Abstract
There is currently no reliable predictive tool for late recurrence in hormone receptor (HR)-positive, human epidermal growth factor receptor 2 (HER2)-positive breast cancer. This study aimed to explore the potential of the clinical treatment score post-5l̥years (CTS5) as a predictive tool for long-term survival beyond 5 years in patients with specifically HR-positive, HER2-positive breast cancer. We collected patient-level data from the HERceptin Adjuvant (HERA) (BIG1-01; ClinicalTrials.gov identifier: NCT00045032) trial. Our investigation focused on assessing the risk of late distant recurrence (DR) and overall survival (OS) according to the CTS5 risk score as continuous value and CTS5 stratification risk groups. A total of 1,818 patients with HR-positive, HER2-positive breast cancer were included in this analysis. The CTS5 score, as a continuous variable, emerged as an independent prognostic factor for both late DR (adjusted HR, 2.05; 95% CI, 1.63-2.58; P < 0.001) and OS (adjusted HR, 2.02; 95% CI, 1.58-2.58; P < 0.001), respectively. In addition, multivariable analysis showed a significant association between the high-risk group and adverse outcomes in late DR (adjusted HR, 2.76; 95% CI, 1.84-4.13; P < 0.001) and OS (adjusted HR, 2.44; 95% CI, 1.59-3.73; P < 0.001) compared to low/intermediate group. Consistent results were observed, regardless of age or administration of HER2-targeted therapy. CTS5 is a useful prognostic tool for predicting late DR and OS in HR-positive, HER2-positive breast cancer patients. Extension of endocrine therapy should be actively considered in patients with CTS5 high-risk group.
Subject terms: Breast cancer, Metastasis, Tumour biomarkers
Introduction
In the treatment of early-stage hormone-receptor (HR)-positive, human epidermal growth factor receptor 2 (HER2)-negative breast cancer, adjuvant endocrine therapy for 5 years has become a standard practice due to its proven efficacy in reducing recurrences and mortality1. Nevertheless, a substantial number of women still face the risk of late recurrences even after completing the adjuvant endocrine therapy with ~50% of recurrences occurring beyond the initial 5 years2,3. Several clinical trials have suggested that patients with a more extensive tumor burden were likely to benefit from extending endocrine therapy after 5 years4,5. However, there are several concerns regarding the endocrine therapy extension, including potential overtreatment and adverse effects, as well as adherence to endocrine therapy.
Currently, several multi-gene assays, including Breast Cancer Index (BCI), MammaPrint, EndoPredict, Oncotype DX, and PAM50, have demonstrated potential for predicting late recurrence in HR-positive, HER2-negative breast cancer6. Particularly, BCI has been specifically validated for its ability to predict the benefit of extended endocrine therapy7–9. However, the routine application of these multi-gene assays for predicting late recurrence in all patients remains challenging in daily clinical practice. The Clinical treatment score post-5 years (CTS5) is one of the available tools, designed to predict the risk of late relapse occurring between 5–10 years after diagnosis for postmenopausal women. CTS5 is based on clinicopathologic parameters, including tumor size, nodal status, age, and histologic grade10. This model was developed and validated using data from two major randomized clinical trials, the ATAC (Arimidex, Tamoxifen, Alone or Combination) trial11 and the BIG (Breast International Group) 1-98 trial12. Low CTS5 scores are indicative of a very low risk of late distant recurrence, suggesting that extended endocrine therapy may not be necessary for this specific subgroup.
Approximately 10% of breast cancers belong to HR-positive, HER2-positive subtype13,14. While patients with HR-positive, HER2-positive breast cancer also tend to experience late relapses compared to those with HR-negative, HER2-positive breast cancer, or triple-negative breast cancer15,16, the optimal duration of endocrine therapy for this subtype remains uncertain. The ATAC and BIG 1-98 trials, which formed the basis for the development and validation of CTS5, enrolled patients during the late 1990s and early 2000s and, as such, did not evaluate HER2 status. Consequently, the clinical utility of CTS5 in this specific context is still questionable.
This study aimed to determine whether the CTS5 could serve as a valuable tool for predicting long-term survival beyond 5 years in patients with HR-positive, HER2-positive breast cancer. To achieve this, we utilized patient-level data from the HERceptin Adjuvant (HERA) (BIG1-01; ClinicalTrials.gov identifier: NCT00045032) trial17. We also assessed the clinical validity of CTS5 in relation to several factors, such as age and administration of trastuzumab.
