Abstract
Probiotics are widely marketed as dietary supplements and dairy products for their purported antimicrobial and immunomodulatory activities, often with limited supporting evidence. We identified and isolated probiotics from commercial dietary supplements and dairy products using metagenomics and cultured-based methods. We assessed their anti-bacterial activity against diverse pathogens and investigated their immunomodulatory effects on phagocytes and natural killer (NK) cells. Metagenomic analysis revealed that Lactobacillus and Bifidobacterium were the predominant genera in dietary supplements, while Streptococcus spp. was dominated in dairy products. However, only 37% of the predominant microorganisms identified by metagenomics were accurately listed on product labels. Among 70 representative probiotic strains, 4.3–17.1% probiotic strains demonstrated strong antibacterial-effects against pathogenic bacteria. Notably, specific strains of Bifidobacterium longum and Lactobacillus plantarum exhibited strong antagonistic activity against extended-spectrum beta-lactamase-producing and carbapenem-resistant Escherichia coli. Some strains of Lactobacillus spp. significantly enhanced phagocytic activity in monocytes and increased IFN-γ production in NK cells, while members of Lactobacillus rhamnosus significantly suppressed TNF-α, IL-6, and IL-8 production in lipopolysaccharide-stimulated macrophages. In contrast, Bifidobacterium animalis stimulated the production of anti-inflammatory cytokines. This study highlights discrepancies in probiotic labeling and demonstrates the antimicrobial and immunomodulatory potential of specific probiotic strains, suggesting their utility in enhancing health and wellness.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-025-95664-w.
Keywords: Probiotics, Antimicrobial activity, Metagenomic, Immune response, Supplements, Dairy products
Subject terms: Innate immunity, Microbiology, Microbial communities
Introduction
Probiotics are live microorganisms, including bacteria and yeast, that promote health benefits to the host when consumed in adequate amounts. Lactic acid bacteria (LAB) are Gram-positive bacteria that produce lactic acid from carbohydrate fermentation, such as Lactobacillus spp., Enterococcus spp., Bifidobacterium spp. and Saccharomyces spp. These microorganisms are common probiotics formulated into dietary supplements and dairy products. Over 11,000 probiotics strains have been reported to exhibit biological activities that may benefit health1. The use of probiotics is increasingly recognized as a strategy to prevent infections and improve human and animal health2–6. The potential antimicrobial properties of probiotics and their products involve inhibiting the growth of pathogens6–9. In addition, probiotics can modulate immune responses, either activating10–13 or suppressing14 specific immune functions. These studies showed that probiotics interact with the host environment and influence the host immune response in various pathways.
Macrophages are a diverse group of immune cells with critical roles in both pro-inflammatory and anti-inflammatory responses. These cells help maintain immune balance and respond to infections and inflammation15. Different probiotic strains can influence cytokine production by macrophages, which can enhance or suppress inflammation. Due to the various effects of probiotic strains on immune activation, it is essential to evaluate the immunomodulatory properties of probiotics to ensure their safety and effectiveness in treating immune-related diseases16.
Natural killer (NK) cells are another important part of the innate immune system. These cells produce cytokines, such as interferon-gamma (IFN-γ), which activate other immune cells like macrophages and dendritic cells17. The secretion of IFN-γ is triggered by cytokines, microbial products, or direct interaction with infected or cancerous cells. Some strains of LAB have been shown to activate IFN-γ production by NK cells, suggesting their potential role in boosting immunity18,19.
Although many commercial probiotic products are available, studies using culture methods have shown inaccuracies in the labeling of their microbial compositions20–22. The mismatch between declared and actual probiotic species raises concerns about product reliability. Accurate methods, such as metagenomics, are needed to identify the whole-microbial genome present in these products and further verified by using standard culturing techniques to determine the viability of all the microbes23. Furthermore, manufacturers often claim that probiotics can restore gut health, support digestion, and modulate the immune system. However, the stability of probiotics can be affected when combined with other bacterial isolates, food ingredients, or supplements24,25.
Despite the potential health benefits of probiotics, little is known about how they interact with immune cells such as macrophages and NK cells. To address this gap, we hypothesized that metagenomics could be used to validate the composition of probiotics in commercial dietary supplements and dairy products. These probiotics might exhibit antimicrobial properties against harmful bacteria, including antibiotic-resistant pathogens, and influence cellular immune functions.
This study aimed to validate microbial species in commercially available dietary supplements and dairy products. We used a metagenomics approach to identify probiotics in 13 dietary supplements and 25 dairy products, as probiotics are commonly formulated into these products. The identified microorganisms were isolated and further analyzed for their antimicrobial properties. Additionally, we assessed their effects on cellular immune functions, including phagocytosis by monocytes, cytokine release by macrophages, and NK cell activation.
Results
Metagenomic detection of probiotics in dietary supplements and dairy products at the genus level
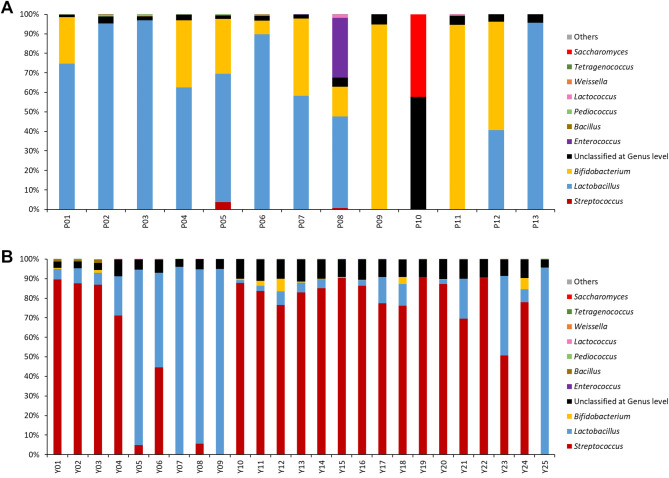
A total of 13 dietary supplements (codes P01-P13) and 25 dairy products (codes Y01-Y25) were analyzed for the probiotic population using metagenomic analysis based on 16 S rRNA gene and ITS sequencing (Fig. 1). The composition of probiotics in each product, as indicated on the label, was compared with the results obtained from metagenomic analysis (Fig. 2, Supplementary Figure S1 and Supplementary Table S1). In this study, references to Lactobacillus at the genus level correspond to the broader lactobacilli group as classified in the GreenGenes database version 13.5, rather than the current taxonomic definition following the 2020 revision26.
Fig. 1.
Metagenomic profiles of probiotics in dietary supplements (A) and dairy products (B).
Fig. 2.
The comparison between the microorganism species declared on the labels of probiotic supplement products and those identified through metagenomic sequencing using bioinformatic analyses and cultivation methods. The comparison was performed from six perspectives. First, microbes present in the supplement that were declared on the label and detected through analysis (green). Second, microbes were declared on the label but detected at a very low abundance in the analysis (less than 1% of the reads) (yellow). Third, microbes were declared on the label but not detected via metagenomic analyses (red). Fourth, microbes were detected within the supplements’ metagenomic but were not declared on the label (purple). The numbers in the table indicated the % abundance of microbe in the metagenomic analysis. Fifth, microbes indicate with a star were detected in the supplement’s metagenomics and grown as a single colony and identified by MALDI-TOF. Finally, microbes indicate with a G were grown as a single colony and identified by MALDI-TOF but were not declared on the label and were undetectable by metagenomics.
Thirteen dietary supplements were indicated with 5 genera of microorganisms on their labels, including Lactobacillus, Bifidobacterium, Saccharomyces, Lactococcus and Streptococcus, which were discovered by metagenomic analysis (Figs. 1 and 2 and Supplementary Tables S1-2). Notably, the metagenomic analysis revealed additional genera not indicated on the product labels (Supplementary Figure S1 and Supplementary Tables S1- 2). For instance, Enterococcus was detected as the most abundant microorganism in dietary supplement P08 but was absent from the product’s label. Other low-abundance genera, such as Acidomyces, Bifidobacterium, Lactobacillus, Lactococcus, Pediococcus, Plectosphaerella and Streptococcus, were detected in dietary supplements P09-P13 without being listed on their labels.
