Abstract
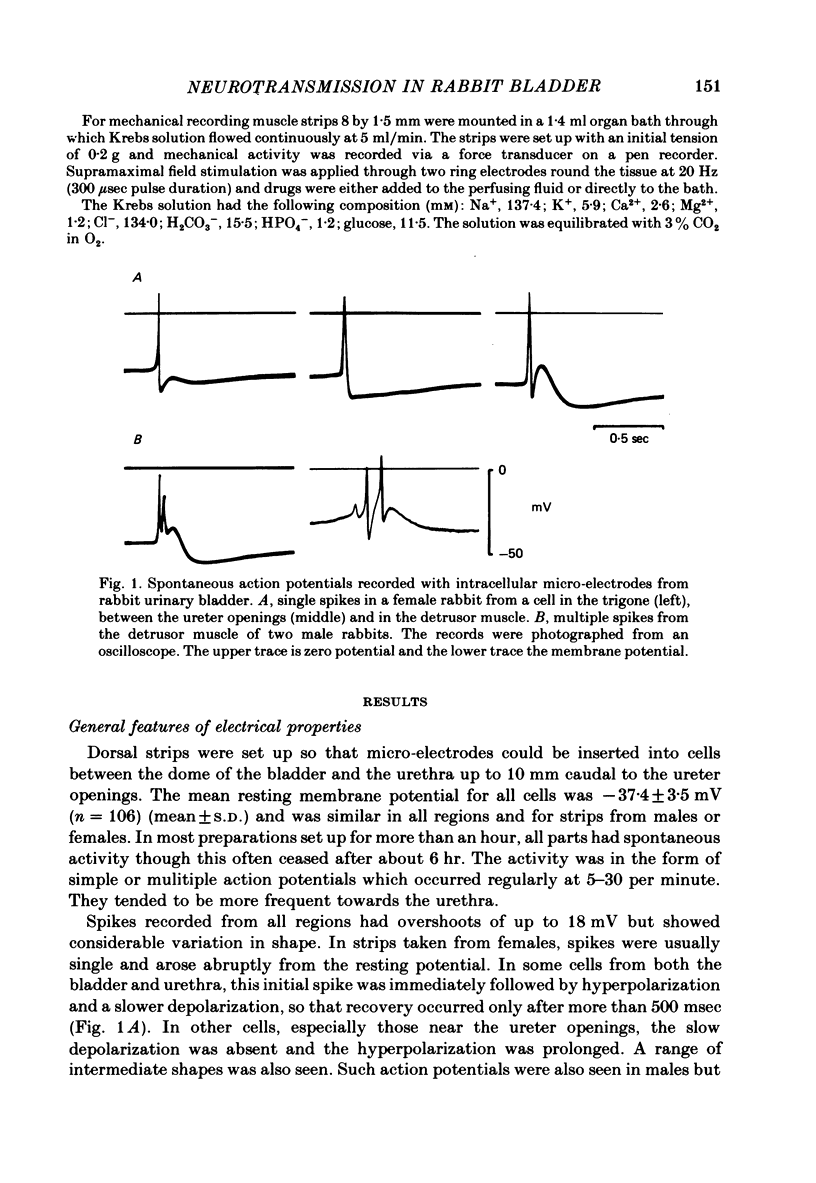
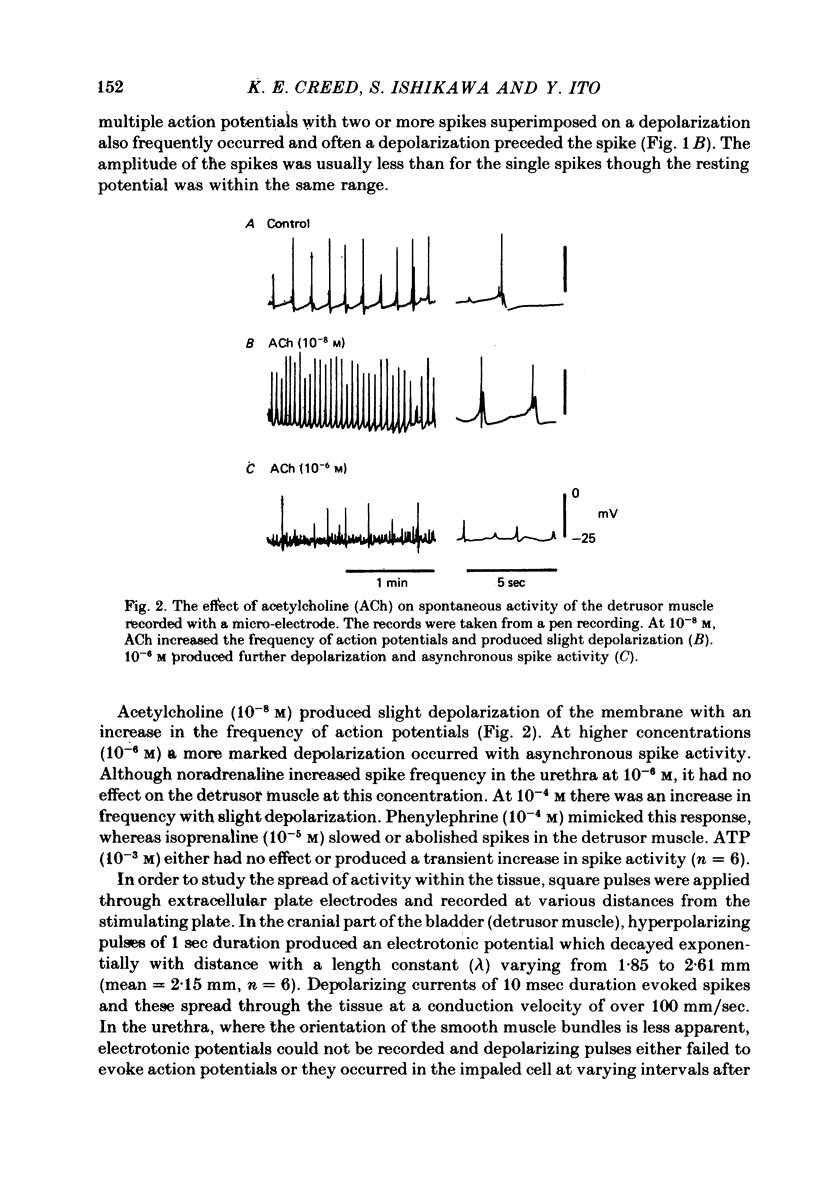
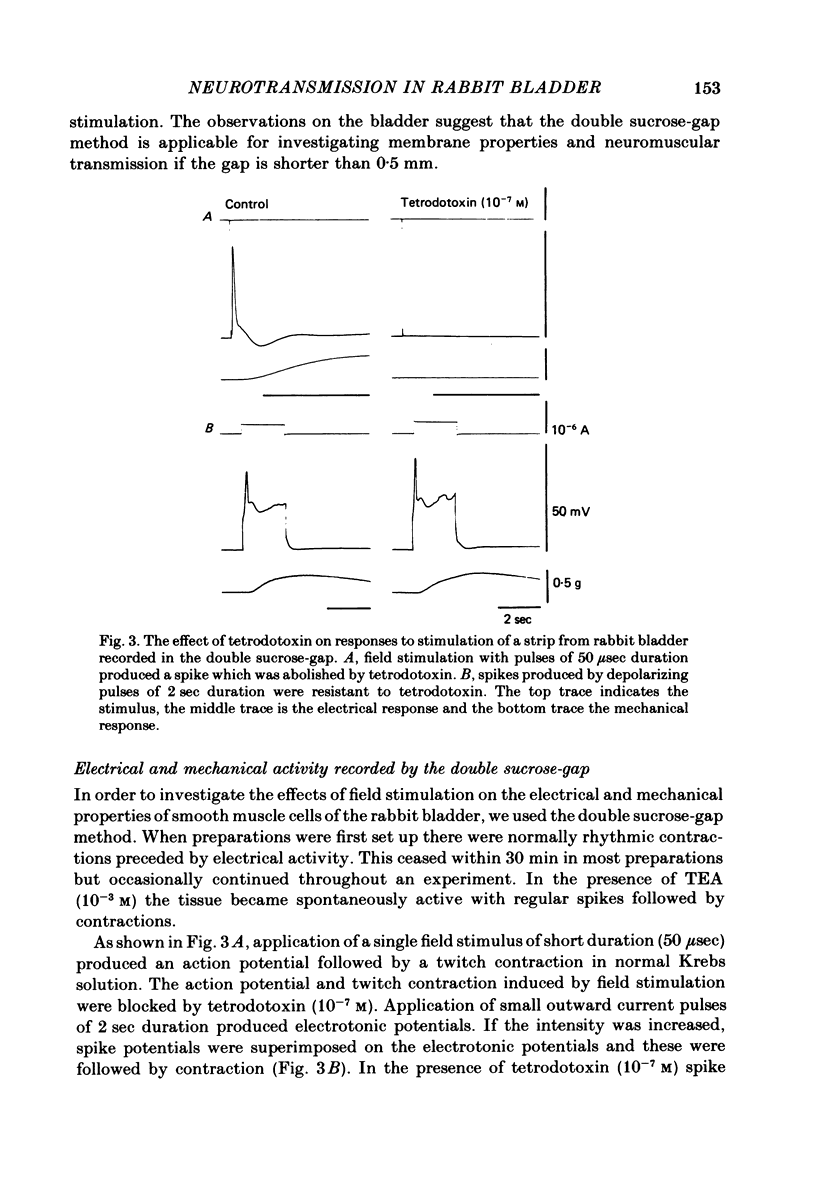
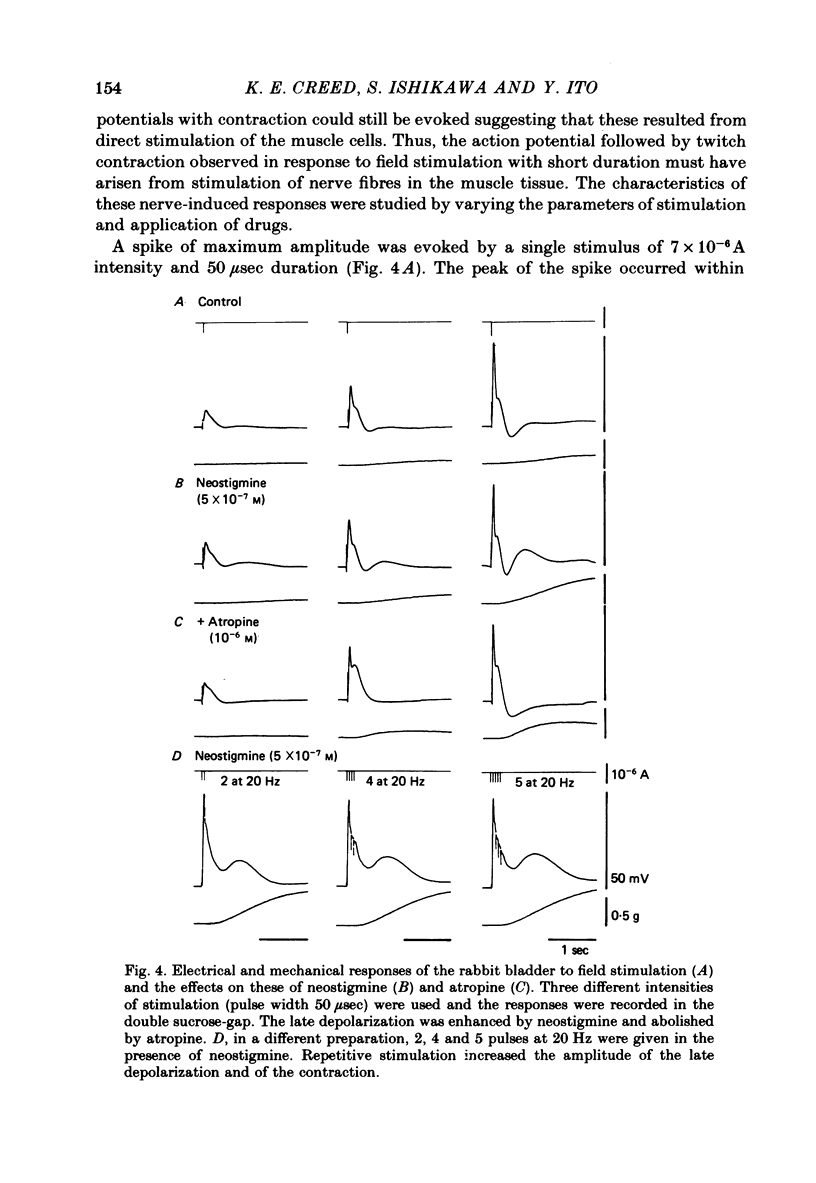
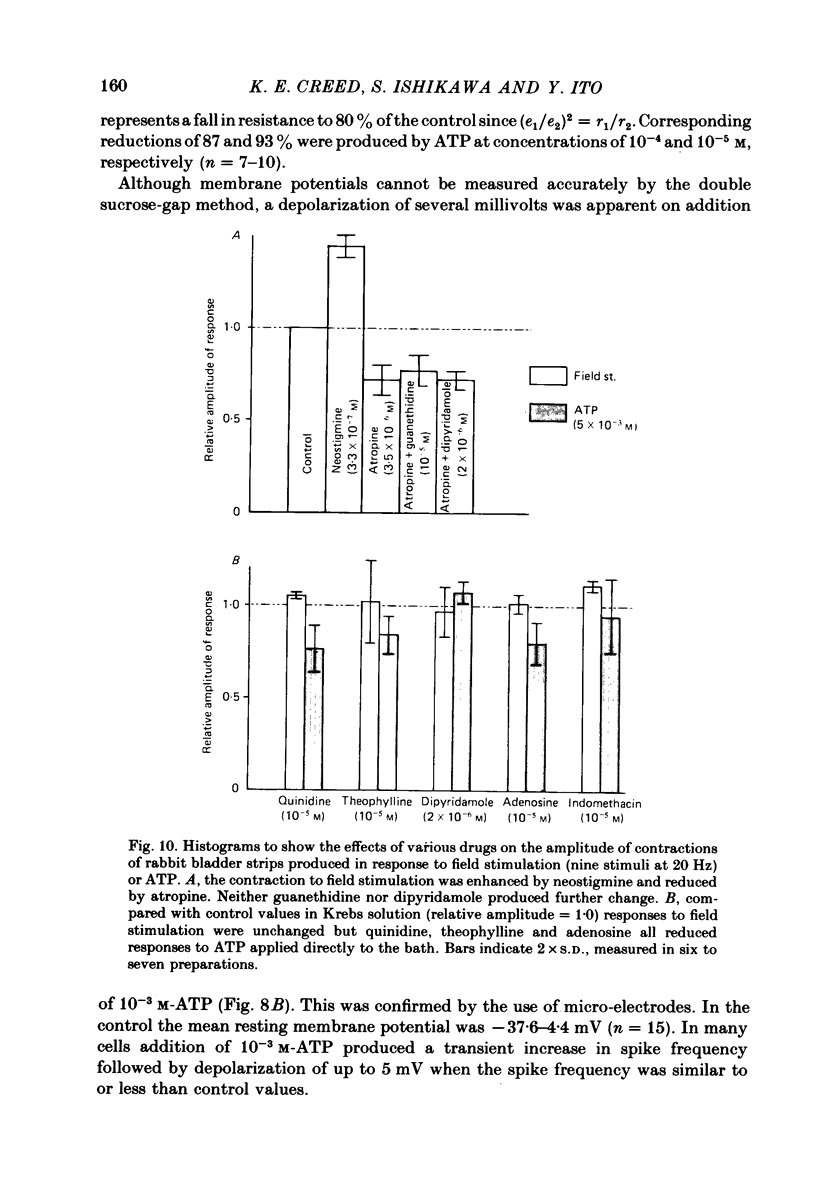
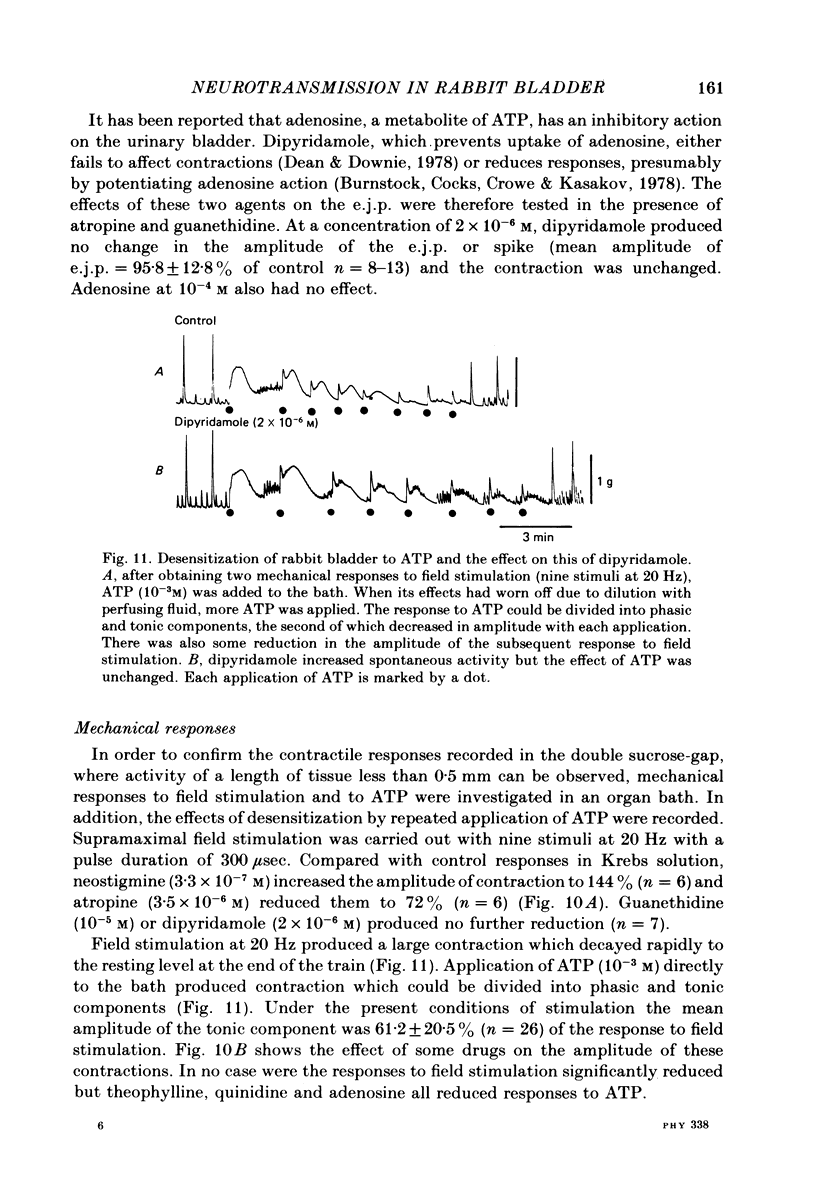
Responses of the smooth muscle membrane of the rabbit bladder to intramuscular nerve stimulation were investigated by the micro-electrode and double sucrose-gap methods. The cell generated regular spontaneous action potentials. Acetylcholine produced a maintained increase in the frequency and ATP a transient increase. Noradrenaline only increased the frequency at very