Results
Baseline characteristics
The study included a total of 1818 patients with HR-positive, HER2-positive breast cancer. The patient demographics are summarized in Table 1. Of total, 1109 (61.0%) patients were aged 50 or less, and 1046 (57.5%) patients had tumor grade III. Most patients (95.5%) had pT1 or T2 stage. In terms of pN stage, 37.8% of patients were categorized as pN0, 36.4% as pN1, and 25.7% as pN2. Besides, 19.7% of patients received ovarian-function suppression, 33.3% received trastuzumab for 1 year, and 34.9% received trastuzumab for 2 years.
Table 1.
Baseline characteristics of study participants according to CTS5 risk group
| Variables | Low (N = 403) | Intermediate (N = 596) | High (N = 819) | Total (N = 1818) | P |
|---|---|---|---|---|---|
| Age | <0.001 | ||||
| ≤ 50 | 305 (75.7) | 376 (63.1) | 428 (52.3) | 1109 (61.0) | |
| > 50 | 98 (24.3) | 220 (31.0) | 391 (47.7) | 709 (39.0) | |
| ER | 0.320 | ||||
| Positive | 368 (91.3) | 535 (89.8) | 754 (92.1) | 1657 (91.1) | |
| Negative | 35 (8.7) | 61 (10.2) | 65 (7.9) | 161 (8.9) | |
| PRa | 0.857 | ||||
| Positive | 281 (72.8) | 404 (71.4) | 550 (44.5) | 1235 (72.2) | |
| Negative | 105 (27.2) | 162 (28.6) | 208 (27.4) | 475 (27.8) | |
| Tumor grade | <0.001 | ||||
| 1 | 26 (6.5) | 14 (2.3) | 7 (0.9) | 47 (2.6) | |
| 2 | 238 (59.1) | 239 (40.1) | 248 (30.3) | 725 (39.9) | |
| 3 | 139 (34.5) | 343 (57.6) | 564 (68.9) | 1046 (57.5) | |
| pT | <0.001 | ||||
| 1 | 372 (92.3) | 317 (53.2) | 232 (28.3) | 921 (50.7) | |
| 2 | 30 (7.4) | 269 (45.1) | 517 (63.1) | 816 (44.9) | |
| 3 | 1 (0.3) | 10 (1.7) | 70 (8.5) | 81 (4.5) | |
| pN | <0.001 | ||||
| 0 | 349 (86.6) | 311 (52.2) | 27 (3.3) | 687 (37.8) | |
| 1 | 54 (13.4) | 266 (44.6) | 343 (41.9) | 663 (36.5) | |
| 2 | 0 | 19 (3.2) | 449 (54.8) | 468 (25.7) | |
| Endocrine therapy | 0.165 | ||||
| No | 31 (7.7) | 51 (8.6) | 49 (6.0) | 131 (7.2) | |
| Yes | 372 (92.3) | 545 (91.4) | 770 (94.0) | 1687 (92.8) | |
| OFS | <0.001 | ||||
| No | 285 (70.7) | 472 (79.2) | 702 (85.7) | 1459 (80.3) | |
| Yes | 118 (29.3) | 124 (20.8) | 117 (14.3) | 359 (19.7) | |
| Trastuzumab use | 0.629 | ||||
| Observation | 126 (31.3) | 199 (33.4) | 253 (30.9) | 578 (31.8) | |
| 1 year | 141 (35.0) | 184 (30.9) | 280 (34.2) | 605 (33.3) | |
| 2 years | 136 (33.7) | 213 (35.7) | 286 (34.9) | 635 (34.9) |
aMissing values.
ER estrogen receptor, PR progesterone receptor, pT pathologic T stage, pN pathologic N stage, OFS ovarian function suppression.
Out of these patients, 403 (22.2%) were classified in the low-risk subgroup, 596 (32.8%) in the intermediate-risk subgroup, and 819 (45.0%) in the high-risk subgroup. As expected, tumor grade, pT stage, and pN stage significantly increased in the order of high-, intermediate-, and low-risk groups. The majority of patients with tumor grade 3 (564 of 1046 [53.9%]), pT3 (70 of 81 [86.4%]), and pN2 (449 of 468 [95.9%]) were classified as high-risk group. Meanwhile, the proportion of patients aged 50 or less and the rate of ovarian function suppression (OFS) application were significantly higher in the order of CTS5 low-, intermediate-, and high-risk groups (P < 0.001, P < 0.001, respectively). The other variables, such as ER and PR status and administration of trastuzumab, did not differ depending on risk groups.