Of the 25 dairy products, 24 indicated probiotics on their labels (Supplementary Tables S1). All 24 products (100%) revealed the presence of Lactobacillus, while Streptococcus and Bifidobacterium were displayed on 19 (76%) and 8 (32%) dairy products, respectively. Metagenomic analysis confirmed the presence of all probiotics indicated on the labels of these products, but in a lower proportion than stated. In addition, a small amount of other bacterial genera not indicated on the labels, such as Bacillus, Bifidobacterium, Pediococcus, Streptococcus and Tetragenococcus, were detected by metagenomic analysis in several dairy products (Y01-09, Y12, Y14, Y16, Y22, Y23 and Y25).
Metagenomic detection of probiotics in dietary supplements and dairy products at species level
Seventeen species of probiotics were listed on the labels of 13 dietary supplements, including 10 species of Lactobacillus, 4 species of Bifidobacterium, and 1 species each of Lactococcus, Streptococcus and Saccharomyces (Supplementary Table S1). Metagenomic analysis at the species level revealed all indicated probiotics on product labels with lower relative abundance (Figs. 1 and 2, Supplementary Figure S2 and Supplementary Tables S1-2). Resemble with the genus classification, Enterococcus faecium, unlisted on the labels, was found in P08.
The dairy products had less diversity of probiotics than dietary supplements. Only 6 species of probiotics were commonly listed in 24 dairy products: Bifidobacterium animallis subsp. lactis, Lactobacillus acidophilus, Lactobacillus delbrueckii subsp. bulgaricus, Lactobacillus casei, Lactobacillus paracasei and Streptococcus salivarius subsp. thermophilus. Almost indicated probiotics on product labels were detected by metagenomic analysis (Figs. 1 and 2, Supplementary Figure S2 and Supplementary Tables S1-2). Only L. casei, was not classified in products Y09 and Y18.
The top ten microorganisms identified by metagenomic analysis of all products are shown in Supplementary Table S2. The median proportion of probiotics in the top ten list was 95.03% (range 52.5–97.65, IQR 94.2–95.6%) of the DNA library. Only 38.6% of microbes in the top ten list from the metagenomic analysis matched with those indicated on the product labels.
Misidentification of probiotics at the species level was more prevalent in dietary supplements (abundance range: 1.5 − 54%) compared to dairy products (abundance range: 0.81 − 18.3%). These findings highlight discrepancies between label information and the actual microbial composition.
Isolation of probiotics from supplemental food products
All 13 dietary supplements and randomly selected 8 of 25 dairy products were cultured and isolated for probiotics (Supplementary Tables S1-3). From these 21 samples, 671 colonies were isolated, and 22 microbial species were identified by MALDI-TOF analysis. Among these, 13 species matched the probiotic listed on the labels of 11 dietary supplements and 4 dairy products. These species included B. animalis (2 products), B. longum (2 products), L. acidophilus (5 products), L. bulgaricus (1 product), L. fermentum (1 product), L. gasseri (1 product), L. paracasei (4 products), L. plantarum (4 products), L. reuteri (2 products), L. rhamnosus (1 product), L. salivarius (2 products), S. thermophilus (3 products) and S. cerevisiae (1 products). However, 4 species (B. bifidum, B. breve, L. casei and L. lactis) were initially isolated but could not be maintained in culturing conditions used in this study. We were unable to isolate the probiotics listed on the labels of 2 dietary supplements (P08 and P12) and 4 dairy products (Y01, Y02, Y15 and Y21). In addition, 5 species were isolated but not listed on the label of any products, including Bacillus cereus and Paenibacillus timonensis from Y18, Bacillus licheniformis from Y21, Enterococcus faecium from P08 and Enterococcus gallinarum from Y15.
Many species such as L. plantarum, L. paracasei, L. rhamnosus, L. reuteri, B. longum were isolated by culture of 10 products (P01, P07, P08, P11, P12, Y01, Y02, Y03, Y20 and Y21) but were not indicated on their labels. These microorganisms were also detected at very low abundance by metagenomic analysis.
From 671 isolates, 70 probiotic strains were randomly selected and used as representative strains to evaluate anti-bacterial and immunomodulation activities. For each species, one isolate per product was selected, and 3 isolates per species were included when multiple strains of the same microorganism were labeled on a product. The selected isolates included B. animalis (N = 12), B. longum (N = 3), E. faecium (N = 1), E. gallinarum (N = 1), L. acidophilus (N = 10), L. delbruckii (N = 1), L. fermentum (N = 1), L, gasseri (N = 1), L. paracasei (N = 8), L. plantarum (N = 9), L. rhamosus (N = 13), L. reuteri (N = 2), L. salivarius (N = 2), S. thermophilus (N = 3) and S. cerevisiae (N = 3).
Antimicrobial activity of probiotic isolates against pathogenic bacteria
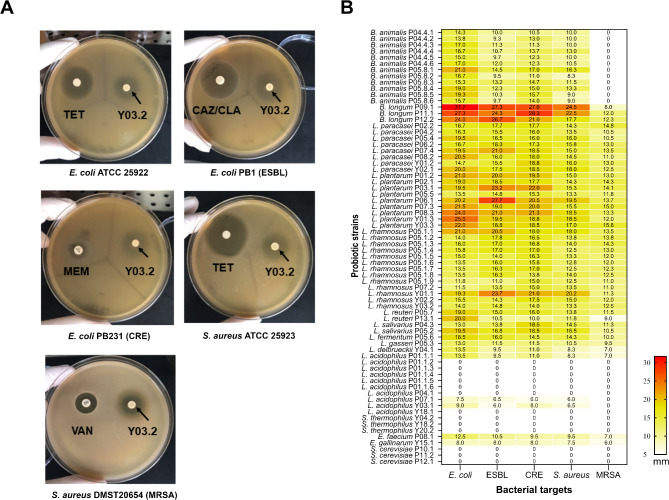
Anti-bacterial activity of 70 live probiotic isolates against five pathogenic bacteria was evaluated using the agar overlay method (Fig. 3A). The diameters of inhibition zones were summarized in a heatmap chart (Fig. 3B) and categorized as follows: strong activity, inhibition zone > 20 mm; moderate activity, inhibition zones 10–20 mm; and weak activity, inhibition zones < 10 mm. The anti-bacterial activities varied depending on the probiotic species and strains. 51.4–64.3% of isolates demonstrated inhibitory effects with moderate levels against pathogenic bacteria. However, the overall effectiveness of the probiotics was lower against Methicillin-resistant Staphylococcus aureus (MRSA). (Table 1). Interestingly, 12.9% and 10.0% of probiotic isolates showed strong antimicrobial activity, with inhibition zone > 20 mm against antibiotic resistant bacteria including E. coli PB1 (Extended-spectrum β-lactamase; ESBL) and E. coli PB231 (Carbapenem-resistant Enterobacterales; CRE). Strong antimicrobial activity was particularly observed in isolates of B. longum (P09.1, P11.1 and P12.2), L. plantarum (P03.1, P06.1 and P08.3) and L. rhamnosus (Y01.1). In contrast, species such as L. acidophilus, S. cerevisae and S. thermophillus showed no anti-bacterial activity against any of the target bacteria (Fig. 3B). These findings suggest that certain probiotic strains, particularly B. longum, L. plantarum, and L. rhamnosus, have promising antimicrobial potential against multidrug-resistant bacteria, whereas others may lack anti-bacterial efficacy.
Fig. 3.
