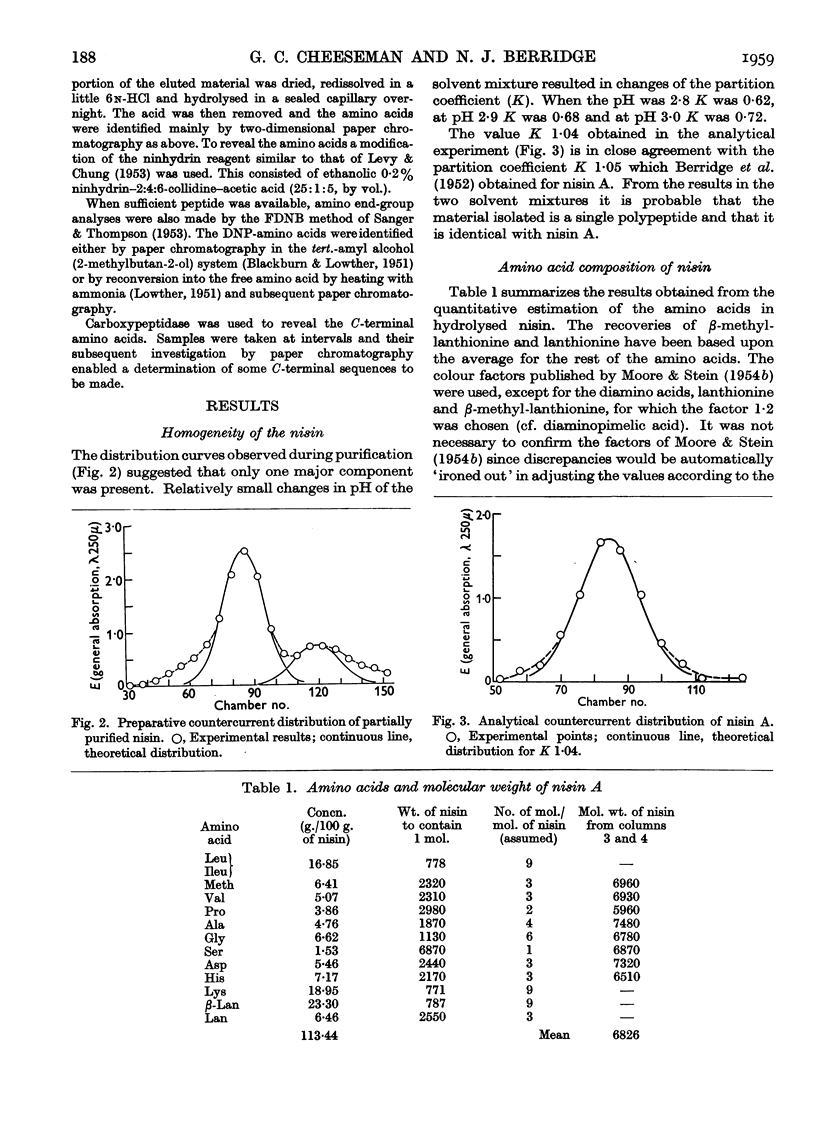
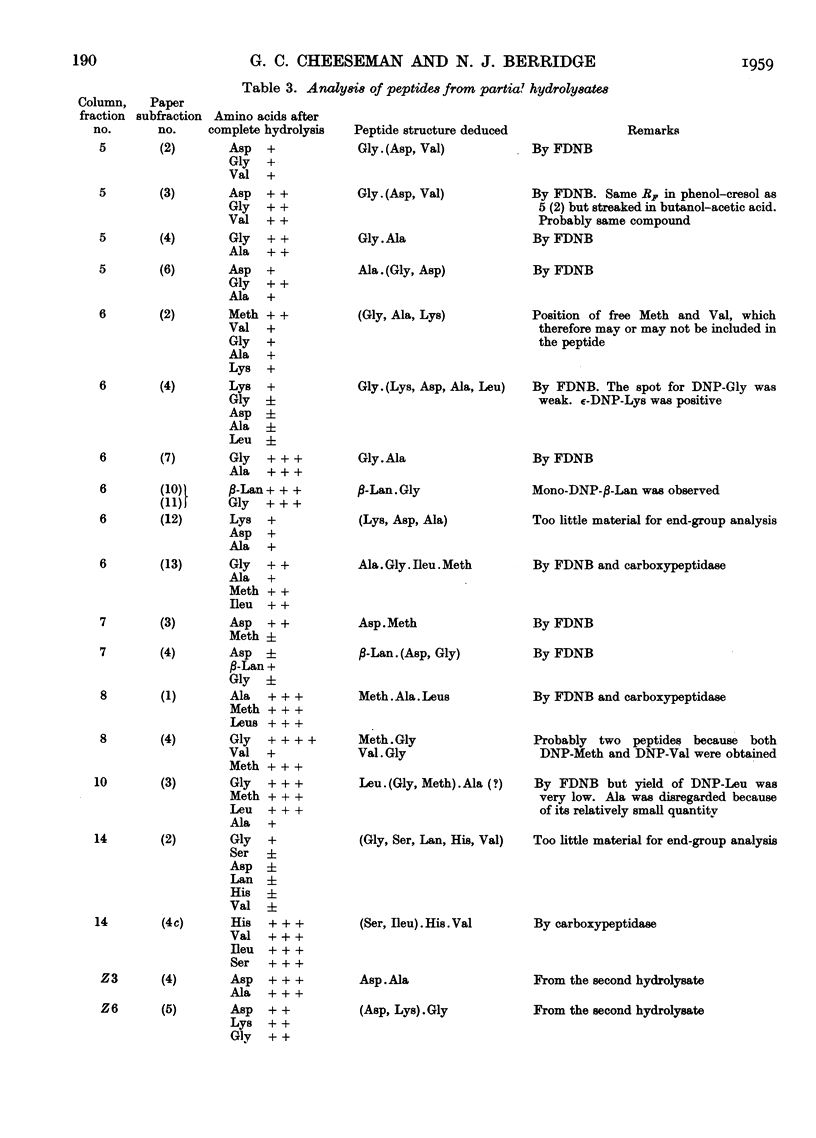
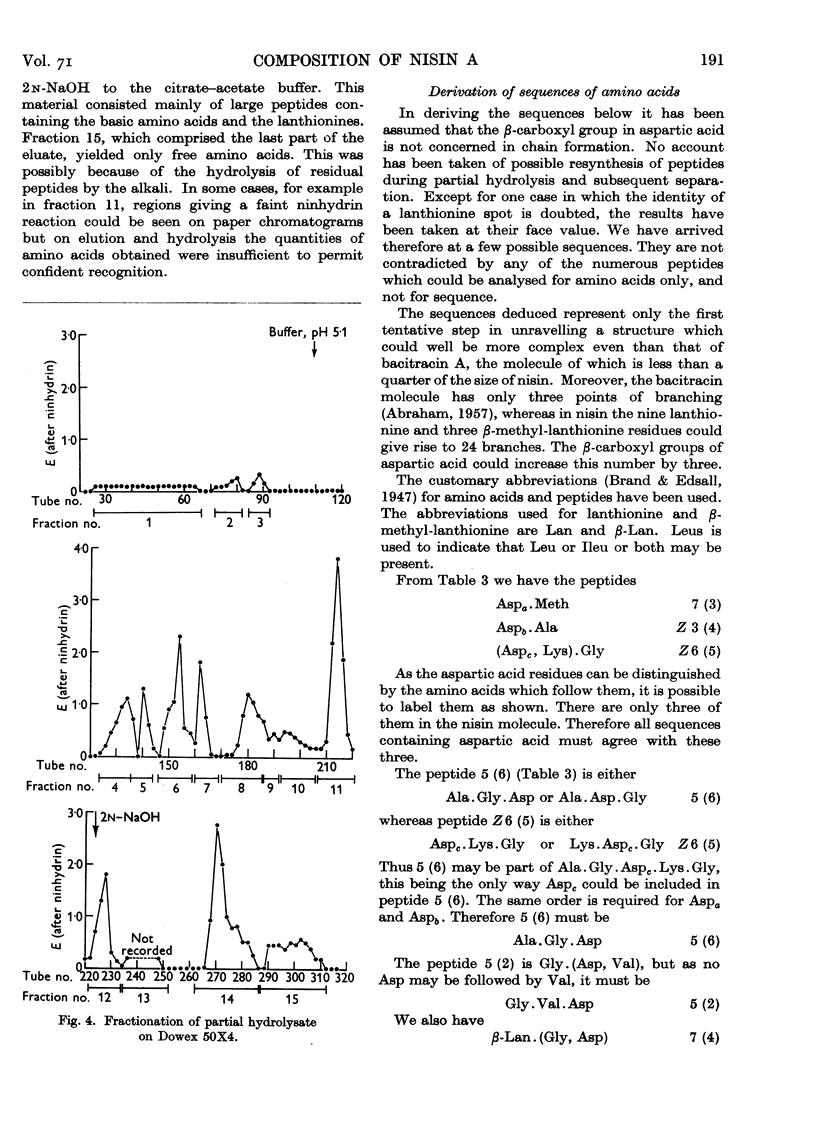
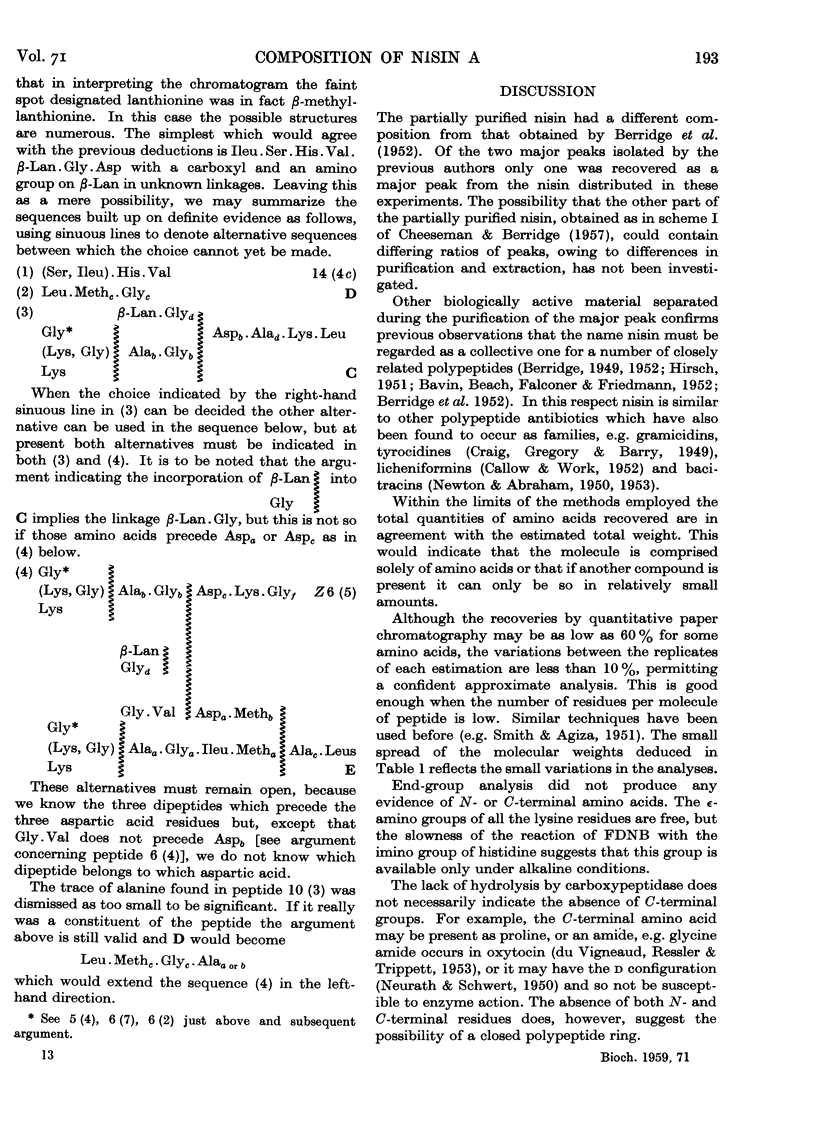
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BAVIN E. M., BEACH A. S., FALCONER R., FRIEDMANN R. Nisin in experimental tuberculosis. Lancet. 1952 Jan 19;1(6699):127–129. doi: 10.1016/s0140-6736(52)92429-x. [DOI] [PubMed] [Google Scholar]
- BERRIDGE N. J. Counter-current distribution of nisins. Nature. 1952 Apr 26;169(4304):707–708. doi: 10.1038/169707b0. [DOI] [PubMed] [Google Scholar]
- BERRIDGE N. J., NEWTON G. G. F., ABRAHAM E. P. Purification and nature of the antibiotic nisin. Biochem J. 1952 Dec;52(4):529–535. doi: 10.1042/bj0520529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BERRIDGE N. J. Preparation of the antibiotic nisin. Biochem J. 1949;45(4):486–493. doi: 10.1042/bj0450486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BLACKBURN S., LOWTHER A. G. The separation of N-2:4-dinitrophenly amino-acids on paper chromatograms. Biochem J. 1951 Jan;48(1):126–128. doi: 10.1042/bj0480126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CALLOW