Prognosis according to CTS5 risk groups
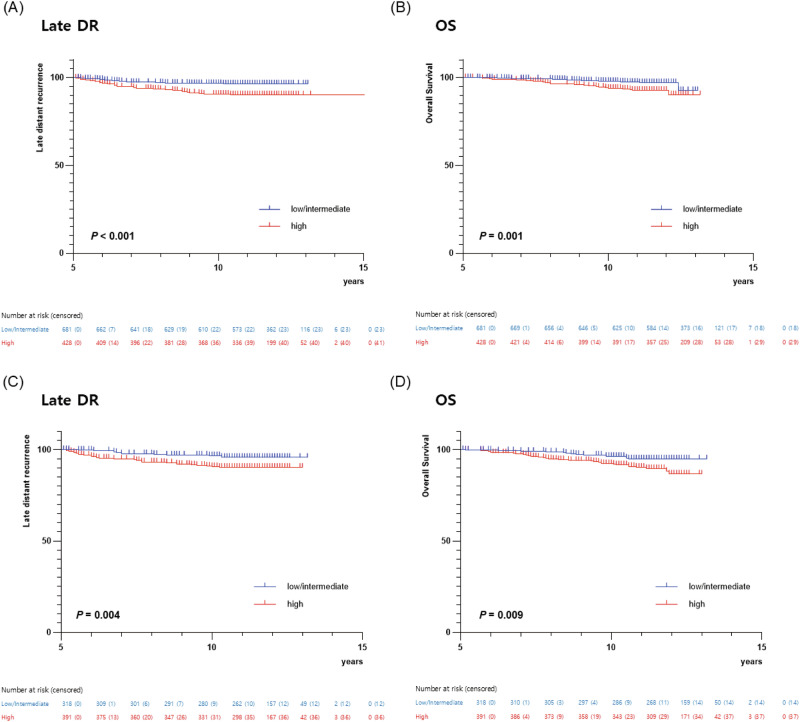
During the median follow-up of 132 months (range 61–158 months), 112 late DR events and 90 deaths occurred. There was a significant difference in the probability of late DR and OS based on the CTS5 risk groups. Kaplan-Meier curves showed that the high-risk group had significantly poor survival outcomes, while the low- and intermediate-risk groups exhibited similar prognosis (Fig. 1). Therefore, we analyzed survival outcomes by dividing the CTS5 risk group into two groups: low/intermediate vs. high.
Fig. 1. Kaplan-Meier estimates of survival according to CTS5 risk group.
Kaplan-Meier estimates of (A) late distant recurrence (DR) and (B) overall survival (OS) according to CTS5 risk group (low vs. intermediate vs. high). Kaplan-Meier estimates of (C) late distant recurrence (DR) and (D) overall survival (OS) according to CTS5 risk group (low/intermediate vs. high).
The high-risk group had a significantly worse prognosis in terms of late DR (log-rank P < 0.001) and OS (log-rank P < 0.001) in all patients (Fig. 1). Multivariable analysis showed that the high-risk group was significantly associated with adverse late DR (adjusted HR, 2.76; 95% CI, 1.84-4.13; P < 0.001) and OS (adjusted HR, 2.44; 95% CI, 1.59-3.73; P < 0.001) compared to low/intermediate group (Table 2). In addition, the CTS5 score as a continuous variable was an independent prognostic factor for late DR (adjusted HR, 2.05; 95% CI, 1.63-2.58; P < 0.001) and OS (adjusted HR, 2.02; 95% CI, 1.58-2.58; P < 0.001), respectively (Table 2).
Table 2.
Cox regression analysis of prognosis according to CTS5 risk group, stratified by age
| Cohort | CTS5 | Late DR | OS | ||
|---|---|---|---|---|---|
| HRa (95% CI) | P | HRa (95% CI) | P | ||
| All patients | CTS5 scoreb | 2.05 (1.63-2.58) | <0.001 | 2.02 (1.58-2.58) | <0.001 |
| CTS5 risk group | |||||
| Low/intermediate | Ref. | Ref. | |||
| High | 2.76 (1.84-4.13) | <0.001 | 2.44 (1.59-3.73) | <0.001 | |
| Age ≤ 50 | CTS5 scoreb | 2.06 (1.52-2.81) | <0.001 | 1.95 (1.37-2.79) | <0.001 |
| CTS5 risk group | |||||
| Low/intermediate | Ref. | Ref. | |||
| High | 2.91 (1.74-4.89) | <0.001 | 2.49 (1.38-4.49) | 0.003 | |
| Age > 50 | CTS5 scoreb | 2.06 (1.44-2.94) | <0.001 | 2.02 (1.44-2.85) | <0.001 |
| CTS5 risk group | |||||
| Low/intermediate | Ref. | Ref. | |||
| High | 2.46 (1.28-4.73) | 0.007 | 2.18 (1.18-4.03) | 0.013 | |
aAdjusted for the administration of endocrine therapy, ovarian function suppression, and trastuzumab (observation vs. 1 year vs. 2 years).
bContinuous value.