Antibacterial activity of probiotic isolates against antibiotic resistance bacteria by agar overlay method. (A) Agar overlay method demonstrated the antibacterial activity of live L. rhamnosus Y03.2 against five bacterial pathogens used as target strains, including E. coli ATCC25922, E. coli PB1 (ESBL), E. coli PB231 (CRE), S. aureus ATCC25923 and S. aureus DMST20654 (MRSA). The inhibition zones were observed surrounding probiotic spots (arrows). The diameter of the inhibition zone (mm), including probiotic spots (approximately 5 mm in diameter) was measured, and reported as means of two independent experiments. (B) The zone of inhibition of 70 tested probiotic strains was summarized in a heatmap chart. The diameter of the inhibition zone was interpreted as follows: >20 mm as a strong activity (red), 10–20 mm as a moderate activity (yellow), and < 10 mm as a weak inhibition (bright yellow).
Table 1.
The number of isolated probiotics showed antibacterial effects against tested organisms.
| Level | Number of isolated probiotics showed antibacterial effects against the tested organisms (% of probiotics) | ||||
|---|---|---|---|---|---|
|
E. coli
ATCC25922 |
E. coli
PB1 (ESBL) |
E. coli
PB231 (CRE) |
S. aureus ATCC25923 | S. aureus DMST20654 (MRSA) | |
| Strong antibacterial activity (> 20 mm) | 12 (17.1%) | 9 (12.9%) | 7 (10.0%) | 3 (4.3%) | 0 (0.0%) |
| Moderate antibacterial activity (10–20 mm) | 41(58.6%) | 39 (55.7%) | 45 (64.3%) | 45 (64.3%) | 36 (51.4%) |
| Weak antibacterial activity (< 10 mm) | 17 (24.3%) | 22 (31.4%) | 18 (25.7%) | 22(31.4%) | 34 (48.6%) |
Effect of probiotics on phagocytosis enhancement in THP-1 monocytes
Several dietary supplements claimed immune-supporting benefit (Supplementary table S4). To evaluate these claims, we determined the effect of probiotics on phagocytosis activity in THP-1 monocyte cells (Fig. 4). THP-1 cells were stimulated with probiotics at an MOI of 200, and their phagocytic activity was assessed using heat-killed, CFSE stained E. coli. Interestingly, stimulation of THP-1 cells with all tested strains of L. rhamnosus and Enterococcus spp. significantly increased phagocytosis of heat-killed E. coli compared to untreated cells (p < 0.0001). However, the enhancement of phagocytosis was strain dependent for L. paracasei, L. plantarum, L. salivarius and L. acidophilus. Moreover, the pre-treatment with Bifidobacterium spp., L. reuteri, L. fermentum, and S. thermophilus suppressed the phagocytosis activity of THP-1 cells. These findings indicate that certain probiotic species, particularly L. rhamnosus, and Enterococcus spp., may enhance innate immune functions, while others, such as Bifidobacterium spp. and L. reuteri, may have an inhibitory effect on monocyte phagocytosis.
Fig. 4.
Effect of probiotics on phagocytic activity. The phagocytic index is defined as % phagocytosis of monocyte cells × mean fluorescence intensity. Monocyte with RPMI medium was used as the negative control (No treatment), and monocyte treated with PMA was the positive control. Each color indicates probiotic species. Data were presented as bar graphs with mean ± standard deviation from three independent experiments. One-way ANOVA was used to test the difference between probiotic bacteria affecting phagocytic activity. (****, P < 0.0001, ***, p < 0.001, **, p < 0.01).
Effect of probiotics on cytokine production from THP-1-derived macrophages stimulated with LPS
To evaluate the immunomodulatory effects of probiotics, we examined the impact on cytokine production in THP-1-derived macrophages. The pro-inflammatory cytokine, IL-6 was determined following stimulation with 1000 ng/ml of LPS as a positive control. Stimulation with LPS alone significantly increased IL-6 levels in THP-1 derived macrophage. However, treatment with probiotics without LPS showed no significant difference in IL-6 production compared to untreated cells (RPMI medium), indicating that these probiotics do not induce inflammation via IL-6 production. (Fig. 5A).
Fig. 5.
Effect of probiotics on cytokine production. Effect of probiotics on cytokine profiles from THP-1-derived macrophages or stimulated with LPS 1 µg/ml for 18 h. The supernatant was collected to measure cytokine profiles using Luminex assay. Cells with RPMI medium was used as a negative control and cells treated with LPS 1 µg/ml alone was used as a positive control. (A) IL-6 production from THP-1-derived macrophages after stimulating with probiotic strains. (B–F) IL-6, IL-8, IL-12p70 and TNF-α levels from cells treated with LPS and probiotic strains for 18 h. (G–H) IL-1RA and IL-10 from cells treated with LPS and probiotic strains for 18 h. (Mann-Whitney test; *, p < 0.05, **p < 0.01). (Ban; B. animalis, Blo; B. longum, Lpa; L. paracacei, L. plantarum, Lrh; L. rhamnosus, Lre; L. reuteri, Lsa; L. salivarius, Lfe; L. fermentum, Lga; L. gasseri, Lde; L. delbrueckii, Lac; L. acidophilus, Sth; S. thermophilus, Efa; E. faecium, Ega; E. gallinarum, Sce; Saccharomyces cerevisiae)
Next, we investigate whether probiotics isolated from dietary supplement and yogurt products could modulate the inflammatory response induced by LPS. THP-1-derived macrophages were co-treated with LPS and probiotics, and levels of pro-inflammatory cytokines (IL-1β, IL-6, IL-8, IL-12p70, and TNF-α) as well as anti-inflammatory cytokines (IL-1RA and IL-10) were measured (Fig. 5B-H). Stimulation with LPS alone led to elevated levels of IL-6, IL-8 and TNF-α and IL-1RA and IL-10 compared with untreated cells. However, co-treatment with probiotics significantly altered the cytokine response. For example, IL-6, IL-8 and TNF- α productions were decreased when co-stimulated with L. rhamnosus while IL-10 production was enhanced especially when co-stimulated with B. animalis. These findings suggest that while the probiotics tested do not directly induce inflammation, they can attenuate LPS-induced inflammatory responses in THP-1-derived macrophages by reducing pro-inflammatory cytokines and promoting anti-inflammatory cytokines.
Enhancement of NK cell activity by probiotics
To assess the impact of probiotics on innate immune response, we evaluated their ability to activate NK cells. NK-92 MI cell lines were co-cultured with 70 probiotic isolates from 16 species for 24 h, and the levels of IFN- γ in the supernatant were measured (Fig. 6). Most probiotic strains tested did not elicit NK-92 MI cells responses. However, 9 isolates of probiotics strains were able to stimulate NK-92 MI cells to secrete low levels of IFN-γ compared to the positive control. These isolates included L. paracasei (P02.2), L. plantarum (P03.1 and P06.1), L. rhamnosus (Y01.1), L. reuteri (P05.7), L. gasseri (P05.3), L. acidophilus (P01.1.1 and P01.1.3) and S. cerevisae (P10.1). The results suggest potential strain-specific immunomodulatory effects of probiotics on NK cells.
Fig. 6.
IFN-γ production by NK-92 MI cells activated with different probiotics. NK-92 MI cells were incubated with 70 isolates of probiotic bacteria from 16 species for 24 h. Mixture of PMA and ionomycin was a positive control and medium was a negative control. The supernatant was collected to measure IFN-γ concentration using ELISA assay. Data were represented as a bar graph with mean ± standard deviation from three independent experiments. One-way ANOVA was used to test the difference between probiotic bacteria affecting NK cell-derived IFN-γ production. ***, p < 0.0001.
Discussion
Dietary supplements and dairy products that contain probiotics frequently claim the health advantages of probiotics for promoting consumption. This study evaluated the microbial composition, antimicrobial activity, and immunomodulatory effects of probiotics from dietary supplements and dairy products using a metagenomics approach, culture-based isolation, and immune function assays. The findings highlight discrepancies between product labels and actual microbial content, provide insights into the antimicrobial properties of specific probiotic strains, and explore their potential to modulate innate immune responses.
Our metagenomic analysis revealed notable discrepancies between the labeled and actual microbial compositions of probiotics in both dietary supplements and dairy products. While the labels accurately listed some genera and species, many products contained additional bacterial genera, including Enterococcus and Bacillus, not mentioned on the labels. Furthermore, some strains listed on the labels, such as L. casei, were not detected in specific products. In addition, the culture method was able to isolate E. faecium from dietary supplement P08, suggesting contamination. These findings align with previous reports of inconsistencies in probiotic labeling20,21 and emphasize the importance of rigorous quality control and accurate product labeling to ensure consumer trust and product efficacy.
