The crystal structure of capsaicin, the natural product responsible for the pungency of chilli peppers, was determined by low-temperature single-crystal X-ray diffraction.
Keywords: capsaicin, capsaicinoid, Capsicum, crystal structure, single-crystal X-ray diffraction, natural product
Abstract
The crystal structure of capsaicin (C18H27NO3), or trans-8-methyl-N-vanillylnon-6-enamide, the natural product responsible for the spiciness of chilli peppers, was determined using low-temperature single-crystal X-ray diffraction. The reported crystal structure is in good agreement with previous determinations based on powder X-ray diffraction data. The localization and free refinement of all H atoms revealed that each capsaicin molecule is hydrogen bonded to four other molecules, with the O—H and N—H groups acting as hydrogen-bond donors, and the C=O group serving as a bifurcated hydrogen-bond acceptor.
Introduction
Capsaicin (Scheme 1) [systematic name (E)-N-[(4-hydroxy-3-methoxyphenyl)methyl]-8-methylnon-6-enamide; CAS: 404-86-4] is the principal bioactive compound from the capsaicinoid family of secondary metabolites found in the fruits of chilli pepper plants, which belong to the genus Capsicum with a very rich diversity of cultivars (Fig. 1 ▸). This natural product is primarily responsible for the spiciness or the heat sensation of hot chillies and acts as a potent agonist of the TRPV1 (transient receptor potential vanilloid 1) heat receptor, eliciting the characteristic burning sensation and making it a strong irritant (Caterina et al., 1997 ▸). Chilli peppers have been cultivated for several millennia and are integral to the culinary traditions of many cultures worldwide, with their consumption and popularity continuing to rise (Spence, 2018 ▸; Bosland & Votava, 2012 ▸). Beyond their culinary use, capsaicin and capsaicinoids have garnered attention for their pharmacological properties and diverse biological activity (Srinivasan, 2016 ▸; Spence, 2018 ▸). The pungency of chillies is quantified using the Scoville Heat Scale, where capsaicin is assigned a value of 16 million Scoville Heat Units (SHU), reflecting its extreme potency (Scoville, 1912 ▸; Collins et al., 1995 ▸; Bosland & Votava, 2012 ▸). Its unique physiological effects and diverse applications have made capsaicin a subject of extensive research.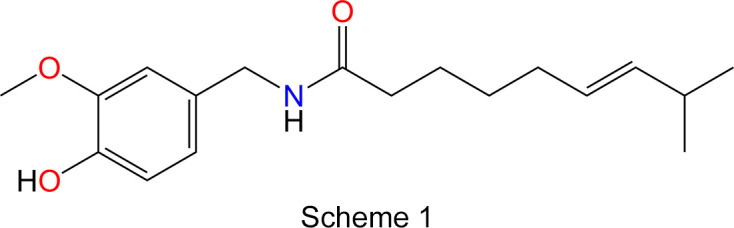
Figure 1.
A colourful variety of capsaicin-containing spicy chilli pepper fruits (Capsicum).
The Cambridge Structural Database (CSD, Version 5.46, November 2024; Groom et al., 2016 ▸) contains two previous structure determinations of the capsaicin crystal structure. The oldest entry, with CSD refcode FABVAF (Oliver, 1985 ▸), reports only the unit-cell parameters, without atomic coordinates. The second entry, FABVAF01, is a structure determination based on synchrotron powder X-ray diffraction (PXRD) data employing simulated annealing (David et al., 1998 ▸); however, the atomic coordinates were not refined, as the model of the capsaicin molecule was constructed using standard bond lengths and angles. The report mentions a single-crystal structure determination, which was used to validate the simulated annealing solution, but the single-crystal data were neither published nor deposited in the CSD. Similarly, unpublished single-crystal data were also used as a reference crystal structure for another structure redetermination of capsaicin via a simulated annealing approach from laboratory monochromatic capillary transmission PXRD data (Florence et al., 2005 ▸).1 Capsaicin has also been employed as a test sample in structure determination from powder diffraction data (Shankland et al., 2013 ▸), utilizing a hybrid Monte Carlo method (Markvardsen et al., 2005 ▸) and a local minimization approach (Shankland et al., 2010 ▸). Furthermore, the crystal structure of an α-fluorinated capsaicin derivative (FOSXOB; Winkler et al., 2009 ▸) and a cocrystal of a zinc coordination complex with a disordered capsaicin guest molecule (SOLZOM; Orton & Coles, 2024 ▸) were reported. The Protein Data Bank (PDB; Berman et al., 2000 ▸) contains several experimentally determined structures of macromolecular complexes with capsaicin (PDB entry 4dy) as a ligand, including 7vek (Maharjan et al., 2022 ▸), 7lr0 (Nadezhdin et al., 2021 ▸), 7lpa, 7lpb, 7lpd and 7lpe (Kwon et al., 2021 ▸), as well as 2n27 (Hetényi et al., 2016 ▸).