CTS5 Clinical Treatment Score post-5 years, DR distant recurrence, OS overall survival.
Performance of CTS5 in subgroups
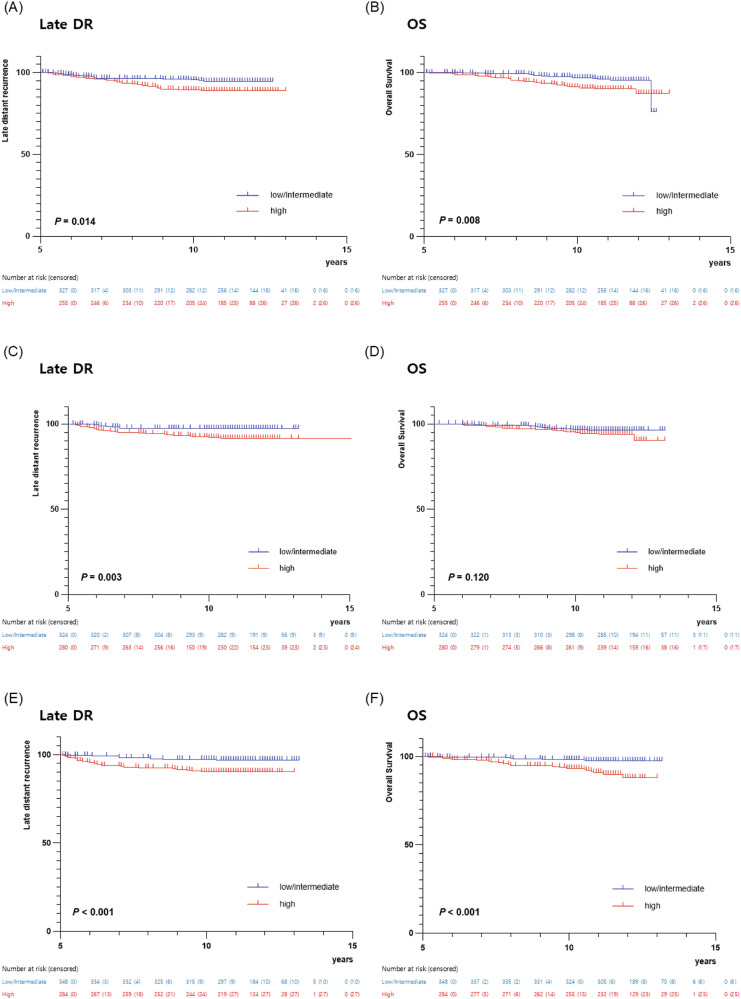
Next, we assessed the performance of CTS5 stratified by several clinicopathologic variables. Similar to the results in all patients, poor late DR and OS were observed in a high-risk group, regardless of age (Fig. 2). The multivariable analysis revealed that the patients within the high-risk group had significantly poor late DR (age ≤ 50: adjusted HR, 2.91; 95% CI, 1.74–4.89; P < 0.001, age > 50: adjusted HR, 2.46; 95% CI, 1.28–4.73; P = 0.003) and OS (age ≤ 50: adjusted HR, 2.49; 95% CI, 1.38–4.49; P = 0.003, age > 50: adjusted HR, 2.18; 95% CI, 1.18–4.03; P = 0.013) compared to those within low/intermediate-risk group (Table 2). Furthermore, the CTS5 score as a continuous value also had a prognostic solid value, irrespective of age (Table 2).
Fig. 2. Kaplan-Meier estimates of survival according to CTS5 risk group.
A Late distant recurrence (DR) and (B) overall survival (OS) in age ≤ 50; (C) Late DR and (D) OS in age > 50.
The difference in clinical outcomes according to the CTS5 risk group remained similar in each patient group divided by administration of trastuzumab (Fig. 3). In line with this, the multivariable analysis revealed that patients within the high-risk group had significantly poor late DR (observation: adjusted HR, 2.00; 95% CI, 1.06–3.76; P = 0.032, trastuzumab for 1 year: adjusted HR, 3.20; 95% CI, 1.47–6.96; P = 0.003, trastuzumab for 2 years: adjusted HR, 3.46; 95% CI, 1.67–7.17; P = 0.001) compared to those in the low/intermediate-risk group. Similarly, poor OS was observed in the high-risk group (observation: adjusted HR, 2.16; 95% CI, 1.10–4.26; P = 0.026, trastuzumab for 1 year: adjusted HR, 1.83; 95% CI, 0.85–3.92; P = 0.122, trastuzumab for 2 years: adjusted HR, 3.69; 95% CI, 1.66–8.20; P = 0.001). Moreover, the CTS5 score as a continuous value consistently demonstrated robust prognostic value for both late DR and OS across all trastuzumab administration groups (Table 3).