The success of isolating probiotics from dietary supplements and dairy products is significantly influenced by the composition of the culture medium. In this study, only 29 of the 70 microbes indicated on the label of 21 products were successfully isolated. Media such as MRSc and M17, commonly used for the cultivation of lactic acid bacteria were insufficient to promote the growth of all probiotics in each product. This limitation aligns with previous studies that suggest the need to optimize media compositions to support the growth of diverse lactic acid bacteria27. Specific nutrients or supplements, such as carbon sources, vitamins, or growth factors may be required to enhance the recovery of certain strains. Furthermore, the blending of several probiotics in a single product can further complicate isolation. Competitive interactions among co-cultured strains may suppress the growth of less dominant species, particularly in nutrient-limited media28. It is possible that the probiotic strains may enter a viable but non-culturable (VBNC) state during production or storage, rendering them difficult to isolate under conventional conditions. Resuscitation of VBNC cells often requires specific environmental conditions, such as changes in temperature, pH, or nutrient availability, which are not provided by standard media28. This highlights the need for advanced culturing techniques to recover these dormant but viable microorganisms28.
The antimicrobial activity of probiotics varies significantly by species and strains. Over half of the isolates exhibited moderate inhibitory effects against a panel of pathogenic bacteria, with strong activity observed in B. longum, L. plantarum, and L. rhamnosus against antibiotic-resistant strains such as E. coli PB1 (ESBL) and E. coli PB231 (CRE). In contrast, species like L. acidophilus, S. cerevisiae, and S. thermophilus showed no anti-bacterial activity. These results highlight the strain-specific nature of antimicrobial properties and suggest that certain strains may serve as promising candidates for combating multidrug-resistant pathogens.
LAB, particularly lactobacilli, have demonstrated the ability to combat bacterial pathogens through various mechanisms. These include producing antimicrobial substances such as hydrogen peroxide, organic acids (mainly lactic acid), and bacteriocins29, interacting with resident microbiota and host, improving gut barrier integrity, and producing enzymes30. Consistent with previous studies, our results revealed that B. longum, L. plantarum, L. rhamnosus, and L. reuteri strains exhibited remarkable antagonistic activity against S. aureus ATCC25923 and E. coli ATCC25922, with the inhibition profile varying by strains31. Furthermore, LAB probiotic strains in our study, especially B. longum and L. plantarum exhibited strongly antagonistic activity against ESBL- and CRE-E. coli. Previous study supports these findings, demonstrating that the cell-free supernatant (CFS) of L. plantarum, L. rhamnosus and L. paracacei strains exhibit significant anti-CRE activity using agar well diffusion and time-kill assay32. Additionally, a recent study revealed that CFS of B. longum FB1-1 inhibits the transfer of drug resistance plasmids among carbapenem-resistant Klebsiella pneumoniae strains33. While in vitro findings may not directly translate to in vivo outcomes, our study suggests that specific Lactobacillus and Bifidobacterium strains hold significant potential for preventing colonization by ESBL and CRE pathogens. Nonetheless, further studies, particularly in animal models, are needed to confirm these promising findings and explore their clinical relevance.
The innate immune system is essential for defending the host against pathogens. Key immune cells in the innate immune response include macrophages, dendritic cells, and natural killer (NK) cells. Several researchers have suggested that intestinal homeostasis can be restored through supplements containing fermented dairy products and beneficial microorganisms, such as Lactobacillus probiotics. For example, a previous study demonstrated that healthy adults consuming 300 g/day of yogurt supplement containing L. acidophilus 74 − 2 and B. lactis 420 showed a significant increase in granulocytes and monocytes phagocytic activity during the probiotic intervention34. Our study demonstrated diverse effects of probiotics on monocyte phagocytic activity. L. rhamnosus, E. faecium and E. gallinarum significantly enhanced phagocytosis, suggesting a role in boosting innate immune defense. Additionally, L. paracasei, L. plantarum, L. salivarius and L. acidophilus were strains dependent on enhancing phagocytosis. In contrast, Bifidobacterium spp. and L. reuteri suppressed phagocytosis. These findings underscore the need to evaluate strain-specific immunomodulatory effects, as different probiotics may have opposing effects on innate immune functions.
A previous study reported that heat-killed L. plantarum KCTC 13314BP enhances the phagocytic activity of RAW264.7 macrophages via activation of MAPK and STAT3 pathways35. Similarly, our results suggest that strains of L. plantarum, L. rhamnosus from dietary supplements and yogurt products may enhance phagocytic activity and have the potential to protect the host from pathogens.
Macrophages play a key role in maintaining intestinal homeostasis as immune sentinels in the gastrointestinal tract. They infiltrate the lamina propria and release cytokines to regulate and activate the innate immune responses. During infections, monocyte-derived macrophages migrate into the intestine, triggering an inflammatory response. Disruptions in macrophage balance contribute to immune damage in chronic inflammatory diseases36,37. In our study, Gram-positive probiotics were shown not to induce IL-6 production, indicating their minimal pro-inflammatory effect. Conversely, E. coli-LPS activated toll-like receptor 4 (TLR4), leading to the secretion of pro-inflammatory cytokines such as TNF-α, IL-1β, IL-6, and IL-8. These cytokines are crucial for the host’s defense against bacterial colonization and invasion38. Additionally, TLR5 recognizes flagellin from both Gram-negative and Gram-positive bacteria, activating the NF-κB pathway to further amplify pro-inflammatory responses39.
Our results revealed that LPS-induced TNF-α, IL-6, and IL-8 secretion by THP-1-derived macrophages was significantly reduced by probiotic strains, particularly L. rhamnosus, which suppressed all three cytokines. L. acidophilus strains suppressed TNF-α and IL-6, while B. animalis, L. paracasei, and L. plantarum selectively suppressed IL-6. These effects are likely to reflect strain-specific alterations in TLR4 expression, as observed in previous studies40.
In addition to reducing pro-inflammatory cytokines, B. animalis significantly induced the production of anti-inflammatory cytokines such as IL-10 and IL-1RA in LPS-stimulated macrophages. Intestinal macrophages consistently produce IL-10, which plays a critical role in maintaining immune tolerance by regulating regulatory T cells (Tregs). This interplay between macrophages and Tregs, both major sources of IL-10, is essential for balancing immune responses and preventing excessive inflammation in the intestinal mucosa36.
Natural killer (NK) cells play a critical role in combating infections and cancer. They destroy infected or abnormal cells by releasing cytolytic granules, such as perforin and granzymes, and secrete cytokines like IFN-γ and TNF-α, which regulate the immune cells. Probiotics have shown the potential to enhance NK cell activity. For instance, L. casei Shirota (LcS) has been demonstrated to boost NK cell activity in vitro by increasing the expression of surface activation markers CD69 and CD25 on CD8+ and CD56+ subsets of peripheral blood mononuclear cells (PBMCs), even without additional stimuli41. Similarly, the consumption of dairy yogurt containing L. paracasei ssp. paracasei, B. animalis ssp. lactis, and heat-treated L. plantarum has been shown to enhance immune function in immunocompromised elderly individuals by increasing NK cell activity and the production of IL-12 and IFN-γ42.
In our study, certain probiotic strains directly stimulated NK cells to secrete higher levels of IFN-γ compared to untreated controls. These strains included L. paracasei P02.2, L. plantarum P03.1, L. plantarum P06.1, L. rhamnosus Y01.1, L. reuteri P05.7, L. gasseri P05.3, L. acidophilus P01.1.1 and P01.1.3, as well as the yeast S. cerevisiae P10.1. Given the critical role of IFN-γ in enhancing macrophage and NK cell activity, this cytokine likely contributes to the anticarcinogenic and anti-infectious effects of these probiotics.