high concentrations. Application of short current pulses (50 microseconds) produced an initial excitatory junction potential (e.j.p.) with a superimposed spike, followed by a late depolarization. On some occasions, hyperpolarization of the membrane appeared between initial e.j.p. and the late depolarization. All these responses were abolished by tetrodotoxin. The late depolarization was enhanced by pre-treatment with neostigmine and abolished by atropine. This means that the delayed depolarization is due to activation of the muscarinic receptor. When the late depolarization was abolished, the amplitude of hyperpolarization was enhanced. The e.j.p. and contraction were unaffected by guanethidine, phentolamine, methysergide, mepyramine, quinidine or theophylline. This means that the e.j.p. is not mediated by activation of adrenergic, tryptaminergic, histaminergic or purinergic receptors. ATP reduced the amplitude of the e.j.p. due to depolarization of the membrane and reduction in the membrane resistance. The amplitude of the e.j.p. was gradually reduced by repetitive stimulation (0.5-2.0 Hz). However, the rate of depression was unchanged in the presence of ATP. Dipyridamole did not change the electrical and mechanical responses to field stimulation. These results do not support the proposal that ATP is the non-cholinergic excitatory transmitter. Apamine and tetraethylammonium (TEA) suppressed the hyperpolarization produced by field stimulation but guanethidine did not inhibit the hyperpolarization. Therefore, the hyperpolarization is due to increased K conductance of the membrane but it is not possible to conclude whether this component is due to the inhibitory action of a neurotransmitter or solely to after hyperpolarization of the spike. It was concluded that the rabbit bladder receives both cholinergic and noncholinergic excitatory neurones.