Fig. 3. Kaplan-Meier estimates of survival according to CTS5 risk group.
A Late distant recurrence (DR) and (B) overall survival (OS) in observation group; (C) Late DR and (D) OS in 1 year trastuzuamb group. (E) Late DR and (F) OS in 2 years trastuzuamb group.
Table 3.
Cox regression analysis of prognosis according to CTS5 risk group, stratified by administration of trastuzumab
| Cohort | CTS5 | Late DR | OS | ||
|---|---|---|---|---|---|
| HRa (95% CI) | P | HRa (95% CI) | P | ||
| Obervation | CTS5 scoreb | 1.72 (1.19-2.47) | 0.004 | 1.94 (1.31-2.87) | 0.001 |
| CTS5 risk group | |||||
| Low / intermediate | Ref. | Ref. | |||
| High | 2.00 (1.06–3.76) | 0.032 | 2.16 (1.10-4.26) | 0.026 | |
| Trastuzumab for 1 year | CTS5 scoreb | 2.20 (1.43-3.39) | <0.001 | 1.74 (1.11-2.72) | 0.016 |
| CTS5 risk group | |||||
| Low / intermediate | Ref. | Ref. | |||
| High | 3.20 (1.47-6.96) | 0.003 | 1.83 (0.85-3.92) | 0.122 | |
| Trastuzumab for 2 year | CTS5 scoreb | 2.40 (1.59-3.61) | <0.001 | 2.43 (1.56-3.76) | <0.001 |
| CTS5 risk group | |||||
| Low / intermediate | Ref. | Ref. | |||
| High | 3.46 (1.67-7.17) | 0.001 | 3.69 (1.66-8.20) | 0.001 | |
aAdjusted for administration of endocrine therapy and ovarian-function suppression.
bContinuous value.
CTS5 Clinical Treatment Score post-5 years, DR distant recurrence, OS overall survival.
Discussion
In recent years, there have been efforts to broaden the application of CTS5, a prognostic tool developed using data from postmenopausal women with HR-positive breast cancer. However, most prior research has predominantly focused on assessing the applicability of CTS5 for premenopausal women with HR-positive, HER2-negative breast cancer18–20. Despite the common clinical scenario of deciding whether to extend endocrine therapy in patients with HR-positive, HER2-positive breast cancer, the precise predictive marker for guiding this decision remains unclear. To our knowledge, only one retrospective cohort study, utilizing the Surveillance, Epidemiology, and End Results database, has demonstrated a moderate prognostic value for CTS5 in late DR in HR-positive, HER2-positive breast cancer21. Moreover, this study had a fatal limitation in that the SEER database did not incorporate accurate treatment information.
For this reason, we explored the clinical validity of CTS5 in HR-positive, HER2-positive breast cancer using long-term outcome data from a large-scale phase III trial comparing the effects of homogeneous anti-HER2 targeted therapy in early HER2-positive breast cancer. We found that CTS5 is a valuable tool for estimating the risk of late DR in patients with HR-positive, HER2-positive breast cancer who remain free of DR during the initial 5 years after randomization. Besides, we evaluated the performance of CTS5 in subgroups stratified by age (≤50 vs. >50) and administration of trastuzumab (observation vs. trastuzumab for 1 year vs. trastuzumab for 2 years), which may impact long-term clinical outcomes. Notably, poor late DR and OS in patients within the CTS5 high-risk group are consistently confirmed in each subgroup. Taken together, our results suggest that HR-positive, HER2-positive breast cancer patients identified as high risk by CTS5 may benefit from the extension of endocrine therapy.
Given the prevailing treatment practices in clinical settings, there are several considerations for identifying the high-risk group using CTS5. First of all, the patients likely to be CTS5-high risk usually receive neoadjuvant systemic therapy rather than upfront surgery22. The present CTS5 model relies on postoperative assessment of pathologic tumor size, the number of involved nodes, and tumor grade, making its application in the neoadjuvant setting challenging. Thus, it is essential to develop a novel model with a combination of factors evaluated from the clinical stage and biopsy samples. Furthermore, treatment response to neoadjuvant systemic therapy, such as pathologic complete response, serves as a surrogate marker for predicting prognosis23,24. Accordingly, it is crucial to evaluate whether predictive models like CTS5 outperform compared to treatment response as an indicator of late DR.
