Abstract
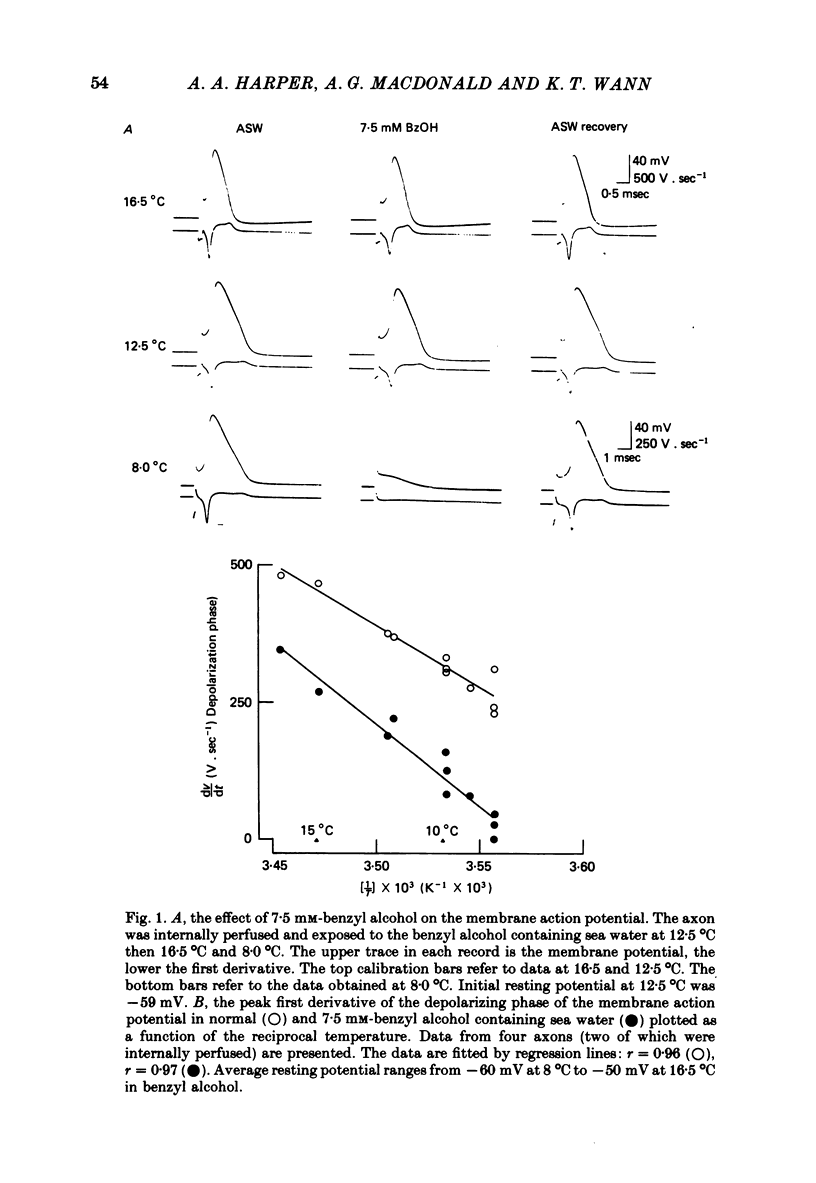
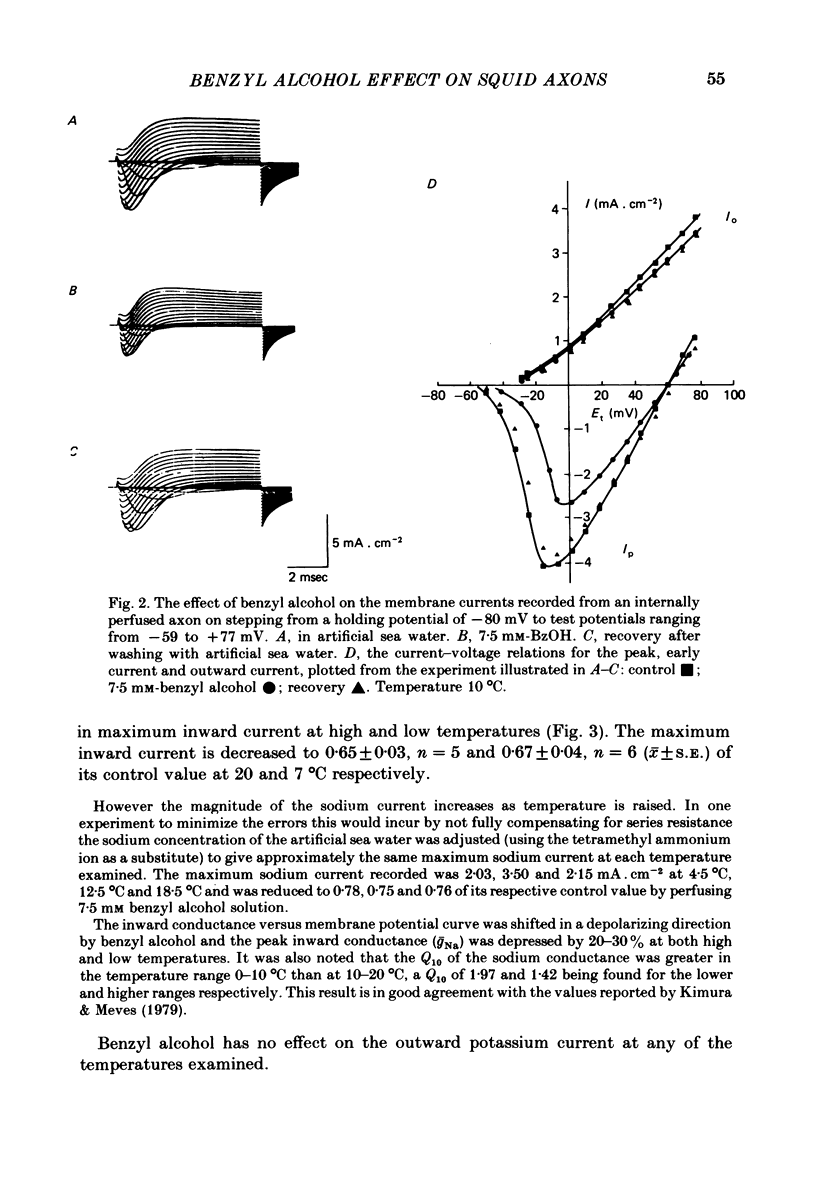
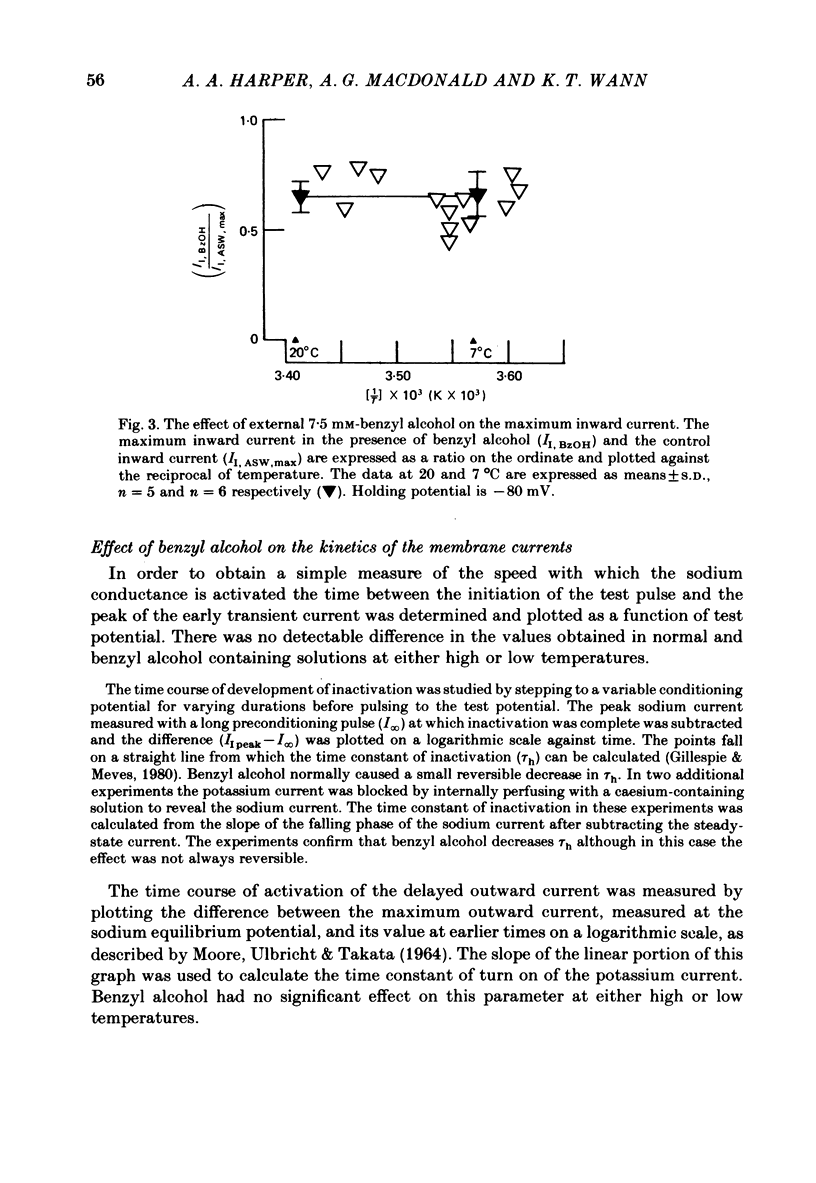
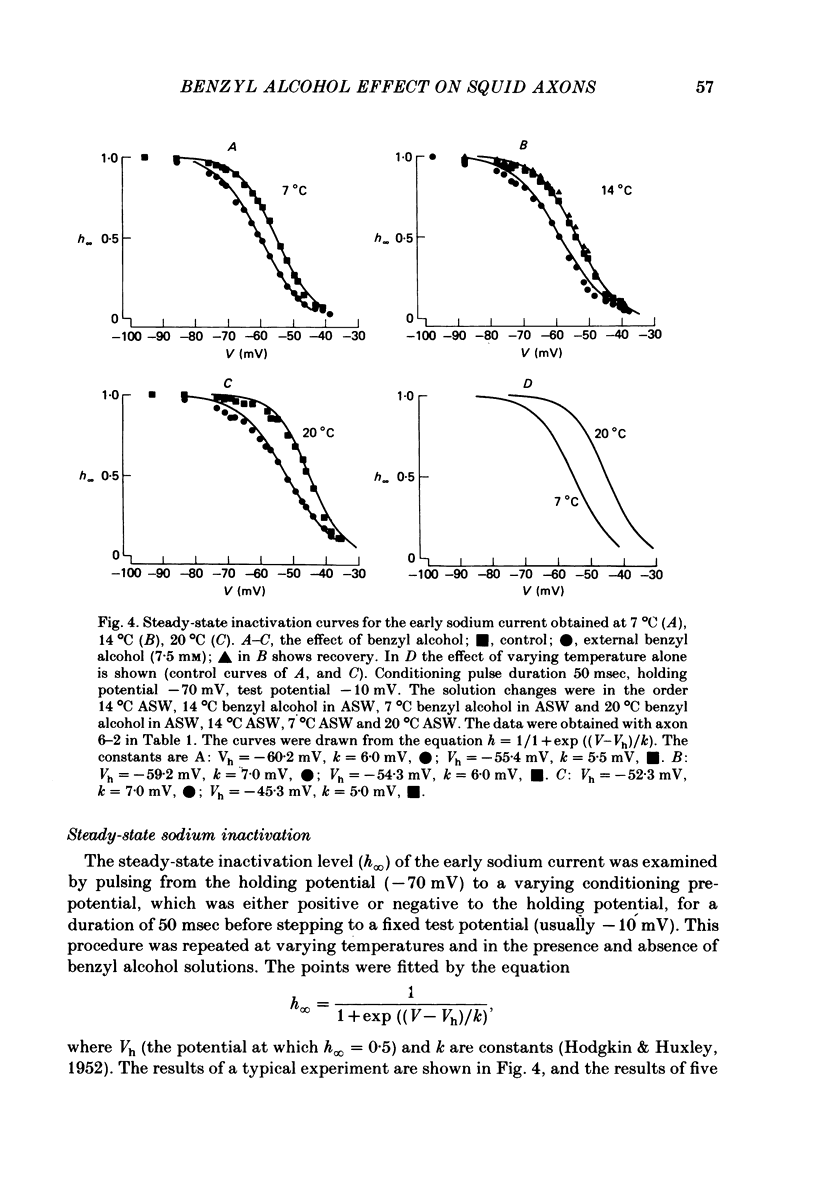
The action of benzyl alcohol was studied on the voltage-clamped giant axons of Loligo forbesi. The depressant effect of 7.5 mM-benzyl alcohol on the action potential amplitude and peak rate of rise was more marked at a low (8 degrees C) than at a high temperature (16.5 degrees C). A small depolarization (approximately 5 mV) was also produced. These effects were usually reversible. Benzyl alcohol (7.5 mM) selectively depressed the amplitude of the peak early current. The maximum inward current was depressed to 0.65 and 0.67 of the control value at 20 degrees C and 7 degrees C respectively. This effect was usually reversible. Benzyl alcohol also depressed the peak inward conductance (gNa but had no effect on the time to peak early current. A small reversible decrease in the time constant of inactivation (tau h) was caused by benzyl alcohol (7.5 mM). This was usually reversible on washout of the anaesthetic. Benzyl alcohol (7.5 mM) had no effect on the time course of activation or the amplitude of the delayed outward current. The curve of the steady-state inactivation parameter (h infinity) for the Na current against the conditioning membrane potential was shifted by benzyl alcohol in a hyperpolarizing direction (at h infinity = 0.5 the shift was an average of 3.3 mV and was reversible). Increasing the temperature from 7 to 20 degrees C shifted the curve in a depolarizing direction by approximately 10 mV. The reason for the increased nerve-blocking action of benzyl alcohol at the lower temperature is discussed.