Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- COX R. I. The interaction of some factors affecting the kinetics of a molluscan beta-glucuronidase. Biochem J. 1959 Apr;71(4):763–768. doi: 10.1042/bj0710763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DODGSON K. S., LLOYD A. G. Studies on sulphatases. XVIII. Preparation of chondroitinase-free chondrosulphatase from extracts of Proteus vulgaris. Biochem J. 1957 Jul;66(3):532–538. doi: 10.1042/bj0660532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DODGSON K. S. Observations on the arylsulphatase of Proteus vulgaris. Enzymologia. 1959 Jul 15;20:301–312. [PubMed] [Google Scholar]
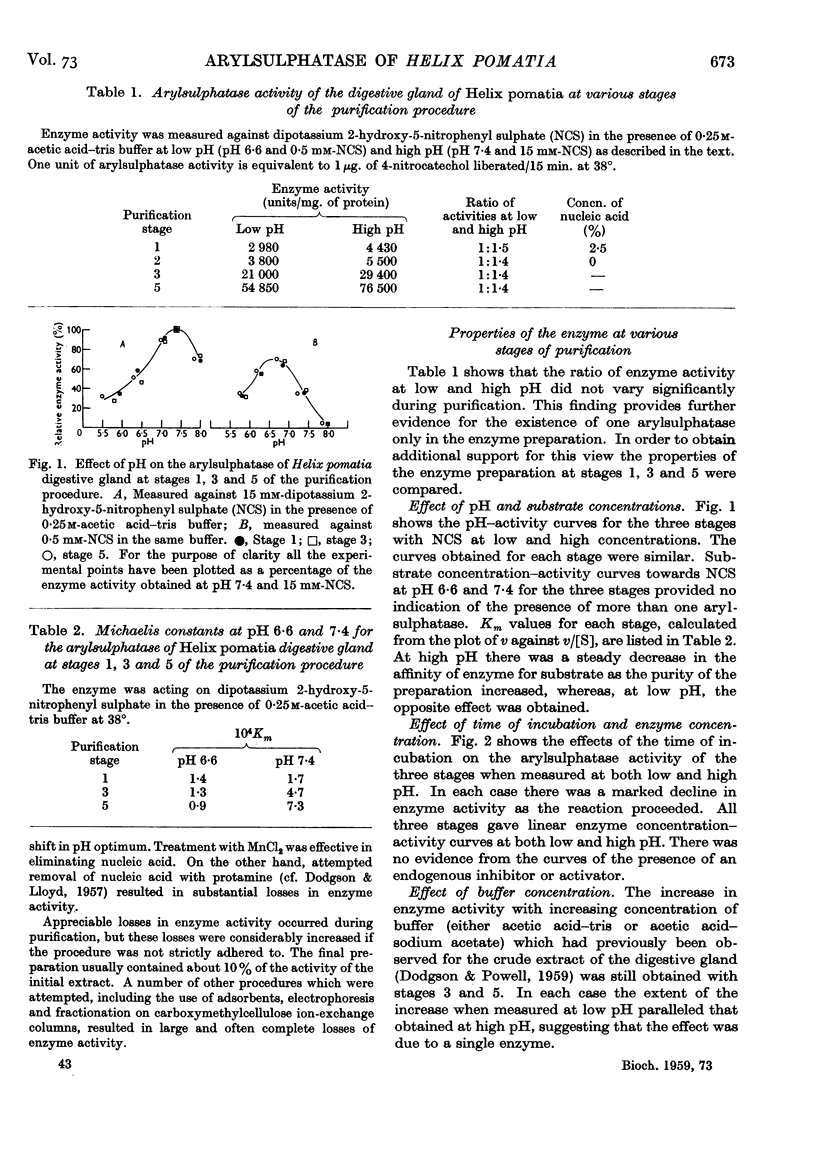
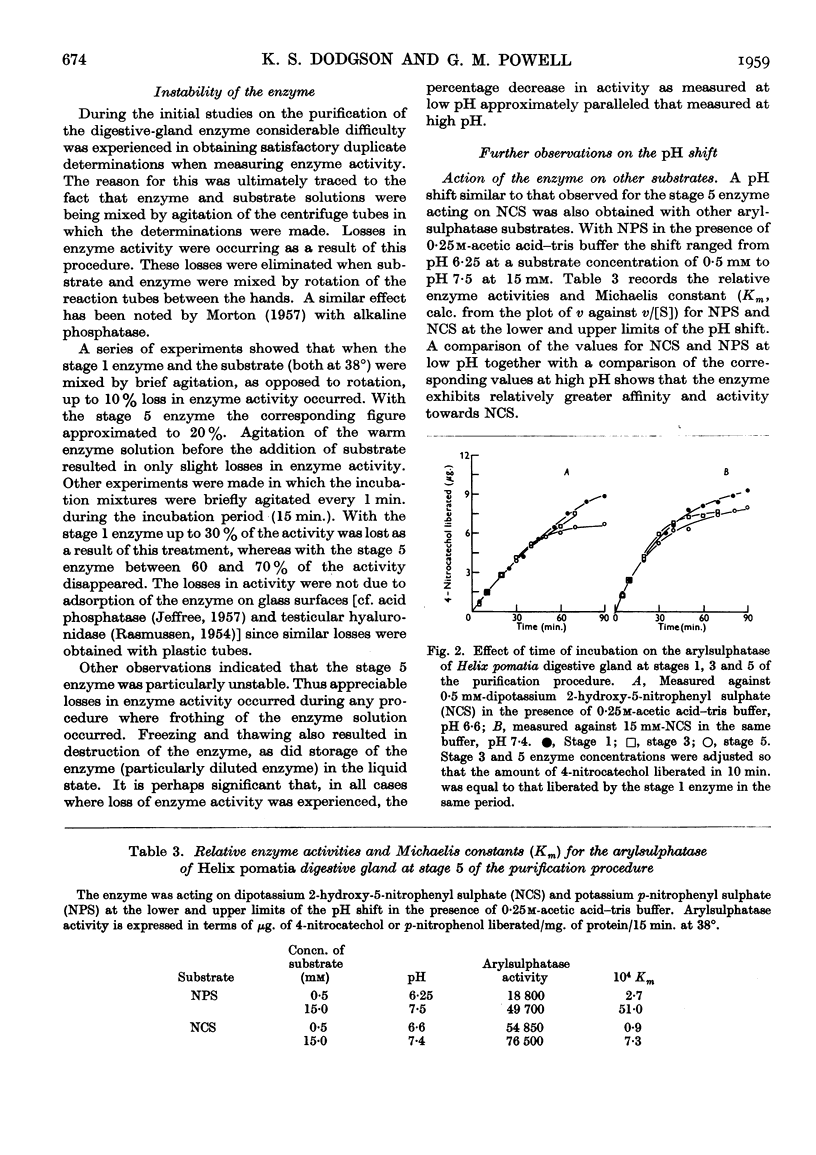
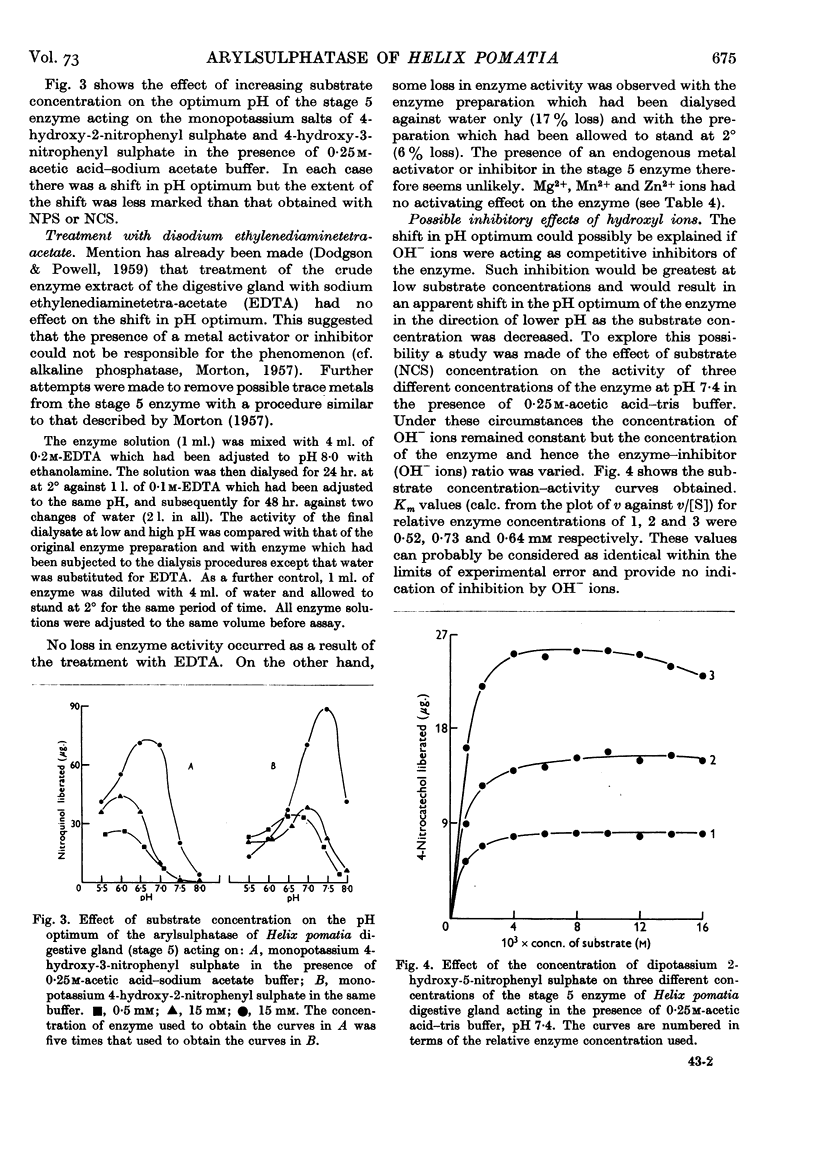
