untreated. The infected plants exhibited various symptoms, such as leaf curling, thickening, stunting, blistering, epinasties, and reduced leaf size. Application of Trichoderma increased the biomass at T1V, T2V, and T3V treatments compared to control plants, resulting in significantly higher fresh weight (FW) and dry weight (DW) in TV plants (Fig. 2). The analysis revealed fresh and dry weight in plants-treated by Trichoderma (T) have a significant difference with plants that was not treated (C). The most disease severity was observed in CV plants than TV plants at 21-, 32-, and 45 days post-inoculation (dpi). The symptoms were decreased in plants treated with 103, 106, and 108 spores ml−1 of Trichoderma at 21, 32, and 45 dpi compared to infected plants not treated with Trichoderma (Fig. 3a). Mild symptoms, including leaf curling and stunting, were observed in young leaves in the CV, T1V, T2V, and T3V treatments (Fig. 4).
Fig. 2.
The biomass analysis in different treatment, fresh weight (a) and dry weight (b) of BCTIV-infected and non-infected tomato plants, compared to untreated control plants (C). C (Control), T1 (103 spores ml−1), T2 (106 spores ml−1), T3 (108 spores ml−1). Grouping was determined by Fisher’s protected LSD test at significance levels P < 0.05, where similar letters indicate non-significant differences among treatments. Vertical bars represent the standard deviation (SD).
Fig. 3.
The impact of Trichoderma application in tomato infected by BCTIV, (a) Plant Disease Severity at 21-, 32, and 45 dpi. Different letters indicate significant differences (P ≤ 0.05) among treatments. (b) the result of qPCR for virus accumulation at 21 dpi. Significant differences in different dpi’s among various treatments were demonstrated by the asterisks, where significance levels are indicated as follows: *** (P < 0.0001), ** (P < 0.001), and * (P < 0.01), based on the LSD test at the 0.05 level of significance. The error bars represent standard deviation.
Fig. 4.

The symptoms of different treatment in this study. (A) Mock plants (not infected- not treated (C), (B) Inoculated by BCTIV not treated by Trichoderma (CV), (C) not infected by BCTIV—treated by 103 spore ml−1 (T1), (D) Inoculated by BCTIV, treated by 103 spore ml−1 (T1V), (E) not infected by BCTIV—treated by 106 spore ml−1 (T2), (F) Inoculated by BCTIV, treated by 106 spore ml−1 (T2V) (G) not-inoculated by BCTIV, treated by 108 spore ml−1 (T3), (H) Inoculated by BCTIV, treated by 108 spore ml−1 (T3V).
Virus accumulation
RT-qPCR analysis of the formed and expanded leaf at the most terminal part of the plant at 21 dpi showed that the rate of virus accumulation decreased in TV plants compared to CV plants. T3V plants exhibited the lowest virus accumulation compared to CV and, T1V and T2V plants (Figs. 3b and 4).
Gene expression
Using RT-qPCR, the expression of some genes involved in defense mechanism in C, V, and TV plants was examined at 21 dpi, when the first BCTIV symptoms appeared. Infected tomato young leaves with BCTIV stimulated the expression of the PR1, HSP90, and AGO2a genes. The PR1, HSP90, and AGO2a genes were clearly expressed more in T1V, T2V, and T3V plants than in control plants, according to the RT-qPCR data. Compared to CV and C plants, TV plants showed a more significant change in the expression of examined genes (Fig. 5).
Fig. 5.
Gene expression in the interaction of Trichoderma and BCTIV infection. The result of qPCR of (A) PR1, (B) HSP90, and (C) AGO2a with different treatments. Significant differences in different dpi’s among various treatments were demonstrated by the asterisks, where significance levels are indicated as follows: **** (P < 0.00001), *** (P < 0.0001), ** (P < 0.001), * (P < 0.01), and ns (non-significant) based on the LSD test at the 0.05 level of significance. The error bars represent standard deviation.
Enzyme activities
The lowest values of total POD and CAT activity were assayed in the plant that not treatment by Trichoderma and infected by BCTIV (CV). The lowest POD and CAT activity values were obtained compared to the highest values of the mentioned enzymes in the T3V and T2V treatments based on a one-way ANOVA-LSD test (P < 0.05). Total POD and CAT activity in CV remained significantly lower compared to TV and T22 treatments. The plants inoculated with BCTIV and treated with T22 as the control plant exhibited the most downward trend of POD and CAT activity, while the highest values were observed at T3V and T2V treatments, respectively (Fig. 6).