Unlike the results from the ATAC and BIG 1-98 trials, we observed no difference in prognosis between the low- and intermediate-risk groups. There are a couple of factors that might explain this discrepancy. The percentage of patients classified as low-risk by CTS5 in our study was 22.2%, which is notably lower compared to the 42% and 42.6% proportions observed in the ATAC and BIG 1-98 trials where the CTS5 model was developed. Furthermore, given that participants who have received chemotherapy are enrolled, the patients with an extremely early stage, such as T1a-b and N0, are unlikely to be included in the HERA trial. Another potential factor is the incomplete documentation of menopausal status for a substantial portion of the HERA trial participants. Notably, 61% of patients were aged 50 years or younger, although it is estimated that about one-third of this subgroup received ovarian function suppression. Further analysis in large-scale cohorts with more extremely early stages is needed to accurately assess the prognostic significance of intermediate-risk by CTS5 in HR-positive, HER2-positive breast cancer.
Our study has several limitations. First, our results should be interpreted with caution because this exploratory study was not prespecified statistical analysis. Additionally, we could not obtain the data related to the extension of endocrine therapy, which could potentially have influenced our findings. Given that the HERA trail was conducted in the early 2000s, it is unlikely that the participants would receive the extension of endocrine therapy. Lastly, in the HERA trial, only trastuzumab was administered as anti-HER2 targeted therapy. Considering the evolving landscape of treatment modalities in the contemporary era, such as the adoption of dual HER2 blockade25 and the utilization of adjuvant trastuzumab emtansine in patients who did not attain a pathological complete response (pCR) following neoadjuvant systemic therapy26, as well as extended adjuvant therapy with neratinib for high-risk HER2-positive breast cancer27, our findings may not fully align with current clinical trends. Accordingly, a new validation study on CTS5 comprising a cohort of HR-positive, HER2-positive patients treated in accordance with current treatment guidelines.
In conclusion, our study provides substantial insights into the utility of the CTS5 in predicting late DR and OS in patients with HR-positive, HER2-positive breast cancer. Notably, the discerning power of CTS5 persists when evaluated as both a continuous risk score and categorical risk groups. The classification of patients into the high-risk group, as defined by CTS5, is robustly associated with a significantly poorer prognosis compared to individuals categorized as low- or intermediate-risk. Importantly, these findings endure regardless of patient age or the administration of anti-HER2 targeted therapy, both of which may exert an influence on long-term clinical outcomes. As a consequence, our results advocate for the consideration of prolonged endocrine therapy beyond the customary five-year period in this specific subpopulation, underscoring the need for personalized therapeutic approaches in HR-positive, HER2-positive breast cancer patients.
Methods
Study populations
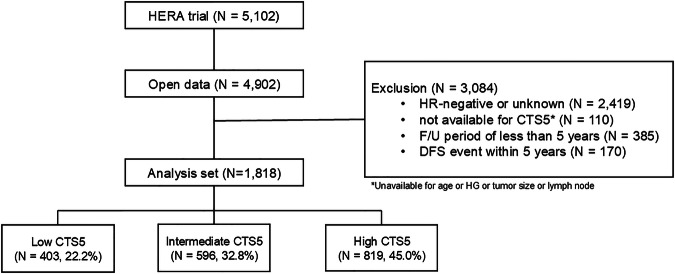
In this study, we utilized Individual participant data from the phase III clinical trial, HERA (NCT00045032). We accessed it according to Roche policy, and it has been made available through Vivli, Inc. (www.vivli.org; 10.25934/PR00006472). The HERA trial included 5102 female patients with HER2-positive invasive breast cancer who were randomly assigned 1:1:1 to observation (without trastuzumab), trastuzumab for 1 year, or trastuzumab for 2 year17,28. Through the open data resource, 4902 individual participant data were available. From these, we excluded 3084 women who (1) had HR-negative breast cancer (n = 2419), (2) lacked clinicopathologic information for calculating CTS5 (n = 110), (3) had a follow-up period of <5 years, or (4) experienced distant recurrence (DR) event within 5 years after randomization. Finally, 1818 women were included in this post hoc analysis (Fig. 4).
Fig. 4.
Flow chart of the study.
The study protocol was reviewed and approved by the Institutional Review Boards of the Gangnam Severance Hospital, Yonsei University, Seoul, Korea (IRB no. 2021-0961-001), and adhered to the principles of the Declaration of Helsinki. The requirement for written informed consent was waived due to the retrospective study design.
