Abstract
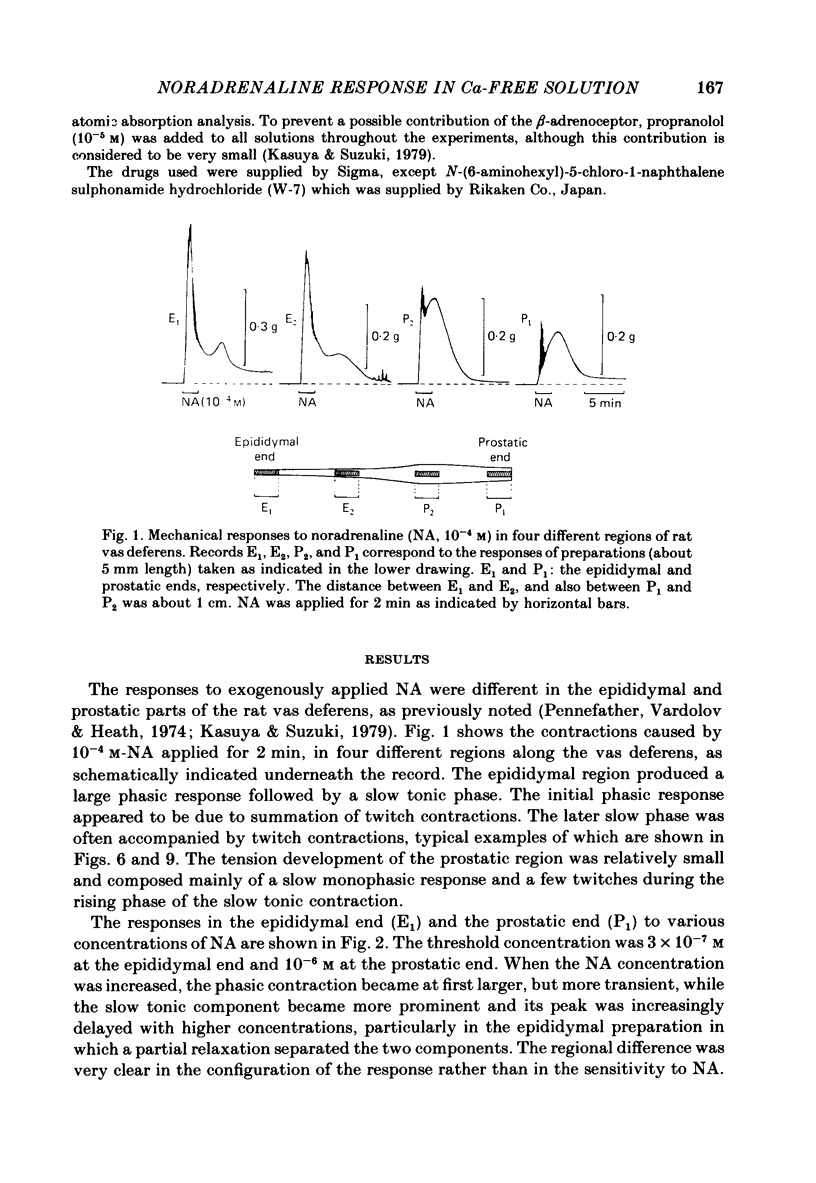
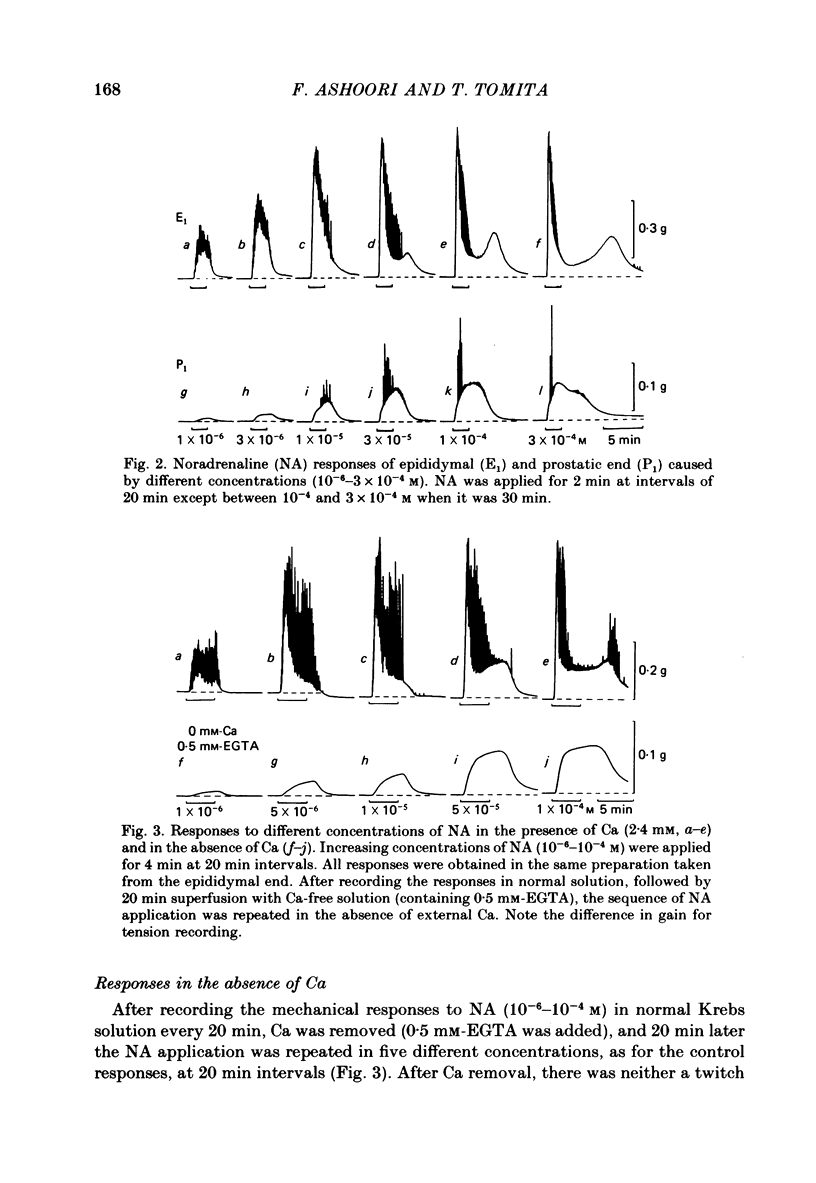
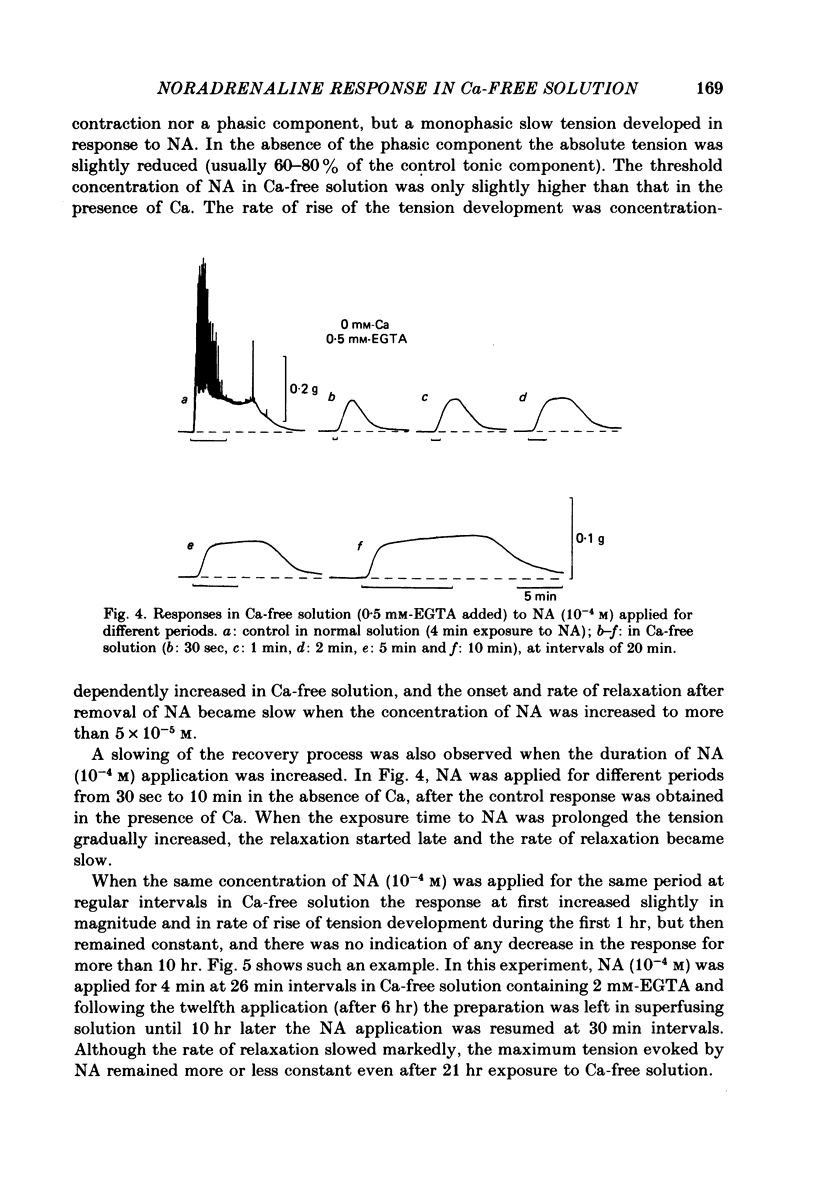
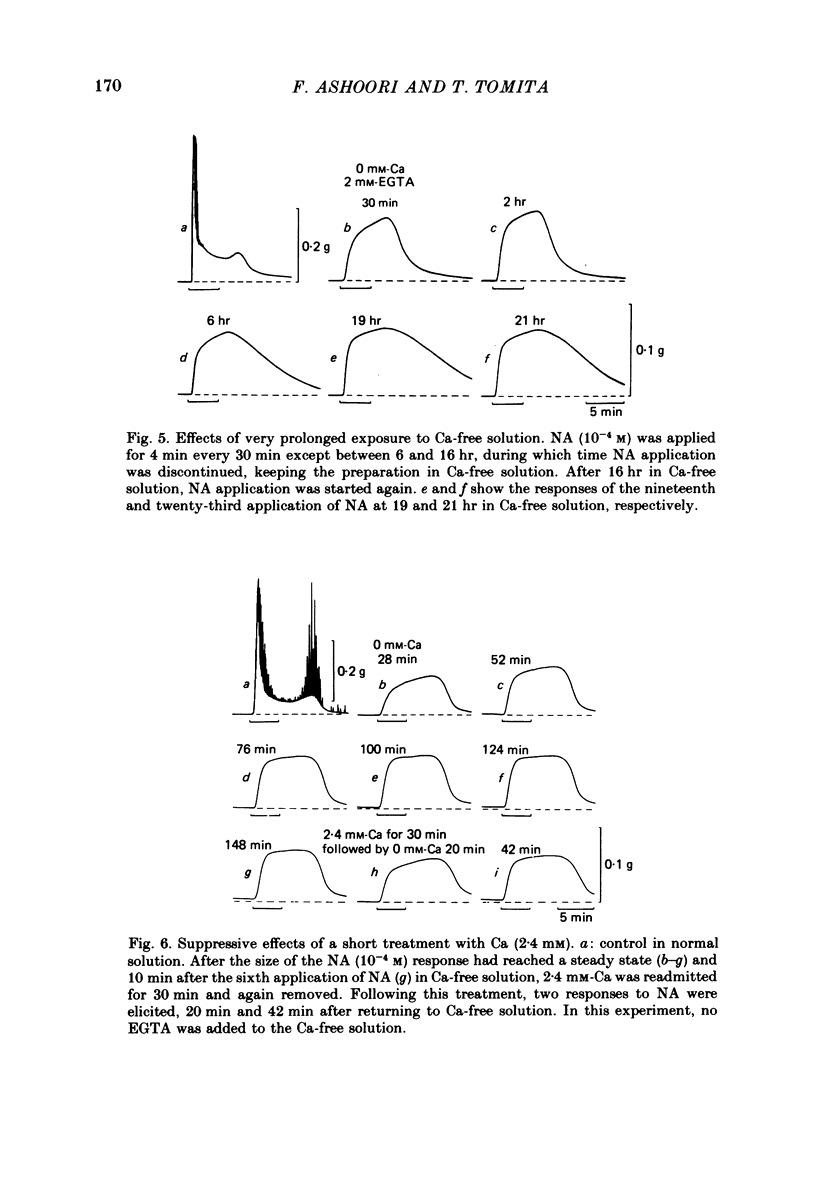
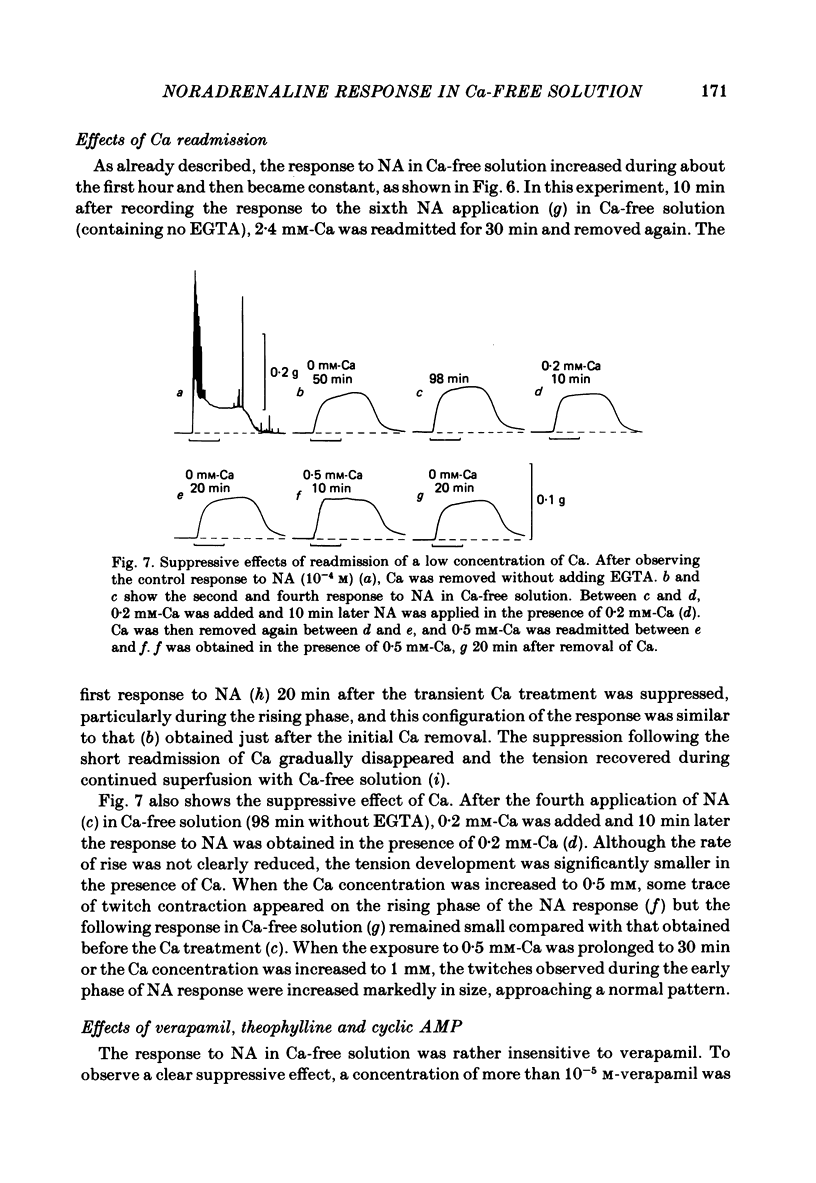
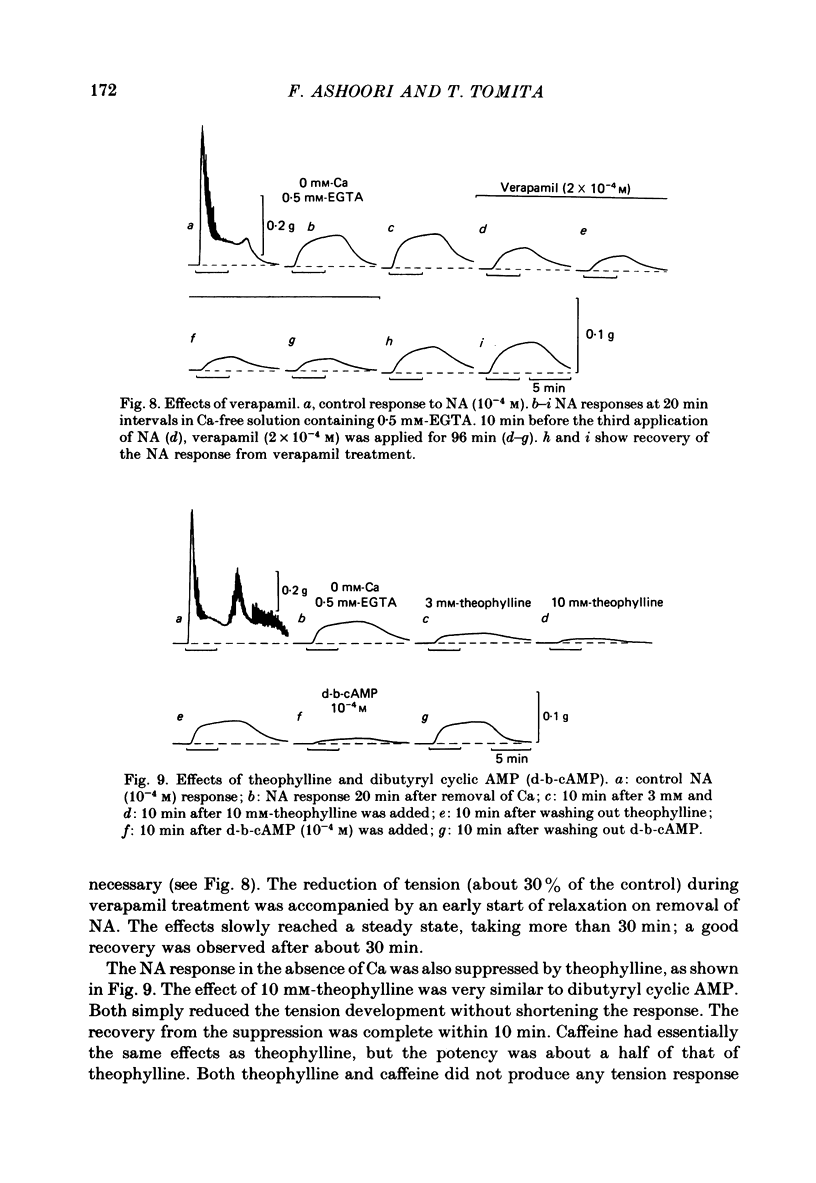
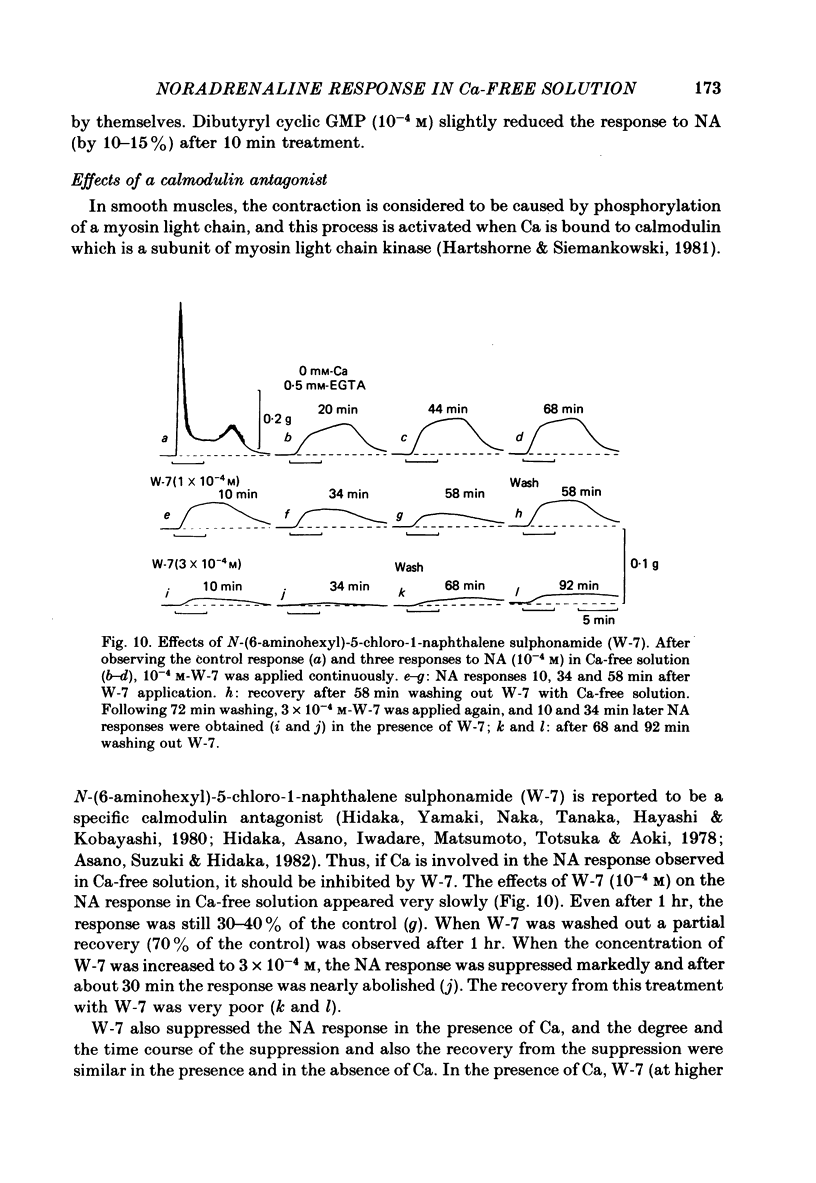
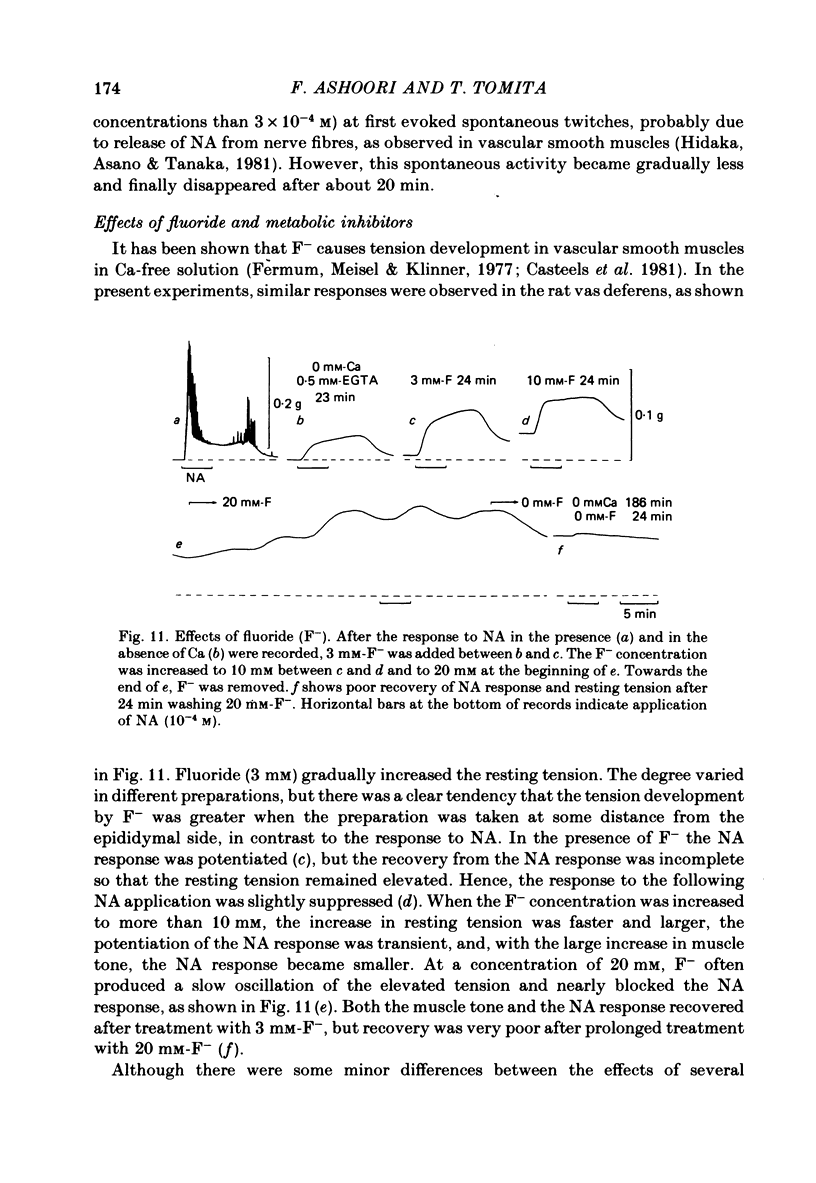
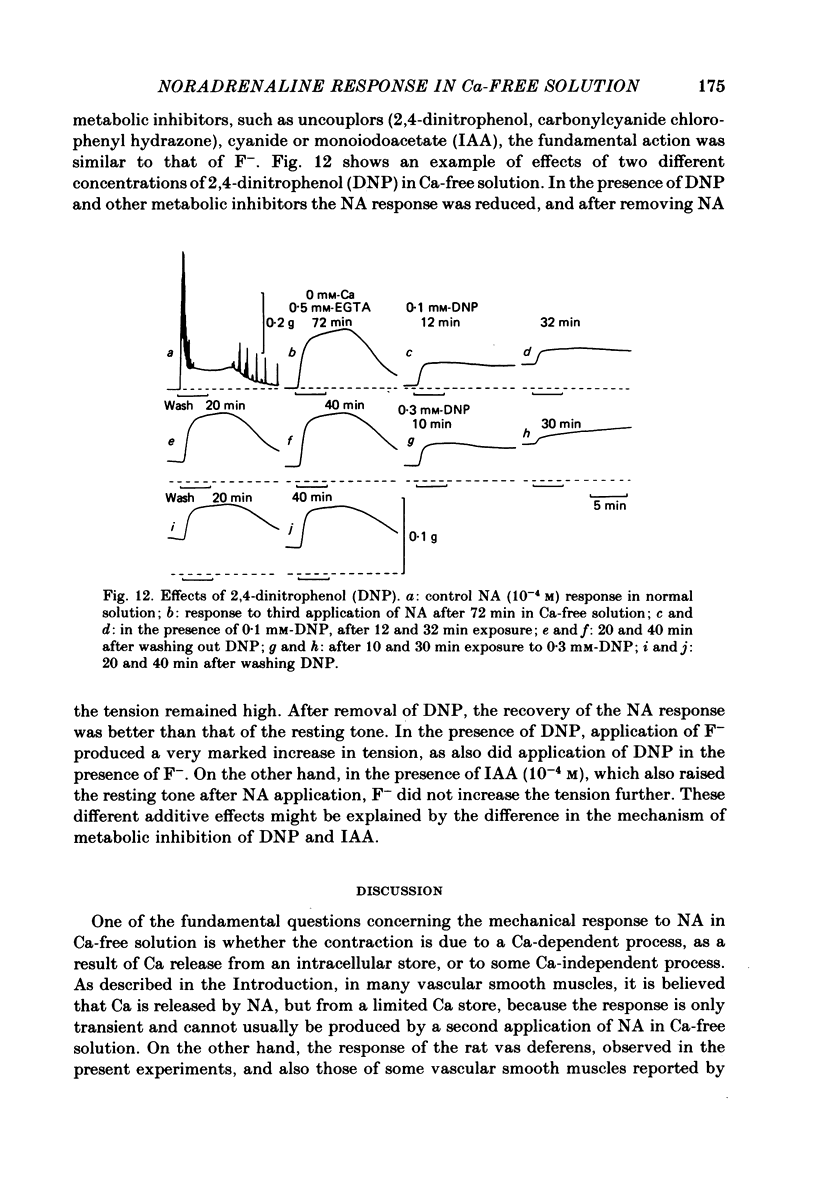
Mechanical responses to noradrenaline (NA) were investigated in the rat vas deferens exposed to Ca-free solution containing 0.5 mM-EGTA. A tonic response was produced in Ca-free solution at the epididymal portion, while almost no response could be observed at the prostatic portion. In most experiments NA (10(-4) M) was applied for 4 min, every 20 min. The absolute tension development in Ca-free solution was usually 60-80% of the control tonic response in the presence of 2.4 mM-Ca. The response could be produced repeatedly, even after exposure to Ca-free solution for more than 20 hr, without a significant decrease. During the first hour of exposure to Ca-free solution, the rate of rise and the magnitude of the NA contraction increased and then remained constant, though the relaxation became slow. Transient treatment