The PXRD crystal structure of capsaicin (David et al., 1998 ▸) has frequently served as a starting point for calculations and as a benchmark in computational studies (Alberti et al., 2008 ▸; Siudem et al., 2017 ▸; Soriano-Correa et al., 2023 ▸).
Single-crystal X-ray diffraction (SCXRD) is considered a ‘gold standard’ (Bond, 2014 ▸) for the structural elucidation of natural products and continues to provide valuable insights into the crystal structures of naturally occurring crystals, with recent examples of such studies including (+)-cedrol hemihydrate (Chakoumakos & Wang, 2024 ▸) and calcium (2R,3R)-tartrate tetrahydrate (Polo et al., 2024 ▸). Increasingly, 3D electron diffraction is gaining prominence in natural product characterization (Delgadillo et al., 2024 ▸), because it enables crystal structure and absolute configuration determination on nanometer-sized crystallites, as demonstrated by recent studies of beauveriolide I (Gurung et al., 2024 ▸) and berkecoumarin (Decato et al., 2024 ▸).
In this work, the crystal structure of capsaicin was determined using low-temperature single-crystal X-ray diffraction, providing a detailed insight into its molecular geometry, conformation and hydrogen-bonding interactions.
Experimental
Single-crystal selection
Capsaicin is a potent irritant and, to minimize exposure to the sample, it was handled as though the compound were air sensitive (Motaln et al., 2024 ▸). The sample of capsaicin was procured from a commercial source (Sigma–Aldrich, ≥95%) and stored in a refrigerator within a nitrogen-filled glovebox (Vigor SG1200/750E). A small amount of the microcrystalline powder was transferred onto a thin layer of Baysilone-Paste (Bayer-Silicone, mittelviskos) on a watch glass inside the glovebox and covered with a layer of perfluorodecaline (Fluorochem, 96.0%). A small crystal, measuring 27 µm × 63 µm × 75 µm, was selected under a polarizing microscope and attached to a MiTeGen Dual-Thickness MicroLoop using the Baysilone-Paste.
X-ray data collection and processing
Low-temperature single-crystal X-ray diffraction data were collected using a Rigaku OD XtaLAB Synergy-S instrument equipped with PhotonJet Ag and Cu microfocus X-ray tubes, a Dectris EIGER2 R CdTe 1M hybrid photon-counting detector and an Oxford Cryosystems Cryostream 800 Plus sample cooler. The crystal was measured at 100 K using Cu Kα radiation (λ = 1.54184 Å). Experimental details on crystal data, data collection, and structure refinement are summarized in Table 1 ▸. CrysAlis PRO software (Rigaku OD, 2024 ▸) was used for data collection and reduction, and the crystal structure was solved and refined within the OLEX2 program (Dolomanov et al., 2009 ▸) using SUPERFLIP (Palatinus & Chapuis, 2007 ▸; Palatinus & van der Lee, 2008 ▸; Palatinus et al., 2012 ▸) and SHELXL (Sheldrick, 2015 ▸), respectively. The measured crystal was an aggregate with two components; however, due to the presence of only a small fraction of overlapped reflections (<5%), data integration was performed on the major component (Bear et al., 2023 ▸). The positions and isotropic displacement parameters (Uiso) of all H atoms were refined freely (Cooper et al., 2010 ▸). Molecular graphics were generated using DIAMOND (Brandenburg, 2018 ▸).