Fig. 6.
Enzyme activity in the interaction of different concentration of Trichoderma spores and BCTIV infection. Left) Peroxidase activity Right) Catalase activity. Significant differences in different dpi’s among various treatments were demonstrated by the asterisks, where significance levels are indicated as follows: **** (P < 0.00001), and ** (P < 0.001), based on the LSD test at the 0.05 level of significance. The error bars represent standard deviation.
Gas chromatography mass spectrometry (GC/MS) analysis
Around 28 compounds were detected in different treatments, and then a few compounds were selected based on their unique nature and relative abundance of the peaks. The identity of the compounds was confirmed through the NIST MS library of spectral programs and the Wiley mass spectral library. Isopropyl stearate, dodecanol, butyl tetradecyl, phthalic acid, isopropyl stearate, octanol, dimethyl benzene, eicosane, pentadecane, and 1,2 octadecanoic acid were observed in the compounds detected in the treatments. Treated plants with 103 spores per ml of Trichoderma and inoculated with the virus (T1V) had the highest detected metabolite of isopropyl stearate, around 14%, while eicosane (0.8%) was the lowest detected metabolite. In addition, the highest and lowest amount of Isopropyl stearate (16.2%) and 1,2-Octadecanoic acid (0.2%) was seen in the T3V treatment, respectively. Additionally, the highest amounts of compounds in T1V and T3V treatments were related to the isopropyl stearate metabolite, possibly due to viral infection.
Discussion
It has been demonstrated that the biocontrol agent known as T. harzianum may increase resistance of plant to several different types of biotic and abiotic stressors25. According to the findings of this research, T. harzianum has the potential to lessen the likelihood that tomato plants will become infected with BCTIV. The results suggested that treatment with Trichoderma considerably decreased the disease’s severity and the pace at which the virus accumulated in tomato plants that had been infected with BCTIV through an increase in the expression of defense-related genes such as HSP90, AGO2a, and PR1, the symbiotic relationship between tomato plants and T. harzianum produced systemic resistance in the tomato plants. According to the findings of this study, treatment with Trichoderma led to enhanced plant development and an increase in both the fresh and dry weight of tomato plants. Additionally, Trichoderma colonization was confirmed in both primary and secondary roots (Fig. 7), supporting its role in plant protection. Treatment with Trichoderma also enhanced plant growth and significantly increased both the fresh and dry weight of tomato plants.
Fig. 7.
Isolation and identification of Trichoderma harzianum from tomato roots. (A,B) Colony and microscopic view of T. harzianum in primary roots. (C,D) Colony and microscopic view of T. harzianum in secondary roots.
Trichoderma species are well-known for their adaptability as biocontrol agents and have been widely used in managing plant diseases39,40. As there are no synthetic chemical compounds available for controlling viral diseases, the T22 strain of T. harzianum and T. viridae has been studied as a novel strategy for controlling viruses40,41. In addition, Trichoderma spp. is responsible for the synthesis of several metabolites that not only have a substantial impact on plant development but also encourage localized and systemic resistance as well as stress tolerance in plants. Within the rhizosphere of plants, some strains of Trichoderma have established themselves as intercellular root colonizers. Trichoderma, like other beneficial microbes, stimulates ISR via pathways dependent on JA and ET and triggers priming responses in the plant. The effects of this stimulation are contingent on some factors, including the strains and concentrations of trichoderma that were used, the plant material, the developmental stage of the plant, and the timing of the interaction. Trichoderma is also responsible for producing the phytohormones ET and IAA, which play an essential role in facilitating the link between the growth of plants and their defensive responses.
Many distinct features of molecular pathways need to be well known regarding how Trichoderma affects plant resistance against viral infection. Trichoderma spp. is known to be capable of producing a wide variety of bioactive compounds, some of which include chitinases, proteases, and polysaccharides, all of which have the potential to either directly or indirectly trigger plant defense responses. These substances have the potential to set off the creation of reactive oxygen species (ROS), plant hormones, and other signaling molecules in plants. These molecules are responsible for regulating the expression of genes associated with plant defense and inducing systemic resistance in plants. Trichoderma’s capacity to colonize plant roots and support the development of helpful microbes is another probable method via which it increases plant resistance to viral infection. This process might be responsible for the fungus’s antiviral effects. Trichoderma may also improve plant nutrient absorption and increase plant development by secreting plant growth-promoting chemicals like auxins and cytokinins. This allows Trichoderma to do both of these things.