with 2.4 mM-Ca slightly suppressed the subsequent NA response in Ca-free solution. Similarly, the NA response was smaller during readmission of 0.2-0.5 mM-Ca than that obtained before Ca readmission. A high concentration of verapamil (2 X 10(-4) M) reversibly reduced the NA response by about 70% after 30 min. Theophylline (10 mM) and dibutyryl cyclic AMP (10(-4) M) also reversibly suppressed the NA response, the suppression being about 80%. None of these substances produced a tension change by themselves. The suppressing effect may be mediated via an increase of intracellular cyclic AMP which reduces phosphorylation of myosin. Caffeine (10 mM) and dibutyryl cyclic GMP (10(-4) M) had similar but much weaker effects than theophylline and dibutyryl cyclic AMP. A calmodulin antagonist, N-(6-aminohexyl)-5-chloro-1-naphthalene sulphonamide (W-7) slowly reduced the NA response. The block was nearly complete after 30 min treatment with 3 X 10(-4) M-W-7, and the recovery was very poor after prolonged exposure. This effect of W-7, which is the same in the presence and absence of Ca, suggests that a Ca-calmodulin reaction is involved in the NA response in Ca-free solution. Fluoride at a concentration higher than 3 mM increased the muscle tone in the absence of external Ca, and transiently potentiated the NA response. In the presence of F-, the relaxation of the NA response was incomplete and the muscle tone increased stepwise after each NA application. When the muscle tone became higher than the NA response in the absence of F-, the NA response was abolished. The action of several metabolic inhibitors (2,4-dinitrophenol, carbonylcyanide chlorophenyl hydrazone, NaCN, monoiodoacetate) was similar to that of F-, suggesting that they release Ca from mitochondria, causing tension development. The observations are consistent with the hypothesis that the contraction of the vas deferens caused by NA in the absence of external Ca depends on the availability of intracellular Ca, stored in mitochondria and released by NA.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Asano M., Suzuki Y., Hidaka H. Effects of various calmodulin antagonists on contraction of rabbit aortic strips. J Pharmacol Exp Ther. 1982 Jan;220(1):191–196. [PubMed] [Google Scholar]
- Babcock D. F., Chen J. L., Yip B. P., Lardy H. A. Evidence for mitochondrial localization of the hormone-responsive pool of Ca2+ in isolated hepatocytes. J Biol Chem. 1979 Sep 10;254(17):8117–8120. [PubMed] [Google Scholar]
