Abstract
Background
The Triglyceride-Glucose (TyG) index, a reliable marker for insulin resistance, is now employed to assess the onset and prognosis of various conditions like acute coronary syndrome, chronic kidney disease, and ischemic stroke. However, whether the TyG index can be used to assess respiratory failure (RF) risk among Chronic obstructive pulmonary disease (COPD) patients remains uncertain. The present study aims to delve into the link between the TyG index and the risk of RF in COPD patients.
Methods
Individuals with COPD were retrospectively acquired from the MIMIC-IV 2.2 (The Medical Information Mart for Intensive Care IV, version 2.2) database. The association between the TyG index and the probability of RF among COPD patients was evaluated using Cox proportional hazards models and restricted cubic spline (RCS) curves. Cumulative incidence curves were generated to appraise the RF risk across the quartile groups. Finally, 1188 patients were recruited from the First Hospital of Jiaxing City to externally validate the Cox modeling results for the primary outcome.
Results
This study incorporated a total of 1,232 participants from MIMIC database. Among these individuals, 134 cases (10.9%) experienced RF. According to Cox regression analysis, a one-unit increment in the TyG index was linked to a 1.821-fold elevated risk of RF in the COPD population (HR, 1.821[95% CI 1.349–2.459], P < 0.001). High TyG index levels were significantly linked to a higher RF risk (HR, 3.510 [95% CI 1.885–6.535], P < 0.001). RCS curve analysis also signaled a linear correlation between the TyG index and RF risk (P-Nonlinear = 0.074).
Conclusion
There exists a certain correlation between high-level TyG index and the risk of RF occurrence in COPD patients, indicating promising prospects for utilizing the TyG index to assess the severity of COPD patients.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12890-025-03597-x.
Keywords: Chronic obstructive pulmonary disorder, Respiratory insufficiency, TyG index, Medical Information Mart for Intensive Care IV (MIMIC-IV) dataset, Externally validate
Introduction
Chronic obstructive pulmonary disease (COPD) represents a notable category of enduring and progressive respiratory disorders, characterized by high rates of disability and mortality. As per the data provided by the World Health Organization (WHO), COPD ranks as the third leading cause of death globally, following ischemic heart disease and stroke. It places a growing economic strain on low- and middle-income nations, with high prevalence and mortality [1, 2]. As COPD advances, patients often develop serious complications such as pulmonary hypertension, cor pulmonale, respiratory failure (RF), and pneumothorax. Among these, RF stands out as the most prevalent risk factor leading to recurrent hospitalization, poor prognosis, and mortality [3–6]. Consequently, clinicians have turned their attention to predicting the RF risk in COPD patients. Early identification of RF risk is critical for preventive and early-stage treatment, as it can significantly mitigate the risk of worsening COPD, improve patient prognosis, and enhance their quality of life.
Insulin resistance (IR) signifies a decline in the responsiveness of peripheral tissues to insulin [7], and it has been correlated with reduced lung function [8]. The conventional method for diagnosing IR is the hyperinsulinemic euglycemic glucose clamp (HEGC) [9], an invasive and time-intensive procedure [10]. Laboratory-based alternatives to this test, including the Homeostasis Model Assessment of Insulin Resistance (HOMA-IR) and the Quantitative Insulin Sensitivity Test Index (QUICKI), necessitate the direct measurement of insulin and have limited applicability in clinical practice [11].
The TyG index, a dependable biomarker and surrogate for IR [10, 12], is calculated utilizing the formula Ln (fasting triglyceride (mg/dl) × fasting blood glucose (mg/dl)/2) [13]. The data required for computing this index are derived from routine biochemistry tests, making it a convenient and easily accessible alternative to traditional detection methods that demand specialized equipment and technology. Over the years, numerous investigations have explored the link between the TyG index and disease prognosis. TyG index has been demonstrated significantly correlated with the onset and prognosis of circulatory system diseases [14], chronic kidney disease [15], stroke [16], fatty liver [17], and other conditions, showcasing its strong potential for application. Furthermore, the TyG index has also been associated with the occurrence and prognosis of certain respiratory diseases. A prior study [18] has revealed that an elevated TyG index may serve as a risk factor for declining lung function, which is a key determinant in the development of RF in COPD patients. The occurrence of RF is closely tied to the prognosis of COPD. Therefore, the present study seeks to explore the correlation between the TyG index and the risk of RF in COPD patients, offering valuable insights for predicting RF in this patient population.
Materials and methods
Data source
This retrospective investigation utilized data extracted from the Medical Information Mart for Intensive Care (MIMIC-IV version 2.2) (https://physionet.org/content/mimiciv/2.2/). It is an expansive, publicly accessible, and freely available database. It was meticulously curated and developed by the Laboratory of Computational Physiology at the Massachusetts Institute of Technology, Beth Israel Deaconess Medical Center (BIDMC) at Harvard Medical School, and Philips Healthcare. MIMIC-IV 2.2 includes clinical records (including data from both general ward and ICU admissions) from over 450,000 hospital admissions involving 299,712 patients treated at BIDMC between 2008 and 2019. [19]. We have successfully completed the requisite training and examination process for database access, we received approval from the Institutional Review Boards of both the Massachusetts Institute of Technology and BIDMC (Certificate Number: 52663507). The detailed certificate information is in Supplementary Material 1. It's essential to note that all sensitive patient information within the MIMIC project has been de-identified, ensuring that the research remains ethically compliant.
Additionally, to verify the generalizability of the results, we conducted a retrospective study of COPD patients hospitalized at the First Hospital of Jiaxing City from January 2021 to December 2023. The study was conducted from February to June 2024. Ethical approval for the external validation participants was obtained from our institution (Ethical Approval No. 2023-KY-607).
Study population
All data pertaining to the study population is sourced from the MIMIC-IV database. Inclusion criteria comprised: (1) Patients diagnosed with COPD based on the ICD codes (ICD-10: J44, J440, J441, J449; ICD-9: 49,120, 49,121, 4912, 496); and (2) Adult patients aged 18 years and older. Exclusion criteria encompassed: (1) Patients without follow-up time; (2)Patients who were not hospitalized; (3) Patients with pre-existing RF prior to admission; and (4) Patients lacking glucose and triglyceride data within 48 h following patient admission. Furthermore, we focused our analysis on the initial admission for patients with multiple admissions. The ultimate study cohort included 1,232 patients, who were split into four groups according to quartiles of the TyG index.
Data collection
Clinical data on eligible participants were acquired from the MIMIC-IV database employing Navicat Premium software (version 16.0, https://navicat.com.cn). Essential patient demographics were obtained, including age, gender, BMI, and ethnicity. Additionally, we systematically compiled the blood test parameters at initial admission, comprising (i) blood indicators: white blood cell count (WBC), neutrophil count (Ncell), lymphocyte count (Lcell), platelet count (PLT), and red blood cell count (RBC)); (ii) biochemical indexes: sodium (Na+), potassium (K+), glucose, triglyceride, serum creatinine (Scr), blood urea nitrogen (BUN), alanine aminotransferase (ALT), aspartate aminotransferase (AST), albumin, total bilirubin (TBil), indirect bilirubin (IBil), total cholesterol (TC), low-density lipoprotein (LDL), high-density lipoprotein (HDL), and hemoglobin A1c (HbA1c); (iii) coagulation parameters: prothrombin time (PT), activated partial thromboplastin time (APTT), and D-dimer; (iv) arterial blood gas analysis: partial pressure of oxygen (PO2) and partial pressure of carbon dioxide (PCO2). We also extracted data on complications, such as asthma, respiratory tract infection (RTI), hypertension, heart failure (HF), coronary heart disease (CHD), diabetes, cerebrovascular disease (CVD), Interstitial lung disease (ILD), and sepsis. Furthermore, we cataloged the administration of pharmaceuticals, including corticosteroids, antibiotics, bronchodilators, and albumin. Various clinical assessment scores were also acquired, namely the Sequential Organ Failure Assessment (SOFA) score, Acute Physiology Score III (APS III), and Simplified Acute Physiology Score II (SAPS II) on Day 1. For all laboratory indicators, we analyzed the data from the initial measurements taken within 48 h after patient admission. It is important to note that data collected subsequent to the occurrence of RF was deemed unsuitable for analysis to mitigate any potential for reverse causality. In this study, the TyG index was calculated as ln(fasting blood glucose (mg/dL) × fasting triglycerides (mg/dL)) / 2.
Primary and secondary outcomes
The follow-up for both study cohorts commenced at the time of admission, with the endpoint being either the occurrence of RF or discharge. The principal endpoint was the likelihood of RF in patients diagnosed with COPD. RF is defined as arterial oxygen pressure (PaO2) < 60 mmHg or the oxygenation index (PaO2/FiO2) ≤ 300 mmHg under oxygen supplementation, with or without hypercapnia. Secondary endpoints encompassed the hospitalization stay and the utilization of invasive mechanical ventilation. Invasive mechanical ventilation encompasses the rates of endotracheal intubation and tracheostomy.
Statistical analysis
Normally distributed continuous variables were depicted as the mean and standard deviation (SD). Meanwhile, if continuous variables were abnormally distributed, they were presented by the median and interquartile range (IQR). Categorical variables were delineated by both quantity and frequency (%).
Comparisons of continuous variables were executed utilizing either Student's t-test or nonparametric testing methods according to their respective distributions. On the other hand, comparative analyses of categorical variables were carried out employing either Pearson's chi-square test or Fisher's exact test. Data with missing values of less than 20% were handled with multiple imputation techniques using the random forest method from the "missForest" package. However, when data displayed missing values exceeding 20%, we utilized dummy variables for data processing.