According to the results, the accumulation of BCTIV in young plant leaves was dramatically reduced when tomato plants were treated with T. harzianum (Fig. 3). At the same time, the total amount of biomass was enhanced in T. harzianum-treated plants. Prior research revealed decreased symptom intensity and lesser accumulation of Cucumber mosaic virus (CMV-Y) in colonized Arabidopsis plants42. Furthermore, a decrease in virus accumulation was observed in Nicotiana tabacum infected with Tobacco mosaic virus43. Because of this, the use of T. harzianum to decrease the virus accumulation and increase plant resistance can still be investigated. Plants infected with BCTIV may display apparent symptoms, including curled, swollen, yellowed, and stunted leaves. According to the findings of our study, the control plants (CV) had more severe symptoms than the TV plants at 21-, 32-, and 45-days post-inoculation (dpi). When compared to control plants, the severity of infection was found to be at its lowest in plants treated with T3V, while it was found to be at its greatest in plants treated with T1V. Based on the recent findings, tomato plants colonized with T. harzianum were less likely to get infected with BCTIV.
Trichoderma strains have successfully colonized the intercellular spaces of plant roots in the rhizosphere, thereby promoting both plant growth and the activation of pathogen defense mechanisms42,44. It has also been demonstrated that expressing Trichoderma genes in plants has positive benefits, particularly in reducing plant diseases and boosting resilience to unfavorable environmental factors. In this study, Trichoderma-inoculated (TV) plants expressed considerably more defense-related genes than control (CV) plants, including HSP90, PR1, and AGO2a. Additionally, these genes were expressed at greater levels in the early leaves of tomato plants that had received Trichoderma inoculation and BCTIV infection. This may partially explain the decreased viral concentration and infectivity in CV plants. Likewise, the expression of defense-related gene PR1 was significantly upregulated in plant treated with Trichoderma and subsequently infected with CMV. These results imply that tomato colonization following viral infection might enhance plant defense responses against BCTIV. This results in enhanced systemic acquired resistance in plant that treated by Trichoderma due to the high expression of defense genes45. The application of T. viride on PVY-infected potatoes leads to significant improvements in plant growth and defense responses, including increased levels of peroxidase, polyphenol oxidase, total proteins, and chlorophyll. Moreover, qPCR analysis reveals a substantial reduction in PVY accumulation and heightened expression of defense-related genes in Tvd44-treated plants, suggesting its potential as an effective agent against PVY infections in potatoes, possibly enhancing crop productivity40.
Plants possess highly efficient enzymatic antioxidant defense systems that work collaboratively to safeguard against oxidative damage by effectively neutralizing reactive oxygen species (ROS). The impact of ROS extends beyond oxidative stress, as it intricately modulates the expression of numerous genes and regulates vital processes such as growth, cell cycle regulation, programmed cell death (PCD), responses to abiotic stress, defense against pathogens, systemic signaling, and overall development44. This research showed that BCTIV dramatically boosted the activity of POD and CAT enzymes in TV plants, either alone or in combination with T. harzianum-T22, compared to control plants, which may induce defense responses, according to the previous studies41,46. The accumulation of CAT and POD in plants exposed to T22 suggests that these enzymes may enhance cell wall resistance and act as signals for the expression of protective genes. This could facilitate the retention of H2O2 within the cell, subsequently promoting SA formation41. In nature, peroxidases are a family of antioxidant enzymes that catalyze the oxidation of numerous electron donor substrates and the breakdown of hydrogen peroxide (H2O2). Throughout a plant’s life cycle, plant peroxidases are essential for the physiological processes of growth and development47. Last but not least, our research found that tomatoes infected with the T1V and T3V pathogens contained an isopropyl stearate substance, one of 28 metabolites with antibacterial characteristics. It has been documented that metabolites are produced as a defense mechanism during interactions between plants and pathogens. One of the molecular processes thought to be involved in the interaction between Trichoderma and BCTIV is the generation of phytohormones by the plant, such as salicylic and jasmonic acids. Trichoderma may stimulate the production of genes essential for SA and JA biosynthesis, activating the plant’s BCTIV defense systems. Because it promotes the expression of genes associated with pathogenesis (PR), which encode proteins with antiviral activity, SA is particularly crucial in the defense against viral infections. Plant cell wall composition regulation has been proposed as another molecular mechanism in the interaction between Trichoderma and BCTIV. Trichoderma creates enzymes that break down cell walls, such as chitinases and glucanases, which can change the structure of plant cell walls and trigger the production of genes that strengthen cell walls. As a result, both the physical barrier against BCTIV infection and the signaling pathways involved in plant defense responses were strengthened13. Trichoderma has been shown to enhance the production of flavonoids and alkaloids, two volatile organic compounds (VOCs) that play a significant role in immune responses and antiviral activity. It has been approved that Trichoderma could stimulate the biosynthesis of these bioactive compounds46,48,49.