R. K., WORK T. S. Antibiotic peptides from Bacillus licheniformis; licheniformins A, B and C. Biochem J. 1952 Jul;51(4):558–568. doi: 10.1042/bj0510558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHEESEMAN G. C., BERRIDGE N. J. An improved method of preparing nisin. Biochem J. 1957 Mar;65(3):603–608. doi: 10.1042/bj0650603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CRAIG L. C., GREGORY J. D., BARRY G. T. Studies on polypeptides and amino acids by countercurrent distribution. Cold Spring Harb Symp Quant Biol. 1950;14:24–31. doi: 10.1101/sqb.1950.014.01.005. [DOI] [PubMed] [Google Scholar]
- DU VIGNEAUD V., RESSLER C., TRIPPETT S. The sequence of amino acids in oxytocin, with a proposal for the structure of oxytocin. J Biol Chem. 1953 Dec;205(2):949–957. [PubMed] [Google Scholar]
- ELLIOTT D. F. A search for specific chemical methods for fission of peptide bonds. I. The N-acyl to O-acyl transformation in the degradation of silk fibroin. Biochem J. 1952 Feb;50(4):542–550. doi: 10.1042/bj0500542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HALSEY Y. D., NEURATH H. The terminal carboxyl groups of denatured yeast triosephosphate dehydrogenase. J Biol Chem. 1955 Nov;217(1):247–252. [PubMed] [Google Scholar]