To investigate the correlation between the risk of RF and various TyG index levels, cumulative incidence curves were utilized to assess the RF risk in each group based on TyG index quartiles. The differences between groups were analyzed using log-rank tests.
The modeling analysis was then performed using both the continuous variable form and the quartile-based categorical form of the TyG index. Following an evaluation of the TyG index's normality, a Cox proportional hazards model was employed to examine the link between the TyG index and the likelihood of experiencing RF among individuals diagnosed with COPD. The outcomes were presented utilizing hazard ratio (HR) accompanied by a 95% confidence interval (CI). Three distinct models were formulated for analysis. No covariates were adjusted in Model 1, while Model 2 adjusted for three key variables: age, gender, and BMI. Building upon Model 2, Model 3 additionally adjusted for covariates that could potentially influence the incidence of RF, encompassing comorbidities (e.g., asthma, hypertension, HF, CHD, diabetes mellitus, and CVD), laboratory parameters (including albumin, potassium, HbA1c, TC, APTT, PT, HDL, LDL, Na +), and medication use (specifically, glucocorticoids antibiotics and bronchodilators). Furthermore, logistic regression was employed to delve into the correlation between the TyG index and the utilization of invasive mechanical ventilation. Multivariate linear regression was utilized to assess the connection between the TyG index and the duration of hospitalization stay.
To explore any potential nonlinear relationships, we harnessed restricted cubic splines (RCSs) to delve into the link between RF risk and TyG index as a continuous variable.
Moreover, we conducted additional subgroup analysis based on gender, age (categorized as < 60 years and ≥ 60 years), and the presence of various comorbidities including diabetes, hypertension, HF, liver cirrhosis, sepsis, and respiratory tract infection. The primary aim was to appraise the uniformity of the TyG index's impact on outcomes across these groups and to delve into potential interactions between the TyG index and the stratification variables.
Data analysis was conducted utilizing R software (available at www.R-project.org; version 4.2.3). All statistical tests were conducted with a two-tailed approach, and P < 0.05 was utilized to define statistical significance.
Chinese cohort
We conducted a retrospective study of COPD patients hospitalized at the First Hospital of Jiaxing City from January 2021 to December 2023. The inclusion and exclusion criteria, data collection, processing, and analysis methods, as well as the definitions of outcomes for the Chinese cohort, were consistent with those in the MIMIC database.
Results
A total of 14,050 individuals diagnosed with COPD were retrospectively acquired from the MIMIC-IV 2.2 database. Ultimately 1,232 patients were eligible for this investigation (Fig. 1). The median age within this cohort stood at 72.0 years [63.0; 80.0], with 640 (51.9%) males and 592 (48.1%) females. The majority of patients belonged to the Caucasian ethnic group. The median TyG index for all enrolled patients equated to 8.76 [8.4, 9.2]. Among these individuals, 134 patients (10.9%) developed RF, while 1098 patients (89.1%) did not suffer from RF.
Fig. 1.
Flowchart of patient screening. MIMIC-IV: Medical Information Mart for Intensive Care-IV; n: Number; COPD: chronic obstructive pulmonary disease
A retrospective review was conducted at the First Hospital of Jiaxing City, including 1,188 patients. The median age was 75.0 years [65.0, 82.0], with 911 (76.7%) being male. For external validation patients, the median TyG index was 8.37 [8.0, 8.7], with a total of 455 (38.3%) cases experiencing RF.
Baseline characteristics
Table 1 delineates patient baseline features in the MIMIC database. Patients with the higher TyG index quartile (Q4) were generally younger (P < 0.001) and exhibited elevated BMI in comparison to those with lower TyG index quartile (Q1). Moreover, the prevalence of diabetes mellitus, RTI, and CHD upon admission was higher in patients with a higher TyG index. They also presented with heightened levels of WBC, liver transaminases, BUN, LDL and TC, while HDL levels were lower. Furthermore, APTT and PT were shorter (P < 0.05).
Table 1.
The baseline clinical characteristics of the study population
| Categories | Overall (n = 1232) | Q1 (n = 308) | Q2 (n = 309) | Q3 (n = 307) | Q4 (n = 308) | P-value | Text |
|---|---|---|---|---|---|---|---|
| Demographic | |||||||
| Age, years | 72.0 [63.0, 80.0] | 75.0 [64.0, 83.0] | 73.0 [65.0, 81.0] | 71.0 [63.0, 79.0] | 68.0 [59.0, 76.0] | < 0.001 | nonnorm |
| Male, n (%) | 640 (51.9) | 157 (51.0) | 173 (56.0) | 155 (50.5) | 155 (50.3) | 0.436 | |
| Ethnicity, n (%) | 0.535 | ||||||
| Asian | 13 (1.1) | 2 (0.6) | 2 (0.6) | 4 (1.3) | 5 (1.6) | ||
| Black | 109 (8.8) | 29 (9.4) | 33 (10.7) | 25 (8.1) | 22 (7.1) | ||
| White | 954 (77.4) | 245 (79.5) | 234 (75.7) | 241 (78.5) | 234 (76.0) | ||
| Other | 156 (12.7) | 32 (10.4) | 40 (12.9) | 37 (12.1) | 47 (15.3) | ||
| BMI,kg/m2 | 27.60 [23.60, 33.15] | 25.90 [22.50, 29.95] | 27.10 [22.10, 33.15] | 28.20 [23.80, 33.40] | 29.30 [26.22, 35.80] | < 0.001 | nonnorm |
| Comorbidities | |||||||
| Asthma, n (%) | 86 (7.0) | 19 (6.2) | 23 (7.4) | 24 (7.8) | 20 (6.5) | 0.835 | |
| RTI, n (%) | 24 (1.9) | 2 (0.6) | 4 (1.3) | 7 (2.3) | 11 (3.6) | 0.049 | |
| CVD, n (%) | 422 (34.3) | 101 (32.8) | 109 (35.3) | 112 (36.5) | 100 (32.5) | 0.671 | |
| CHD, n (%) | 510 (41.4) | 105 (34.1) | 128 (41.4) | 127 (41.4) | 150 (48.7) | 0.004 | |
| Hypertension, n (%) | 641 (52.0) | 150 (48.7) | 164 (53.1) | 155 (50.5) | 172 (55.8) | 0.309 | |
| HF, n (%) | 411 (33.4) | 96 (31.2) | 100 (32.4) | 113 (36.8) | 102 (33.1) | 0.484 | |
| DM, n (%) | 419 (34.0) | 47 (15.3) | 83 (26.9) | 99 (32.2) | 190 (61.7) | < 0.001 | |
| Liver cirrhosis, n (%) | 35 (2.8) | 7 (2.3) | 11 (3.6) | 10 (3.3) | 7 (2.3) | 0.684 | |
| Viral hepatitis, n (%) | 35 (2.8) | 7 (2.3) | 11 (3.6) | 10 (3.3) | 7 (2.3) | 0.684 | |
| Sepsis, n (%) | 37 (3.0) | 4 (1.3) | 9 (2.9) | 12 (3.9) | 12 (3.9) | 0.188 | |
| Laboratory tests | |||||||