The current study showed that T. harzianum forms symbiotic relationships with host plants that colonize the roots of plants and boost crop yield and stress tolerance. In addition, there has been an increased interest in using Trichoderma-based biocontrol agents to manage plant diseases sustainably and environmentally, lessening the need for chemical pesticides. More research is necessary to improve the use of Trichoderma-based products in various agro-ecosystems and clarify the fundamental processes behind Trichoderma-mediated interactions between plants and microorganisms.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
We would like to appreciate from the University of Zanjan.
Author contributions
D.K. and O.E. conceived and designed the study. S.S. conducted the experiments and collected the data. D.K. and S.S. analyzed the data and wrote the manuscript. R.H advised for a part of project and data analysis. All authors contributed to interpreting the results, reviewing and editing the manuscript, and approving the final version.
Funding
This work financially supported by University of Zanjan.
Data availability
The datasets generated and/ during the current study are available in the NCBI repository and received accession numbers. Accession Numbers, PQ031222, PQ031223, PQ031224.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Debbi, A., Boureghda, H., Monte, E. & Hermosa, R. Distribution and genetic variability of Fusarium oxysporum associated with tomato diseases in Algeria and a biocontrol strategy with indigenous Trichoderma spp.. Front. Microbiol.9, 282 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Harman, G. E. Myths and dogmas of biocontrol changes in perceptions derived from research on Trichoderma harzinum T-22. Plant Dis.84, 377–393 (2000). [DOI] [PubMed] [Google Scholar]
- 3.Harman, G. E., Howell, C. R., Viterbo, A., Chet, I. & Lorito, M. Trichoderma species—Opportunistic, avirulent plant symbionts. Nat. Rev. Microbiol.2, 43–56 (2004). [DOI] [PubMed] [Google Scholar]
- 4.Coppola, M. et al. Trichoderma harzianum enhances tomato indirect defense against aphids. Insect Sci.24, 1025–1033 (2017). [DOI] [PubMed] [Google Scholar]
- 5.López-Bucio, J., Pelagio-Flores, R. & Herrera-Estrella, A. Trichoderma as biostimulant: Exploiting the multilevel properties of a plant beneficial fungus. Sci. Hortic.196, 109–123 (2015). [Google Scholar]
- 6.Sood, M. et al. Trichoderma: The “secrets” of a multitalented biocontrol agent. Plants9, 762 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vinale, F. et al. A novel role for Trichoderma secondary metabolites in the interactions with plants. Physiol. Mol. Plant Pathol.72, 80–86 (2008). [Google Scholar]
- 8.Carrero-Carrón, I. et al. Interactions between Trichoderma harzianum and defoliating Verticillium dahliae in resistant and susceptible wild olive clones. Plant Pathol.67, 1758–1767 (2018). [Google Scholar]
- 9.Hermosa, R., Viterbo, A., Chet, I. & Monte, E. Plant-beneficial effects of Trichoderma and of its genes. Microbiology158, 17–25 (2012). [DOI] [PubMed] [Google Scholar]
- 10.Morán-Diez, E. et al. The ThPG1 endopolygalacturonase is required for the Trichoderma harzianum–plant beneficial interaction. Mol. Plant Microbe Interact.22, 1021–1031 (2009). [DOI] [PubMed] [Google Scholar]
- 11.Mendoza-Mendoza, A. et al. Molecular dialogues between Trichoderma and roots: Role of the fungal secretome. Fungal Biol. Rev.32, 62–85 (2018). [Google Scholar]
- 12.Shoresh, M., Yedidia, I. & Chet, I. Involvement of jasmonic acid/ethylene signaling pathway in the systemic resistance induced in cucumber by Trichoderma asperellum T203. Phytopathology95, 76–84 (2005). [DOI] [PubMed] [Google Scholar]