Full text
PDF


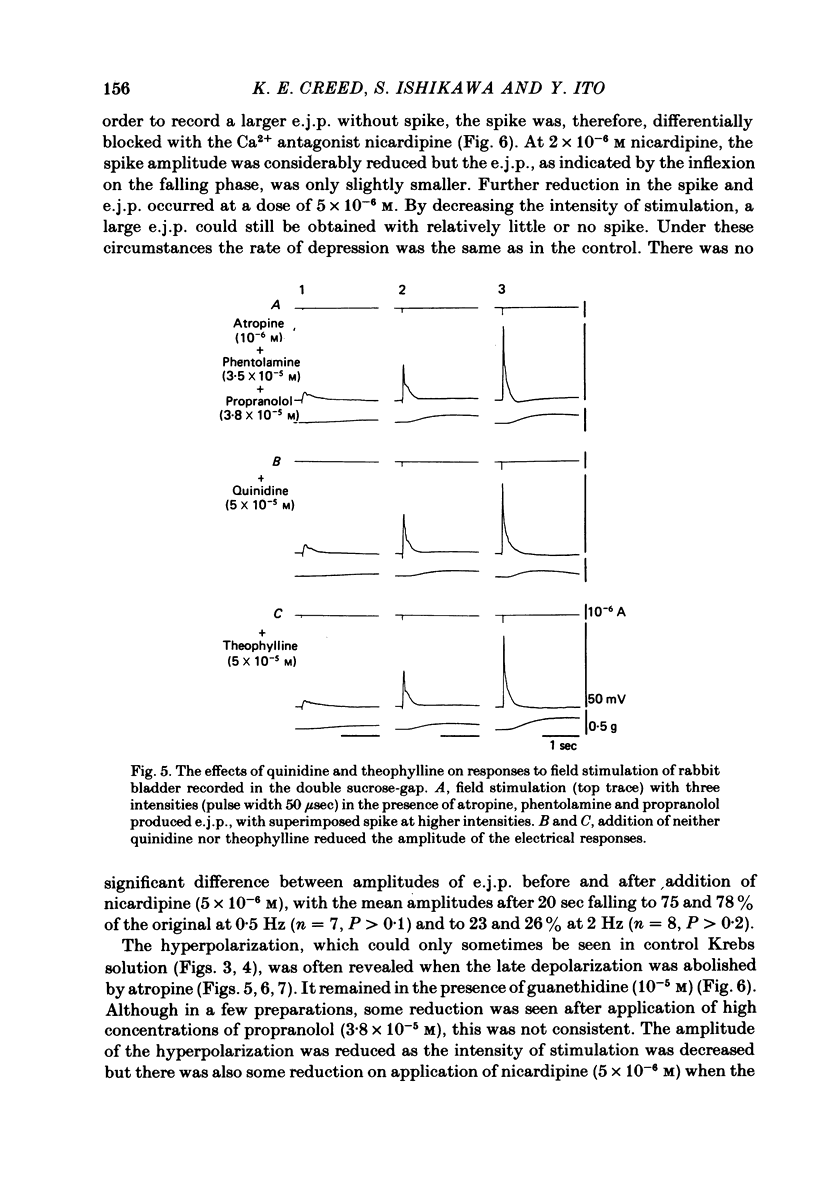
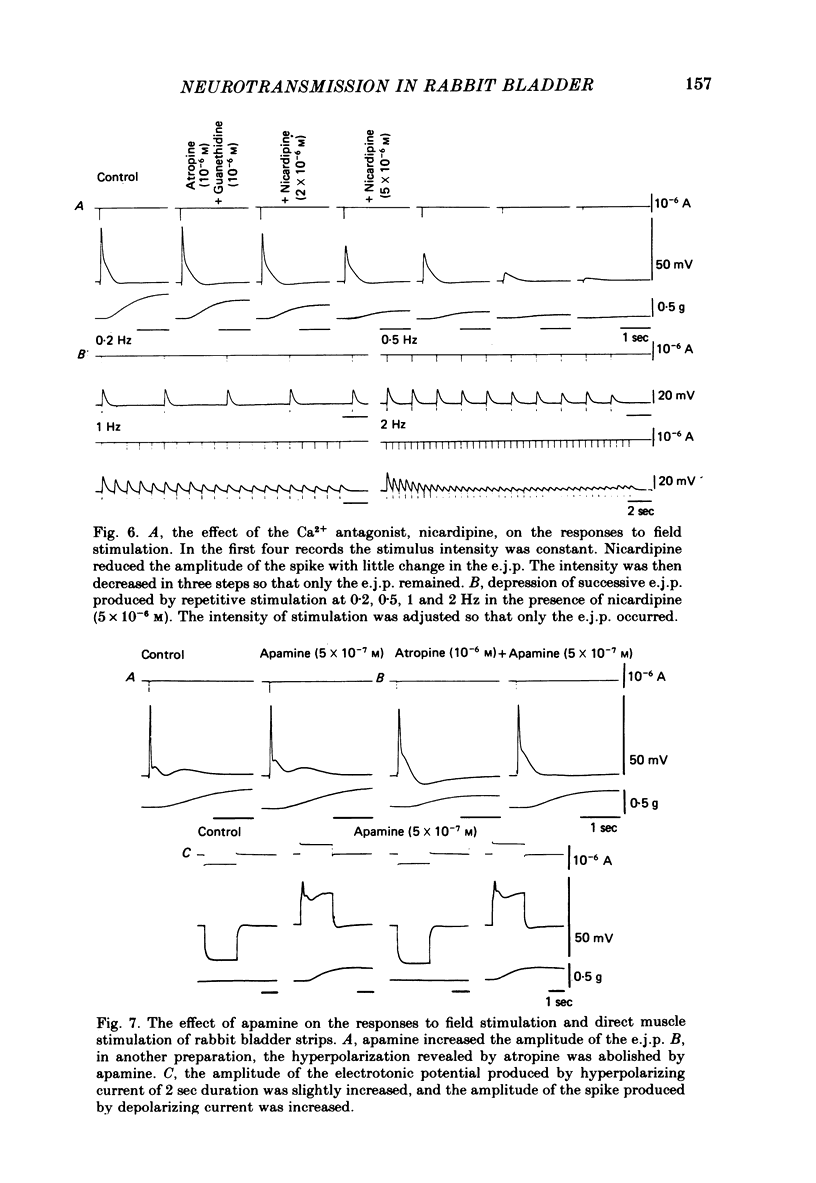
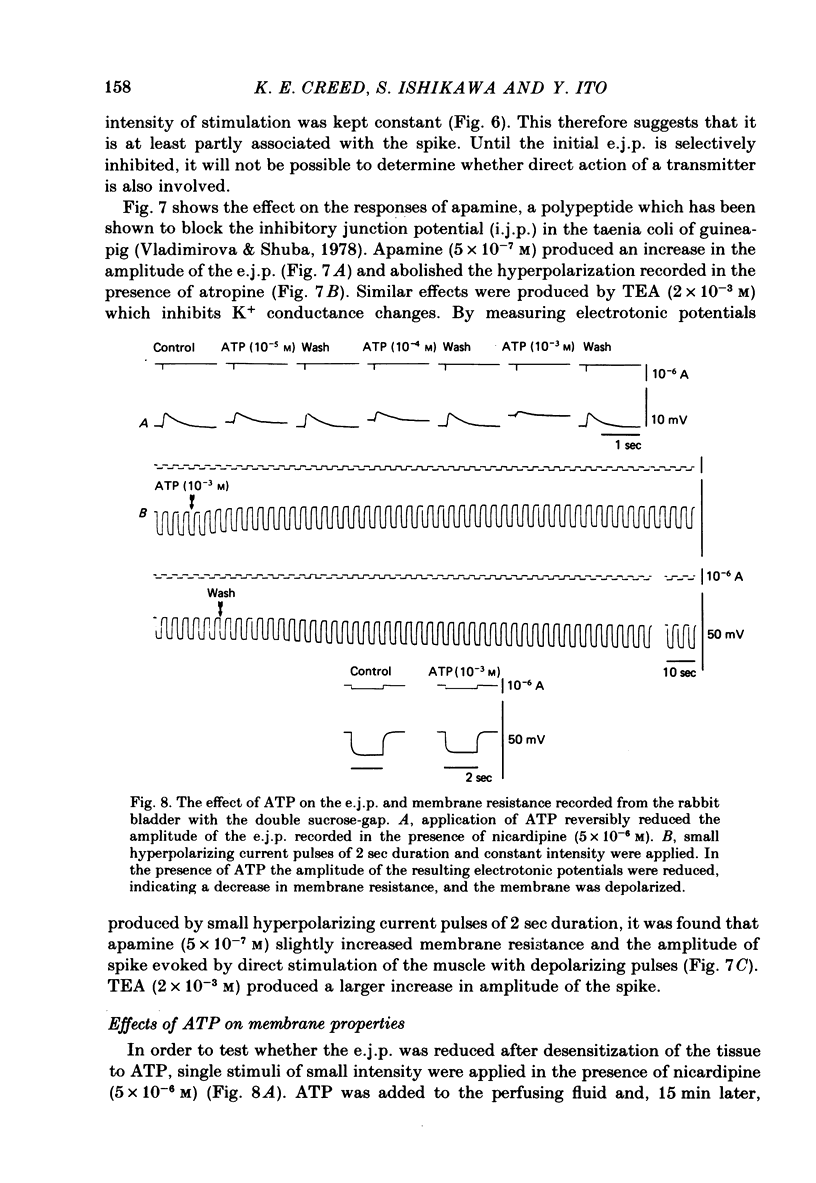
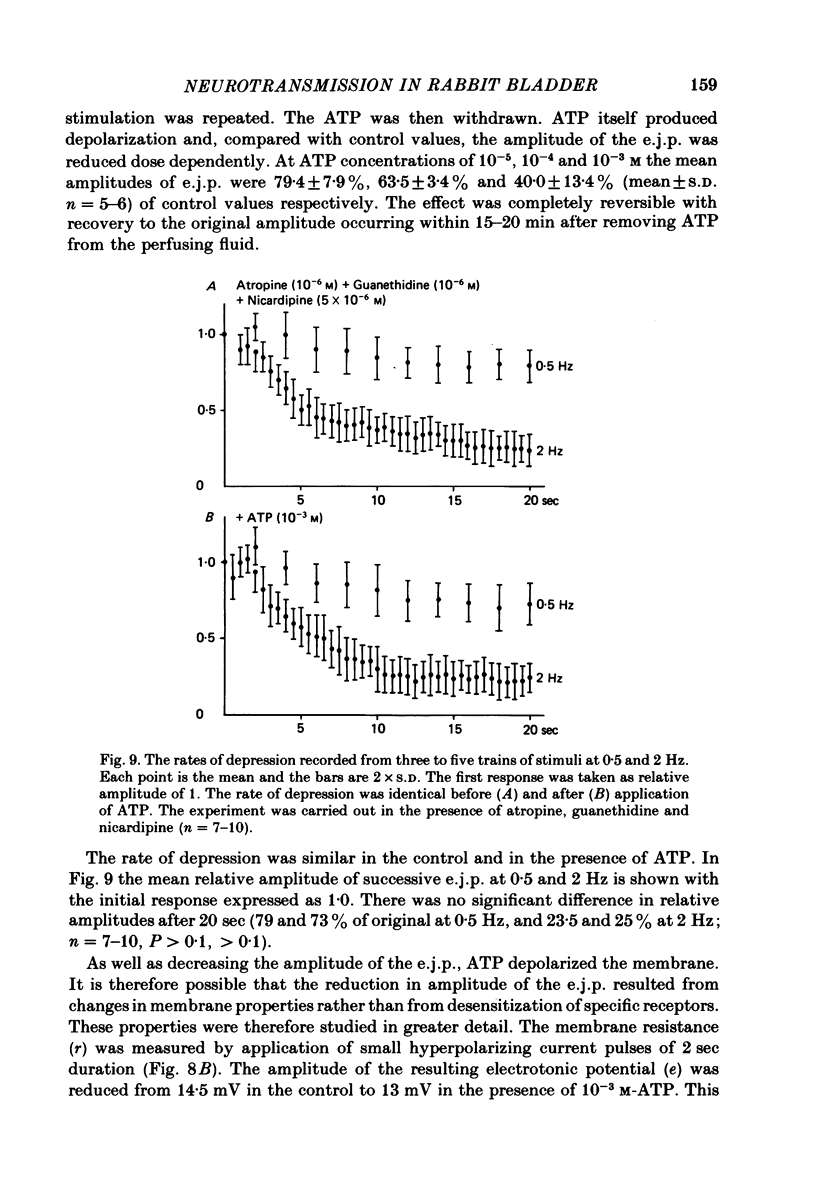



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AMBACHE N. The use and limitations of atropine for pharmacological studies on autonomic effectors. Pharmacol Rev. 1955 Dec;7(4):467–494. [PubMed] [Google Scholar]
- Abe Y., Tomita T. Cable properties of smooth muscle. J Physiol. 1968 May;196(1):87–100. doi: 10.1113/jphysiol.1968.sp008496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambache N., Zar M. A. Non-cholinergic transmission by post-ganglionic motor neurones in the mammalian bladder. J Physiol. 1970 Oct;210(3):761–783. doi: 10.1113/jphysiol.1970.sp009240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer V., Kuriyama H. Evidence for non-cholinergic, non-adrenergic transmission in the guinea-pig ileum. J Physiol. 1982 Sep;330:95–110. doi: 10.1113/jphysiol.1982.sp014331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnstock G., Cocks T., Crowe R., Kasakov L. Purinergic innervation of the guinea-pig urinary bladder. Br J Pharmacol. 1978 May;63(1):125–138. doi: 10.1111/j.1476-5381.1978.tb07782.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnstock G., Dumsday B., Smythe A. Atropine resistant excitation of the urinary bladder: the possibility of transmission via nerves releasing a purine nucleotide. Br J Pharmacol. 