- Batra S. The role of mitochondrial calcium uptake in contraction and relaxation of the human myometrium. Biochim Biophys Acta. 1973 May 30;305(2):428–432. doi: 10.1016/0005-2728(73)90188-6. [DOI] [PubMed] [Google Scholar]
- Blackmore P. F., El-Refai M. F., Exton J. H. alpha-Adrenergic blockade and inhibition of A23187 mediated Ca2+ uptake by the calcium antagonist verapamil in rat liver cells. Mol Pharmacol. 1979 May;15(3):598–606. [PubMed] [Google Scholar]
- Blackmore P. F., Hughes B. P., Shuman E. A., Exton J. H. alpha-Adrenergic activation of phosphorylase in liver cells involves mobilization of intracellular calcium without influx of extracellular calcium. J Biol Chem. 1982 Jan 10;257(1):190–197. [PubMed] [Google Scholar]
- Carafoli E. The interaction of Ca2+ with mitochondria, with special reference to the structural role of Ca2+ in mitochondrial and other membranes. Mol Cell Biochem. 1975 Sep 30;8(3):133–140. doi: 10.1007/BF01792764. [DOI] [PubMed] [Google Scholar]
- Casteels R., Raeymaekers L., Suzuki H., Van Eldere J. Tension response and 45Ca release in vascular smooth muscle incubated in Ca-free solution. Pflugers Arch. 1981 Dec;392(2):139–145. doi: 10.1007/BF00581262. [DOI] [PubMed] [Google Scholar]
- Deth R., van Breemen C. Agonist induced release of intracellular Ca2+ in the rabbit aorta. J Membr Biol. 1977 Jan 28;30(4):363–380. doi: 10.1007/BF01869677. [DOI] [PubMed] [Google Scholar]
- Deth R., van Breemen C. Relative contributions of Ca2+ influx and cellular Ca2+ release during drug induced activation of the rabbit aorta. Pflugers Arch. 1974 Apr 4;348(1):13–22. doi: 10.1007/BF00587735. [DOI] [PubMed] [Google Scholar]
- Droogmans G., Raeymaekers L., Casteels R. Electro- and pharmacomechanical coupling in the smooth muscle cells of the rabbit ear artery. J Gen Physiol. 1977 Aug;70(2):129–148. doi: 10.1085/jgp.70.2.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein P. M., Fiss K., Hachisu R., Andrenyak D. M. Interaction of calcium antagonists with cyclic AMP phosphodiesterases and calmodulin. Biochem Biophys Res Commun. 1982 Apr 14;105(3):1142–1149. doi: 10.1016/0006-291x(82)91089-0. [DOI] [PubMed] [Google Scholar]
