Abstract
Glioblastoma (GBM) the most common and most aggressive primary brain tumor has a five-year survival rate of less than 5%. The onset of GBM is very complicated and has always been the focus of researchers. This study analyzed data from 637 GBM and 20 normal tissues from The Cancer Genome Atlas (TCGA), and patients were categorized into high and low EIF2S2 expression groups. The Overall survival and disease-free survival of GBM patients in low expression of EIF2S2 group were significantly higher than those in high expression of EIF2S2 group (p < 0.001), and the expression level of EIF2S2 was significantly correlated with tumor grade (p < 0.001) and tumor recurrence (p < 0.001). The study designed three different short hairpin RNA (shRNA) sequence vectors, identifying shEIF2S2-1 as the most effective. This vector significantly reduced EIF2S2 expression, cell proliferation, and migration while increasing apoptosis in SHG-44 and U251 cells (p < 0.01). By detecting SHG-44 cells infected with shEIF2S2 vector and shCtrl with human whole gene expression chip, we identified WNT5A that is a downstream target gene of EIF2S2. Interfering with WNT5A and overexpressing EIF2S2 in SHG-44 and U251 cells revealed that EIF2S2 regulates WNT5A expression. This regulation led to an increased apoptosis rate (p < 0.05) and a significant reduction in cell migration (p < 0.05) in both the EIF2S2 overexpression and shWNT5A interference groups, confirming that WNT5A is a downstream regulatory target of EIF2S2. This study revealed the key role of EIF2S2 in GBM and its potential molecular mechanism.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12935-025-03762-6.
Keywords: GBM, EIF2S2, WNT5A, SHG-44, U251
Introduction
Cerebral glioma (CG) is the most common central nervous system tumor, accounting for about 85–90% of all primary central nervous system tumors [1]. CG occurs in important brain areas such as the central sulcus, basal ganglia, thalamus and brainstem [2]. About 55% of gliomas can deteriorate into glioblastoma (GBM) [3], and the average survival of patients who deteriorate into glioblastoma is less than one year. Most brain tumor lesions are cancer metastases outside the central nervous system [3], and the incidence is 5–10 times that of primary brain tumors. Among them, glioma is the most common primary brain tumor [4], accounting for 30% of all primary brain tumors and 80% of malignant tumors which are the cause of death from most primary brain tumors. And GBM is highly aggressive, which leads to the recurrence of residual tumor cells after surgical removal [5]. The blood–brain barrier [6] also restricts many anticancer drugs from entering the brain tissue that can reduce the effectiveness of chemotherapy.
The EIF2S2 (eukaryotic translation initiation factor 2 subunit 2) gene encodes a subunit of eukaryotic translation initiation factor 2 [7] which plays a key role in the initiation stage of protein synthesis. EIF2S2 plays an important role in regulating translation initiation and cell stress response and its abnormal expression is closely related to the occurrence, development and prognosis of various cancers [8], such as hepatocellular carcinoma [7], breast cancer [9] and colorectal cancer [10]. In hepatocellular carcinoma, overexpression of EIF2S2 promotes the proliferation and anti-apoptosis ability of tumor cells and is associated with poor prognosis of patients [7]. In breast cancer research, EIF2S2 can promote tumor growth and metastasis by regulating the cell cycle and inhibiting cell apoptosis [9]. EIF2S2 regulates protein synthesis by regulating the activity of translation initiation factors, which can help cells adapt and survive [11]. In cancer cells, this mechanism is often hijacked to promote the survival and growth of tumor cells. EIF2S2 can also affect tumor progression through multiple signaling pathways, such as EIF2S2 regulating protein synthesis and cell growth in the mTOR signaling pathway [12].
Wingless-Type MMTV Integration Site Family, Member 5A (WNT5A) [13] is an important member of the Wnt signaling pathway and plays a key role in biological processes such as embryonic development, cell proliferation, migration and differentiation [14]. Studies have found that WNT5A may play a dual role in different types of cancer [15] that can act as both a tumor suppressor gene and an oncogene, and its specific function depends on the type of cancer and the cell environment. In some types of cancer, WNT5A exhibits the characteristics of a tumor suppressor gene [16]. Its low expression or loss is closely related to the occurrence, development and poor prognosis of cancer, such as colorectal cancer [16] and gastric cancer [17]. In other types of cancer, WNT5A exhibits the characteristics of an oncogene. Its high expression promotes the growth, migration and invasion of tumor cells, such as breast cancer [18] and melanoma [19]. Some studies have shown that WNT5A is highly expressed in GBM and is associated with increased tumor invasiveness and metastasis [20].
In this study, we downloaded data of 155 GBM and 5 normal paracancerous tissues from The Cancer Genome Atlas (TCGA) database and divided GBM patients into high expression of EIF2S2 group and low expression of EIF2S2 group according to the average expression level of EIF2S2 gene to determine the relationship between EIF2S2 gene expression level and Overall survival and disease-free survival of GBM patients. Three different shRNA sequence vectors (shEIF2S2-1, shEIF2S2-2, and shEIF2S2-3) were designed, and the shRNA sequence vector with the highest silencing efficiency was screened in SHG-44 cells. By analyzing the EIF2S2 mRNA and protein expression levels, cell proliferation, apoptosis, cell cycle, and cell mobility of SHG-44 and U251 cells infected with shEIF2S2 vectors, we explored the specific mechanism of action of EIF2S2 in GBM. In addition, this study identified the downstream target gene WNT5A of EIF2S2 by detecting SHG-44 cells through human whole gene expression chip, and further explored the interaction between WNT5A and EIF2S2 in GBM by interfering with WNT5A and overexpressing EIF2S2. This study reveals the key role of EIF2S2 in GBM and provides new perspectives and potential targets for future GBM treatment.
Method
Cell and data sources
U251 Human Glioblastoma Cell Line, SHG-44 Human Glioblastoma Cell Line), U373 Human Glioblastoma Cell Line, U87 Human Glioblastoma Cell Line, HEB Human Normal Brain Cell Line. All cell lines in this study were derived from the Chinese Academy of Sciences Cell Bank (Shanghai, China). RNAseq data of 617 GBM and 20 normal para-cancerous tissues were downloaded from The Cancer Genome Atlas (TCGA) database [21], and the phenotypic data of the corresponding patients, such as the demographic information (age) and clinical opathological characteristics (tumor grade, stage).
PCA analysis and differential expression analysis
The R package TCGAbiolinks [21] was used to preprocess the RNA-seq data of GBM patients and normal para-cancerous tissues. The estimate the dispersion method in DEseq2 [22] was used to standardize the expression profiles of GBM patients and normal para-cancerous tissues. In order to avoid the errors caused by inappropriate sample grouping, principal component analysis (PCA) analysis [23] was performed on the gene expression levels of 637 patients. The R package DEseq2 was used to identify differentially expressed genes (DEGs) in 617 cases and normal para-cancerous tissues. For the DEseq2 results, genes with Fold Change greater than or equal to 1.3 or less than or equal to − 1.3 were screened.
EIF2S2 gene expression level and patients’ clinical data
The mean expression levels of EIF2S2 gene in 139 GBM patients were calculated, and GBM patients were divided into high EIF2S2 expression group and low EIF2S2 expression group according to the mean expression levels of EIF2S2 gene. We used to Chi-square test [24] evaluate the relationship between EIF2S2 gene expression level and tumor characteristics of glioma patients, namely age, gender, tumor recurrence and tumor grade.
Overall survival and disease-free survival analysis
The R packages survival [25] and survminer [26] used the Kaplan–Meier method [27] to plot the overall survival curve and disease-free survival curve of the EIF2S2 high expression group and the low EIF2S2 expression group. The Log-Rank test [28] was used to evaluate the effect of EIF2S2 expression level on patient survival time.