This study highlighted significant strain-specific differences in antimicrobial and immunomodulatory effects. However, there are several limitations. First, the analysis was limited to a subset of strains isolated from the products. It is possible that other strains present in low abundance or VBNC state were not captured, potentially underestimating the full scope of probiotic diversity and functionality. Second, conventional culture methods, such as MRSc and M17 media, were insufficient to isolate all the probiotics listed on product labels. The inability to cultivate certain strains or resuscitate VBNC cells limits the comprehensiveness of the analysis and highlights the need for optimized or alternative culturing techniques. Third, the antimicrobial and immunomodulatory activities of probiotics were evaluated using in vitro models, which do not fully replicate the complexity of the human gut environment. The observed effects may not directly translate to in vivo outcomes due to differences in microbiota interactions, immune system dynamics, and environmental conditions. Fourth, while metagenomics provided detailed insights into microbial composition, the resolution was limited at the strain level for some genera. This may have affected the accuracy of identifying specific strains and their relative abundances. Lastly, variability in manufacturing processes, storage conditions, and product formulations may have influenced the viability and functionality of probiotics. These factors were not systematically controlled or analyzed, potentially affecting reproducibility.
In conclusion, our study demonstrated significant discrepancies between product labels and actual microbial content, emphasizing the need for quality control and accurate labeling to ensure product efficacy and consumer trust. However, the accuracy and reliability of metagenomic studies in dietary supplements and dairy products depend on multiple factors. Metagenomic sequencing alone is unable to differentiate viable and non-viable microbes in samples. Many probiotic strains are not well-represented in public databases, leading to misclassification or incomplete identification. The algorithm used for DNA analysis may have limitations in distinguishing between closely related species. In multi-strain products, some probiotics may overcome others during culture on medium, leading to dominance in culture-dependent identification. The antimicrobial activity of probiotics varied by strain. These findings highlight the potential of specific probiotics in combating multidrug-resistant pathogens through different mechanisms. In addition, probiotics exhibited diverse effects on immune modulation, and we demonstrated that several strains directly stimulated NK cells to secrete higher levels of IFN-γ. Overall, this study underscores the strain-specific nature of probiotics and their potential to support antimicrobial and immunomodulatory therapies. Further research is needed, particularly in vivo studies, to validate these findings and explore their clinical applications in infection control and immune health.
Methods
Probiotic products
Thirteen dietary supplements (referred to as product code P01-P13) and 25 dairy products (referred to as product codes Y01-Y25) (Supplementary Table S1) were randomly selected and analyzed in this study. Each product was purchased in a single package from drug stores, hypermarkets, and retailers in Bangkok, Thailand, and other countries. The dietary supplements (P01-P13) were in tablet and powder forms which indicated probiotics with or without quantity on the labels. The dietary supplements were stored at room temperature as recommended by the manufacturers. The dairy products were all yogurt with indicated probiotics on the labels. The yogurt products were transported in the cooling bag and stored at 4 °C as recommended by the manufacturers.
16 S and internal transcribed spacer (ITS) sequencing analyses
DNA was extracted from 250 µl of dietary products using DNeasy PowerSoil Pro (Qiagen). The powder of dietary supplements was suspended in 2 ml sterile PBS prior to DNA extraction.
DNA quality was determined by measuring the 260/280 nm absorbance rationo using NanoDrop spectrophotometer (Thermo Fisher Scientific). The quantity of extracted DNA was assessed using a Qubit fluorometer with a Qubit dsDNA High Sensitivity Assay kit (Thermo Fisher Scientific), which provides accurate quantitation within a detection range of 0.1–120 ng. Populations of microbiota were identified by 16 S and ITS ribosomal RNA (rRNA) sequencing. For taxonomic identification, DNA library was prepared using a Swift amplicon 16 S and ITS panel (Swift Biosciences, MI, USA). This panel amplifies all nine hypervariable regions (V1-V9) of 16 S rRNA gene for bacterial identification, and both ITS1 and ITS2 regions for fungal identification. Library concentration was quantified using TaqMan-based qPCR assay on a CFX Opus 96 Real-Time PCR system (Bio-Rad Laboratories, CA, USA). The quantification was performed based on a library size of 475 bp, and the final library pool was diluted to 2 nM.The library pool was denatured with 0.2 N NaOH and further diluted to 10 pM with pre-chilled HT1 buffer. The denatured library (600 µl) was loaded onto with a MiSeq v2 cartridge and sequenced using the MiSeq platform (Illumina). The sequencing analysis of 16 S rRNA gene and ITS regions was performed Using Illumina’s BaseSpace platform version 5.36.1. Quality control and sequence processing were performed following standard bioinformatics procedures. Raw sequence data were initially assessed using FastQC, followed by adapter trimming using Trimmomatic. Reads were filtered based on quality thresholds: Phred quality score ≥ 20, minimum read length of 50 bp after trimming, and removal of reads containing ambiguous bases. Error correction was performed using AfterQC. For taxonomic classification, sequences were clustered into Operational Taxonomic Units (OTUs) at 97% similarity threshold for 16 S rRNA data, while dynamic thresholds were applied for ITS data using the UNITE database. Chimeric sequences were removed using UCLUST. Only OTUs with a minimum abundance of 0.005% of total reads were retained for downstream analysis. Alpha and beta diversity analyses were performed using QIIME 2. For taxonomic classification, the GreenGenes database version 13_5 (last updated in May 2013) was used for 16 S rRNA gene sequence, while UNITE Fungal ITS Database v7.2 (Version 01.12.2017) was employed for ITS regions.
Isolation of bacteria and culture conditions
Microorganisms were isolated from dietary supplements and dairy products using De Man Rogosa & Sharpe medium supplemented with 0.05% L-cysteine (MRSc)43. The powder of dietary supplements was suspended in 2 ml sterile phosphate buffered saline, pH 7.4 (PBS containing 0.137 M NaCl, 2.683 mM KCl, 8.101 mM Na2HPO4, 1.470 mM KH2PO4; Oxoid, Hampshire, England) and thoroughly mixed. Twenty microliters of the suspension or dairy products was inoculated into 13 ml of MRSc broth and incubated under both aerobic and anaerobic conditions overnight to recover different types of probiotics from the products. The microorganisms were sub-cultured on MRSc agar and incubated at 37oC for 2 days. Identification of the probiotics was performed using matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF MS) as previously described44. Bacterial protein was extracted by formic acid method. Then, 1 µl of the solution was spotted onto a MSP-384 polished steel target plate (Bruker Daltonics, Germany). After drying in air, each spot was overlaid with 1 µl of formic acid. After drying in air, each spot will be overlaid with 1 µl of matrix, α-cyano-4-hydroxycinnamic acid (HCCA) (Bruker Daltonics, Germany) dissolved in a solution of 50% acetonitrile, 2.5% trifluoroacetic acid and 47.5% water. Each spot was measured in 200 shot steps for a total of 1200 laser shots using a MALDI-TOF Mass Spectrometer Autoflex speed (Bruker Daltonics, Germany) and FlexControl software (version 3.4.135, Bruker Daltonics, Germany). Spectra were analyzed within a mass range of 2,000–20,000 Da. Identification was achieved using the MALDI-Biotyper software (version 3.1, Bruker Daltonics, Germany). Interpretation will be performed according to the manufacturer’s recommendation. The cut-off scores were ≥ 2.00 and 1.700–1.900 for the species and genus levels, respectively.
16 S rRNA gene (for bacterial identification) or ITS regions (for fungal identification) were amplified and sequenced using the Sanger sequencing method to confirm identification of the isolated microorganisms. The probiotic bacteria and yeast were suspended in MRSc broth with 20% glycerol, except for Streptococcus thermophilus, which was suspended in M17 broth with 0.1% skim milk and 25% glycerol to enhance cell viability and cryoprotection efficiency, and then stored at -80 ºC for further experiments. The isolates were sub-cultured twice in the same media before performing assays.