1972 Mar;44(3):451–461. doi: 10.1111/j.1476-5381.1972.tb07283.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bywater R. A., Holman M. E., Taylor G. S. Atropine-resistant depolarization in the guinea-pig small intestine. J Physiol. 1981 Jul;316:369–378. doi: 10.1113/jphysiol.1981.sp013794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CLARK B. B., URSILLO R. C. The action of atropine on the urinary bladder of the dog and on the isolated nerve-bladder strip preparation of the rabbit. J Pharmacol Exp Ther. 1956 Nov;118(3):338–347. [PubMed] [Google Scholar]
- Creed K. E. The role of the hypogastric nerve in bladder and urethral activity of the dog. Br J Pharmacol. 1979 Mar;65(3):367–375. doi: 10.1111/j.1476-5381.1979.tb07840.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creed K. E., Tulloch A. G. The effect of pelvic nerve stimulation and some drugs on the urethra and bladder of the dog. Br J Urol. 1978 Oct;50(6):398–405. doi: 10.1111/j.1464-410x.1978.tb04218.x. [DOI] [PubMed] [Google Scholar]
- Dave K. C., Dhattiwala A. S. Adrenoreceptors of the guinea-pig urinary bladder. Br J Pharmacol. 1976 Sep;58(1):37–41. doi: 10.1111/j.1476-5381.1976.tb07690.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean D. M., Downie J. W. Contribution of adrenergic and "purinergic" neurotransmission to contraction in rabbit detrusor. J Pharmacol Exp Ther. 1978 Nov;207(2):431–445. [PubMed] [Google Scholar]
- GILLESPIE J. S. The electrical and mechanical responses of intestinal smooth muscle cells to stimulation of their extrinsic parasympathetic nerves. J Physiol. 1962 Jun;162:76–92. doi: 10.1113/jphysiol.1962.sp006915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito Y., Kuriyama H. The properties of the rectal smooth muscle membrane of the guinea-pig in relation to the nervous influences. Jpn J Physiol. 1971 Jun;21(3):277–294. doi: 10.2170/jjphysiol.21.277. [DOI] [PubMed] [Google Scholar]
- Ito Y., Tajima K. Actions of indomethacin and prostaglandins on neuro-effector transmission in the dog trachea. J Physiol. 1981;319:379–392. doi: 10.1113/jphysiol.1981.sp013915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito Y., Takeda K. Non-adrenergic inhibitory nerves and putative transmitters in the smooth muscle of cat trachea. J Physiol. 1982 Sep;330:497–511. doi: 10.1113/jphysiol.1982.sp014355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langley J. N., Anderson H. K. The Innervation of the Pelvic and adjoining Viscera: Part II. The Bladder. Part III. The External Generative Organs. Part IV. The Internal Generative Organs. Part V. Position of the Nerve Cells on the Course of the Efferent Nerve Fibres. J Physiol. 