Real-time qPCR detection
Total RNA was extracted from five cell samples using Sigma’s Trizol method. The RNA was reverse transcribed into cDNA following Vazyme’s Hiscript QRT supermix instructions. The qPCR reaction mix included SYBR Green mastermix, primers, Dye2, cDNA and RNase-Free H2O with 5 replicates per group. The reaction was run in triplicate on a qPCR plate, with the program set to 95 °C for 10 min for initial denaturation, followed by 45 cycles of 95 °C for 15 s and 60 °C for 60 s.
Construction of EIF2S2 gene RNA interference lentiviral vector
The EIF2S2 gene was successfully cloned into the BR-V108 vector using TOP10 E. coli competent cells. RNA interference target sequences of 19–21 nucleotides were designed based on the EIF2S2 gene template (Table S1), following standard RNAi design principles. These sequences were optimized for efficient silencing and incorporated restriction sites for vector construction. Additionally, a TTTTT termination signal was added at the 3′ end of the positive strand, with a complementary sequence at the 5′ end of the reverse strand to form double-stranded shRNA. The designed sequences were synthesized by Biotech and annealed to form double-stranded DNA (Table S2).
For vector preparation, the BR-V108 vector was linearized by double digestion with Age I and EcoR I enzymes, and the target fragment was recovered by gel extraction. The double-stranded DNA insert was ligated into the linearized vector using T4 DNA ligase. The ligation product was transformed into competent E. coli cells, followed by heat shock to facilitate uptake of the plasmid. The transformed cells were cultured on selective LB agar plates containing Ampicillin to identify positive clones. Colonies that showed correct insertion were selected, and plasmid DNA was extracted from the positive clones. The plasmids were sequenced to confirm the correct integration of the target sequence. Correctly sequenced clones were cultured in LB medium containing Ampicillin, and plasmids were purified using the EndoFree Maxi Plasmid Kit for further use in downstream experiments.
Infection of target cells by lentivirus
SHG-44 and U251 cells (1 × 105) in the logarithmic growth phase were infected with lentiviral vectors containing shEIF2S2 (shEIF2S2-1 plasmid) and shCtrl (EIF2S2 plasmid without RNA interference sequence). Infection efficiency and fluorescence were observed under a microscope 72 h later.
Western Blot
SHG-44 and U251 cells infected with shEIF2S2 and shCtrl vectors were first washed three times with PBS to remove any residual culture medium or viral particles. The cells were then lysed using Western and IP cell lysis buffer, supplemented with PMSF (100:1 ratio) to prevent protease activity during protein extraction. The cell lysates were incubated on ice for 10–15 min to ensure complete lysis. After incubation, the samples were centrifuged at 4 °C at 12,000 rpm for 10 min to remove cell debris, and the supernatant was carefully collected. The protein samples were mixed with loading buffer and heated at 100 °C for 20 min to denature the proteins. After a brief centrifugation to spin down any condensed material, the samples were stored at − 20 °C for future use.
For protein analysis, the samples were separated by SDS-PAGE and transferred to a PVDF membrane at 4 °C, with a constant current of 300 mA for 90 min. After the transfer, the membrane was blocked with 5% skim milk in TBST for 1 h at room temperature to prevent non-specific binding. The membrane was then incubated overnight at 4 °C with primary antibodies: Rabbit polyclonal anti-EIF2S2 and Mouse monoclonal anti-GAPDH. Following incubation, the membrane was washed three times with TBST, then incubated with secondary antibodies (EIF2S2: Goat Anti-Rabbit HRP, GAPDH: Mouse monoclonal) for 1 h at room temperature. After further washing, the membrane was developed using the Millipore Immobilon Western Chemiluminescent HRP Substrate Kit, and protein bands were visualized using a chemiluminescence detection system.
Celigo system for detecting cell proliferation
The cells in the logarithmic growth phase were digested with trypsin and resuspended in complete medium into a cell suspension. The number of cells per well was set to 2000 cells/well, with 3 replicates per group, and the culture system was 100 μL/well. The cells were cultured in a 37 °C, 5% CO2 incubator. Starting from the second day after plating, the Celigo assay was read once a day for 5 consecutive days.
Cell cycle detection
Cells were grown to ~ 80% confluence, collected after trypsin digestion and centrifuged. After washing with cold PBS, the cells were fixed in 70% ethanol for 1 h. Following centrifugation and PBS wash with 3 replicates per group, the cells were stained with cell staining solution (0 × PI (propidium iodide) stock solution (2 mg/ml): 100 × RNase stock solution (10 mg/ml): 1 × PBS = 25: 10: 1000) and resuspended for analysis.
Wound-healing assay
Cells in the logarithmic growth phase were digested, resuspended, and plated at 50,000 cells/well in a 96-well plate with 100 μL of medium per well. After 24 h, the medium was replaced with low-serum medium. A scratch was made using a scratch instrument, rinsed with serum-free medium, and then cultured in low-serum medium. The plate was scanned using Cellomics to analyze the migration area over time.
Transwell migration experiment
Prepare a 24-well plate by adding 100 μL serum-free medium to the chambers and incubate for 1–2 h. Digest cells in the logarithmic phase, create a suspension, and count the cells. Remove the medium from the chambers, add 600 μL of 30% FBS medium to the lower chamber, and add 100 μL of diluted cell suspension (100,000–200,000 cells) to each chamber. Place the chambers in the lower chamber and incubate for 24 h. Stain the transferred cells by soaking the chamber in staining solution for 5 min, then rinse and capture images under a microscope.
Apoptosis detection
Cells in each group were grown to ~ 70% confluence, digested and resuspended. Drug-induced apoptosis was initiated, and cells were collected with the supernatant. After centrifugation and washing, the cell pellet was resuspended in 200 μL binding buffer, stained with Annexin V-APC, incubated in the dark for 10–15 min and finally detect on the machine, with 5 replicates per group.
Detection of downstream genes of shEIF2S2 by human whole gene expression microarray
Three SHG-44 cell samples from the shCtrl (NC) and shEIF2S2 (KD) groups were collected (Table S3). Total RNA was extracted and quality tested, then synthesized into cDNA and hybridized to a human gene expression chip. The oligo package [29] in R was used for background correction and normalization. DEseq2a constructed the design and comparison matrices, and the empirical Bayesian linear model calculated p-values, with FDR correction by the Benjamini–Hochberg method. DEG were identified with |Fold Change| ≥ 1.3 and FDR < 0.05, and clustered using hierarchical clustering. Functional and disease enrichment analyses were performed with clusterProfiler [30] and DisGeNET [31], respectively. A heatmap of DEG was created with Complex Heatmap [32], and STRINGdb [33] was used for EIF2S2 protein interaction analysis.
Reaction experiment to verify downstream genes
This experiment used the LV-013 vector and the experimental strain was TOP10 Escherichia coli competent cells (TIANGEN, Cat. #CB104-03). Using the WNT5A gene as a template, three interference target sequences were designed (shWNT5A-1, shWNT5A-2, shWNT5A-3). Four cell groups were established for investigating the roles of WNT5A and EIF2S2 in GBM: NC(OE+KD): Cells infected with empty vectors (LV-013 and BR-V108), EIF2S2+NC(KD): Cells infected with EIF2S2 and NC-shWNT5A lentivirus to over-express EIF2S2, shWNT5A+NC(OE): Cells infected with shWNT5A and NC-EIF2S2 lentivirus to down-regulate WNT5A, EIF2S2+shWNT5A: Cells infected with lentiviruses to simultaneously down-regulate WNT5A and over-express EIF2S2.
Statistical analysis
SPSS 21.0 (Statistical Package for Social Sciences, Inc., Chicago, IL, USA) (George and Mallery, 2019) and Graphpad prism5 (Statistical and Graphing Software, China) (Motulsky, 2007) were used for data analysis and graphing. The t test was used to evaluate whether there were significant differences in the two groups. Statistical significance was defined as p < 0.05.