| WBC, K/μL | 8.6 [6.6, 11.1] | 7.8 [5.9, 10.3] | 8.2 [6.6, 11.0] | 8.7 [6.9, 11.4] | 9.4 [7.2, 11.9] | < 0.001 | nonnorm |
| Lcell, K/μL | 1.3 [0.8, 1.9] | 1.2 [0.8, 1.6] | 1.1 [0.7, 1.8] | 1.3 [0.9, 2.0] | 1.4 [0.9, 2.3] | 0.230 | nonnorm |
| Ncell, K/μL | 7.3 [4.7, 9.9] | 7.4 [3.5, 9.5] | 8.1 [5.8, 9.2] | 7.1 [5.1, 9.6] | 7.3 [3.9, 12.5] | 0.729 | nonnorm |
| RBC, K/μL | 4.0 [3.6, 4.5] | 3.9 [3.5, 4.4] | 4.1 [3.6, 4.4] | 4.0 [3.6, 4.5] | 4.0 [3.6, 4.6] | 0.030 | nonnorm |
| PLT, K/μL | 224.0 [175.0, 282.3] | 225.0[182.0, 276.0] | 226.0 [176.0, 279.3] | 217.5 [171.0, 278.8] | 228.0 [175.5, 297.0] | 0.532 | nonnorm |
| CRP, mg/L | 32.2 [6.3, 87.9] | 31.0 [11.6, 56.0] | 15.4 [4.5, 78.6] | 35.6 [6.5, 87.0] | 44.7 [9.4, 107.8] | 0.421 | nonnorm |
| HbA1c, g/dL | 12.0 [10.6, 13.4] | 11.9 [10.6, 13.0] | 12.2 [10.7, 13.4] | 12.0 [10.5, 13.5] | 12.0 [10.6, 13.5] | 0.420 | nonnorm |
| TBil, mg/dL | 0.5 [0.4, 0.8] | 0.5 [0.4, 0.9] | 0.5 [0.4, 0.7] | 0.6 [0.3, 0.9] | 0.5 [0.3, 0.8] | 0.341 | nonnorm |
| IBil, mg/dL | 0.6 [0.4, 1.6] | 0.6 [0.4, 0.8] | 0.8 [0.4, 1.8] | 0.8 [0.5, 1.6] | 0.7 [0.2, 1.6] | 0.792 | nonnorm |
| ALT, IU/L | 21.0 [14.0, 36.0] | 19.0 [13.0, 32.0] | 19.0 [14.0, 33.0] | 19.0 [14.0, 32.0] | 27.0 [16.0, 48.0] | < 0.001 | nonnorm |
| AST, IU/L | 25.0 [17.0, 41.0] | 24.0 [18.0, 40.0] | 22.5 [17.0, 35.3] | 23.0 [16.0, 41.0] | 27.0 [20.0, 47.5] | 0.040 | nonnorm |
| Scr, mg/dL | 4.0 [3.6, 4.5] | 3.9 [3.5, 4.4] | 4.1 [3.6, 4.4] | 4.0 [3.6, 4.5] | 4.0 [3.6, 4.6] | 0.030 | nonnorm |
| K+, mEq/L | 4.1 [3.8, 4.4] | 4.0 [3.8, 4.4] | 4.1 [3.8, 4.4] | 4.0 [3.7, 4.4] | 4.2 [3.8, 4.5] | 0.007 | nonnorm |
| Na+, mEq/L | 139.0 [137.0, 141.0] | 140.0[137.0, 141.0] | 139.0 [137.0, 142.0] | 140.0 [138.0, 142.0] | 139.0 [136.0, 141.0] | 0.013 | nonnorm |
| Albumin, g/dL | 3.5 [3.0, 3.9] | 3.5 [3.1, 3.9] | 3.5 [3.0, 3.9] | 3.4 [3.0, 3.8] | 3.5 [3.0, 3.9] | 0.338 | nonnorm |
| BUN, mg/Dl | 18.0 [14.0, 27.0] | 17.0 [13.0, 25.0] | 18.0 [13.0, 27.0] | 19.0 [14.0, 27.0] | 20.5 [15.0, 31.3] | < 0.001 | nonnorm |
| Glucose, mg/dl | 111.0 [94.0, 143.0] | 93.0 [84.0, 108.0] | 103.0 [94.0, 121.0] | 118.0 [102.0, 146.0] | 154.5 [118.8, 215.3] | < 0.001 | nonnorm |
| Triglycerides, mg/dl | 112.0 [83.0, 154.0] | 71.0 [58.0, 84.0] | 101.0 [88.0, 115.0] | 133.0 [106.5, 157.0] | 198.5 [150.0, 252.0] | < 0.001 | nonnorm |
| TC, mg/dL | 150.0 [126.0, 180.0] | 142.0[118.0, 171.5] | 146.0 [123.0, 170.0] | 151.5 [126.8, 182.3] | 165.0 [139.5, 202.5] | < 0.001 | nonnorm |
| LDL, mg/dL | 79.0 [57.0, 102.0] | 75.0 [55.8, 95.5] | 77.0 [56.5, 99.0] | 82.0 [60.0, 104.0] | 83.0 [59.0, 112.0] | 0.015 | nonnorm |
| HDL, mg/dL | 44.0 [35.0, 56.0] | 51.0 [41.0, 64.3] | 46.0 [36.5, 57.5] | 41.0 [34.0, 52.0] | 39.0 [31.0, 47.0] | < 0.001 | nonnorm |
| APTT, sec | 30.6 [27.4, 38.1] | 31.2 [228.0, 39.7] | 30.6 [27.6, 37.7] | 30.7 [27.2, 38.6] | 29.7 [26.7, 37.0] | 0.041 | nonnorm |
| PT, sec | 13.2 [12.0, 14.7] | 13.4 [12.4, 15.6] | 13.2 [11.9, 14.6] | 13.1 [11.9, 14.6] | 12.9 [11.8, 14.2] | 0.005 | nonnorm |
| D-Dimer, ng/ml | 1177.5 [598.0, 2429.0] | 577.0[382.0, 598.0] | 1652.0 [797.8, 1915.0] | 3437.0[1465.0,5013.88] | 878.0 [253.0, 1144.0] | 0.013 | nonnorm |
| TyG index | 8.8 [8.4, 9.2] | 8.1 [88.0, 8.3] | 8.6 [8.5, 8.7] | 99.0 [8.99, 9.1] | 9.66 [9.44, 9.9] | < 0.001 | nonnorm |
| PO2, mmHg | 112.0 [74.0, 223.0] | 122.0 [74.88, 245.5] | 117.5 [80.0, 226.33] | 105.0 [73.0, 213.0] | 112.0 [72.88, 221.5] | 0.704 | nonnorm |
| PCO2, mmHg | 42.0 [36.0, 49.0] | 41.0 [35.0, 46.33] | 40.0 [35.33, 47.88] | 42.0 [37.0, 50.0] | 44.0 [38.88, 50.0] | 0.172 | nonnorm |
| OI, mmHg | 231.0 [137.0, 320.0] | 218.0 [123.0, 355.0] | 240.0 [138.0, 326.0] | 225.0 [127.0, 280.0] | 236.0 [160.0, 328.0] | 0.900 | nonnorm |
| ICU admission | |||||||
| APSIII | 38.5 [28.0, 49.0] | 39.5 [27.5, 47.88] | 38.0 [27.0, 48.5] | 37.0 [28.5, 45.0] | 40.5 [30.88, 53.33] | 0.114 | nonnorm |
| SAPSII | 33.0 [28.0, 41.0] | 32.0 [29.0, 39.88] | 34.0 [28.0, 41.0] | 32.0 [27.0, 38.0] | 33.0 [26.0, 42.0] | 0.864 | nonnorm |
| SOFA | 3.0 [2.0, 5.0] | 3.0 [1.0, 4.0] | 3.0 [2.0, 4.5] | 3.0 [2.0, 4.0] | 3.0 [2.0, 6.0] | 0.281 | nonnorm |
| Medicine | |||||||
| Glucocorticoid, n(%) | 379 (30.8) | 97(31.5) | 97 (31.4) | 85 (27.7) | 100(32.5) | 0.590 | |
| Bronchodilators, n(%) | 405 (32.9) | 84 (27.3) | 98 (31.7) | 107 (34.9) | 116 (37.7) | 0.040 | |
| Antibiotic, n(%) | 514 (41.7) | 117 (38.0) | 138 (44.7) | 131 (42.7) | 128 (41.6) | 0.395 | |
| Usealbumin, n (%) | 70 (5.7) | 24 (7.8) | 16 (5.2) | 16 (5.2) | 14 (4.5) | 0.311 | |
| Events | |||||||
| LOS hospital, days | 5.0 [3.0, 11.0] | 4.0 [3.0, 9.0] | 5.0 [3.0, 12.0] | 6.0 [3.0, 12.0] | 6.0 [3.0, 12.0] | 0.006 | |
| RF, n (%) | 134 (10.9) | 19 (6.2) | 34 (11.0) | 32 (10.4) | 49 (15.9) | 0.002 | |
| Mechanical ventilation,n(%) | 88(7.1) | 14(4.5) | 16(5.2) | 19(6.2) | 39(12.7) | < 0.001 | |
TyG index: Q1 (≤ 8.408048, Q2 (8.408048 < TyG ≤ 8.759355), Q3 (8.759355 < TyG ≤ 9.205240), Q4 (> 9.205240)
Abbreviations: BMI Body Mass Index, RTI Respiratory tract infection, CVD Cerebrovascular disease, CHD Coronary heart disease, HF Heart failure, DM Diabetes mellitus, WBC White blood cell, Lcell Lymphocyte, Ncell Neutrophile granulocyte, RBC Red blood cell, PLT Platelet, CRP C-reactive protein, HbA1c Glycosylated hemoglobin, Tbil Total bilirubin, Ibil Indirect bilirubin, ALT Glutamic-pyruvic transaminase, AST Glutamic oxalacetic transaminase, Scr Serum creatinine, K+ Potassium, Na + Sodium, BUN Blood urea nitrogen, TC Total cholesterol, LDL Low Density Lipoprotein, HDL High density lipoprotein, APTT Activated partial thromboplastin time, PT Prothrombin time, TyG index Triglyceride glucose index, PO2 pressure of oxygen, PCO2 Pressure of Carbon Dioxide, OI Oxygenation Index, APSIII Acute physiology score III, SAPSII Simplified acute physiological score II, SOFA Sequential organ failure assessment, LOS length of stay, ICU Intensive care unit, RF Respiratory failure, n Number
In the Chinese cohort, baseline characteristics stratified by quartiles of the TyG index are shown in Table 2. Compared to the low TyG index level group (Q1), patients with high TyG index levels (Q4) had higher levels of BUN, D-Dimer, HbA1c, LDL, and Ncell, while APTT and PT levels were lower.
Table 2.