- 13.Martínez-Medina, A. et al. Deciphering the hormonal signalling network behind the systemic resistance induced by Trichoderma harzianum in tomato. Front. Plant Sci.4, 206 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tucci, M., Ruocco, M., De Masi, L., De Palma, M. & Lorito, M. The beneficial effect of Trichoderma spp. on tomato is modulated by the plant genotype. Mol. Plant Pathol.12, 341–354 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Contreras-Cornejo, H. A., Macías-Rodríguez, L., Cortés-Penagos, C. & López-Bucio, J. Trichoderma virens, a plant beneficial fungus, enhances biomass production and promotes lateral root growth through an auxin-dependent mechanism in Arabidopsis. Plant Physiol.149, 1579–1592 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Perez, E. et al. The importance of chorismate mutase in the biocontrol potential of Trichoderma parareesei. Front. Microbiol.6, 1181 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yuan, M. et al. Involvement of jasmonic acid, ethylene and salicylic acid signaling pathways behind the systemic resistance induced by Trichoderma longibrachiatum H9 in cucumber. BMC Genom.20, 1–13 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Viterbo, A., Landau, U., Kim, S., Chernin, L. & Chet, I. Characterization of ACC deaminase from the biocontrol and plant growth-promoting agent Trichoderma asperellum T203. FEMS Microbiol. Lett.305, 42–48 (2010). [DOI] [PubMed] [Google Scholar]
- 19.Medeiros, H. A. D. et al. Tomato progeny inherit resistance to the nematode Meloidogyne javanica linked to plant growth induced by the biocontrol fungus Trichoderma atroviride. Sci. Rep.7, 40216 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Martínez-Medina, A. et al. Shifting from priming of salicylic acid-to jasmonic acid-regulated defences by Trichoderma protects tomato against the root knot nematode Meloidogyne incognita. New Phytol.213, 1363–1377 (2017). [DOI] [PubMed] [Google Scholar]
- 21.Korolev, N., Rav David, D. & Elad, Y. The role of phytohormones in basal resistance and Trichoderma-induced systemic resistance to Botrytis cinerea in Arabidopsis thaliana. Biocontrol53, 667–683 (2008). [Google Scholar]
- 22.Brotman, Y. et al. Trichoderma-plant root colonization: Escaping early plant defense responses and activation of the antioxidant machinery for saline stress tolerance. PLoS Pathog.9, e1003221 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Segarra, G. et al. Proteome, salicylic acid, and jasmonic acid changes in cucumber plants inoculated with Trichoderma asperellum strain T34. Proteomics7, 3943–3952 (2007). [DOI] [PubMed] [Google Scholar]
- 24.Truman, W., Bennett, M. H., Kubigsteltig, I., Turnbull, C. & Grant, M. Arabidopsis systemic immunity uses conserved defense signaling pathways and is mediated by jasmonates. Proc. Natl. Acad. Sci.104, 1075–1080 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Abdelkhalek, A., Al-Askar, A. A., Arishi, A. A. & Behiry, S. I. Trichoderma hamatum strain Th23 promotes tomato growth and induces systemic resistance against tobacco mosaic virus. J. Fungi8, 228 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vitti, A. et al. Beneficial effects of Trichoderma harzianum T-22 in tomato seedlings infected by Cucumber mosaic virus (CMV). Biocontrol60, 135–147 (2015). [Google Scholar]
- 27.Harman, G. E., Herrera-Estrella, A. H., Horwitz, B. A. & Lorito, M. Trichoderma–from basic biology to biotechnology. Microbiology158, 1–2 (2012). [DOI] [PubMed] [Google Scholar]
- 28.Aamir, M. et al. Transcriptomic characterization of Trichoderma harzianum T34 primed tomato plants: Assessment of biocontrol agent induced host specific gene expression and plant growth promotion. BMC Plant Biol.23, 552 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mota, T. M. et al. Hsp genes are differentially expressed during Trichoderma asperellum self-recognition, mycoparasitism and thermal stress. Microbiol. Res.227, 126296 (2019). [DOI] [PubMed] [Google Scholar]
- 30.Khoshnazar, F. & Eini, O. Response of tomato cultivars to agroinfection with Beet curly top Iran virus. J. Crop Prot.5, 473–482 (2016). [Google Scholar]
- 31.Heydarnejad, J., Keyvani, N., Razavinejad, S., Massumi, H. & Varsani, A. Fulfilling Koch’s postulates for beet curly top Iran virus and proposal for consideration of new genus in the family Geminiviridae. Adv. Virol.158, 435–443 (2013). [DOI] [PubMed] [Google Scholar]
- 32.GharouniKardani, S. et al. Diversity of Beet curly top Iran virus isolated from different hosts in Iran. Virus Genes46, 571–575 (2013). [DOI] [PubMed] [Google Scholar]
- 33.Eini, O., Sahraei, G. E. & Behjatnia, S. A. A. Molecular characterization and construction of an infectious clone of a pepper isolate of Beet curly top Iran virus. Mol. Biol. Res. Commun.5, 101 (2016). [PMC free article] [PubMed] [Google Scholar]