Table 1. Experimental details.
| Crystal data | |
| Chemical formula | C18H27NO3 |
| M r | 305.40 |
| Crystal system, space group | Monoclinic, P21/c |
| Temperature (K) | 100 |
| a, b, c (Å) | 12.2165 (3), 14.7791 (4), 9.4719 (2) |
| β (°) | 94.035 (2) |
| V (Å3) | 1705.89 (8) |
| Z | 4 |
| Radiation type | Cu Kα |
| μ (mm−1) | 0.64 |
| Crystal size (mm) | 0.08 × 0.06 × 0.03 |
| Data collection | |
| Diffractometer | Rigaku XtaLAB Synergy-S Dualflex diffractometer with an Eiger2 R CdTe 1M detector |
| Absorption correction | Multi-scan (CrysAlis PRO; Rigaku OD, 2024 ▸) |
| Tmin, Tmax | 0.683, 1.000 |
| No. of measured, independent and observed [I > 2σ(I)] reflections | 18993, 3508, 2644 |
| R int | 0.054 |
| (sin θ/λ)max (Å−1) | 0.630 |
| Refinement | |
| R[F2 > 2σ(F2)], wR(F2), S | 0.044, 0.112, 1.04 |
| No. of reflections | 3508 |
| No. of parameters | 307 |
| H-atom treatment | All H-atom parameters refined |
| Δρmax, Δρmin (e Å−3) | 0.20, −0.21 |
Computer programs: CrysAlis PRO (Rigaku OD, 2024 ▸), SUPERFLIP (Palatinus & Chapuis, 2007 ▸; Palatinus & van der Lee, 2008 ▸; Palatinus et al., 2012 ▸), SHELXL2019 (Sheldrick, 2015 ▸), DIAMOND (Brandenburg, 2018 ▸), OLEX2 (Dolomanov et al., 2009 ▸), Mercury (Macrae et al., 2020 ▸) and publCIF (Westrip, 2010 ▸).
Results and discussion
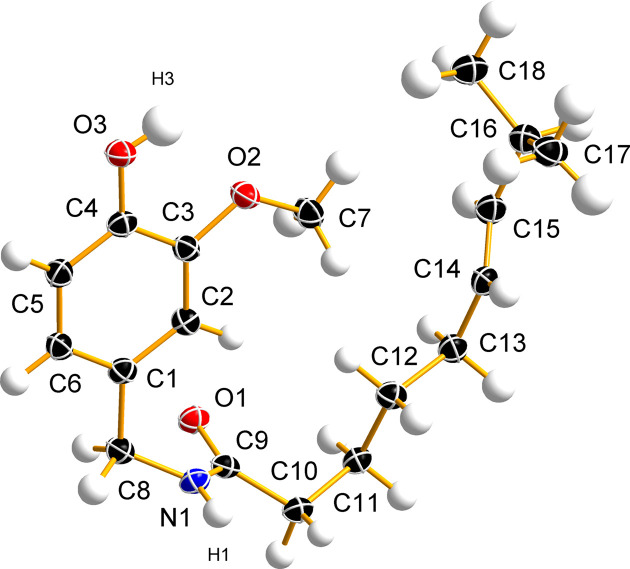
Capsaicin crystallizes in the monoclinic space group P21/c, with one molecule in the asymmetric unit (Fig. 2 ▸) and four molecules in the unit cell (Table 1 ▸). The unit-cell parameters determined at 100 K in this study are in good agreement with those obtained previously by powder X-ray diffraction at 100 K (David et al., 1998 ▸; Shankland et al., 2010 ▸) (Table 2 ▸), with observed differences smaller than 0.1%. Similarly, the conformation of the capsaicin molecule observed in the present SCXRD determination and the previous PXRD determination (David et al., 1998 ▸) are very similar, with root-mean-square deviations (RMSDs) for their alignment of 0.162 and 0.276 Å calculated in Mercury (Macrae et al., 2020 ▸) and OLEX2 (Dolomanov et al., 2009 ▸), respectively. The most notable conformational differences involve the positions of H atoms and specific C atoms, namely, C11, C12, C15 and C18, which are displaced by 0.31, 0.21, 0.36 and 0.27 Å, respectively (Fig. 3 ▸).