The baseline clinical characteristics of the study population in Chinese cohort
| Categories | Overall (N = 1188) | Q1 (N = 297) | Q2 (N = 297) | Q3 (N = 297) | Q4 (N = 297) | P-value | Text |
|---|---|---|---|---|---|---|---|
| Demographic | |||||||
| Age, years | 75.0 [69.0, 82.0] | 76.0 [69.0, 82.0] | 74.0 [69.0, 81.0] | 75.0 [70.0, 82.0] | 75.0 [69.0, 81.0] | 0.628 | nonnorm |
| Male, n (%) | 911(76.7) | 250(84.2) | 238(80.1) | 211(71.0) | 212(71.4) | < 0.001 | |
| Ethnicity, n (%) | |||||||
| Asian | 1188(100%) | 297 (100) | 297 (100) | 297 (100) | 297 (100) | ||
| BMI, kg/m2 | 21.4 [19.5, 23.6] | 19.8 [18.1, 22.6] | 21.3 [20.2, 23.5] | 20.9 [19.3, 23.8] | 22.9 [21.0, 24.4] | 0.012 | nonnorm |
| Comorbidities | |||||||
| Asthma, n (%) | 92 (7.7) | 24 (8.1) | 20 (6.7) | 28 (9.4) | 20 (6.7) | 0.557 | |
| RTI, n (%) | 702 (59.1) | 164 (55.2) | 179 (60.3) | 177 (59.6) | 182 (61.3) | 0.452 | |
| CHD, n (%) | 88 (7.4) | 27 (9.1) | 19 (6.4) | 25 (8.4) | 17 (5.7) | 0.342 | |
| Hypertension, n (%) | 561 (47.2) | 123 (41.4) | 132 (44.4) | 143 (48.1) | 163 (54.9) | 0.007 | |
| HF, n (%) | 341 (28.7) | 94 (31.6) | 79 (26.6) | 86 (29.0) | 82 (27.6) | 0.555 | |
| DM, n (%) | 122 (10.3) | 16 (5.4) | 21 (7.1) | 22 (7.4) | 63 (21.2) | < 0.001 | |
| Liver cirrhosis, n (%) | 60 (5.1) | 18 (6.1) | 15 (5.1) | 15 (5.1) | 12 (4.0) | 0.738 | |
| Viral hepatitis, n (%) | 60 (5.1) | 18 (6.1) | 15 (5.1) | 15 (5.1) | 12 (4.0) | 0.738 | |
| Sepsis, n (%) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | ||
| Laboratory tests | |||||||
| WBC, K/μL | 7.1 [5.5, 9.5] | 6.7 [5.2, 8.7] | 7.1 [5.4, 9.8] | 6.9 [5.4, 9.4] | 7.6 [6.1, 10.4] | < 0.001 | nonnorm |
| Lcell, K/μL | 0.9 [0.6, 1.4] | 0.9 [0.6, 1.4] | 0.9 [0.6, 1.4] | 1.0 [0.6, 1.4] | 0.9 [0.6, 1.4] | 0.829 | nonnorm |
| Ncell, K/μL | 5.5 [3.8, 7.6] | 5.0 [3.6, 7.2] | 5.2 [3.8, 7.5] | 5.3 [3.8, 7.5] | 6.0 [4.4, 8.5] | < 0.001 | nonnorm |
| RBC, K/μL | 4.3 [3.9, 4.7] | 4.3 [3.8, 4.7] | 4.3 [3.9, 4.7] | 4.3 [4.0, 4.8] | 4.4 [4.0, 4.8] | 0.111 | nonnorm |
| PLT, K/μL | 177.0[131.6,232.0] | 174.0 [127.8, 223.3] | 176.0[123.0, 236.5] | 187.0[136.0, 238.0] | 175.0[134.5,233.0] | 0.384 | nonnorm |
| CRP, mg/L | 14.8 [4.0, 61.0] | 13.2 [3.4, 35.9] | 17.1 [4.6, 62.9] | 13.7 [3.9, 60.7] | 15.3 [4.4, 87.2] | 0.017 | nonnorm |
| HbA1c, g/dL | 6.1 [5.7, 6.5] | 5.8 [5.6, 6.1] | 6.0 [5.7, 6.3] | 6.2 [5.8, 6.5] | 6.4 [6.1, 7.5] | < 0.001 | nonnorm |
| TBil, mg/dL | 0.6 [0.4, 0.9] | 0.6 [0.5, 0.9] | 0.6 [0.4, 0.9] | 0.6 [0.4, 0.9] | 0.6 [0.5, 0.9] | 0.161 | nonnorm |
| IBil, mg/dL | 0.4 [0.3, 0.5] | 0.4[0.3,0.5] | 0.4[0.3,0.5] | 0.3[0.2,0.5] | 0.3[0.2,0.5] | 0.007 | nonnorm |
| ALT, IU/L | 17.0 [12.0, 27.0] | 15.0 [11.0, 24.0] | 18.0 [12.0, 28.0] | 19.0 [13.0, 27.0] | 17.0 [12.0, 28.0] | 0.01 | nonnorm |
| AST, IU/L | 24.0 [18.0, 33.5] | 23.0 [17.0, 32.0] | 24.0 [19.0, 33.0] | 24.0 [18.0, 35.0] | 24.0 [18.0, 35.3] | 0.27 | nonnorm |
| Scr, mg/dL | 0.8[0.7,1.0] | 0.8[0.7,1.0] | 0.8[0.7,1.0] | 0.8[0.7,1.0] | 0.9[0.7,1.1] | 0.085 | nonnorm |
| K+, mEq/L | 4.0 [3.7, 4.3] | 4.0 [3.7, 4.3] | 4.0 [3.8, 4.4] | 4.0 [3.6, 4.3] | 3.8 [3.6, 4.2] | < 0.001 | nonnorm |
| Na+, mEq/L | 139.3[136.7,141.3] | 139.4[136.3, 141.3] | 138.8[136.4,141.0] | 139.6[137.0,141.4] | 139.4[137.3,141.4] | 0.087 | nonnorm |
| Albumin, g/dL | 38.3 [35.1, 41.1] | 37.7 [34.6, 40.6] | 37.9 [35.2, 41.0] | 38.5 [35.3, 41.7] | 38.9 [35.2, 41.2] | 0.051 | nonnorm |
| BUN, mg/Dl | 6.2 [4.8, 8.4] | 5.9 [4.7, 7.5] | 6.0 [4.7, 8.0] | 6.3 [5.0, 8.5] | 6.4 [4.7, 9.5] | 0.037 | nonnorm |
| Glucose, mg/dl | 110.9 [90.2, 140.2] | 88.9 [78.7, 104.4] | 105.3 [89.5, 126.7] | 118.3 [97.4, 139.5] | 151.2[117.5,190.4] | < 0.001 | nonnorm |
| Triglycerides, mg/dl | 74.4 [58.5, 96.6] | 54.9 [45.2, 63.8] | 68.2 [56.7, 81.5] | 85.0 [70.9, 104.5] | 106.3 [85.0, 139.9] | < 0.001 | nonnorm |
| TC, mg/dL | 148.9 [125.3, 172.5] | 143.5 [121.8, 163.2] | 143.5 [120.3, 165.1] | 155.8 [129.9, 175.6] | 158.6 [133.8, 182.1] | < 0.001 | nonnorm |
| LDL, mg/dL | 84.0 [67.0, 101.6] | 77.4[61.9,89.0] | 81.3[65.8,96.8] | 89.0[69.7,104.5] | 92.9[73.5,112.2] | < 0.001 | nonnorm |
| HDL, mg/dL | 45.7 [37.5, 54.8] | 46.4[38.7,58.1] | 46.4[38.7,58.1] | 42.6[38.7,54.2] | 42.6[38.7,54.2] | 0.007 | nonnorm |
| APTT, sec | 36.9 [34.3, 40.6] | 38.2 [35.3, 42.0] | 37.5 [34.6, 40.5] | 36.3 [34.0, 40.4] | 35.9 [33.5, 39.5] | < 0.001 | nonnorm |
| PT, sec | 13.5 [12.9, 14.2] | 13.6 [13.0, 14.4] | 13.5 [13.0, 14.3] | 13.4 [12.8, 14.2] | 13.3 [12.7, 14.1] | 0.001 | nonnorm |
| D-Dimer, ng/ml | 700.0[380.0,1425.0] | 625.0[310.0,1172.5] | 720.0[400.0,1410.0] | 760.0[422.5,1577.5] | 785.0[387.5,1562.5] | 0.004 | nonnorm |
| TyG index | 8.4 [8.0, 8.7] | 7.8 [7.7, 8.0] | 8.2 [8.1, 8.3] | 8.5 [8.4, 8.6] | 8.9 [8.8, 9.1] | < 0.001 | nonnorm |
| PO2, mmHg | 80.0 [65.6, 106.1] | 83.4 [68.0, 101.1] | 84.7 [69.2, 112.6] | 81.2 [64.5, 108.6] | 74.5 [62.5, 95.4] | 0.015 | nonnorm |
| PCO2, mmHg | 51.1 [41.7, 66.8] | 48.8 [41.8, 63.4] | 50.9 [41.6, 66.0] | 51.1 [42.1, 67.4] | 54.7 [41.8, 69.7] | 0.631 | nonnorm |
| OI, mmHg | 269.0 [211.0, 366.0] | 277.0 [225.0, 372.0] | 292.0 [229.0, 386.0] | 250.0 [203.0, 350.0] | 258.0 [202.0, 346.0] | 0.071 | nonnorm |
| Medicine | |||||||
| Glucocorticoid, n(%) | 742 (62.5) | 171 (57.6) | 173 (58.2) | 193 (65.0) | 205 (69.0) | 0.009 | |
| Bronchodilators,n(%) | 976 (82.2) | 250 (84.2) | 240 (80.8) | 247 (83.2) | 239 (80.5) | 0.578 | |
| Antibiotic, n(%) | 1137 (95.7) | 287 (96.6) | 284 (95.6) | 280 (94.3) | 286 (96.3) | 0.502 | |
| Usealbumin, n (%) | 53 (4.5) | 17 (5.7) | 9 (3.0) | 11 (3.7) | 16 (5.4) | 0.316 | |
| Events | |||||||
| LOS hospital, days | 8.0 [6.0, 10.0] | 8.0 [6.0, 10.0] | 7.0 [6.0, 10.0] | 8.0 [6.0, 10.0] | 8.0 [6.0, 11.0] | 0.236 | |
| RF, n (%) | 455 (38.3) | 96 (32.3) | 91 (30.6) | 103 (34.7) | 165 (55.6) | < 0.001 | |
| Mechanicalventilation, n(%) | 73 (6.1) | 11 (3.7) | 20 (6.7) | 18 (6.1) | 24 (8.1) | 0.159 | |
TyG index: Q1 (≤ 8.038636, Q2 (8.038636 < TyG ≤ 8.371388), Q3 (8.371388 < TyG ≤ 8.702957), Q4 (> 8.702957)
Abbreviations: BMI Body Mass Index, RTI Respiratory tract infection, CHD Coronary heart disease, HF Heart failure, DM Diabetes mellitus, WBC White blood cell, Lcell Lymphocyte, Ncell Neutrophile granulocyte, RBC Red blood cell, PLT Platelet, CRP C-reactive protein, HbA1c Glycosylated hemoglobin, Tbil Total bilirubin, Ibil Indirect bilirubin, ALT Glutamic-pyruvic transaminase, AST Glutamic oxalacetic transaminase, Scr Serum creatinine, K+ Potassium, Na + Sodium, BUN Blood urea nitrogen, TC Total cholesterol, LDL Low Density Lipoprotein, HDL High density lipoprotein, APTT Activated partial thromboplastin time, PT Prothrombin time, TyG index Triglyceride glucose index, PO2 pressure of oxygen, PCO2 Pressure of Carbon Dioxide, OI Oxygenation Index, LOS length of stay, ICU Intensive care unit, RF Respiratory failure, HR hazard ratio, CI confidence interval, TyG index Triglyceride glucose index
The baseline comparison between the two cohorts is presented in Table S1.