- 34.Friedmann, M., Lapidot, M., Cohen, S. & Pilowsky, M. A novel source of resistance to tomato yellow leaf curl virus exhibiting a symptomless reaction to viral infection. J. Am. Soc. Hortic. Sci.123, 1004–1007 (1998). [Google Scholar]
- 35.El Gamal, A. et al. Silver nanoparticles as a viricidal agent to inhibit plant-infecting viruses and disrupt their acquisition and transmission by their aphid vector. Arch. Virol.167, 85–97 (2021). [DOI] [PubMed]
- 36.Livak, K. J. & Schmittgen, T. D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods25, 402–408 (2001). [DOI] [PubMed] [Google Scholar]
- 37.Gapińska, M., Skłodowska, M. & Gabara, B. Effect of short-and long-term salinity on the activities of antioxidative enzymes and lipid peroxidation in tomato roots. Acta Physiol. Plant.30, 11–18 (2008). [Google Scholar]
- 38.Cai, F. et al. Colonization of Trichoderma harzianum strain SQR-T037 on tomato roots and its relationship to plant growth, nutrient availability and soil microflora. Plant Soil388, 337–350 (2015). [Google Scholar]
- 39.Mukherjee, P. K., Horwitz, B. A., Herrera-Estrella, A., Schmoll, M. & Kenerley, C. M. Trichoderma research in the genome era. Annu. Rev. Phytopathol.51, 105–129 (2013). [DOI] [PubMed] [Google Scholar]
- 40.Aseel, D. G. et al. Trichoderma viride isolate Tvd44 enhances potato growth and stimulates the defense system against potato virus Y. Horticulturae9, 716 (2023). [Google Scholar]
- 41.Vitti, A. et al. Trichoderma harzianum T-22 induces systemic resistance in tomato infected by Cucumber mosaic virus. Front. Plant Sci.7, 1520 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Elsharkawy, M. M., Shimizu, M., Takahashi, H., Ozaki, K. & Hyakumachi, M. Induction of systemic resistance against Cucumber mosaic virus in Arabidopsis thaliana by Trichoderma asperellum SKT-1. Plant Pathol. J.29, 193 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Luo, Y. et al. Antimicrobial peptaibols induce defense responses and systemic resistance in tobacco against tobacco mosaic virus. FEMS Microbiol. Lett.313, 120–126 (2010). [DOI] [PubMed] [Google Scholar]
- 44.Gill, S. S. & Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem.48, 909–930 (2010). [DOI] [PubMed] [Google Scholar]
- 45.Mathioudakis, M. M. et al. Pepino mosaic virus triple gene block protein 1 (TGBp1) interacts with and increases tomato catalase 1 activity to enhance virus accumulation. Mol. Plant Pathol.14, 589–601 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bai, B. et al. Trichoderma species from plant and soil: An excellent resource for biosynthesis of terpenoids with versatile bioactivities. J. Adv. Res.49, 81–102 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Inaba, J.-I., Kim, B. M., Shimura, H. & Masuta, C. Virus-induced necrosis is a consequence of direct protein–protein interaction between a viral RNA-silencing suppressor and a host catalase. Plant Physiol.156, 2026–2036 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lombardi, N. et al. Effect of Trichoderma bioactive metabolite treatments on the production, quality, and protein profile of strawberry fruits. J. Agric. Food Chem.68, 7246–7258 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Khan, R. A. A., Najeeb, S., Hussain, S., Xie, B. & Li, Y. Bioactive secondary metabolites from Trichoderma spp. against phytopathogenic fungi. Microorganisms8, 817 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ebrahimi, S., Eini, O., Koolivand, D. & Valerman, M. The Rep and C1 of Beet curly top Iran virus represent pathogenicity factors and induce hypersensitive response in Nicotiana benthamiana plants. Virus Genes.58, 550–559 (2022). [DOI] [PubMed]
- 51.Tokhmechi, K., Eini, O., El Gamal, A., & Koolivand, D. Eco-friendly chitosan polymer mitigates disease severity and mediates plant resistance against Beet curly top Iran virus in tomato. Ann. Appl. Biol.184, 326–338 (2024).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated and/ during the current study are available in the NCBI repository and received accession numbers. Accession Numbers, PQ031222, PQ031223, PQ031224.