Result
PCA analysis and differential expression analysis
This study first performed PCA analysis on the gene expression profiles of RNA-seq data from 617 cases of GBM patient cancer tissues and 20 cases of normal para-cancerous tissues (Fig. 1A). PC1 and PC2 accounted for more than 45% of the variation in the principal components, of which PC1 accounted for 31.9%. PCA results show that Normal (para-cancerous tissues) and Tumor (cancer) samples can be clearly separated at PC1, proving that the sample data of different batches are highly stable. The DEG results between the Tumor group and the Normal group (Fig. 1B) showed that the expression levels of 2302 genes were up-regulated and the expression levels of 1907 genes were down-regulated in cancer tissues.
Fig. 1.
Bioinformatics analysis of RNA-seq data of GBA patients and paracancer tissue in the TGCA database. A PCA analysis of the tumor group (cancer tissue) and normal group (normal paracancer tissue) of GBM in the TCGA database. B Analysis of DEG between tumor group and normal group. C Overall survival of GBM patients with high expression of EIF2S2 group and low expression of EIF2S2 group. D Disease-free survival of GBM patients with high expression of EIF2S2 group and low expression of EIF2S2 group
The expression of EIF2S2 gene in glioma tissue is significantly higher than that in normal tissue (p < 0.001) (Table S4) which can be used for subsequent statistical analysis of clinic pathological data. The expression level of EIF2S2 gene was not significantly related to the age (p = 0.060) and gender (p = 0.229) of GBM patients. And there was a significant association between tumor grade (p < 0.001) and tumor recurrence (p < 0.001) and EIF2S2 expression level (Table S5). These results indicate that the higher the expression level of EIF2S2, the greater the probability of tumor recurrence and the higher the tumor grade. Kaplan–Meier survival analysis found that the expression of EIF2S2 gene was significantly related to the overall survival (Fig. 1C) and disease-free survival (Fig. 1D) of GBM (p < 0.05). Patients with high expression levels of the EIF2S2 gene have significantly shorter overall survival and disease-free survival than patients with low expression levels of the EIF2S2 gene. Therefore, EIF2S2 could potentially serve as a prognostic biomarker in GBM, with higher expression levels predicting worse clinical outcomes.
Effect of RNA interference on EIF2S2 gene expression
This study used qRT-PCR to measure EIF2S2 mRNA levels in GBM cells (U251, SHG-44, U373, U87) and normal brain cells (HEB) (Fig. 2A). EIF2S2 expression was significantly higher in GBM cells than in HEB cells (p < 0.001), consistent with previous findings that EIF2S2 is elevated in GBM tissues compared to para-cancerous tissues. To assess shRNA-mediated silencing, three shRNA sequences (shEIF2S2-1, shEIF2S2-2, shEIF2S2-3) were tested in SHG-44 cells. ShEIF2S2-1 and shEIF2S2-2 effectively reduced EIF2S2 expression by 57.4 and 74.0%, respectively (p < 0.001) (Fig. 2B), while shEIF2S2-3 was ineffective. ShEIF2S2-1 exhibited the highest silencing efficiency, so we chose the shEIF2S2-1 plasmid for further experimental validation.
Fig. 2.
Analysis of EIF2S2 expression and lentiviral infection efficiency in various cell lines. A Ratio of EIF2S2/GAPDH mRNA expression levels in HEB, U251, SHG-44, U373 and U87 cells. B Effect of three different shRNA sequences on the Ratio of EIF2S2/GAPDH mRNA expression levels. C Fluorescence infection efficiency of lentiviral vector in SHG-44 cells at 0 h (Left) and 72 h (Right). D Fluorescence infection efficiency of lentiviral vector in U251 cells at 0 h (Left) and 72 h (Right). E Ratio of EIF2S2/GAPDH mRNA expression levels in SHG-44 cells containing shCtrl and shEIF2S2. F Ratio of EIF2S2/GAPDH mRNA expression levels in U251 cells containing shCtrl and shEIF2S2. G Protein expression levels of EIF2S2 and GAPDH in SHG-44 cells containing shCtrl and shEIF2S2. H Protein expression levels of EIF2S2 and GAPDH in U251 cells containing shCtrl and shEIF2S2
To investigate the infection efficiency of lentiviral vectors (shCtrl or shEIF2S2) in GBM cells, microscopy was performed 72 h post-infection in SHG-44 and U251 cells (Fig. 2C, D). Fluorescence microscopy revealed an infection efficiency exceeding 80%, indicating effective vector function and successful introduction of shRNA. qRT-PCR analysis showed that EIF2S2/GAPDH mRNA levels were significantly lower in the shEIF2S2 group compared to the shCtrl group (p < 0.001) in both SHG-44 (Fig. 2E) and U251 (Fig. 2F) cells. Silencing efficiencies were 87.00% in SHG-44 and 91.21% in U251 cells. Western blot results further confirmed significant down-regulation of EIF2S2 protein in the shEIF2S2 group relative to the shCtrl group (Fig. 2G, H), validating the effectiveness of shRNA-mediated EIF2S2 silencing.
EIF2S2 interference impairs GBM cell proliferation, induces apoptosis and inhibits migration
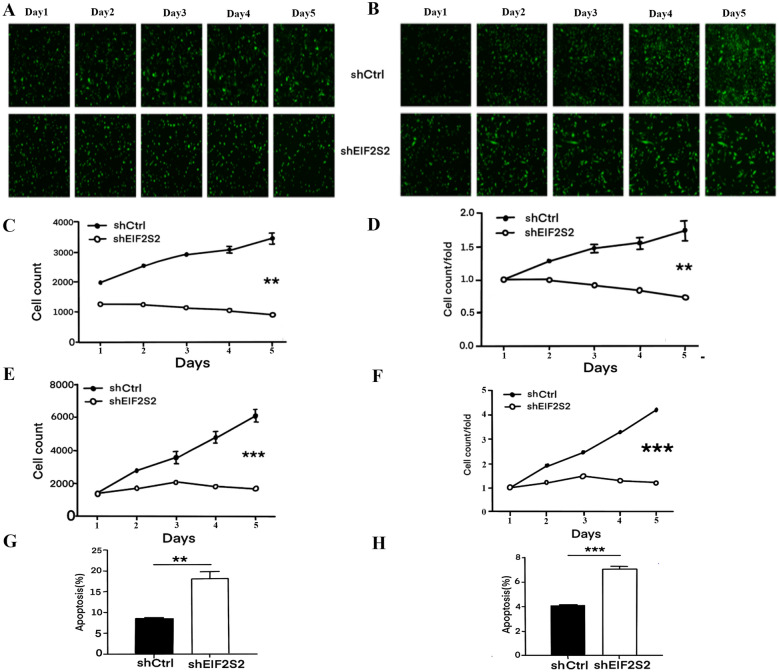
Previous research demonstrated that shEIF2S2 significantly impacts the mRNA and protein expression levels of EIF2S2 in SHG-44 and U251 cells. This study further explored the role of EIF2S2 in GBM cells through cell proliferation experiments (Fig. 3A–F). SHG-44 and U251 cells infected with shEIF2S2 displayed a significantly slower proliferation rate compared to the shCtrl group. Specifically, SHG-44 cells showed a fold change of 2.398 (p < 0.01), and U251 cells exhibited a fold change of 3.468 (p < 0.001). Additionally, flow cytometry revealed that both early and late apoptotic cell populations were significantly increased in the shEIF2S2 group compared to the shCtrl group (p < 0.01) (Fig. 3G, H, Figure S1A–B). These results suggested that interference with EIF2S2 inhibits cell proliferation by promoting apoptosis.
Fig. 3.