Probiotic growth condition and inoculum Preparation
In brief, a single colony was picked and inoculated into 2 ml of an appropriate broth medium: MRSc for all probiotics, except S. thermophilus was cultured in M17 with 0.1% skim milk, Enterococcus spp. was cultured in LB medium, and yeast was culture in yeast extract–peptone dextrose (YPD) broth. The broth culture was incubated at optimal temperature and oxygen requirements as follows: Bifidobacterium sp. and L. delbrueckii were grown in an anaerobic jar with anaerobic gas generating sachet (O2 < 0.1% and CO2 7–15%; Oxoid) for 48 h; Lactobacillus spp. was grown at 37oC for 18 h without shaking; S. thermophilus was grown at 42 ºC for 18 h without shaking; Enterococcus spp. and yeast were grown at 37oC for 18 h with shaking at 200 rpm. Microbes were harvested by centrifugation at 16,2000 ×g for 5 min and the culture supernatant was discarded. The probiotic pellets were re-suspended in PBS and adjusted an optical density at 600 nm to 0.4 with approximately 1 × 108 CFU/ml.
Cell lines and culture conditions
THP-1 cells (TIB-202, American Type Culture Collection), a human monocytic cell line, were maintained in RPMI 1640 medium supplemented with 10% heat-inactivated fetal bovine serum (FBS) and 100 U/ml penicillin and 100 µg/ml streptomycin (Gibco) at 37 ºC with 5% CO2 for 2 days. 4 × 105 cells were seeded into each well of 24-well tissue culture plate and activated with 50 ng/ml of phorbol-12-myristate-13-acetate (PMA; Sigma) for 2 days for the differentiation into macrophages. After removing PMA, the THP-1 monocyte-derived macrophages were incubated with fresh medium at 37 ºC with 5% CO2 for 2 days. Prior to performing the experiments, the macrophages were washed twice with 1 ml of Dulbecco’s phosphate-buffered saline (DPBS; Hyclone) and maintained in 0.5 ml of RPMI medium without FBS and antibiotics45.
A human NK cell line, NK-92MI (CRL-2408, American Type Culture Collection), was maintained in minimum essential medium (MEM-α) without nucleosides and supplemented with 2.2 g/l sodium bicarbonate, 2 mM L-glutamine, 100U/ml penicillin and 100 µg/ml streptomycin, 0.02 mM folic acid (Sigma-Aldrich), 0.2 mM inositol (Sigma-Aldrich), 0.1 mM 2-mercaptoethanol (Sigma-Aldrich), 12.5% fetal bovine serum (Hyclone) and 12.5% horse serum (Gibco) at 37 ºC with 5% CO2. Cells were subcultured every 2–3 days. The cells at a concentration of 6 × 105 cells/ml for 0.5 ml without supplements and antibiotics were seeded into each well of a 24-well tissue culture plate and incubated at 37 ºC, 5% CO2 before performing the experiments.
Antimicrobial activity
Probiotics from dietary supplements and dairy products were examined for antimicrobial activity against 5 pathogenic bacteria by agar overlay method as previously described29. The target bacteria were susceptible and resistant to several antibiotics. These included S. aureus ATCC25923, S. aureus DMST20654 (MRSA), Escherichia coli ATCC25922 and E. coli PB1(ESBL) and PB231 (CRE)46. In brief, the standardized inoculation method, micropipette, dispensed one microliter of probiotic suspension at a concentration of approximately 1 × 108 CFU/ml onto the dry surface of MRSc agar. The plate was incubated with appropriate and consistent growth conditions for 24 h to minimize variability. The target bacteria were cultured in LB broth at 37 ºC with shaking at 200 rpm overnight and adjusted OD at 600 nm to 1.0 (approximately 109 CFU/ml) before mixing 1 ml of bacterial suspension with 9 ml of 0.8% Muller-Hinton agar (MHA) to obtain a final concentration of 1 × 108 CFU/ml. Ten milliliters of each pathogen in soft agar were gently overlaid on MRSc agar plates, which contained probiotic growth in a spot (5 mm diameter) on the surface. The plate was incubated at 37 ºC for 24 h. The clear zones around the probiotics spot were measured. The diameter of the inhibition zone was interpreted as follows: >20 mm as strong activity, 10–20 mm as moderate activity, < 10 mm as weak activity, and no inhibition zone as no activity. Disk diffusion of standard antibiotics: tetracycline (TET), ceftazidime/clavulanic acid (CAZ/CLA), meropenem (MEM) and vancomycin (VAN) (Oxoid, UK) were performed as positive controls.
Phagocytosis assay
To determine whether probiotics can enhance phagocytic activity, carboxyfluorescein diacetate succinimidyl ester (CFSE), a fluorescent membrane permeable ester that activates by intracellular esterase was applied to detect phagocytosed E. coli ATCC25922 in THP-1 monocytes47. First, E. coli ATCC25922 was grown in 10 ml of LB broth at 37℃ with shaking at 200 rpm for 18 h. The bacteria were harvested by centrifugation in 4000 ×g at room temperature for 10 min. After discarding the supernatant, the pellet was washed twice with PBS (pH 7.4) and resuspended in PBS to an OD at 600 nm to 1.0 (approximately 109 CFU/ml). The bacteria were concentrated 25 times by centrifugation and resuspended with PBS. The bacterial suspensions were serially diluted and plated on LB agar plates to enumerate the colony-forming units before inactivation. The inactivation step was applied by placing the obtained solutions in the water bath at 70 ℃ for 40 min. To ensure that all bacterial cells were killed, a cultured plate of bacteria was provided and incubated overnight48. Heat-killed E. coli was adjusted concentration to 2.5 × 1010 CFU/ml according to the bacteria plate count before heat-inactivation. Five microliters of the bacterial suspension then were incubated at 37 °C for 30 min in the dark with an equal volume of a 10 µM solution of CFSE (BD Horizon). The bacteria were collected, and excess dye was removed by centrifugation at 13,800 ×g for 5 min, washed twice with PBS and resuspended in 0.5 ml RPMI medium without FBS.
One hundred microliters of THP-1 monocyte cells at a concentration of 5 × 105 cells/ml in RPMI were seeded into each well of a 96-well plate. The cells were stimulated with 100 µl of probiotics in RPMI at MOI of 200 at 37 ºC with 5% CO2 for 1 h. Culture supernatant was aspirated after centrifugation at 600 ×g for 5 min and washed twice with 200 µl of DPBS. Heat-killed E. coli stained with CFSE was added into each well to obtain the final concentration of E. coli at 5 × 108 CFU/ml in a final volume of 200 µl. The plate was incubated at 37 ºC with 5% CO2 for 2 h. The phagocytosis was stopped by incubation on ice for 5 min. After washing twice with cold DPBS, 100 µl of cells in DPBS were mixed with 100 µl of 0.04% trypan blue quenching solution and then placed on ice for 15 min. Cells were washed twice with DPBS and resuspended in 200 µl of cold DPBS. The phagocytosis was assessed using a FACSCalibur flow cytometer (Becton Dickinson, Franklin Lakes, NJ, USA). Three independent experiments in triplicate were performed. The phagocytic index was defined as the percentage of monocyte cells containing E. coli stained with CFSE (% phagocytosis) multiplied by the mean fluorescence intensity of the cells.
Macrophages stimulation with probiotics and E. coli lipopolysaccharide.
The THP-1 monocyte-derived macrophages were treated with probiotics at 4 × 106 CFU alone or 1 µg/ml lipopolysaccharide of E. coli (E. coli-LPS) (Sigma-Aldrich) alone, or 1 µg/ml of E. coli-LPS together with probiotics at 4 × 106 CFU45. Untreated macrophages in the RPMI medium were used as a negative control. Five hundred microliters of probiotic suspension at a concentration of 8 × 106 CFU/ml in RPMI medium was added into the cells to obtain MOI of 10 and incubated at 37 ºC with 5% CO2 for 18 h. The culture supernatant was collected after centrifugation at 12,000 rpm for 5 min and stored at − 80 ºC until use. The experiments were performed once in duplicate. The concentrations of IL-1β, IL-1RA, IL-6, IL-8, IL-10, IL-12p70, and TNF-α cytokines in the supernatant were evaluated using a Milliplex®MAP Human High Sensitivity T cell magnetic bead panel kit (Merck KGaA, Darmstadt, Germany). The cytokine levels were analyzed using the Luminex analyzer MAGPIX® system with xPOTENT® software.