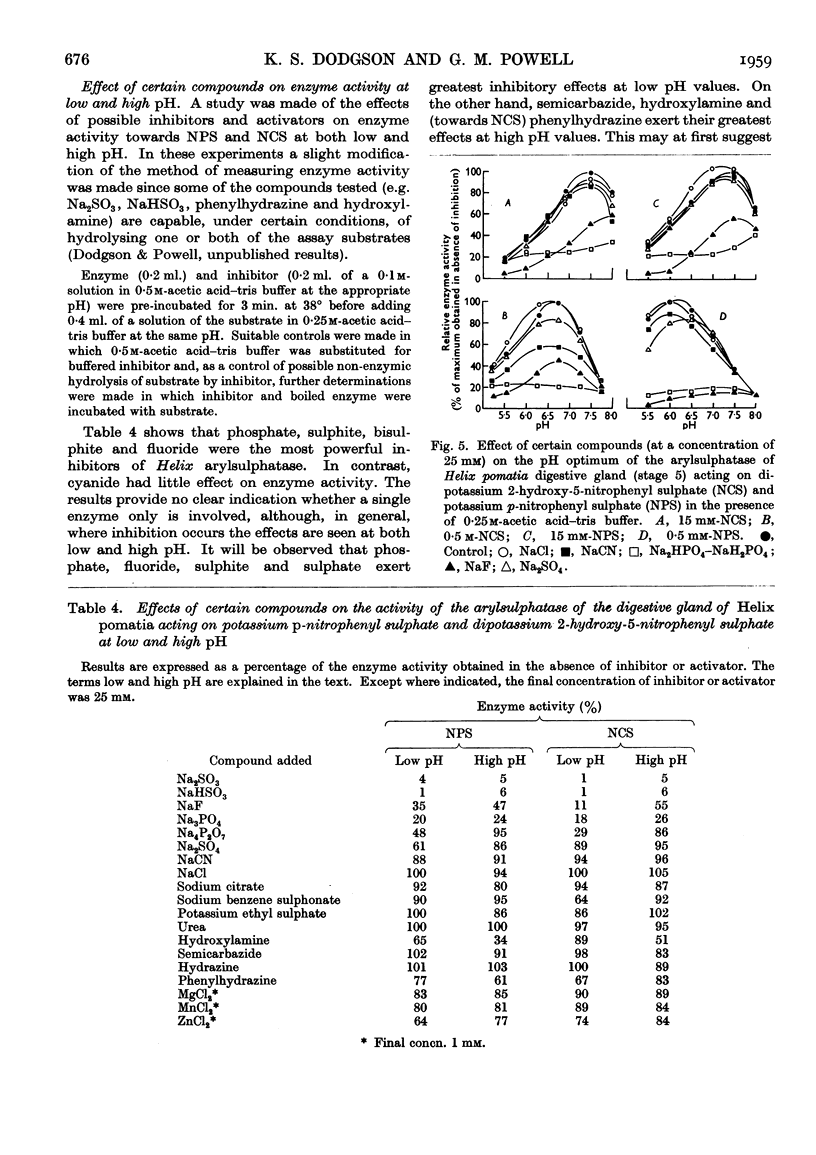
- DODGSON K. S., POWELL G. M. Studies on sulphatases. 26. Arylsulphatase activity in the digestive juice and digestive gland of Helix pomatia. Biochem J. 1959 Dec;73:666–671. doi: 10.1042/bj0730666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DODGSON K. S., SPENCER B. Assay of sulfatases. Methods Biochem Anal. 1957;4:211–255. doi: 10.1002/9780470110201.ch6. [DOI] [PubMed] [Google Scholar]
- DODGSON K. S., SPENCER B., WILLIAMS K. Studies on sulphatase. II. The purification and properties of the arylsulphatase of Alcaligenes metalcaligenes. Biochem J. 1955 Nov;61(3):374–380. doi: 10.1042/bj0610374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DODGSON K. S., WYNN C. H. Studies on sulphatases. 