A 1-5d Celigo fluorescence images of SHG-44 cells with shCtrl and shEIF2S2. B 1-5d Celigo fluorescence images of U251 cells with shCtrl and shEIF2S2. C 1-5d cell count of SHG-44 cells with shCtrl and shEIF2S2. D 1-5d cell count/fold of SHG-44 cells with shCtrl and shEIF2S2. E 1-5d cell count of U251 cells with shCtrl and shEIF2S2. F 1-5d cell count/fold of U251 cells with shCtrl and shEIF2S2. G Apoptosis ratio of SHG-44 in shCtrl and shEIF2S2 groups. H Apoptosis ratio of U251 cells in shCtrl and shEIF2S2 groups
Further analysis of the cell cycle distribution (Fig. 4A, B) indicated that EIF2S2 silencing in SHG-44 and U251 cells resulted in cell cycle arrest, with a significant reduction in the proportion of cells in the S phase and an increase in the proportion of cells in the G2 phase (p < 0.05). This cell cycle arrest was a critical factor contributing to the reduced proliferation observed. Wound healing (Fig. 4C–F) and Transwell assays (Figure S2A–D) demonstrated that EIF2S2 interference significantly inhibited the migration ability of SHG-44 and U251 cells. SHG-44 cells in the shEIF2S2 group showed an 87% reduction in migration rate (p < 0.001) in the wound healing assay, and a 44% reduction in the Transwell assay (p < 0.01). U251 cells exhibited a 36% reduction in migration rate in both assays (p < 0.01 and p < 0.001, respectively). These findings suggest that EIF2S2 plays a crucial role in the migration and invasiveness of GBM cells, with a more pronounced effect in SHG-44 cells.
Fig. 4.
Analysis of cell cycle and wound healing assay in SHG-44 and U251 cells with shCtrl and shEIF2S2. A The proportion of cells in different cell cycles of SHG-44 with shCtrl and shEIF2S2. B The proportion of cells in different cell cycles of U251 with shCtrl and shEIF2S2. C Fluorescence detection of The Wound healing Assay of SHG-44 with shCtrl and shEIF2S2 (Units: um). D Fluorescence detection of the wound healing assay of U251 with shCtrl and shEIF2S2 (Units: um). E The migration rate of cells in shCtrl group and shEIF2S2 group of SHG-44. F The migration rate of cells in shCtrl group and shEIF2S2 group of U251
Analysis of the expression level of the chip
To investigate downstream target genes regulated by EIF2S2, this study used human whole gene expression chips to analyze SHG-44 cells with shCtrl and shEIF2S. The relative logarithmic signal intensities for the NC and KD groups (Fig. 5A) showed that the median Z-scores were below 2, confirming good repeatability of the chip experiment. Intra-group correlation coefficients for KD and NC were over 0.99 (Fig. 5B), indicating high similarity in gene expression within each group, while inter-group coefficients were significantly lower, highlighting large group differences. PCA analysis showed PC1 and PC2 explained over 90% of the variation (Figure S3), with clear clustering of KD and NC samples. A total of 5630 differentially expressed genes (DEGs) were identified (Fig. 5C), with 2787 upregulated and 2843 downregulated. The heat map of the cluster analysis of significantly DEG in the chips of the NC group and the KD group (Fig. 5D) showed the differences between the shEIF2S2 group and the shCtrl group. This suggests that DEGs are likely associated with EIF2S2’s cellular functions.
Fig. 5.
Chip expression profiles of SHG-44 cells with shCtrl and shEIF2S2. A Distribution of signal strength of the relative logarithm of the chip. B Correlation level of signal strength between chips. C Chip significant difference genes between NC group and KD group. D Cluster analysis of significantly different genes in NC group and KD group
Classical path analysis of IPA
Enrichment analysis of DEGs using Ingenuity Pathway Analysis (IPA) revealed that these genes are primarily involved in pathways such as Cholesterol Biosynthesis (I, II, and III), Aryl Hydrocarbon Receptor Signaling, Cell Cycle Control, NF-kB Signaling, and Xenobiotic Metabolism CAR Signaling (Fig. 6A).These results reveal that the EIF2S2 gene is shown to exert its role in cellular function through these key biological processes and signaling pathways.
Fig. 6.
Classic pathway analysis of IPA. A Enrichment analysis of significantly different genes in NC group and KD group. B Disease and functional enrichment analysis of significantly different genes in NC group and KD group. C Disease and function heat map analysis
Disease and function enrichment analysis (Fig. 6B) identified major categories including cancer, organismal injury, endocrine system disorders, gastrointestinal and reproductive system diseases, neurological diseases, and cell survival. These findings suggest that EIF2S2 interference affects crucial biological processes and disease-related pathways, notably cancer. A network relationship analysis (Figure S4) showed that EIF2S2 interacts with various signaling pathways such as ATM Signaling, EIF2 Signaling, ERK/MAPK Signaling, mTOR Signaling, Senescence Pathway and AMPK Signaling. EIF2S2 influences genes like ATF2, CDK1, DLD, EIF2B5 and EIF3J by affecting the expression of genes involved in these pathways, including APP, BIRC3, CUL7, EIF2B5, EIF3M, and G3BP1.
Analysis of downstream targeted genes
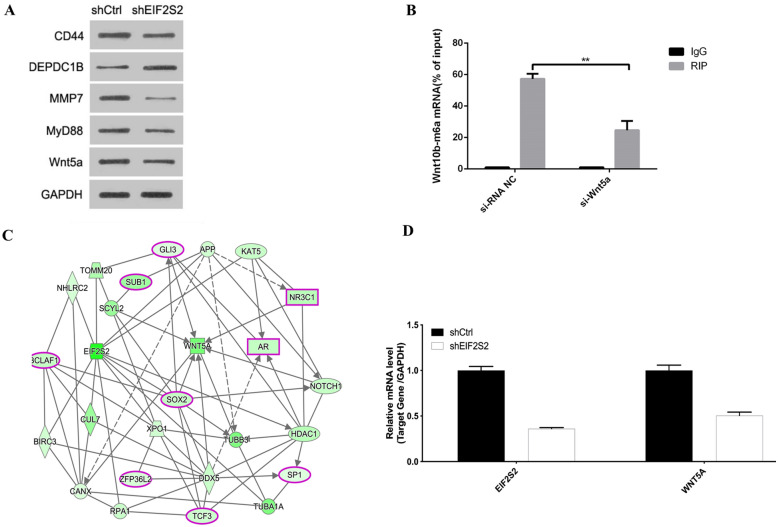
This study identified five potential EIF2S2 downstream target genes through IPA pathway analysis, namely Cluster of Differentiation 44 (CD44), DEP Domain Containing 1B (DEPDC1B), Matrix Metalloproteinase 7 (MMP7), Myeloid Differentiation Primary Response 88 (MYD88), Wingless-Type MMTV Integration Site Family, Member 5A (WNT5A). Western blot results (Fig. 7A) showed the protein expression levels of CD44, MMP7, MYD88 and WNT5A were decreased in the EIF2S2 group, while the protein expression level of DEPDC1B was increased (Fig. 7A). MeRIP-qRT-PCR experiments of these five genes found that WNT5A gene interference significantly reduced the m6A level of the EIF2S2 gene (Fig. 7B). And the interaction diagram between EIF2S2 and APP, BIRC3, CUL7, EIF2B5, EIF3M and WNT5A genes (Fig. 7C) also shows that the WNT5A gene is a key downstream target gene of the EIF2S2 gene. The mRNA expression levels of WNT5A gene and EIF2S2 gene were observed in SHG-44 cells with shEIF2S2 group and control group (Fig. 7D). These results indicated that WNT5A was a downstream target gene of the EIF2S2 gene.
Fig. 7.