Probiotics activation of NK cells
Five hundred microliters of probiotic suspension at a concentration of 6 × 106 CFU/ml was added into NK-92 MI cells to obtain MOI of 10 and incubated at 37 oC with 5% CO2 for 24 h. NK-92 MI cells treated with 20 ng/ml PMA plus 1 µg/ml ionomycin were used as a positive control. The experiments were performed in three independent experiments in triplicate. Cell culture supernatants were collected by centrifugation at 1,000 ×g for 5 min and 12,000 ×g for 5 min and stored at − 80 oC until use. Cell-free supernatant was diluted 1:20 before IFN-γ measurement using a Human IFN-γ ELISA kit (BD Biosciences, Franklin Lakes, NJ, USA). The detection limit for IFN-γ assay was 4.7 pg/ml.
Statistical analysis
All statistical data were analyzed using GraphPad Prism version 7.0 (GraphPad Software Inc, San Diego, CA, USA). Continuous data were reported as mean ± SD. Comparison between groups was analyzed using a one-way ANOVA test. The Mann-Whitney U test tested the median difference of non-normally distributed data between groups. P value < 0.05 was considered statistically significant.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
We are grateful to the staff at the Department of Microbiology and Immunology, Faculty of Tropical Medicine, Mahidol University.
Author contributions
PC, ST, WC and NC conceptualized and designed the experiments. PC, ST, NS, AP, NT and IS performed experiments. PC, ST, AP and NC analyzed data and wrote the manuscript. NC supervised the project. All authors participated in the final editing of the manuscript.
Funding
This research was partially funded by a grant from the Office of the Higher Education Commission of Thailand. Piyaorn Chornchoem was supported by a Commission on Higher Education Ph.D. Scholarship. This research was funded in part, by the Wellcome Trust [220211]. For the purpose of Open Access, the author has applied a CC BY public copyright license to any Author Accepted Manuscript version arising from this submission. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Data availability
The metagenomic data generated during the current study is available in the SRA database under BioProject accession number PRJNA1203583. The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Piyaorn Chornchoem and Sarunporn Tandhavanant contributed equally to this work.
References
- 1.Tsifintaris, M. et al. Probio-Ichnos: A database of microorganisms with in vitro probiotic properties. Microorganisms10.3390/microorganisms12101955 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Piewngam, P. et al. Pathogen elimination by probiotic Bacillus via signalling interference. Nature562, 532–537. 10.1038/s41586-018-0616-y (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kamada, N., Chen, G. Y., Inohara, N. & Nunez, G. Control of pathogens and pathobionts by the gut microbiota. Nat. Immunol.14, 685–690. 10.1038/ni.2608 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gourbeyre, P., Denery, S. & Bodinier, M. Probiotics, prebiotics, and synbiotics: Impact on the gut immune system and allergic reactions. J. Leukoc. Biol.89, 685–695. 10.1189/jlb.1109753 (2011). [DOI] [PubMed] [Google Scholar]
- 5.Macpherson, A. J. & Harris, N. L. Interactions between commensal intestinal bacteria and the immune system. Nat. Rev. Immunol.4, 478–485. 10.1038/nri1373 (2004). [DOI] [PubMed] [Google Scholar]
- 6.Bazireh, H., Shariati, P., Azimzadeh Jamalkandi, S., Ahmadi, A. & Boroumand, M. A. Isolation of novel probiotic Lactobacillus and Enterococcus strains from human salivary and fecal sources. Front. Microbiol.11, 597946. 10.3389/fmicb.2020.597946 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Servin, A. L. Antagonistic activities of lactobacilli and bifidobacteria against microbial pathogens. FEMS Microbiol. Rev.28, 405–440. 10.1016/j.femsre.2004.01.003 (2004). [DOI] [PubMed] [Google Scholar]
- 8.Cheikhyoussef, A., Pogori, N., Chen, W. & Zhang, H. Antimicrobial proteinaceous compounds obtained from bifidobacteria: from production to their application. Int. J. Food Microbiol.125, 215–222. 10.1016/j.ijfoodmicro.2008.03.012 (2008). [DOI] [PubMed] [Google Scholar]
- 9.Jabbari, V. et al. Lactobacillus plantarum as a probiotic potential from Kouzeh cheese (Traditional Iranian cheese) and its antimicrobial activity. Probiotics Antimicrob. Proteins. 9, 189–193. 10.1007/s12602-017-9255-0 (2017). [DOI] [PubMed] [Google Scholar]
- 10.Perez-Cano, F. J., Dong, H. & Yaqoob, P. In vitro Immunomodulatory activity of Lactobacillus fermentum CECT5716 and Lactobacillus salivarius CECT5713: two probiotic strains isolated from human breast milk. Immunobiology215, 996–1004. 10.1016/j.imbio.2010.01.004 (2010). [DOI] [PubMed] [Google Scholar]
- 11.Dong, H., Rowland, I. & Yaqoob, P. Comparative effects of six probiotic strains on immune function in vitro. Br. J. Nutr.108, 459–470. 10.1017/S0007114511005824 (2012). [DOI] [PubMed] [Google Scholar]
- 12.Quinteiro-Filho, W. M., Brisbin, J. T., Hodgins, D. C. & Sharif, S. Lactobacillus and Lactobacillus cell-free culture supernatants modulate chicken macrophage activities. Res. Vet. Sci.103, 170–175. 10.1016/j.rvsc.2015.10.005 (2015). [DOI] [PubMed] [Google Scholar]
- 13.Taha-Abdelaziz, K. et al. In vitro assessment of Immunomodulatory and anti-Campylobacter activities of probiotic lactobacilli. Sci. Rep.9, 17903. 10.1038/s41598-019-54494-3 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Holowacz, S., Guinobert, I., Guilbot, A., Hidalgo, S. & Bisson, J. F. A mixture of five bacterial strains attenuates skin inflammation in mice. Antiinflamm Antiallergy Agents Med. Chem.17, 125–137. 10.2174/1871523017666180813123823 (2018). [DOI] [PubMed] [Google Scholar]
- 15.Viola, A., Munari, F., Sanchez-Rodriguez, R., Scolaro, T. & Castegna, A. The metabolic signature of macrophage responses. Front. Immunol.10, 1462. 10.3389/fimmu.2019.01462 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Drago, L., Nicola, L., Iemoli, E., Banfi, G. & De Vecchi, E. Strain-dependent release of cytokines modulated by Lactobacillus salivarius human isolates in an in vitro model. BMC Res. Notes. 3, 44. 10.1186/1756-0500-3-44 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Romero, C. R. et al. The role of interferon-gamma in the pathogenesis of acute intra-abdominal sepsis. J. Leukoc. Biol.88, 725–735. 10.1189/jlb.0509307 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fink, L. N., Zeuthen, L. H., Ferlazzo, G. & Frokiaer, H. Human antigen-presenting cells respond differently to gut-derived probiotic bacteria but mediate similar strain-dependent NK and T cell activation. FEMS Immunol. Med. Microbiol.51, 535–546. 10.1111/j.1574-695X.2007.00333.x (2007). [DOI] [PubMed] [Google Scholar]
- 19.Fink, L. N. & Frokiaer, H. Dendritic cells from Peyer’s patches and mesenteric lymph nodes differ from spleen dendritic cells in their response to commensal gut bacteria. Scand. J. Immunol.68, 270–279. 10.1111/j.1365-3083.2008.02136.x (2008). [DOI] [PubMed] [Google Scholar]
- 20.Huys, G. et al. Accuracy of species identity of commercial bacterial cultures intended for probiotic or nutritional use. Res. Microbiol.157, 803–810. 10.1016/j.resmic.2006.06.006 (2006). [DOI] [PubMed] [Google Scholar]
- 21.Drago, L., Rodighiero, V., Celeste, T., Rovetto, L. & De Vecchi, E. Microbiological evaluation of commercial probiotic products available in the USA in 2009. J. Chemother.22, 373–377. 10.1179/joc.2010.22.6.373 (2010). [DOI] [PubMed] [Google Scholar]