CTS5 calculation
We calculated the CTS5 score for each patient using the final CTS5 algorithm, incorporating coefficients from the ATAC and BIG 1-98 datasets (https://www.cts5-calculator.com). The clinicopathologic variables used for calculation included age, tumor size, nodal status, and tumor grade: the equation of CTS5 = 0.438 × number of involved nodes + 0.988 × (0.093 × tumor size—0.001 × tumor size2 + 0.375 × tumor grade + 0.017 × age)10. All tumor sizes exceeding 30 mm were replaced with a value of 30 mm, and nodal status was converted to five-point categories: 0 and 1 as 0, 1 respectively, 2 ~ 3 as 2, 4 ~ 9 as 3, and >9 as 4. Patients were divided into three risk groups using the cutoff points: low-(CTS5 < 3.13 for risk < 5%), intermediate-(3.13 to 3.86 for risk of 5%–10%), and high-risk (CTS5 > 3.86 for risk > 10%)10.
Statistical analysis
The primary objective was to evaluate whether CTS5 could predict the late DR in patients with HR-positive, HER2-positive breast cancer. Time to late DR was defined as the period from 5 years after randomization to the date of the first tumor recurrence that occurred 5 years after randomization, excluding contralateral disease and locoregional recurrences, with deaths before recurrence of distant breast cancer considered censoring events. In addition, we examined whether there was a difference in overall survival (OS) according to CTS5 stratification risk groups. OS was defined as the time interval from 5 years after randomization to the first death event for any reason.
Clinicopathologic features such as age, estrogen-receptor (ER) positivity, progesterone-receptor (PR) positivity, tumor grade, pathologic tumor (pT) stage, pathologic nodal (pN) stage, and the administration of endocrine therapy, OFS, and trastuzumab between the groups were compared using the chi-squared test. Kaplan-Meier method was used to estimate the survival rate, and the results between the groups were compared using the log-rank test. Hazard ratios (HR) with its associated 95% confidence intervals (CI) were estimated using Cox regression analysis adjusted for clinicopathologic variables related to prognosis. All statistical analyses were performed using SPSS statistical software version 26 (SPSS; Chicago, IL, USA), with significance tests being two-sided, and a P-value < 0.05 considered statistically significant.
Acknowledgements
This study is based on research using data from data contributors Roche that has been made available through Vivli, Inc. Vivli has not contributed to or approved, and is not in any way responsible for, the contents of this publication. This research was also supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (grant number: NRF-2022R1I1A1A01065696). The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Author contributions
S.J.B.: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Writing—original draft and Writing—review and editing. S.M.: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Writing—original draft and Writing—review and editing. Y.K.: Data curation, Formal analysis, Investigation, and Methodology. Seung Ho Baek: Data curation, Formal analysis, Investigation, and Methodology. M.L.: Data curation, Formal analysis, Investigation, and Methodology. J.H.K.: Data curation, Formal analysis, Investigation, and Methodology. S.G.A.: Data curation, Formal analysis, Investigation, Methodology, and Supervision. J.J.: Conceptualization, Formal analysis, Investigation, Methodology, Supervision, Writing—original draft and Writing—review and editing.
Data availability
No datasets were generated or analysed during the current study.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Soong June Bae, Sohyun Moon.
References
- 1.Davies, C. et al. Relevance of breast cancer hormone receptors and other factors to the efficacy of adjuvant tamoxifen: patient-level meta-analysis of randomised trials. Lancet378, 771–784 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Goss, P. E. et al. Extending aromatase-inhibitor adjuvant therapy to 10 years. N. Engl. J. Med.375, 209–219 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pan, H. et al. 20 year risks of breast-cancer recurrence after stopping endocrine therapy at 5 years. N. Engl. J. Med377, 1836–1846 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pistilli, B., Lohrisch, C., Sheade, J. & Fleming, G. F. Personalizing adjuvant endocrine therapy for early-stage hormone receptor-positive breast cancer. Am. Soc. Clin. Oncol. Educ. Book42, 1–13 (2022). [DOI] [PubMed] [Google Scholar]
- 5.Burstein, H. J. et al. Adjuvant endocrine therapy for women with hormone receptor-positive breast cancer: ASCO clinical practice guideline focused update. J. Clin. Oncol.37, 423–438 (2019). [DOI] [PubMed] [Google Scholar]