Analysis of downstream target of EIF2S2 gene and its regulatory network. A Western Blot of 5 potential EIF2S2 downstream target genes. B MeRIP-qRT-PCR detection of eIF2S2 m6A changes in si-Wnt5 and si-RNA NC groups. C Map of gene interaction network. D Ratio of WNT5A/GAPDH mRNA expression levels in SHG-44 cells with shCtrl and shEIF2S2
Reaction experiment to verify downstream genes
This study examined the effects of three shRNA sequences (shWNT5A-1, shWNT5A-2, shWNT5A-3) on WNT5A expression in SHG-44 cells (Figure S5). Post-lentiviral infection, WNT5A knockout rates were 93.5% for shWNT5A-1, 75.1% for shWNT5A-2, and 87.6% for shWNT5A-3 (p < 0.001). The highest knockout efficiency was observed with shWNT5A-1, which was selected for further experiments. To explore the infection efficiency of shWNT5A-1 and EIF2S2 plasmids in GBM cells, SHG-44 and U251 cells were observed under a microscope after 72 h of infection (Fig. 8A, B). Fluorescence indicated over 80% infection efficiency, confirming the functionality of the lentiviral vectors. MRNA expression levels of EIF2S2/GAPDH and WNT5A/GAPDH were assessed in SHG-44 and U251 cells (Fig. 8C–F). In the EIF2S2+NC (KD) group, EIF2S2 and WNT5A mRNA levels significantly increased (p < 0.001), while in the shWNT5A+NC (OE) group, both decreased significantly (p < 0.05). In the EIF2S2+shWNT5A group, WNT5A mRNA levels significantly decreased (p < 0.001), but EIF2S2 levels remained unchanged. Conversely, in the shWNT5A+NC (OE) group, EIF2S2 mRNA levels increased significantly, while WNT5A decreased (p < 0.05). These results suggest a mutual regulatory relationship between EIF2S2 and WNT5A. Western blot analysis (Fig. 8G, H) showed that in SHG-44 cells, EIF2S2 protein levels significantly increased in the EIF2S2+NC (KD) group, while WNT5A decreased. In the shWNT5A+NC (OE) group, WNT5A decreased significantly, with no significant change in EIF2S2. In U251 cells, the EIF2S2+NC (KD) group showed a significant increase in EIF2S2 protein without a significant change in WNT5A. In the shWNT5A+NC (OE) group, both proteins decreased significantly. These findings reinforce the mutual regulatory relationship between EIF2S2 and WNT5A.
Fig. 8.
Analysis of EIF2S2 and WNT5A expression and lentiviral infection efficiency of four groups (NC(OE+KD), EIF2S2+NC(KD), shWNT5A+NC(OE), EIF2S2+shWNT5A) in SHG-44 and U251 cell. A Fluorescence infection efficiency of four groups in SHG-44 cells at 0 h (Left) and 72 h (Right). B Fluorescence infection efficiency of four groups in U251 cells at 0 h (Left) and 72 h (Right). C Ratio of EIF2S2/GAPDH mRNA expression levels of four groups in SHG-44 cells. D Ratio of EIF2S2/GAPDH mRNA expression levels of four groups in U251 cells. E Ratio of WNT5A/GAPDH mRNA expression levels of four groups in SHG-44 cells. F Ratio of WNT5A/GAPDH mRNA expression levels of four groups in U251 cells. G Protein expression levels of EIF2S2, WNT5A and GAPDH of four groups in SHG-44 cells. H. Protein expression levels of EIF2S2, WNT5A and GAPDH of four groups in U251 cells
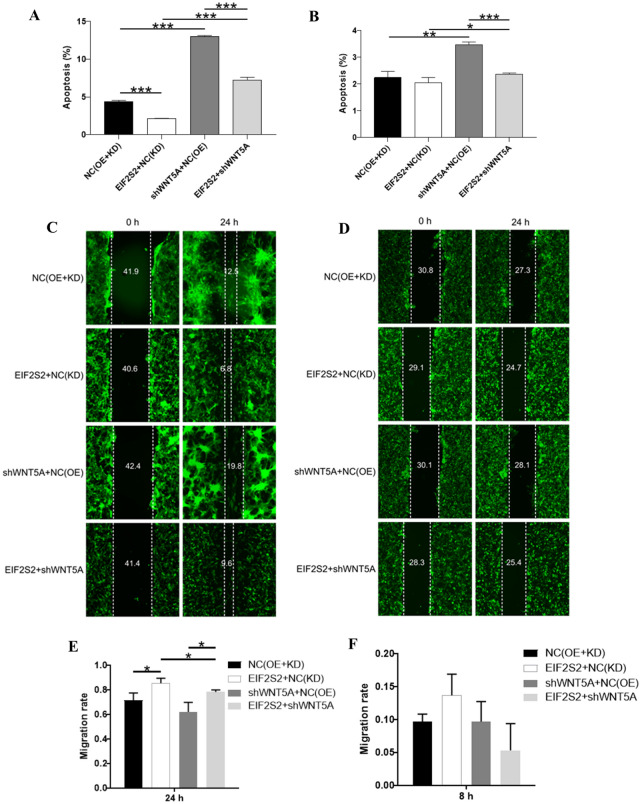
To further explore the effects of EIF2S2 and WNT5A genes on GBM cells, this study conducted apoptosis and wound-healing assay experiments (Fig. 9). In SHG44 cells (Fig. 9A) and U251 cells (Fig. 9B), compared with the NC (OE+KD) group, the cell apoptosis in the EIF2S2+NC (KD) group was reduced (P < 0.001). Compared with the NC (OE)+KD group, the cell apoptosis in the shWNT5A+NC (OE) group was significantly increased (p < 0.05). Compared with the EIF2S2+NC (KD) group, the cell apoptosis in the EIF2S2+shWNT5A group was significantly increased (p < 0.001). Compared with the shWNT5A+NC (OE) group, the cell apoptosis in the EIF2S2+shWNT5A group was reduced (p < 0.001). These results reveal the complex interaction between EIF2S2 and WNT5A in regulating apoptosis, providing important clues for further understanding their roles in cell biological functions.
Fig. 9.
Analysis of fluorescence detection and wound-healing assay of four groups (NC(OE+KD), EIF2S2+NC(KD), shWNT5A+NC(OE), EIF2S2+shWNT5A) in SHG-44 and U251 cell. A Apoptosis ratio of four groups in SHG-44 cells. B Apoptosis ratio of four groups in U251 cells. C Fluorescence detection of wound-healing assay of four groups in SHG-44 cells. D Fluorescence detection of wound healing assay of four groups in U251 cells. E The migration rate of cells wound-healing assay of four groups in SHG-44 cells. F The migration rate of cells of four groups in U251 cells
The scratch healing assay in SHG-44 cells (Fig. 9C, E) showed that the cell migration rate in the EIF2S2+NC (KD) group was significantly increased compared with the NC (OE+KD) group (p < 0.05). There was no significant difference in the cell migration rate in the shWNT5A+NC (OE) group compared with the NC (OE+KD) group. Further comparison showed that the cell migration rate in the EIF2S2+shWNT5A group was significantly decreased compared with the shWNT5A+NC (OE) group (p < 0.05). The cell migration rate in the EIF2S2+shWNT5A group (24 h) was significantly increased compared with the EIF2S2+NC(KD) group (p < 0.05). The scratch healing assay in U251 cells (Fig. 9D, F) found that there was no significant difference in the cell migration rate among the groups.