- 22.Goldstein, E. J., Citron, D. M., Claros, M. C. & Tyrrell, K. L. Bacterial counts from five over-the-counter probiotics: are you getting what you paid for? Anaerobe 25, 1–4 (2014). 10.1016/j.anaerobe.2013.10.005 [DOI] [PubMed]
- 23.Patro, J. N. et al. Culture-Independent Metagenomic Surveillance of Commercially Available Probiotics with High-Throughput Next-Generation Sequencing. mSphere 1 (2016). 10.1128/mSphere.00057-16 [DOI] [PMC free article] [PubMed]
- 24.Champagne, C. P., Ross, R. P., Saarela, M., Hansen, K. F. & Charalampopoulos, D. Recommendations for the viability assessment of probiotics as concentrated cultures and in food matrices. Int. J. Food Microbiol.149, 185–193. 10.1016/j.ijfoodmicro.2011.07.005 (2011). [DOI] [PubMed] [Google Scholar]
- 25.Terpou, A. et al. Probiotics in food systems: significance and emerging strategies towards improved viability and delivery of enhanced beneficial value. Nutrients1110.3390/nu11071591 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zheng, J. et al. A taxonomic note on the genus Lactobacillus: Description of 23 novel genera, emended description of the genus Lactobacillus Beijerinck 1901, and union of Lactobacillaceae and Leuconostocaceae. Int. J. Syst. Evol. Microbiol.70, 2782–2858. 10.1099/ijsem.0.004107 (2020). [DOI] [PubMed] [Google Scholar]
- 27.Hayek, S. A., Gyawali, R., Aljaloud, S. O., Krastanov, A. & Ibrahim, S. A. Cultivation media for lactic acid bacteria used in dairy products. J. Dairy. Res.86, 490–502. 10.1017/S002202991900075X (2019). [DOI] [PubMed] [Google Scholar]
- 28.Boyte, M. E., Benkowski, A., Pane, M. & Shehata, H. R. Probiotic and postbiotic analytical methods: A perspective of available enumeration techniques. Front. Microbiol.14, 1304621. 10.3389/fmicb.2023.1304621 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Halder, D., Mandal, M., Chatterjee, S. S., Pal, N. K. & Mandal, S. Indigenous probiotic Lactobacillus isolates presenting antibiotic like activity against human pathogenic bacteria. Biomedicines510.3390/biomedicines5020031 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sanders, M. E., Merenstein, D. J., Reid, G., Gibson, G. R. & Rastall, R. A. Probiotics and prebiotics in intestinal health and disease: From biology to the clinic. Nat. Rev. Gastroenterol. Hepatol.16, 605–616. 10.1038/s41575-019-0173-3 (2019). [DOI] [PubMed] [Google Scholar]
- 31.Lee, J. Vitro evaluation of probiotic properties and anti-pathogenic effects of Lactobacillus and Bifidobacterium strains as potential probiotics. Foods10.3390/foods13142301 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen, C. C. et al. Antimicrobial activity of Lactobacillus species against carbapenem-resistant Enterobacteriaceae. Front. Microbiol.10, 789. 10.3389/fmicb.2019.00789 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang, J. et al. Inhibitory potential of Bifidobacterium longum FB1-1 cell-rree supernatant against carbapenem-resistant Klebsiella pneumoniae drug resistance spread. Microorganisms10.3390/microorganisms12061203 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Klein, A., Friedrich, U., Vogelsang, H. & Jahreis, G. Lactobacillus acidophilus 74 – 2 and Bifidobacterium animalis subsp lactis DGCC 420 modulate unspecific cellular immune response in healthy adults. Eur. J. Clin. Nutr.62, 584–593. 10.1038/sj.ejcn.1602761 (2008). [DOI] [PubMed] [Google Scholar]
- 35.Jeong, M. et al. Heat-killed Lactobacillus plantarum KCTC 13314BP enhances phagocytic activity and Immunomodulatory effects via activation of MAPK and STAT3 pathways. J. Microbiol. Biotechnol.29, 1248–1254. 10.4014/jmb.1905.05066 (2019). [DOI] [PubMed] [Google Scholar]
- 36.Hirayama, D., Iida, T. & Nakase, H. The phagocytic function of macrophage-enforcing innate immunity and tissue homeostasis. Int. J. Mol. Sci.10.3390/ijms19010092 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rocha-Ramirez, L. M. et al. Probiotic Lactobacillus strains stimulate the inflammatory response and activate human macrophages. J Immunol Res 4607491 (2017). (2017). 10.1155/2017/4607491 [DOI] [PMC free article] [PubMed]
- 38.Sun, K. Y. et al. Lactobacillus paracasei modulates LPS-induced inflammatory cytokine release by monocyte-macrophages via the up-regulation of negative regulators of NF-kappaB signaling in a TLR2-dependent manner. Cytokine92, 1–11. 10.1016/j.cyto.2017.01.003 (2017). [DOI] [PubMed] [Google Scholar]
- 39.Laute-Caly, D. L. et al. The Flagellin of candidate live biotherapeutic Enterococcus gallinarum MRx0518 is a potent immunostimulant. Sci. Rep.9, 801. 10.1038/s41598-018-36926-8 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zielinska, D., Dlugosz, E. & Zawistowska-Deniziak, A. Functional properties of food origin Lactobacillus in the Gastrointestinal ecosystem-in vitro study. Probiotics Antimicrob. Proteins. 11, 820–829. 10.1007/s12602-018-9458-z (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dong, H., Rowland, I., Tuohy, K. M., Thomas, L. V. & Yaqoob, P. Selective effects of Lactobacillus casei Shirota on T cell activation, natural killer cell activity and cytokine production. Clin. Exp. Immunol.161, 378–388. 10.1111/j.1365-2249.2010.04173.x (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lee, A. et al. Consumption of dairy yogurt containing Lactobacillus paracasei Ssp. paracasei, Bifidobacterium animalis Ssp. Lactis and heat-treated Lactobacillus plantarum improves immune function including natural killer cell activity. Nutrients10.3390/nu9060558 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Davis, C. Enumeration of probiotic strains: Review of culture-dependent and alternative techniques to quantify viable bacteria. J. Microbiol. Methods. 103, 9–17. 10.1016/j.mimet.2014.04.012 (2014). [DOI] [PubMed] [Google Scholar]
- 44.Angelakis, E., Million, M., Henry, M. & Raoult, D. Rapid and accurate bacterial identification in probiotics and yoghurts by MALDI-TOF mass spectrometry. J. Food Sci.76, M568–572. 10.1111/j.1750-3841.2011.02369.x (2011). [DOI] [PubMed] [Google Scholar]
- 45.Gebremariam, H. G. et al. Lactobacillus gasseri suppresses the production of Proinflammatory cytokines in Helicobacter pylori-infected macrophages by inhibiting the expression of ADAM17. Front. Immunol.10, 2326. 10.3389/fimmu.2019.02326 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Runcharoen, C. et al. Whole genome sequencing of ESBL-producing Escherichia coli isolated from patients, farm waste and canals in Thailand. Genome Med.9, 81. 10.1186/s13073-017-0471-8 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vander Top, E. A., Perry, G. A. & Gentry-Nielsen, M. J. A novel flow cytometric assay for measurement of in vivo pulmonary neutrophil phagocytosis. BMC Microbiol.6, 61. 10.1186/1471-2180-6-61 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rabiei, P., Mohabatkar, H. & Behbahani, M. Studying the effects of several heat-inactivated bacteria on colon and breast cancer cells. Mol. Biol. Res. Commun.8, 91–98. 10.22099/mbrc.2019.33958.1413 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The metagenomic data generated during the current study is available in the SRA database under BioProject accession number PRJNA1203583. The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.