19. The purification and properties of arylsulphatase B of human liver. Biochem J. 1958 Mar;68(3):387–395. doi: 10.1042/bj0680387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FISHMAN W. H., GREEN S. Enzymic catalysis of glucuronyl transfer. J Biol Chem. 1957 Mar;225(1):435–452. [PubMed] [Google Scholar]
- HOFSTEE B. H. Direct and continuous spectrophotometric assay of beta-glucosidase. Arch Biochem Biophys. 1955 Dec;59(2):398–404. doi: 10.1016/0003-9861(55)90507-5. [DOI] [PubMed] [Google Scholar]
- JEFFREE G. M. Some effects of hydroxyl compounds on prostatic acid phosphatase. Biochim Biophys Acta. 1957 Jan;23(1):155–161. doi: 10.1016/0006-3002(57)90298-6. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MORTON R. K. The kinetics of hydrolysis of phenyl phosphate by alkaline phosphatases. Biochem J. 1957 Apr;65(4):674–682. doi: 10.1042/bj0650674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MORTON R. K. The phosphotransferase activity of phosphatases. 1. Spectrophotometric methods for the estimation of some phosphate esters and other compounds. Biochem J. 1958 Sep;70(1):134–139. doi: 10.1042/bj0700134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MOTZOK I., BRANION H. D. Studies on alkaline phosphatases. 2. Factors influencing pH optima and Michaelis constant. Biochem J. 1959 May;72(1):177–183. doi: 10.1042/bj0720177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WEBB E. C., MORROW P. F. The activation of an arysulphatase from ox liver by chloride and other anions. Biochem J. 1959 Sep;73:7–15. doi: 10.1042/bj0730007. [DOI] [PMC free article] [PubMed] [Google Scholar]