Discussion
EIF2S2 and cancer
The role of EIF2S2 in cancer is mainly achieved through its role in translation regulation [8]. The activity regulation of the eIF2 complex is a key link in protein synthesis, EIF2S2 as an important subunit of this complex which plays an important role in this process [34]. In addition, EIF2S2 promotes the growth and survival of cancer cells by affecting the selective translation of specific mRNAs and regulating key genes related to cell cycle, apoptosis and metabolism [35]. High expression of EIF2S2 is associated with tumor invasiveness and poor prognosis in breast cancer [36]. By inhibiting EIF2S2, the proliferation and migration ability of breast cancer cells is significantly reduced, while the sensitivity of cells to chemotherapeutic drugs is enhanced. This suggests that EIF2S2 may promote the malignant behavior of breast cancer cells by regulating cell stress response and translation initiation process. High expression of EIF2S2 in hepatocellular carcinoma is closely related to tumor grade and patient survival rate [37]. Studies have found that by regulating the expression of EIF2S2, the proliferation and apoptosis of liver cancer cells can be significantly affected [7]. Studies have found that EIF2S2 is highly expressed in GBM which is associated with tumor invasiveness and poor prognosis of patients [38]. Highly expressed EIF2S2 may promote the growth and proliferation of GBM cells. This is consistent with the results of this study.
EIF2S2 affects the expression of multiple downstream genes involved in cell growth, apoptosis, metabolism, and stress response by regulating translation initiation [39, 40]. EIF2S2 can indirectly regulate the stability and function of certain proteins by affecting their translation speed and folding process [41]. Moreover, EIF2S2 regulates the expression of certain non-coding RNAs (such as miRNA and lncRNA) which play an important role in post-transcriptional regulation of gene expression [42]. EIF2S2 regulates the expression of activating transcription factor 4 (ATF4) through selective translation which can help cells cope with various stress conditions, such as oxidative stress and amino acid deficiency [36]. EIF2S2 participates in the apoptosis process triggered by endoplasmic reticulum stress by regulating the expression of CHOP, a pro-apoptotic transcription factor [43].
EIF2S2 affects cell proliferation, survival, and metabolism not only through its basic translation function, but also through interactions with multiple signaling pathways, such as ATM Signaling, EIF2 Signaling [44], ERK/MAPK Signaling [45], mTOR Signaling [38], Senescence Pathway and AMPK Signaling [44]. Studies have found that EIF2S2 can affect the ATM signaling pathway by regulating stress responses during translation [44]. The translation inhibition of EIF2S2 under stress conditions may reduce protein synthesis and reduce the cell’s ability to repair DNA damage that increasing the cell’s sensitivity to DNA damage. The interaction between EIF2S2 and the ERK/MAPK signaling pathway may affect cell behavior by regulating protein synthesis and stress responses [45]. Studies have found that high expression of EIF2S2 can activate the ERK/MAPK pathway which can promote cell proliferation and survival [45]. The mTOR signaling pathway plays a key role in cell growth, metabolism, and survival. Abnormal expression of EIF2S2 can affect cell proliferation and survival through the mTOR pathway [38].
WNT5A and cancer
WNT5A is involved in a variety of biological processes, such as embryonic development, tissue regeneration, and tumorigenesis and development [46, 47]. In cancer, the role of WNT5A is complex and diverse, and it may promote tumor development or inhibit tumor growth [48]. WNT5A has been found to promote cancer cell invasion and migration in certain types of cancer, such as breast cancer [18] and melanoma [19]. Moreover, high expression of WNT5A in breast cancer and melanoma is associated with the malignancy and prognosis of the tumor. WNT5A can affect the development of tumors by affecting the formation and function of the tumor microenvironment. Studies have found that WNT5A can regulate the activation state and infiltration of tumor-associated macrophages [49] that can affect the immune escape and treatment sensitivity of tumors. WNT5A has also been found to inhibit tumor growth and metastasis in some cases [17]. In colorectal cancerand lung cancer, the expression of WNT5A is associated with a better prognosis, and may inhibit the development of tumors by inhibiting the proliferation and invasion of tumor cells [16].
WNT5A can affect tumor progression by regulating cell senescence [50]. Studies have found that in the epithelial ovarian cancer cell line OVCAR5, primary human ovarian cancer epithelial cells and mouse in vivo models, the expression of WNT5A was upregulated by retroviral transduction to increase the recruitment of histone repressor A (HIR A) to promyelocytic bodies (PML) to induce cell senescence [51]. The WNT5A signaling pathway regulates the interaction between tumor cells and tumor microenvironment cells [18]. In colorectal cancer, WNT5A is mainly expressed in its stroma, especially in tumor-associated macrophages in the tumor microenvironment [52]. Studies have found that WNT5A promotes the secretion of IL-10, induces tumor-associated macrophages to polarize to M2, and ultimately promotes tumor growth and metastasis of colorectal cancer by regulating the CaKMII-ERK1/2-STAT3 pathway [49]. In malignant peripheral nerve sheath tumors (MPNST), WNT5A mRNA is significantly upregulated in tumor cells and tissues [53]. After WNT5A knockdown, mRNAs related to extracellular matrix remodeling and immune cell communication are significantly upregulated, the secretion of proinflammatory cytokines is increased, and the occurrence and development of tumors are promoted, indicating that Wnt5a inhibits the formation of MPNST tumors by regulating the MPNST microenvironment.
The development and metastasis of tumor cells is a series of continuous events, including tumor cell proliferation, increased migration, invasion of basement membrane, entry into the circulatory system, and finally tumor cell invasion of distant organs [54]. In some cancers, WNT5A has been found to promote cell proliferation and migration to enhance tumor cell invasion [55]. WNT5A was found to be overexpressed and was associated with the proliferation, migration and invasion of tumor cells in GBM [56]. In colorectal cancer (CSC), compared with CRC patients with WNT5A deletion, CRC patients with WNT5A overexpression in tumor tissue have a longer survival time, which may be related to the upregulation of 15-PGDH mediated by WNT5A [57]. WNT5A has different effects on the proliferation, migration and invasion of different types of tumor cells which may depend on the differential expression of Wnt5a receptors or different downstream pathways.
Conclusion
This study revealed the key role of EIF2S2 gene in the pathogenesis of glioblastoma (GBM) and its potential molecular mechanism through in-depth study of the EIF2S2 gene in GBM. In cell experiments, we designed three different shRNA sequence vectors and identified shEIF2S2-1 with the highest silencing efficiency. Cell experiments showed that the mRNA and protein expression levels of EIF2S2 were significantly decreased in cells infected with shEIF2S2 vector, accompanied by a slowdown in cell proliferation, an increase in apoptosis rate, and a decrease in migration rate. In addition, through whole gene expression chip detection, we identified the downstream target gene WNT5A of EIF2S2 and confirmed the interaction between them. The findings of this study not only deepen our understanding of the pathogenesis of GBM, but also provide important inspiration for the development of new therapeutic strategies. This study provides a new perspective for understanding the pathogenesis of GBM, and EIF2S2 and WNT5A may become potential targets for the treatment of GBM.
Supplementary Information
Additional file 1: Figure S1: A. Flow cytometry detection of apoptosis of SHG-44 cells with shCtrl and shEIF2S2. B. Flow cytometry detection of apoptosis of U251 cells with shCtrl and shEIF2S2
Additional file 2: Figure S2: A. Transwell migration assay images of SHG-44 cells with shCtrl and shEIF2S2. B. Transwell migration assay images of U251 cells with shCtrl and shEIF2S2. C. The migration cell per fieldand migration fold changeof SHG-44 with shCtrl and shEIF2S2. D. The migration cell per fieldand migration fold changeof U251 with shCtrl and shEIF2S2
Additional file 3: Figure S3: PCA analysis of chip signal strength in NC group and KD group
Additional file 4: Figure S4: Gene interaction network diagram
Additional file 5: Figure S5: Effect of three different shRNA sequences on the Ratio of WNT5A/GAPDH mRNA expression levels
Additional file 6: Table S1: Three 19-21 nt RNA interference target sequences of EIF2S2 gene
Additional file 7: Table S2: Three 19-21nt RNA interference target sequences of EIF2S2 gene single-stranded DNA oligo
Additional file 8: Table S3: The samples Negative Control groupand Knockdown group
Additional file 9: Table S4: LogFC value and p value of EIF2S2 gene in cancer tissues and normal tissues
Additional file 10: Table S5: Relationship between EIF2S2 expression and tumor characteristics in patients with glioma cancer
Acknowledgements
Not applicable.