- Fermum R., Meisel P., Klinner U. Versuche zum Wirkungsmechanismus von Gefässspasmolytika. 3. Wirkung von Nitroprussid-Natrium, Nitroglyzerin, Prenylamin und Verapamil an der Fluorid-induzierten Kontraktur isolierter Koronararterien. Acta Biol Med Ger. 1977;36(2):245–255. [PubMed] [Google Scholar]
- Hartshorne D. J., Siemankowski R. F. Regulation of smooth muscle actomyosin. Annu Rev Physiol. 1981;43:519–530. doi: 10.1146/annurev.ph.43.030181.002511. [DOI] [PubMed] [Google Scholar]
- Heaslip R. J., Rahwan R. G. Evidence for the existence of two distinct pools of intracellular calcium in the rat aorta accessible to mobilization by norepinephrine. J Pharmacol Exp Ther. 1982 Apr;221(1):7–13. [PubMed] [Google Scholar]
- Hidaka H., Asano M., Iwadare S., Matsumoto I., Totsuka T., Aoki N. A novel vascular relaxing agent, N-(6--aminohexyl)-5-chloro-1-naphthalensulfonamide which affects vascular smooth muscle actomyosin. J Pharmacol Exp Ther. 1978 Oct;207(1):8–15. [PubMed] [Google Scholar]
- Hidaka H., Asano M., Tanaka T. Activity-structure relationship of calmodulin antagonists, Naphthalenesulfonamide derivatives. Mol Pharmacol. 1981 Nov;20(3):571–578. [PubMed] [Google Scholar]
- Hidaka H., Yamaki T., Naka M., Tanaka T., Hayashi H., Kobayashi R. Calcium-regulated modulator protein interacting agents inhibit smooth muscle calcium-stimulated protein kinase and ATPase. Mol Pharmacol. 1980 Jan;17(1):66–72. [PubMed] [Google Scholar]