Figure 2.
The asymmetric unit and selected atom labels of the capsaicin crystal structure, with displacement ellipsoids plotted at the 50% probability level.
Table 2. Comparison of the unit-cell parameters of capsaicin crystal structures derived from previous structural determinations and the present work.
| CSD refcode | FABVAF | FABVAF01 | – | – | – |
| Reference | Oliver (1985 ▸) | David et al. (1998 ▸) | Florence et al. (2005 ▸) | Shankland et al. (2010 ▸) | This work |
| Space group | P21/c | P21/c | P21/c | P21/c | P21/c |
| a (Å) | 12.380 (4) | 12.2234 (1) | 12.672 | 12.224 | 12.2165 (3) |
| b (Å) | 14.814 (8) | 14.7900 (1) | 14.980 | 14.787 | 14.7791 (4) |
| c (Å) | 9.491 (3) | 9.4691 (1) | 9.426 | 9.468 | 9.4719 (2) |
| β (°) | 93.63 (3) | 93.9754 (3) | 93.69 | 93.972 | 94.035 (2) |
| V (Å3) | 1737.13 | 1707.30 | 1785.6 | 1707.3 | 1705.89 (8) |
| T (K) | 173 | 100 | Room temperature | 100 | 100 |
Figure 3.
Molecular overlap comparison of capsaicin molecular conformations from SCXRD crystal structure determination (red; this work) and PXRD simulated annealing (blue; David et al., 1998 ▸).
In contrast to the typical representation of the capsaicin molecule (Scheme 1), where the 8-methylnon-6-enamide side chain is depicted pointing away from the benzene ring, the crystal structure reveals that it bends back towards the vanillyl group and lies roughly parallel to the plane of the ring (Fig. 2 ▸). Atoms C15 and C16 are positioned 0.913 (5) and 0.612 (6) Å above the benzene-ring plane, respectively. The H16—C16—C15—H15 torsion angle is −66.4 (18)°, placing atom C17 0.825 (6) Å below and atom C18 1.578 (6) Å above the benzene-ring plane. The OH group is oriented parallel to the arene ring, while the methyl group (C7) is displaced by 0.109 (3) Å from the plane of the benzene ring. The dihedral angle between the plane of the amide group [–(O=)CNH–] and that of the benzene ring is 75.9 (4)°. The length of the C=C double bond, which adopts a trans configuration, is 1.325 (2) Å. Bond distances involving heteroatom functional groups [C—OH = 1.363 (2) Å, C—OCH3 = 1.369 (2) Å, O—CH3 = 1.423 (2) Å, C=O = 1.2459 (19) Å, N—CO = 1.334 (2) Å and N—CH2 = 1.452 (2) Å] are within the expected ranges (Allen et al., 1987 ▸).
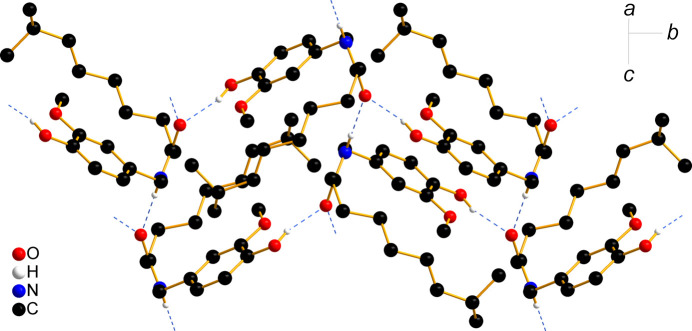
In the crystal structure, each capsaicin molecule forms hydrogen bonds with four others, with the O—H and N—H groups functioning as hydrogen-bond donors and the C=O group acting as a bifurcated hydrogen-bond acceptor (Table 3 ▸). The resulting conjoined tetrameric hydrogen-bonded rings, described by graph-set notations 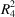 (20) and
(20) and 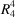 (28) (Etter, 1990 ▸) (Fig. 4 ▸), link the capsaicin molecules into a double layer with a herringbone pattern extending within the bc plane (Fig. 5 ▸). The distance between the benzene-ring planes of neighbouring stacked molecules is 3.370 (3) Å in the smaller hydrogen-bonded ring and 4.671 (5) Å in the larger one. The double layers, with the hydrogen-bonded vanillyl and amide groups at the centre and the alkenyl chains on the exterior, are stacked along the crystallographic a direction (Fig. 5 ▸).