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BAKER P. F., HODGKIN A. L., SHAW T. I. Replacement of the axoplasm of giant nerve fibres with artificial solutions. J Physiol. 1962 Nov;164:330–354. doi: 10.1113/jphysiol.1962.sp007025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHERKIN A., CATCHPOOL J. F. TEMPERATURE DEPENDENCE OF ANESTHESIA IN GOLDFISH. Science. 1964 Jun 19;144(3625):1460–1462. doi: 10.1126/science.144.3625.1460. [DOI] [PubMed] [Google Scholar]
- Colton C. A., Colton J. S. Depression of glutamate-mediated synaptic transmission by benzyl alcohol. Can J Physiol Pharmacol. 1977 Aug;55(4):917–922. doi: 10.1139/y77-122. [DOI] [PubMed] [Google Scholar]
- Gillespie J. I., Meves H. The time course of sodium inactivation in squid giant axons. J Physiol. 1980 Feb;299:289–307. doi: 10.1113/jphysiol.1980.sp013125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., HUXLEY A. F. A quantitative description of membrane current and its application to conduction and excitation in nerve. J Physiol. 1952 Aug;117(4):500–544. doi: 10.1113/jphysiol.1952.sp004764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haydon D. A., Hendry B. M., Levinson S. R., Requena J. Anaesthesia by the n-alkanes. A comparative study of nerve impulse blockage and the properties of black lipid bilayer membranes. Biochim Biophys Acta. 1977 Oct 3;470(1):17–34. doi: 10.1016/0005-2736(77)90058-x. [DOI] [PubMed] [Google Scholar]