Author contributions
Zhongjie Yan conceived and designed the research. Bo Fan analyzed the transcriptome data and wrote the original draft. Qing Pan, Xiaokai Yuan and Du Wei revised the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
Not applicable.
Data availability
No datasets were generated or analysed during the current study.
Declarations
Ethics approval and Consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no conflict of interest.
Institutional review board statement
All animal experiments were conducted following the guidelines of the Institutional Animal Care and Use Committee (IACUC) of The Second Hospital of Hebei Medical University.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Raab P, Hattingen E, Franz K, Zanella FE, Lanfermann H. Cerebral gliomas: diffusional kurtosis imaging analysis of microstructural differences. Radiology. 2010;254:876–81. [DOI] [PubMed] [Google Scholar]
- 2.Rooney AG, Carson A, Grant R. Depression in cerebral glioma patients: a systematic review of observational studies. J Natl Cancer Inst. 2011;103:61–76. [DOI] [PubMed] [Google Scholar]
- 3.Omuro A, DeAngelis LM. Glioblastoma and other malignant gliomas: a clinical review. JAMA. 2013;310:1842–50. [DOI] [PubMed] [Google Scholar]
- 4.Schaff LR, Mellinghoff IK. Glioblastoma and other primary brain malignancies in adults: a review. JAMA. 2023;329:574–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Putavet DA, de Keizer PL. Residual disease in glioma recurrence: a dangerous liaison with senescence. Cancers. 2021;13:1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mo F, Pellerino A, Soffietti R, Rudà R. Blood–brain barrier in brain tumors: biology and clinical relevance. Int J Mol Sci. 2021;22:12654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ji P, Wang H, Cheng Y, Liang S. Prognostic prediction and gene regulation network of EIF2S2 in hepatocellular carcinoma based on data mining. J Gastrointest Oncol. 2021;12:3061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu J, Liu T, Zhang C, He J, Zhou D, Wang Z, Wang R. EIF2S2 is a novel independent prognostic biomarker and correlated with immune infiltrates in hepatocellular carcinoma. Front Genet. 2022;13: 992343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guo M, Ying Y, Chen Y, Miao X, Cui H, Yu Z, Wang X. Eukaryotic Translation initiation factor 2 subunit β as a prognostic biomarker associates with immune cell infiltration in breast cancer. J Surg Res. 2024;295:753–62. [DOI] [PubMed] [Google Scholar]
- 10.Yang J-W, Yuan L-L, Gao Y, Liu X-S, Wang Y-J, Zhou L-M, Kui X-Y, Li X-H, Ke C-B, Pei Z-J. 18F-FDG PET/CT metabolic parameters correlate with EIF2S2 expression status in colorectal cancer. J Cancer. 2021;12:5838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rahardjo AK. Effects of eukaryotic initiation factor 2A upregulation on protein synthesis in insulin producing cells. University of British Columbia, 2022.
- 12.Schatz C, Sprung S, Schartinger V, Codina-Martínez H, Lechner M, Hermsen M, Haybaeck J. Dysregulation of translation factors EIF2S1, EIF5A and EIF6 in intestinal-type adenocarcinoma (ITAC). Cancers. 2021;13:5649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bueno MLP, Saad STO, Roversi FM. WNT5A in tumor development and progression: a comprehensive review. Biomed Pharmacother. 2022;155: 113599. [DOI] [PubMed] [Google Scholar]
- 14.Arredondo SB, Guerrero FG, Herrera-Soto A, Jensen-Flores J, Bustamante DB, Oñate-Ponce A, Henny P, Varas-Godoy M, Inestrosa NC, Varela-Nallar L. Wnt5a promotes differentiation and development of adult-born neurons in the hippocampus by noncanonical Wnt signaling. Stem cells. 2020;38:422–36. [DOI] [PubMed] [Google Scholar]
- 15.Sun G, Wu L, Sun G, Shi X, Cao H, Tang W. WNT5a in colorectal cancer: research progress and challenges. Cancer Manag Res. 2021;2483–98. [DOI] [PMC free article] [PubMed]
- 16.Hirashima T, Karasawa H, Aizawa T, Suzuki T, Yamamura A, Suzuki H, Kajiwara T, Musha H, Funayama R, Shirota M. Wnt5a in cancer-associated fibroblasts promotes colorectal cancer progression. Biochem Biophys Res Commun. 2021;568:37–42. [DOI] [PubMed] [Google Scholar]
- 17.Astudillo P. Wnt5a signaling in gastric cancer. Front Cell Dev Biol. 2020;8:110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lopez-Bergami P, Barbero G. The emerging role of Wnt5a in the promotion of a pro-inflammatory and immunosuppressive tumor microenvironment. Cancer Metastasis Rev. 2020;39:933–52. [DOI] [PubMed] [Google Scholar]
- 19.Douglass SM, Fane ME, Sanseviero E, Ecker BL, Kugel CH III, Behera R, Kumar V, Tcyganov EN, Yin X, Liu Q. Myeloid-derived suppressor cells are a major source of Wnt5A in the melanoma microenvironment and depend on Wnt5A for full suppressive activity. Can Res. 2021;81:658–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen T, Zhang F, Liu J, Huang Z, Zheng Y, Deng S, Liu Y, Wang J, Sun X. Dual role of WNT5A in promoting endothelial differentiation of glioma stem cells and angiogenesis of glioma derived endothelial cells. Oncogene. 2021;40:5081–94. [DOI] [PubMed] [Google Scholar]
- 21.Weinstein JN, Collisson EA, Mills GB, Shaw KR, Ozenberger BA, Ellrott K, Shmulevich I, Sander C, Stuart JM. The cancer genome atlas pan-cancer analysis project. Nat Genet. 2013;45:1113–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Love M, Anders S, Huber W. Differential analysis of count data—the DESeq2 package. Genome Biol. 2014;15:10–1186. [Google Scholar]
- 23.Abdi H, Williams LJ. Principal component analysis. Wiley Interdiscip Rev Comput Stat. 2010;2:433–59. [Google Scholar]
- 24.McHugh ML. The chi-square test of independence. Biochemia medica. 2013;23:143–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Therneau TM, Lumley T. Package ‘survival.’ R Top Doc. 2015;128:28–33. [Google Scholar]
- 26.Pawar A, Chowdhury OR, Salvi O. A narrative review of survival analysis in oncology using R. Cancer Res Stat Treat. 2022;5:554–61. [Google Scholar]
- 27.Bland JM, Altman DG. Survival probabilities (the Kaplan-Meier method). BMJ. 1998;317:1572–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kleinbaum DG, Klein M, Kleinbaum DG, Klein M. Kaplan-Meier survival curves and the log-rank test. Survival analysis: a self-learning text. 2012;55–96.
- 29.Carvalho B, Irizarry R, Bolstad B, Carey V, Huber W, Jaffee H, MacDonald J, Settles M, Hooiveld G, Carvalho MB. Package ‘oligo’. 2013.