- Kasuya Y., Suzuki N. Variation of postjunctional natures along the length of the rat vas deferens as a cause of regional difference in the sensitivity to norepinephrine. Arch Int Pharmacodyn Ther. 1979 Sep;241(1):24–31. [PubMed] [Google Scholar]
- Malmström K., Carafoli E. The interaction of Ga2+ with mitochondria from human myometrium. Arch Biochem Biophys. 1977 Aug;182(2):657–666. doi: 10.1016/0003-9861(77)90546-x. [DOI] [PubMed] [Google Scholar]
- McGuffee L. J., Hurwitz L., Little S. A., Skipper B. E. A 45Ca autoradiographic and stereological study of freeze-dried smooth muscle of the guinea pig vas deferens. J Cell Biol. 1981 Jul;90(1):201–210. doi: 10.1083/jcb.90.1.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy E., Coll K., Rich T. L., Williamson J. R. Hormonal effects on calcium homeostasis in isolated hepatocytes. J Biol Chem. 1980 Jul 25;255(14):6600–6608. [PubMed] [Google Scholar]
- SLATER E. C., BORNER W. D., Jr The effect of fluoride on the succinic oxidase system. Biochem J. 1952 Oct;52(2):185–196. doi: 10.1042/bj0520185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saad K. H., Huddart H. Influence of noradrenaline and KCl on calcium translocation in rat vas deferens smooth muscle and its subcellular fractions. Gen Pharmacol. 1981;12(5):373–380. doi: 10.1016/0306-3623(81)90094-x. [DOI] [PubMed] [Google Scholar]
- Silver P. J., DiSalvo J. Adenosine 3':5'-monophosphate-mediated inhibition of myosin light chain phosphorylation in bovine aortic actomyosin. J Biol Chem. 1979 Oct 25;254(20):9951–9954. [PubMed] [Google Scholar]
- Steinsland O. S., Furchgott R. F., Kirpekar S. M. Biphasic vasoconstriction of the rabbit ear artery. Circ Res. 1973 Jan;32(1):49–58. doi: 10.1161/01.res.32.1.49. [DOI] [PubMed] [Google Scholar]
- Van Breemen C., Farinas B. R., Gerba P., McNaughton E. D. Excitation-contraction coupling in rabbit aorta studied by the lanthanum method for measuring cellular calcium influx. Circ Res. 1972 Jan;30(1):44–54. doi: 10.1161/01.res.30.1.44. [DOI] [PubMed] [Google Scholar]
- Whiting J. A., Barritt G. J. On the mechanism by which hormones induce the release of Ca2+ from mitochondria in the liver cell. Biochem J. 1982 Jul 15;206(1):121–129. doi: 10.1042/bj2060121. [DOI] [PMC free article] [PubMed] [Google Scholar]