- Haydon D. A., Kimura J. E. Some effects of n-pentane on the sodium and potassium currents of the squid giant axon. J Physiol. 1981 Mar;312:57–70. doi: 10.1113/jphysiol.1981.sp013615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura J. E., Meves H. The effect of temperature on the asymmetrical charge movement in squid giant axons. J Physiol. 1979 Apr;289:479–500. doi: 10.1113/jphysiol.1979.sp012748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee A. G. Model for action of local anaesthetics. Nature. 1976 Aug 12;262(5569):545–548. doi: 10.1038/262545a0. [DOI] [PubMed] [Google Scholar]
- MOORE J. W., ULBRICHT W., TAKATA M. EFFECT OF ETHANOL ON THE SODIUM AND POTASSIUM CONDUCTANCES OF THE SQUID AXON MEMBRANE. J Gen Physiol. 1964 Nov;48:279–295. doi: 10.1085/jgp.48.2.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metcalfe J. C., Seeman P., Burgen A. S. The proton relaxation of benzyl alcohol in erythrocyte membranes. Mol Pharmacol. 1968 Jan;4(1):87–95. [PubMed] [Google Scholar]
- Oxford G. S., Swenson R. P. n-Alkanols potentiate sodium channel inactivation in squid giant axons. Biophys J. 1979 Jun;26(3):585–590. doi: 10.1016/S0006-3495(79)85273-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oxford G. S., Wu C. H., Narahashi T. Removal of sodium channel inactivation in squid giant axons by n-bromoacetamide. J Gen Physiol. 