Risk of RF by quartiles
We utilized cumulative incidence curves to assess the RF risk across four TyG groups (Fig. 2). It is noteworthy that a significant difference in RF risk was observed among these groups (P = 0.005).
Fig. 2.

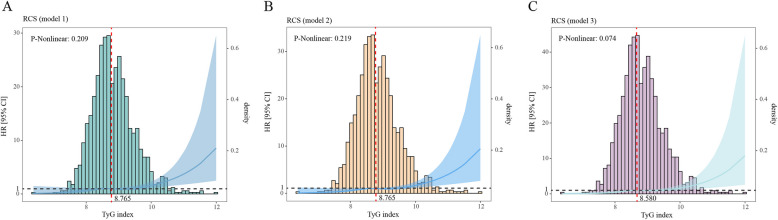
Restricted cubic spline analysis of TyG index with respiratory failure incidence (Model 1, Model 2 and Model 3). HR: hazard ratio, CI: confidence interval, TyG index: Triglyceride-glucose index. Heavy central lines represent the estimated adjusted hazard ratios, with shaded ribbons denoting 95% confidence intervals. TyG index 8.765 was selected as the reference level represented by the vertical dotted lines in model 1and model 2, TyG index 8.580 was selected as the reference level represented by the vertical dotted lines in model 3. The horizontal dotted lines represent the hazard ratio of 1.0
Correlation between TyG index and outcomes
We subsequently incorporated both forms of the TyG index, continuous and quartile-based variables, for distinct modeling analyses. In the MMIC database, the Cox proportional hazards analysis (Table 3) unveiled that, in the unadjusted model (Model 1), the risk of RF exhibited a substantial association with the TyG index (HR, 1.561[95% CI 1.219–2.000], P < 0.001). Furthermore, this association was notably maintained in Model 3 (HR: 1.821 [95% CI 1.349–2.459], P < 0.001); for each one-unit increase in the TyG index, the risk of developing RF increases by a factor of 1.821. Moreover, in comparison to the Q1 group, the Q4 group demonstrated a significant increase in the risk of RF; the likelihood of RF occurrence in the Q4 group is 3.51 times greater than that in the Q1 group. (Model 1: HR:2.400 [95% CI 1.413–4.078], P = 0.001; Model 2: HR: 2.551 [95% CI 1.485–4.381], P < 0.001; Model 3: HR: 3.510 [95% CI 1.885–6.535], P < 0.001).
Table 3.
Cox regression analysis for TyG index with respiratory failure in MIMIC database
| Categories | Events (%) | Model 1 | Model 2 | Model 3 | |||
|---|---|---|---|---|---|---|---|
| HR [95%CI] | P-value | HR [95%CI] | P-value | HR [95%CI] | P-value | ||
| Incidence of respiratory failure | |||||||
| Continuous variable per 1 unit | 134 (10.9) | 1.561 [1.219–2.000] | < 0.001 | 1.632 [1.263–2.107] | < 0.001 | 1.821 [1.349–2.459] | < 0.001 |
| Quartilea | p-value: 0.004 | p-value: 0.001 | p-value: < 0.001 | ||||
| Q1 (N = 308) | 19 (6.2) | Ref | Ref | Ref | |||
| Q2 (N = 309) | 34 (11.0) | 1.638 [0.934–2.873] | 0.085 | 1.674 [0.954–2.939] | 0.073 | 2.372 [1.317–4.273] | 0.004 |
| Q3 (N = 307) | 32 (10.4) | 1.405 [0.795–2.480] | 0.242 | 1.481 [0.836–2.625] | 0.178 | 1.853 [1.017–3.376] | 0.044 |
| Q4 (N = 308) | 49 (15.9) | 2.400 [1.413–4.078] | 0.001 | 2.551 [1.485–4.381] | < 0.001 | 3.510 [1.885–6.535] | < 0.001 |
Model 1: unadjusted
Model 2: adjusted for Age, Gender, BMI
Model 3: adjusted for Age, Gender, BMI, Cerebrovascular disease, Coronary heart disease, Heart failure, Diabetes mellitus, Hypertension, Asthma, use Bronchodilators, use Glucocorticoid, use Antibiotic, Albumin, High Density Lipoprotein, Low Density Lipoprotein, Total cholesterol, Activated partial thromboplastin time, Blood urea nitrogen, Potassium, Sodium, Glycosylated Hemoglobin, Type A1C
HR hazard ratio, CI confidence interval, TyG index Triglyceride glucose index
aTyG index: Q1 (≤ 8.408048, Q2 (8.408048 < TyG ≤ 8.759355), Q3 (8.759355 < TyG ≤ 9.205240), Q4 (> 9.205240)
In the Chinese cohort, Cox proportional hazards analysis yielded similar results (Table 4). Both in the unadjusted model (Model 1: HR, 1.568 [95% CI 1.350–1.822], P < 0.001) and the fully adjusted model (Model 3: HR, 1.786 [95% CI 1.513–2.108], P < 0.001), there was a significant association between the risk of RF occurrence and the TyG index; the risk of developing RF increases by 1.786 times for each one-unit increase in the TyG index. Additionally, compared to the low level of TyG index (Q1), the high level of TyG index (Q4) was also associated with a higher risk of RF occurrence; the risk of RF in the Q4 group is 1.911 times higher compared to the Q1 group (Model 1: HR, 1.598 [95% CI 1.241–2.058], P < 0.001; Model 2: HR, 1.611 [95% CI 1.250–2.077], P < 0.001; Model 3: HR, 1.911 [95% CI 1.445–2.526], P < 0.001).
Table 4.
Cox regression analysis for TyG index with respiratory failure in Chinese cohort
| Categories | Events (%) | Model 1 | Model 2 | Model 3 | |||
|---|---|---|---|---|---|---|---|
| HR [95%CI] | P-value | HR [95%CI] | P-value | HR [95%CI] | P-value | ||
| Incidence of respiratory failure | |||||||
| Continuous variable per 1 unit | 341 (28.7) | 1.568 [1.350–1.822] | < 0.001 | 1.581 [1.362–1.836] | < 0.001 | 1.786 [1.513–2.108] | < 0.001 |
| Quartilea | p-value: < 0.001 | p-value: < 0.001 | p-value: < 0.001 | ||||
| Q1 (N = 297) | 94 (31.6) | Ref | Ref | Ref | |||
| Q2 (N = 297) | 79 (26.6) | 0.797 [0.596–1.065] | 0.125 | 0.796 [0.594–1.066] | 0.125 | 0.833 [0.620–1.120] | 0.226 |
| Q3 (N = 297) | 86 (29.0) | 1.064 [0.805–1.406] | 0.663 | 1.026 [0.775–1.358] | 0.857 | 1.102 [0.822–1.478] | 0.514 |
| Q4 (N = 297) | 82 (27.6) | 1.598 [1.241–2.058] | < 0.001 | 1.611 [1.250–2.077] | < 0.001 | 1.911 [1.445–2.526] | < 0.001 |
Model 1: unadjusted
Model 2: adjusted for Age, Gender, BMI
Model 3: adjusted for Age, Gender, BMI, Coronary heart disease, Heart failure, Diabetes mellitus, Hypertension, Asthma, use Bronchodilators, use Glucocorticoid, use Antibiotic, Albumin, High Density Lipoprotein, Low Density Lipoprotein, Total cholesterol, Activated partial thromboplastin time, Blood urea nitrogen, Potassium, Sodium, Glycosylated Hemoglobin, Type A1C
HR hazard ratio, CI confidence interval, TyG index Triglyceride glucose index, Ref. reference
aTyG index: Q1 (≤ 8.038636, Q2 (8.038636 < TyG ≤ 8.371388), Q3 (8.371388 < TyG ≤ 8.702957), Q4 (> 8.702957)
The RCS curves indicated a nearly linear relationship between the TyG index and the RF risk in COPD patients across all models (Fig. 3) (Model 1: P-non-linear = 0.209), Model 2: P-non-linear = 0.219, Model 3: P-non-linear = 0.074). Furthermore, heightened levels of the TyG index (TyG index > 8.765 in Model 1 and Model 2, TyG index > 8.580 in Model 3) were positively associated with the RF risk in COPD patients. It is noteworthy that the majority of RF patients had a TyG index of 8 to 10.