- 6.Bottosso, M. et al. Gene expression assays to tailor adjuvant endocrine therapy for HR+/HER2- breast cancer. Clin. Cancer Res.30, 2884–2894 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sgroi, D. C. et al. Prediction of late disease recurrence and extended adjuvant letrozole benefit by the HOXB13/IL17BR biomarker. J. Natl. Cancer Inst.105, 1036–1042 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bartlett, J. M. S. et al. Breast cancer index and prediction of benefit from extended endocrine therapy in breast cancer patients treated in the adjuvant tamoxifen-to offer more? (aTTom) trial. Ann. Oncol.30, 1776–1783 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Noordhoek, I. et al. Breast cancer index predicts extended endocrine benefit to idividualize selection of patients with HR(+) Early-stage Breast Cancer for 10 years of endocrine therapy. Clin. Cancer Res.27, 311–319 (2021). [DOI] [PubMed] [Google Scholar]
- 10.Dowsett, M. et al. Integration of clinical variables for the prediction of late distant recurrence in patients with estrogen receptor-positive breast cancer treated with 5 years of endocrine therapy: CTS5. J. Clin. Oncol.36, 1941–1948 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cuzick, J. et al. Effect of anastrozole and tamoxifen as adjuvant treatment for early-stage breast cancer: 10 year analysis of the ATAC trial. Lancet Oncol.11, 1135–1141 (2010). [DOI] [PubMed] [Google Scholar]
- 12.Coates, A. S. et al. Five years of letrozole compared with tamoxifen as initial adjuvant therapy for postmenopausal women with endocrine-responsive early breast cancer: update of study BIG 1-98. J. Clin. Oncol.25, 486–492 (2007). [DOI] [PubMed] [Google Scholar]
- 13.Howlader, N. et al. US incidence of breast cancer subtypes defined by joint hormone receptor and HER2 status. J. Natl. Cancer Inst.106, dju055 (2014). [DOI] [PMC free article] [PubMed]
- 14.DeSantis, C. E. et al. Breast cancer statistics, 2019. CA Cancer J. Clin.69, 438–451 (2019). [DOI] [PubMed] [Google Scholar]
- 15.García Fernández, A. et al. Survival and clinicopathological characteristics of breast cancer patient according to different tumour subtypes as determined by hormone receptor and Her2 immunohistochemistry. a single institution survey spanning 1998 to 2010. Breast21, 366–373 (2012). [DOI] [PubMed] [Google Scholar]
- 16.Blows, F. M. et al. Subtyping of breast cancer by immunohistochemistry to investigate a relationship between subtype and short and long term survival: a collaborative analysis of data for 10,159 cases from 12 studies. PLoS Med.7, e1000279 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cameron, D. et al. 11 years’ follow-up of trastuzumab after adjuvant chemotherapy in HER2-positive early breast cancer: final analysis of the HERceptin adjuvant (HERA) trial. Lancet389, 1195–1205 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee, J. et al. Validation of clinical treatment score post-5 years (CTS5) risk stratification in premenopausal breast cancer patients and Ki-67 labelling index. Sci. Rep.10, 16850 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Richman, J., Ring, A., Dowsett, M. & Sestak, I. Clinical validity of clinical treatment score 5 (CTS5) for estimating risk of late recurrence in unselected, non-trial patients with early oestrogen receptor-positive breast cancer. Breast Cancer Res Treat.186, 115–123 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Villasco, A. et al. Validation of CTS5 on a retrospective cohort of real-life pre- and postmenopausal patients diagnosed with estrogen receptor-positive breast cncers: is it prognostic?. Clin. Breast Cancer21, e53–e62 (2021). [DOI] [PubMed] [Google Scholar]
- 21.Wang, C. et al. Validation of CTS5 model in large-scale breast cancer population and the impact of menopausal and HER2 status on its prognostic value. Sci. Rep.10, 4660 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sharma, P., Connolly, R. M., Roussos Torres, E. T. & Thompson, A. Best foot forward: neoadjuvant systemic therapy as standard of care in triple-negative and HER2-positive breast cancer. Am. Soc. Clin. Oncol. Educ. Book40, 1–16 (2020). [DOI] [PubMed] [Google Scholar]
- 23.Cortazar, P. et al. Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet384, 164–172 (2014). [DOI] [PubMed] [Google Scholar]
- 24.Yau, C. et al. Residual cancer burden after neoadjuvant chemotherapy and long-term survival outcomes in breast cancer: a multicentre pooled analysis of 5161 patients. Lancet Oncol.23, 149–160 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Piccart, M. et al. Adjuvant pertuzumab and trastuzumab in early HER2-positive breast cancer in the APHINITY trial: 6 Years’ follow-up. J. Clin. Oncol.39, 1448–1457 (2021). [DOI] [PubMed] [Google Scholar]
- 26.von Minckwitz, G. et al. Trastuzumab emtansine for residual ivasive HER2-positive breast cancer. N. Engl. J. Med.380, 617–628 (2019). [DOI] [PubMed] [Google Scholar]
- 27.Martin, M. et al. Neratinib after trastuzumab-based adjuvant therapy in HER2-positive breast cancer (ExteNET): 5 year analysis of a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol.18, 1688–1700 (2017). [DOI] [PubMed] [Google Scholar]
- 28.Piccart-Gebhart, M. J. et al. Trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer. N. Engl. J. Med.353, 1659–1672 (2005). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No datasets were generated or analysed during the current study.