1978 Mar;71(3):227–247. doi: 10.1085/jgp.71.3.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyes J., Latorre R. Effect of the anesthetics benzyl alcohol and chloroform on bilayers made from monolayers. Biophys J. 1979 Nov;28(2):259–279. doi: 10.1016/S0006-3495(79)85175-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards C. D., Martin K., Gregory S., Keightley C. A., Hesketh T. R., Smith G. A., Warren G. B., Metcalfe J. C. Degenerate perturbations of protein structure as the mechanism of anaesthetic action. Nature. 1978 Dec 21;276(5690):775–779. doi: 10.1038/276775a0. [DOI] [PubMed] [Google Scholar]
- Trudell J. R. A unitary theory of anesthesia based on lateral phase separations in nerve membranes. Anesthesiology. 1977 Jan;46(1):5–10. doi: 10.1097/00000542-197701000-00003. [DOI] [PubMed] [Google Scholar]
- Trudell J. R., Hubbell W. L., Cohen E. N. Pressure reversal of inhalation anesthetic-induced disorder in spin-labeled phospholipid vesicles. Biochim Biophys Acta. 1973 Jan 26;291(2):328–334. doi: 10.1016/s0005-2736(73)80001-x. [DOI] [PubMed] [Google Scholar]
- Turner G. L., Oldfield E. Effect of a local anaesthetic on hydrocarbon chain order in membranes. Nature. 1979 Feb 22;277(5698):669–670. doi: 10.1038/277669a0. [DOI] [PubMed] [Google Scholar]
- Wang C. M., Narahashi T., Scuka M. Mechanism of negative temperature coefficient of nerve blocking action of allethrin. J Pharmacol Exp Ther. 1972 Sep;182(3):442–453. [PubMed] [Google Scholar]