(28) (Etter, 1990 ▸) (Fig. 4 ▸), link the capsaicin molecules into a double layer with a herringbone pattern extending within the bc plane (Fig. 5 ▸). The distance between the benzene-ring planes of neighbouring stacked molecules is 3.370 (3) Å in the smaller hydrogen-bonded ring and 4.671 (5) Å in the larger one. The double layers, with the hydrogen-bonded vanillyl and amide groups at the centre and the alkenyl chains on the exterior, are stacked along the crystallographic a direction (Fig. 5 ▸).
Table 3. Hydrogen-bond geometry (Å, °).
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| O3—H3⋯O1i | 0.88 (3) | 1.93 (3) | 2.7621 (17) | 158 (2) |
| N1—H1⋯O1ii | 0.85 (2) | 2.13 (2) | 2.9769 (18) | 175.7 (19) |
Symmetry codes: (i) 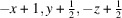 ; (ii)
; (ii) 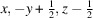 .
.
Figure 4.
Hydrogen-bonding motifs in the crystal structure of capsaicin. H atoms not involved in hydrogen bonding have been omitted for clarity.
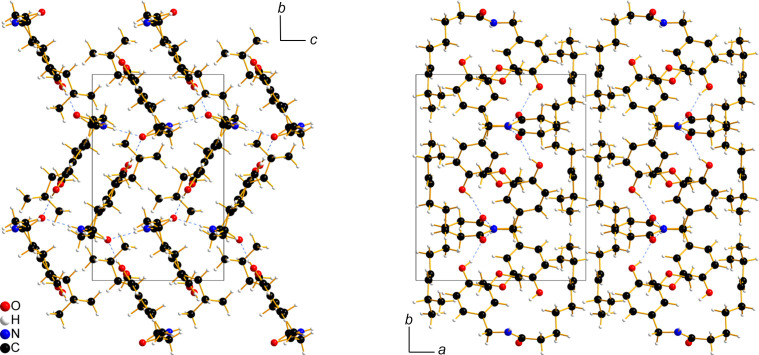
Figure 5.
Packing diagrams and the unit cell of the capsaicin crystal structure viewed along the crystallographic a axis (left) and the crystallographic c axis (right).
Conclusion
A low-temperature single-crystal X-ray diffraction study of capsaicin, the natural product responsible for the pungency of chilli peppers, was reported for the first time. The determined crystal structure aligns well with the previous simulated annealing structure solution based on powder X-ray diffraction data. In the present model, all H atoms were precisely localized and refined freely, enabling an accurate description of the hydrogen-bonding interactions. Each capsaicin molecule forms hydrogen bonds with four other molecules, with the O—H and N—H groups acting as hydrogen-bond donors, and the C=O group serving as a bifurcated hydrogen-bond acceptor, resulting in the formation of double layers.
Supplementary Material
Crystal structure: contains datablock(s) I, global. DOI: 10.1107/S2053229625001706/oc3025sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S2053229625001706/oc3025Isup2.hkl
Supporting information file. DOI: 10.1107/S2053229625001706/oc3025Isup3.cml
CCDC reference: 2426261
Acknowledgments
The author is grateful to Assistant Professor Mirela Dragomir for inspiring his enthusiasm for chilli cultivation and spicy food.
Funding Statement
Funding for this research was provided by: European Research Council (ERC) under the European Union’s Horizon 2020 Research and Innovation Programme (grant No. 950625); Jožef Stefan Institute Director’s Fund.