1895 Dec 30;19(1-2):71–139. doi: 10.1113/jphysiol.1895.sp000587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purves R. D. Muscarinic excitation: a microelectrophoretic study on cultured smooth muscle cells. Br J Pharmacol. 1974 Sep;52(1):77–86. doi: 10.1111/j.1476-5381.1974.tb09689.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shuba M. F., Vladimirova I. A. Effect of apamin on the electrical responses of smooth muscle to adenosine 5'-triphosphate and to non-adrenergic, non-cholinergic nerve stimulation. Neuroscience. 1980;5(5):853–859. doi: 10.1016/0306-4522(80)90154-2. [DOI] [PubMed] [Google Scholar]
- Taira N. The autonomic pharmacology of the bladder. Annu Rev Pharmacol. 1972;12:197–208. doi: 10.1146/annurev.pa.12.040172.001213. [DOI] [PubMed] [Google Scholar]
- URSILLO R. C. Electrical activity of the isolated nerve-urinary bladder strip preparation of the rabbit. Am J Physiol. 1961 Sep;201:408–412. doi: 10.1152/ajplegacy.1961.201.3.408. [DOI] [PubMed] [Google Scholar]
- Vladimirova I. A., Shuba M. F. Vliianie strikhnina, gidrastina i apamina na sinapticheskuiu peredachu v gladkomyshechnykh kletkakh. Neirofiziologiia. 1978;10(3):295–299. [PubMed] [Google Scholar]