Fig. 3.
Cumulative event rate analysis curves for respiratory failure. TyG index: Q1 (≤ 8.408048, Q2 (8.408048 < TyG ≤ 8.759355), Q3 (8.759355 < TyG ≤ 9.205240), Q4 (> 9.205240)
The logistic regression analysis unveiled a conspicuous link between the TyG index and the utilization of invasive mechanical ventilation (Table 5) (Model 1: OR, 1.665[95% CI 1.227, 2.243], P < 0.001; Model 3: OR, 1.611[95% CI 1.086, 2.392], P = 0.018). Moreover, elevated levels of the TyG index were linked to a heightened frequency of invasive mechanical ventilation use (Model 1: OR, 3.045[95% CI 1.655, 5.921], P < 0.001; Model 2: OR, 2.802[95% CI 1.494, 5.539], P = 0.002; Model 3: OR, 2.887[95% CI 1.38, 6.315], P = 0.006). It is notable that each incremental unit in the TyG index was linked to a 1.611-fold elevated risk of invasive mechanical ventilation use (OR,1.611[95% CI 1.086, 2.392], P = 0.018)]).
Table 5.
Logistics regression analysis for TyG index with invasive mechanical ventilation
| Categories | Events (%) | Model 1 | Model 2 | Model 3 | |||
|---|---|---|---|---|---|---|---|
| OR [95%CI] | P-value | OR [95%CI] | P-value | OR [95%CI] | P-value | ||
| Whether using mechanical ventilate | |||||||
| Continuous variable per 1 unit | 88(7.1) | 1.665[1.227, 2.243] | < 0.001 | 1.629[1.177, 2.244] | 0.003 | 1.611[1.086, 2.392] | 0.018 |
| Quartilea | |||||||
| Q1 (N = 308) | 14(4.5) | Ref | Ref | Ref | |||
| Q2 (N = 309) | 16(5.2) | 1.147[0.549, 2.423] | 0.715 | 1.121[0.534, 2.379] | 0.762 | 1.183[0.546, 2.592] | 0.670 |
| Q3 (N = 307) | 19(6.2) | 1.385[0.685, 2.867] | 0.368 | 1.35[0.663, 2.812] | 0.411 | 1.266[0.593, 2.758] | 0.544 |
| Q4 (N = 308) | 39(12.7) | 3.045[1.655, 5.921] | < 0.001 | 2.802[1.494, 5.539] | 0.002 | 2.887[1.38, 6.315] | 0.006 |
Model 1: unadjusted
Model 2: adjusted for Age, Gender, BMI
Model 3: adjusted for Age, Gender, BMI, Cerebrovascular disease, Coronary heart disease, Heart failure, Diabetes mellitus, Hypertension, Asthma, use Bronchodilators, use Glucocorticoid, use Antibiotic, Albumin, High Density Lipoprotein, Low Density Lipoprotein, Total cholesterol, Activated partial thromboplastin time, Blood urea nitrogen, Potassium, Sodium, Glycosylated Hemoglobin, Type A1C
HR hazard ratio, CI confidence interval, TyG index Triglyceride glucose index
aTyG index: Q1 (≤ 8.408048, Q2 (8.408048 < TyG ≤ 8.759355), Q3 (8.759355 < TyG ≤ 9.205240), Q4 (> 9.205240)
The multiple linear regression implied a positive relationship between the TyG index and the duration of hospitalization (Table 6) (Model 1: Beta, 0.922[95% CI 0.066, 1.777], P = 0.035; Model 3: Beta, 0.972[95% CI 0.104, 1.840], P = 0.028). Furthermore, in Models 1, 2, and 3, heightened TyG index levels were also markedly linked to prolonged hospital stay (Model 1: Beta, 1.779[95% CI 0.179, 3.379], P = 0.029; Model 2: Beta, 1.777[95% CI 0.154, 3.400], P = 0.032; Model 3: Beta, 1.642 [95% CI 0.073, 3.212], P = 0.041).
Table 6.
Linear regression analysis for TyG index with Days in hospital
| Categories | Model 1 | Model 2 | Model 3 | |||
|---|---|---|---|---|---|---|
| Beta [95% CI] | P-value | Beta [95% CI] | P-value | Beta [95% CI] | P-value | |
| Days in hospital | ||||||
| Continuous variable per 1 unit | 0.922[0.066, 1.777] | 0.035 | 0.962[0.081, 1.842] | 0.033 | 0.972[0.104, 1.840] | 0.028 |
| Quartilea | ||||||
| Q1 (N = 308) | Ref | Ref | Ref | |||
| Q2 (N = 309) | 1.146[−0.452, 2.745] | 0.200 | 1.007[−0.577, 2.591] | 0.200 | 0.727[0.646, 2.099] | 0.300 |
| Q3 (N = 307) | 1.830[0.229, 3.431] | 0.025 | 1.872[0.280, 3.463] | 0.021 | 1.477[0.070, 2.884] | 0.040 |
| Q4 (N = 308) | 1.779[0.179, 3.379] | 0.029 | 1.777[0.154, 3.400] | 0.032 | 1.642 [0.073, 3.212] | 0.041 |
Model 1: unadjusted
Model 2: adjusted for Age, Gender, BMI
Model 3: adjusted for Age, Gender, BMI, Cerebrovascular disease, Coronary heart disease, Heart failure, Diabetes mellitus, Hypertension, Asthma, use Bronchodilators, use Glucocorticoid, use Antibiotic, Albumin, High Density Lipoprotein, Low Density Lipoprotein, Total cholesterol, Activated partial thromboplastin time, Blood urea nitrogen, Potassium, Sodium, Glycosylated Hemoglobin, Type A1C
HR hazard ratio, CI confidence interval, TyG index Triglyceride glucose index
aTyG index: Q1 (≤ 8.408048, Q2 (8.408048 < TyG ≤ 8.759355), Q3 (8.759355 < TyG ≤ 9.205240), Q4 (> 9.205240)
Subgroup analysis
Subgroup analysis was executed by gender, age (< 60 years and ≥ 60 years), and the presence or absence of diabetes, hypertension, heart failure, cirrhosis, sepsis, and respiratory tract infection Fig. 4). The results indicated that an elevated continuous TyG index was markedly linked to an increased RF risk in most subgroups, we also calculated the hazard ratio (HR) and confidence intervals (per one unit of TyG index), such as patients under the age of 60 years [HR(95% CI) 1.376(1.020–1.855)], patients aged 60 years and above [HR(95% CI) 2.023(1.281–3.193)], females [HR (95% CI) 2.049(1.384–3.034)], patients with diabetes [HR(95% CI) 1.606(1.090–2.366), patients without diabetes [HR (95% CI) 1.613(1.104–2.356)], patients without HF [HR(95% CI) 1.570(1.134–2.175)], patients with HF [HR(95% CI) 1.520(1.037–2.228)], patients without hypertension [HR(95% CI) 1.446(1.038–2.014)], patients with hypertension [HR(95% CI) 1.775(1.229–2.562)], patients without liver cirrhosis [HR (95% CI) 1.572(1.218–2.030)], patients without sepsis [HR(95% CI) 1.461(1.115–1.914)], patients without RTI [HR (95% CI)1.504(1.164–1.943)] (P < 0.05). Interaction analysis revealed that except for gender, which suggested an interaction (P for interaction = 0.089), the differences in effects among the various subgroups were not statistically significant (P for interaction > 0.1).
Fig. 4.
Subgroup analysis of the relationship between TyG index and respiratory failure. HR hazard ratio, CI confidence interval, TyG index Triglyceride-glucose index, HF Heart Failure, RTI Respiratory tract infection
Discussion
The present study firstly probes into the link between the TyG index and the risk of RF in patients with COPD. The data on patients were acquired from the MIMIC-IV 2.2 database, and 1232 COPD patients were eligible and included. The substantial sample size has the potential to enhance the generalizability of these findings. Our analysis results unequivocally indicate that elevated TyG index levels serve as independent risk factors for RF in COPD patients. Besides, a direct association is noted between higher TyG index levels and an augmented risk of RF, as well as increased utilization of invasive mechanical ventilation, prolonged hospitalization. The cumulative incidence curves further corroborate that individuals with heightened TyG index values face a heightened risk of RF, thereby underscoring the clinical significance of a high TyG index in predicting the likelihood of RF occurrence in COPD patients. Furthermore,we performed external validation of the primary outcome Cox model, which yielded similar results.