Footnotes
References
- Alberti, A., Galasso, V., Kovač, B., Modelli, A. & Pichierri, F. (2008). J. Phys. Chem. A, 112, 5700–5711. [DOI] [PubMed]
- Allen, F. H., Kennard, O., Watson, D. G., Brammer, L., Orpen, A. G. & Taylor, R. (1987). J. Chem. Soc. Perkin Trans. 2, pp. S1–S19.
- Bear, J. C., Terzoudis, N. & Cockcroft, J. K. (2023). IUCrJ, 10, 720–728. [DOI] [PMC free article] [PubMed]
- Berman, H. M., Westbrook, J., Feng, Z., Gilliland, G., Bhat, T. N., Weissig, H., Shindyalov, I. N. & Bourne, P. E. (2000). Nucleic Acids Res.28, 235–242. [DOI] [PMC free article] [PubMed]
- Bond, A. D. (2014). Resonance, 19, 1087–1092.
- Bosland, P. W. & Votava, E. J. (2012). Peppers: Vegetable and Spice Capsicums, 2nd ed., Crop Production Science in Horticulture Series. Cambridge, MA, USA: CABI.
- Brandenburg, K. (2018). DIAMOND. Crystal Impact GbR, Bonn, Germany.
- Caterina, M. J., Schumacher, M. A., Tominaga, M., Rosen, T. A., Levine, J. D. & Julius, D. (1997). Nature, 389, 816–824. [DOI] [PubMed]
- Chakoumakos, B. C. & Wang, X. (2024). Acta Cryst. C80, 43–48. [DOI] [PubMed]
- Collins, M. D., Wasmund Mayer, L. & Bosland, P. W. (1995). HortScience, 30, 137–139.
- Cooper, R. I., Thompson, A. L. & Watkin, D. J. (2010). J. Appl. Cryst.43, 1100–1107.
- David, W. I. F., Shankland, K. & Shankland, N. (1998). Chem. Commun. pp. 931–932.
- Decato, D., Palatinus, L., Stierle, A. & Stierle, D. (2024). Acta Cryst. C80, 143–147. [DOI] [PMC free article] [PubMed]
- Delgadillo, D. A., Burch, J. E., Kim, L. J., de Moraes, L. S., Niwa, K., Williams, J., Tang, M. J., Lavallo, V. G., Khatri Chhetri, B., Jones, C. G., Hernandez Rodriguez, I., Signore, J. A., Marquez, L., Bhanushali, R., Woo, S., Kubanek, J., Quave, C., Tang, Y. & Nelson, H. M. (2024). ACS Cent. Sci.10, 176–183. [DOI] [PMC free article] [PubMed]
- Dolomanov, O. V., Bourhis, L. J., Gildea, R. J., Howard, J. A. K. & Puschmann, H. (2009). J. Appl. Cryst.42, 339–341.
- Etter, M. C. (1990). Acc. Chem. Res.23, 120–126.
- Florence, A. J., Shankland, N., Shankland, K., David, W. I. F., Pidcock, E., Xu, X., Johnston, A., Kennedy, A. R., Cox, P. J., Evans, J. S. O., Steele, G., Cosgrove, S. D. & Frampton, C. S. (2005). J. Appl. Cryst.38, 249–259.