- 30.Yu G, Wang L-G, Han Y, He Q-Y. clusterProfiler: an R package for comparing biological themes among gene clusters. Omics J Integr Biol. 2012;16:284–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Piñero J, Queralt-Rosinach N, Bravo A, Deu-Pons J, Bauer-Mehren A, Baron M, Sanz F, Furlong LI. DisGeNET: a discovery platform for the dynamical exploration of human diseases and their genes. Database. 2015;2015:bav028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gu Z. Complex heatmap visualization. Imeta. 2022;1: e43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Szklarczyk D, Morris JH, Cook H, Kuhn M, Wyder S, Simonovic M, Santos A, Doncheva NT, Roth A, Bork P. The STRING database in 2017: quality-controlled protein–protein association networks, made broadly accessible. Nucleic Acids Res. 2016;gkw937. [DOI] [PMC free article] [PubMed]
- 34.Lu J, Chen S, Tan H, Huang Z, Li B, Liu L, Chen Y, Zeng X, Zou Y, Xu L. Eukaryotic initiation factor-2, gamma subunit, suppresses proliferation and regulates the cell cycle via the MAPK/ERK signaling pathway in acute myeloid leukemia. J Cancer Res Clin Oncol. 2021;147:3157–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Huang L, Xu Z, Xie Y, Jiang S, Han W, Tang Z, Zhu Q. Comprehensive characterization of ageing-relevant subtypes associated with different tumorigenesis and tumor microenvironment in prostate cancer. Front Mol Biosci. 2022;9: 803474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cao Z, Jing Y, Cheng C, Wang F, Guan M, Zhang K, Jiao J, Ruan L, Chen Z. EIF2Ss, a novel c-Myc-correlated gene family, is associated with poor prognosis and immune infiltration in pancreatic adenocarcinoma. Front Biosci Landmark. 2024;29:119. [DOI] [PubMed] [Google Scholar]
- 37.Wang W-J, Tang L, Wei C, Wang B, Xiao H, Tao X-f, Yang J-L, Yu T-Z, Guan J-F, Yuan R-F. EIF2S3 is a prognostic biomarker correlated with immune infiltration in hepatocellular carcinoma. 2022.
- 38.Lei D, Chen Y, Zhou Y, Hu G, Luo F. A starvation-based 9-mRNA signature correlates with prognosis in patients with hepatocellular carcinoma. Front Oncol. 2021;11: 716757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.English AM, Green KM, Moon SL. A (dis) integrated stress response: genetic diseases of eIF2α regulators. Wiley Interdiscip Rev RNA. 2022;13: e1689. [DOI] [PubMed] [Google Scholar]
- 40.Panzhinskiy E, Skovsø S, Cen HH, Chu KY, MacDonald K, Soukhatcheva G, Dionne DA, Hallmaier-Wacker LK, Wildi JS, Marcil S. Eukaryotic translation initiation factor 2A protects pancreatic beta cells during endoplasmic reticulum stress while rescuing translation inhibition. bioRxiv; 2021:2021.2002. 2017.431676.
- 41.Sun L, Yu F, Xu Z, Zeng X, Ferreri M, Han B. Alteration of osteocalcin mRNA expression in ovine osteoblasts in dependence of sodium fluoride and sodium selenite medium supplementation. Acta Biol Hung. 2010;61:52–63. [DOI] [PubMed] [Google Scholar]
- 42.Trakman L. Long non-coding RNA directed modulation of phenotypic plasticity and viability in HEK-293 cells. UNSW Sydney, 2020.
- 43.Han J, Back SH, Hur J, Lin Y-H, Gildersleeve R, Shan J, Yuan CL, Krokowski D, Wang S, Hatzoglou M. ER-stress-induced transcriptional regulation increases protein synthesis leading to cell death. Nat Cell Biol. 2013;15:481–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Barresi V. The crucial findings derived from the special issue “Inside Cancer Genomics: From Structure to Therapy”. Cancers. 2023;15:3488. MDPI; 2023:3488. [DOI] [PMC free article] [PubMed]
- 45.Rabalski AJ. Quantitative Proteomic Characterization of CX-4945, a Clinical Stage Inhibitor of Protein Kinase CK2. The University of Western Ontario (Canada), 2017.
- 46.van Amerongen R, Fuerer C, Mizutani M, Nusse R. Wnt5a can both activate and repress Wnt/β-catenin signaling during mouse embryonic development. Dev Biol. 2012;369:101–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kumawat K, Gosens R. WNT-5A: signaling and functions in health and disease. Cell Mol Life Sci. 2016;73:567–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Asem MS, Buechler S, Wates RB, Miller DL, Stack MS. Wnt5a signaling in cancer. Cancers. 2016;8:79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liu Q, Yang C, Wang S, Shi D, Wei C, Song J, Lin X, Dou R, Bai J, Xiang Z. Wnt5a-induced M2 polarization of tumor-associated macrophages via IL-10 promotes colorectal cancer progression. Cell Commun Signal. 2020;18:1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bitler BG, Nicodemus JP, Li H, Cai Q, Wu H, Hua X, Li T, Birrer MJ, Godwin AK, Cairns P. Wnt5a suppresses epithelial ovarian cancer by promoting cellular senescence. Can Res. 2011;71:6184–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Asem M, Young AM, Oyama C, Claure De La Zerda A, Liu Y, Yang J, Hilliard TS, Johnson J, Harper EI, Guldner I. Host Wnt5a potentiates microenvironmental regulation of ovarian cancer metastasis. Cancer Res. 2020;80:1156–1170 [DOI] [PMC free article] [PubMed]
- 52.Huang D, Du X. Crosstalk between tumor cells and microenvironment via Wnt pathway in colorectal cancer dissemination. World J Gastroenterol: WJG. 1823;2008:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Thomson CS, Pundavela J, Perrino MR, Coover RA, Choi K, Chaney KE, Rizvi TA, Largaespada DA, Ratner N. WNT5A inhibition alters the malignant peripheral nerve sheath tumor microenvironment and enhances tumor growth. Oncogene. 2021;40:4229–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Liotta LA, Kleinerman J, Saidel GM. Quantitative relationships of intravascular tumor cells, tumor vessels, and pulmonary metastases following tumor implantation. Can Res. 1974;34:997–1004. [PubMed] [Google Scholar]
- 55.Cheng R, Sun B, Liu Z, Zhao X, Qi L, Li Y, Gu Q. Wnt5a suppresses colon cancer by inhibiting cell proliferation and epithelial–mesenchymal transition. J Cell Physiol. 2014;229:1908–17. [DOI] [PubMed] [Google Scholar]
- 56.Yu JM, Jun ES, Jung JS, Suh SY, Han JY, Kim JY, Kim KW, Jung JS. Role of Wnt5a in the proliferation of human glioblastoma cells. Cancer Lett. 2007;257:172–81. [DOI] [PubMed] [Google Scholar]
- 57.Ying J, Li H, Yu J, Ng KM, Poon FF, Wong SCC, Chan AT, Sung JJ, Tao Q. WNT5A exhibits tumor-suppressive activity through antagonizing the Wnt/β-catenin signaling, and is frequently methylated in colorectal cancer. Clin Cancer Res. 2008;14:55–61. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Figure S1: A. Flow cytometry detection of apoptosis of SHG-44 cells with shCtrl and shEIF2S2. B. Flow cytometry detection of apoptosis of U251 cells with shCtrl and shEIF2S2
Additional file 2: Figure S2: A. Transwell migration assay images of SHG-44 cells with shCtrl and shEIF2S2. B. Transwell migration assay images of U251 cells with shCtrl and shEIF2S2. C. The migration cell per fieldand migration fold changeof SHG-44 with shCtrl and shEIF2S2. D. The migration cell per fieldand migration fold changeof U251 with shCtrl and shEIF2S2
Additional file 3: Figure S3: PCA analysis of chip signal strength in NC group and KD group
Additional file 4: Figure S4: Gene interaction network diagram
Additional file 5: Figure S5: Effect of three different shRNA sequences on the Ratio of WNT5A/GAPDH mRNA expression levels
Additional file 6: Table S1: Three 19-21 nt RNA interference target sequences of EIF2S2 gene
Additional file 7: Table S2: Three 19-21nt RNA interference target sequences of EIF2S2 gene single-stranded DNA oligo
Additional file 8: Table S3: The samples Negative Control groupand Knockdown group
Additional file 9: Table S4: LogFC value and p value of EIF2S2 gene in cancer tissues and normal tissues
Additional file 10: Table S5: Relationship between EIF2S2 expression and tumor characteristics in patients with glioma cancer
Data Availability Statement
No datasets were generated or analysed during the current study.