In previous research, Wang et al. noted that the TyG index is associated with poor prognostic outcomes, such as hospital and ICU mortality, in patients with acute exacerbations of COPD [20]. Similarly, Zhou et al. reached a similar conclusion, showing a significant association between the TyG index and all-cause mortality in patients with asthma and COPD [21]. In our study, Cox proportional hazard model analysis clearly demonstrates that a high TyG index is an independent risk factor for respiratory failure in COPD patients, even after adjusting for confounding factors. This finding is consistent with and reinforces the results from earlier studies.
The relationship between the TyG index and the risk of RF in COPD patients is still not fully understood. The TyG index is a reliable biomarker for insulin resistance (IR), which may be its primary mechanism. Previous studies have shown a high prevalence of IR in individuals with chronic obstructive pulmonary disease (COPD) [22], with more severe breathlessness [23] and a higher incidence of poor prognostic outcomes [24] in those who have IR. Elevated insulin levels induced by IR have been implicated in triggering laminin expression in airway smooth muscle through phosphatidylinositol 3 kinase (PI3K) and Rho-kinase dependent pathways. This, in turn, leads to heightened contraction of airway smooth muscle [25]. Moreover, increased insulin levels can provoke vagus nerve-mediated bronchoconstriction and the impairment of parasympathetic inhibitory muscarinic receptor 2 function [26, 27]. These effects lead to diminished lung function, which worsens the condition of COPD patients and ultimately results in respiratory failure. Furthermore, oxidative stress and inflammation are considered key factors in the progression of COPD. Studies have shown a persistent inflammatory response in the airways and lungs of COPD patients, which is closely linked to oxidative stress [28]. The increased levels of reactive oxygen species exceed the body's antioxidant defenses, causing cellular damage and dysfunction [29]. In the context of COPD pathophysiology, oxidative stress not only intensifies the inflammatory response but may also contribute to airway remodeling and a decline in lung function, thus highlighting its critical role in the disease's progression [30]. Prior research has also indicated that elevated blood glucose levels result in elevated glucose content and advanced glycation end products (AGEs) in airway secretions, thereby fostering local inflammation, respiratory tract infections, and consequent impairment of lung function [31, 32]. Elevated triglyceride levels can incite inflammatory responses in macrophages and various immune cells [33]. The TyG index, serving as a dependable biomarker for IR, integrates both glucose, reflecting hepatic IR, and triglyceride, indicating adipocyte IR. Consequently, it provides a more comprehensive representation of the interplay between IR and COPD patients.
An extended hospital stay may elevate the risk of complications like nosocomial infections, deep vein thrombosis, and pressure sores, potentially impacting a patient's rehabilitation progress [34]. Additionally, the duration of hospitalization can serve as an indirect indicator of disease severity. Hence, we examined the link between TyG index and hospital stay. Our findings indicated that the hospital stay were prolonged in tandem with higher TyG index levels. As a result, we surmised that the TyG index level might be employed to forecast the duration of hospitalization in COPD patients. This finding assists in early detection of COPD patients, and individuals with a high TyG index should be given additional care upon admission, in order to enhance disease management, reduce hospital stay, and prevent complications associated with prolonged hospitalization. Drawing from previous studies, the use of invasive mechanical ventilation has been associated with increased exacerbations in COPD patients [35], while noninvasive assisted ventilation has significantly reduced mortality in acute exacerbation of COPD (AECOPD) [36]. Therefore, pinpointing the indications for invasive mechanical ventilation and minimizing unnecessary usage can potentially enhance the prognosis for COPD patients. Our study discovered a significant increase in invasive mechanical ventilation usage in individuals with high TyG index levels. Consequently, for patients with elevated TyG index, optimizing the respiratory support treatment strategy and using non-invasive ventilation, instead of invasive treatment, can effectively reduce exacerbations and enhance the prognosis for COPD patients.
According to the baseline characteristics of COPD patients enrolled in the present study, younger patients tend to have higher TyG index levels, aligning with prior research findings [37]. This underscores the importance of focusing on the younger COPD population, as they might be at a heightened risk for developing RF. Additionally, we uncovered that patients with RF exhibited elevated levels of white blood cells (WBC), neutrophils, and C-reactive protein (CRP), further underscoring the role of the inflammatory response in the progression of COPD. We also observed that the BMI of the study population in the MIMIC database was notably higher than that in the Chinese cohort, which can be ascribed to several factors. Research has indicated that the American diet is characterized by a higher proportion of high-fat, high-sugar, and high-calorie foods, largely due to the widespread fast food culture, resulting in excessive calorie intake and an elevated risk of obesity [38]. In contrast, the traditional Chinese diet primarily consists of rice, vegetables, and legumes, with relatively lower meat consumption [39]. Furthermore, a sedentary lifestyle and insufficient physical activity are common in the United States [40]. Although China is experiencing lifestyle changes due to urbanization, the overall level of physical activity remains higher in comparison [41]. Additionally, there may be variations in the carrier frequency of obesity-related genes between the two populations [42], which could further contribute to the significant BMI difference.
Nonetheless, this study has several limitations. Firstly, our research did not encompass data related to lifestyle, nutritional status, and the use of antidiabetic or lipid-lowering medications. These factors could potentially influence the baseline TyG levels. Secondly, it's important to acknowledge that this is a retrospective study, retrospective data may be subject to selection bias, and the quality and completeness of the data cannot be intervened or controlled, making it susceptible to statistical issues like selection bias and limited control of confounding variables. Third, there are variations in BMI and other characteristics between the study populations in the Chinese cohort and the MIMIC cohort. Consequently, the generalizability of the findings from this study may be limited when applied to different populations. In conclusion, the TyG index holds promise as an effective biomarker for predicting RF in COPD patients, with potential clinical utility. However, its validity still needs to be verified by prospective and multicenter investigations.
Conclusion
The TyG index can forecast the RF risk in COPD patients to some extent. Individuals with elevated TyG index levels tend to face a higher risk of RF. Additionally, it serves as an indicator for increased usage of invasive mechanical ventilation, and extended stays in the hospital. Our results unveil that TyG index is correlated with the risk of RF in COPD patients, and it is worthy of further exploration and practical application in clinical settings. Further studies are desired to investigate whether reducing TyG index levels can mitigate the risk of RF in COPD patients, and whether the TyG index can be integrated into the comprehensive evaluation of COPD.
Supplementary Information
Supplementary Material 1: Table S1. Baseline comparison between the database population and the Chinese cohort population.
Acknowledgements
Not applicable.
Abbreviations
- TyG
Triglyceride-Glucose
- RF
Respiratory failure
- COPD
Chronic obstructive pulmonary disease
- RCS
Restricted cubic spline
- QUICKI
Quantitative Insulin Sensitivity Test Index
- BIDMC
Beth Israel Deaconess Medical Center
Authors' contributions
Conceptualization: SH, YZ(2), ZC. Data curation: SH, YZ(2), ZC. Formal analysis: SH, WC. Funding acquisition: SH. Investigation: XT, JW. Methodology: XT, WC. Project administration: XT. Resources: XT, WC. Software: YZ(1), JW. Supervision: YZ(1), ZC,YZ(2). Validation: YZ, WC.Visualization: YZ(1). Writing -original draft: SH, XT, YZ(1), YZ(2), JW, WC. Writing-review & editing: SH, XT, YZ(1), YZ(2), JW, WC.
Funding
This study was supported by Key Construction Disciplines of Provincial and Municipal Co construction of Zhejiang [NO.2023-SSGJ-002].
The fund was not involved in any study design, data collection, analysis and interpretation, report writing, and article submission for publication.
Data availability
This retrospective investigation utilized data extracted from the Medical Information Mart for Intensive Care (MIMIC-IV version 2.2) (https://physionet.org/content/mimiciv/2.2/).
Declarations
Ethics approval and consent to participate
Ethical approval for the external validation participants was obtained from our institution (Ethical Approval No. 2023-KY-607).
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Shiyu Hu and Ye Zhang contributed equally to this work and share first authorship.
References
- 1.Venkatesan P. GOLD COPD report: 2023 update. Lancet Respir Med. 2023;11(1):18. [DOI] [PubMed] [Google Scholar]
- 2.Chronic obstructive pulmonary disease (COPD). https://www.who.int/news-room/fact-sheets/detail/chronic-obstructive-pulmonary-disease-(copd).