- HIRSCH A. Various antibiotics from one strain of Streptococcus lactis. Nature. 1951 Jun 23;167(4260):1031–1032. doi: 10.1038/1671031a0. [DOI] [PubMed] [Google Scholar]
- LOWTHER A. G. Identification of N-2: 4-dinitrophenylamino-acids. Nature. 1951 May 12;167(4254):767–768. doi: 10.1038/167767b0. [DOI] [PubMed] [Google Scholar]
- MOORE S., STEIN W. H. A modified ninhydrin reagent for the photometric determination of amino acids and related compounds. J Biol Chem. 1954 Dec;211(2):907–913. [PubMed] [Google Scholar]
- MOORE S., STEIN W. H. Chromatography of amino acids on sulfonated polystyrene resins. J Biol Chem. 1951 Oct;192(2):663–681. [PubMed] [Google Scholar]
- MOORE S., STEIN W. H. Procedures for the chromatographic determination of amino acids on four per cent cross-linked sulfonated polystyrene resins. J Biol Chem. 1954 Dec;211(2):893–906. [PubMed] [Google Scholar]
- NEWTON G. G. F., ABRAHAM E. P. Ayfivin and bacitracin: resolution of crude products into similar series of peptides. Biochem J. 1950 Sep;47(3):257–267. doi: 10.1042/bj0470257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NEWTON G. G. F., ABRAHAM E. P. Some properties of the bacitracin polypeptides. Biochem J. 1953 Mar;53(4):597–604. doi: 10.1042/bj0530597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PARTRIDGE S. M., BRIMLEY R. C. Displacement chromatography on synthetic ion-exchange resins. VIII. A systematic method for the separation of amino-acids. Biochem J. 1952 Aug;51(5):628–639. doi: 10.1042/bj0510628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SANGER F., THOMPSON E. O. P. The amino-acid sequence in the glycyl chain of insulin. II. The investigation of peptides from enzymic hydrolysates. Biochem J. 1953 Feb;53(3):366–374. doi: 10.1042/bj0530366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SANGER F. The terminal peptides of insulin. Biochem J. 1949;45(5):563–574. doi: 10.1042/bj0450563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F. The free amino groups of insulin. Biochem J. 1945;39(5):507–515. doi: 10.1042/bj0390507. [DOI] [PMC free article] [PubMed] [Google Scholar]