- Groom, C. R., Bruno, I. J., Lightfoot, M. P. & Ward, S. C. (2016). Acta Cryst. B72, 171–179. [DOI] [PMC free article] [PubMed]
- Gurung, K., Šimek, P., Jegorov Jr, A. & Palatinus, L. (2024). Acta Cryst. C80, 56–61. [DOI] [PMC free article] [PubMed]
- Hetényi, A., Németh, L., Wéber, E., Szakonyi, G., Winter, Z., Jósvay, K., Bartus, E., Oláh, Z. & Martinek, T. A. (2016). FEBS Lett.590, 2768–2775. [DOI] [PubMed]
- Kwon, D. H., Zhang, F., Suo, Y., Bouvette, J., Borgnia, M. J. & Lee, S.-Y. (2021). Nat. Struct. Mol. Biol.28, 554–563. [DOI] [PMC free article] [PubMed]
- Macrae, C. F., Sovago, I., Cottrell, S. J., Galek, P. T. A., McCabe, P., Pidcock, E., Platings, M., Shields, G. P., Stevens, J. S., Towler, M. & Wood, P. A. (2020). J. Appl. Cryst.53, 226–235. [DOI] [PMC free article] [PubMed]
- Maharjan, R., Fukuda, Y., Nakayama, T., Nakayama, T., Hamada, H., Ozaki, S.-i. & Inoue, T. (2022). Acta Cryst. D78, 379–389. [DOI] [PMC free article] [PubMed]
- Markvardsen, A. J., Shankland, K., David, W. I. F. & Didlick, G. (2005). J. Appl. Cryst.38, 107–111.
- Motaln, K., Gurung, K., Brázda, P., Kokalj, A., Radan, K., Dragomir, M., Žemva, B., Palatinus, L. & Lozinšek, M. (2024). ACS Cent. Sci.10, 1733–1741. [DOI] [PMC free article] [PubMed]
- Nadezhdin, K. D., Neuberger, A., Nikolaev, Y. A., Murphy, L. A., Gracheva, E. O., Bagriantsev, S. N. & Sobolevsky, A. I. (2021). Nat. Commun.12, 2154. [DOI] [PMC free article] [PubMed]
- Oliver, J. D. (1985). Am. Crystallogr. Assoc. Abstr. Pap. (Winter), 13, 57.
- Orton, J. B. & Coles, S. J. (2024). CSD Communication, CCDC 2339733, https://dx.doi.org/10.5517/ccdc.csd.cc2jjp81.
- Palatinus, L. & Chapuis, G. (2007). J. Appl. Cryst.40, 786–790.
- Palatinus, L., Prathapa, S. J. & van Smaalen, S. (2012). J. Appl. Cryst.45, 575–580.
- Palatinus, L. & van der Lee, A. (2008). J. Appl. Cryst.41, 975–984.
- Polo, A., Soriano-Jarabo, A., Rodríguez, R., Macías, R., García-Orduña, P. & Sanz Miguel, P. J. (2024). Acta Cryst. C80, 681–684. [DOI] [PMC free article] [PubMed]
- Rigaku OD (2024). CrysAlis PRO. Rigaku Corporation, Wrocław, Poland.
- Scoville, W. L. (1912). J. Am. Pharm. Assoc.1, 453–454.
- Shankland, K., Markvardsen, A. J., Rowlatt, C., Shankland, N. & David, W. I. F. (2010). J. Appl. Cryst.43, 401–406.
- Shankland, K., Spillman, M. J., Kabova, E. A., Edgeley, D. S. & Shankland, N. (2013). Acta Cryst. C69, 1251–1259. [DOI] [PubMed]
- Sheldrick, G. M. (2015). Acta Cryst. C71, 3–8.
- Siudem, P., Paradowska, K. & Bukowicki, J. (2017). J. Mol. Struct.1146, 773–781.
- Soriano-Correa, C., Pérez de la Luz, A. & Sainz-Díaz, C. I. (2023). J. Pharm. Sci.112, 798–807. [DOI] [PubMed]
- Spence, C. (2018). Int. J. Gastron. Food. Sci.12, 16–21.
- Srinivasan, K. (2016). Crit. Rev. Food Sci. Nutr.56, 1488–1500. [DOI] [PubMed]
- Westrip, S. P. (2010). J. Appl. Cryst.43, 920–925.
- Winkler, M., Moraux, T., Khairy, H. A., Scott, R. H., Slawin, A. M. Z. & O’Hagan, D. (2009). ChemBioChem, 10, 823–828. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablock(s) I, global. DOI: 10.1107/S2053229625001706/oc3025sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S2053229625001706/oc3025Isup2.hkl
Supporting information file. DOI: 10.1107/S2053229625001706/oc3025Isup3.cml
CCDC reference: 2426261