- 3.Suissa S, Dell’Aniello S, Ernst P. Long-term natural history of chronic obstructive pulmonary disease: severe exacerbations and mortality. Thorax. 2012;67(11):957–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Viegi G, Pistelli F, Sherrill DL, Maio S, Baldacci S, Carrozzi L. Definition, epidemiology and natural history of COPD. Eur Respir J. 2007;30(5):993–1013. [DOI] [PubMed] [Google Scholar]
- 5.Foucher P, Baudouin N, Merati M, Pitard A, Bonniaud P, Reybet-Degat O, Jeannin L. Relative survival analysis of 252 patients with COPD receiving long-term oxygen therapy. Chest. 1998;113(6):1580–7. [DOI] [PubMed] [Google Scholar]
- 6.Sukumalchantra Y, Dinakara P, Williams MH Jr. Prognosis of patients with chronic obstructive pulmonary disease after hospitalization for acute ventilatory failure: a three-year follow-up study. Am Rev Respir Dis. 1966;93(2):215–22. [DOI] [PubMed] [Google Scholar]
- 7.Hunter SJ, Garvey WT. Insulin action and insulin resistance: diseases involving defects in insulin receptors, signal transduction, and the glucose transport effector system. Am J Med. 1998;105(4):331–45. [DOI] [PubMed] [Google Scholar]
- 8.Sagun G, Gedik C, Ekiz E, Karagoz E, Takir M, Oguz A. The relation between insulin resistance and lung function: a cross sectional study. BMC Pulm Med. 2015;15:139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Greenfield MS, Doberne L, Kraemer F, Tobey T, Reaven G. Assessment of insulin resistance with the insulin suppression test and the euglycemic clamp. Diabetes. 1981;30(5):387–92. [DOI] [PubMed] [Google Scholar]
- 10.Du T, Yuan G, Zhang M, Zhou X, Sun X, Yu X. Clinical usefulness of lipid ratios, visceral adiposity indicators, and the triglycerides and glucose index as risk markers of insulin resistance. Cardiovasc Diabetol. 2014;13:146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Singh B, Saxena A. Surrogate markers of insulin resistance: A review. World J Diabetes. 2010;1(2):36–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guerrero-Romero F, Simental-Mendía LE, González-Ortiz M, Martínez-Abundis E, Ramos-Zavala MG, Hernández-González SO, Jacques-Camarena O, Rodríguez-Morán M. The product of triglycerides and glucose, a simple measure of insulin sensitivity. Comparison with the euglycemic-hyperinsulinemic clamp. J Clin Endocrinol Metab. 2010;95(7):3347–3351. [DOI] [PubMed]
- 13.Simental-Mendía LE, Rodríguez-Morán M, Guerrero-Romero F. The product of fasting glucose and triglycerides as surrogate for identifying insulin resistance in apparently healthy subjects. Metab Syndr Relat Disord. 2008;6(4):299–304. [DOI] [PubMed] [Google Scholar]
- 14.Tao LC, Xu JN, Wang TT, Hua F, Li JJ. Triglyceride-glucose index as a marker in cardiovascular diseases: landscape and limitations. Cardiovasc Diabetol. 2022;21(1):68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ren X, Jiang M, Han L, Zheng X. Association between triglyceride-glucose index and chronic kidney disease: A cohort study and meta-analysis. Nutr Metab Cardiovasc Dis. 2023;33(6):1121–8. [DOI] [PubMed] [Google Scholar]
- 16.Yan F, Yan S, Wang J, Cui Y, Chen F, Fang F, Cui W. Association between triglyceride glucose index and risk of cerebrovascular disease: systematic review and meta-analysis. Cardiovasc Diabetol. 2022;21(1):226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang J, Yan S, Cui Y, Chen F, Piao M, Cui W. The diagnostic and prognostic value of the triglyceride-glucose index in Metabolic Dysfunction-Associated Fatty Liver Disease (MAFLD): a systematic review and meta-analysis. Nutrients. 2022;14(23):4969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wu TD, Fawzy A, Brigham E, McCormack MC, Rosas I, Villareal DT, Hanania NA. Association of Triglyceride-Glucose Index and Lung Health: A Population-Based Study. Chest. 2021;160(3):1026–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang Y, Tan X, Hu S, Cui Z, Chen W. Relationship Between Systemic Immune-Inflammation Index and Risk of Respiratory Failure and Death in COPD: A Retrospective Cohort Study Based on the MIMIC-IV Database. Int J Chron Obstruct Pulmon Dis. 2024;19:459–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang X, Cui X, Fan H, Hu T. Elevated Triglyceride-Glucose (TyG) Index Predicts Poor Clinical Outcomes in Critically Ill AECOPD Patients: A Retrospective Study. Int J Chron Obstruct Pulmon Dis. 2024;19:2217–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhou WQ, Song X, Dong WH, Chen Z. Independent effect of the triglyceride-glucose index on all-cause mortality in critically ill patients with chronic obstructive pulmonary disease and asthma: A retrospective cohort study. Chron Respir Dis. 2024;21:14799731241245424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zuberi FF, Bader N, Rasheed T, Zuberi BF. Association between insulin resistance and BMI with FEV(1) in non-hypoxemic COPD out-patients. Clin Respir J. 2021;15(5):513–21. [DOI] [PubMed] [Google Scholar]
- 23.Díez-Manglano J, Barquero-Romero J, Almagro P, Cabrera FJ, López García F, Montero L, Soriano JB. COPD patients with and without metabolic syndrome: clinical and functional differences. Intern Emerg Med. 2014;9(4):419–25. [DOI] [PubMed] [Google Scholar]
- 24.Keeratichananont W, Kaenmuang P, Geater SL, Manoret P, Thanapattaraborisuth B. Prevalence, associated factors, and clinical consequences of metabolic syndrome in chronic obstructive pulmonary disease patients: a 5-year prospective observational study. Ther Adv Respir Dis. 2023;17:17534666231167342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dekkers BG, Schaafsma D, Tran T, Zaagsma J, Meurs H. Insulin-induced laminin expression promotes a hypercontractile airway smooth muscle phenotype. Am J Respir Cell Mol Biol. 2009;41(4):494–504. [DOI] [PubMed] [Google Scholar]
- 26.Lee H, Kim SR, Oh Y, Cho SH, Schleimer RP, Lee YC. Targeting insulin-like growth factor-I and insulin-like growth factor-binding protein-3 signaling pathways. A novel therapeutic approach for asthma. Am J Respir Cell Mol Biol. 2014;50(4):667–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nie Z, Jacoby DB, Fryer AD. Hyperinsulinemia potentiates airway responsiveness to parasympathetic nerve stimulation in obese rats. Am J Respir Cell Mol Biol. 2014;51(2):251–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zinellu E, Zinellu A, Fois AG, Pau MC, Scano V, Piras B, Carru C, Pirina P. Oxidative stress biomarkers in chronic obstructive pulmonary disease exacerbations: a systematic review. Antioxidants (Basel). 2021;10(5):710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Białas AJ, Sitarek P, Miłkowska-Dymanowska J, Piotrowski WJ, Górski P. The Role of Mitochondria and Oxidative/Antioxidative Imbalance in Pathobiology of Chronic Obstructive Pulmonary Disease. Oxid Med Cell Longev. 2016;2016:7808576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wiegman CH, Michaeloudes C, Haji G, Narang P, Clarke CJ, Russell KE, Bao W, Pavlidis S, Barnes PJ, Kanerva J, et al. Oxidative stress-induced mitochondrial dysfunction drives inflammation and airway smooth muscle remodeling in patients with chronic obstructive pulmonary disease. J Allergy Clin Immunol. 2015;136(3):769–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McKeever TM, Weston PJ, Hubbard R, Fogarty A. Lung function and glucose metabolism: an analysis of data from the Third National Health and Nutrition Examination Survey. Am J Epidemiol. 2005;161(6):546–56. [DOI] [PubMed] [Google Scholar]
- 32.Sparvero LJ, Asafu-Adjei D, Kang R, Tang D, Amin N, Im J, Rutledge R, Lin B, Amoscato AA, Zeh HJ, et al. RAGE (Receptor for Advanced Glycation Endproducts), RAGE ligands, and their role in cancer and inflammation. J Transl Med. 2009;7:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Baffi CW, Wood L, Winnica D, Strollo PJ Jr, Gladwin MT, Que LG, Holguin F. Metabolic Syndrome and the Lung. Chest. 2016;149(6):1525–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Coca DJ, Castelblanco SM, Chavarro-Carvajal DA, Venegas-Sanabria LC. In-hospital complications in an acute care geriatric unit. Biomedica. 2021;41(2):293–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gadre SK, Duggal A, Mireles-Cabodevila E, Krishnan S, Wang XF, Zell K, Guzman J. Acute respiratory failure requiring mechanical ventilation in severe chronic obstructive pulmonary disease (COPD). Medicine (Baltimore). 2018;97(17): e0487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Osadnik CR, Tee VS, Carson-Chahhoud KV, Picot J, Wedzicha JA, Smith BJ. Non-invasive ventilation for the management of acute hypercapnic respiratory failure due to exacerbation of chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2017;7(7):Cd004104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liao Y, Zhang R, Shi S, Zhao Y, He Y, Liao L, Lin X, Guo Q, Wang Y, Chen L, et al. Triglyceride-glucose index linked to all-cause mortality in critically ill patients: a cohort of 3026 patients. Cardiovasc Diabetol. 2022;21(1):128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Popkin BM, Adair LS, Ng SW. Global nutrition transition and the pandemic of obesity in developing countries. Nutr Rev. 2012;70(1):3–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hu XF, Zhang R, Chan HM. Identification of Chinese dietary patterns and their relationships with health outcomes: a systematic review and meta-analysis. Public Health Nutr. 2024;27(1): e209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yang L, Cao C, Kantor ED, Nguyen LH, Zheng X, Park Y, Giovannucci EL, Matthews CE, Colditz GA, Cao Y. Trends in Sedentary Behavior Among the US Population, 2001–2016. JAMA. 2019;321(16):1587–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li X, Zhang W, Zhang W, Tao K, Ni W, Wang K, Li Z, Liu Q, Lin J. Level of physical activity among middle-aged and older Chinese people: evidence from the China health and retirement longitudinal study. BMC Public Health. 2020;20(1):1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stryjecki C, Alyass A, Meyre D. Ethnic and population differences in the genetic predisposition to human obesity. Obes Rev. 2018;19(1):62–80. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material 1: Table S1. Baseline comparison between the database population and the Chinese cohort population.
Data Availability Statement
This retrospective investigation utilized data extracted from the Medical Information Mart for Intensive Care (MIMIC-IV version 2.2) (https://physionet.org/content/mimiciv/2.2/).