Abstract
Evidence is presented for the existence of a localization of monosynaptic Ia excitatory post-synaptic potentials (e.p.s.p.s) in the motor nucleus of a cat hind limb muscle. Intracellular recordings from biceps femoris motoneurones were made in anaesthetized low spinal cats of the effects of stimuli to the nerve branches supplying the anterior, middle, and posterior portions of the biceps femoris muscle. Recordings were also made during stimulation of nerves to semimembranosus and semitendinosus in order to provide a means of categorizing middle biceps cells as 'extensors' (middle biceps-extensor; i.e. like anterior biceps cells) or as 'flexors' (middle biceps-flexor; like posterior biceps). Homonymous nerve-branch (i.e. from anterior, middle or posterior biceps) monosynaptic Ia e.p.s.p.s were compared within unifunctional (flexor or extensor) groups of motoneurones. In three of four comparisons (anterior biceps nerve branch onto anterior and middle biceps-extensor cells, middle biceps onto middle biceps-flexor and posterior biceps, posterior biceps onto middle biceps-flexor and posterior biceps) the anterior, middle and posterior biceps nerve branches contributed larger e.p.s.p.s to their 'own' motoneurones than to motoneurones supplying other 'compartments' of the muscle. In the fourth case, middle biceps's input appeared to have similar effects onto anterior biceps and middle biceps-extensor cells. A normalization was performed to eliminate the possibility that the differences in e.p.s.p. sizes were due to differences in cell type within the four cell groupings (i.e. differences in the number of cells supplying FF, F(int.), FR and S muscle units). This normalization confirmed that the localization in the first three comparisons was not a consequence of differences in motoneurone type and, in addition, suggested that middle biceps may indeed have greater effects on middle biceps-extensor than anterior biceps cells. In addition to the asymmetrical effects of anterior and middle biceps nerve branches onto anterior biceps and middle biceps-extensor motoneurones, it was shown that while semitendinosus and posterior biceps contributed larger e.p.s.p.s to middle biceps-flexor than to middle biceps-extensor cells, the anterior biceps nerve branch and semimembranosus nerve contributed equally to the two middle biceps groups. Analysis of cell location in the spinal cord and rostro-caudal differences in group I volley sizes gave evidence of a topographic organization of the biceps femoris motor nucleus which could contribute to the observed localization. However, localization was also evident when comparing e.p.s.p. amplitudes in pairs of neighbouring cells of different category, indicating a role for neuronal recognition factors.
Full text
PDF






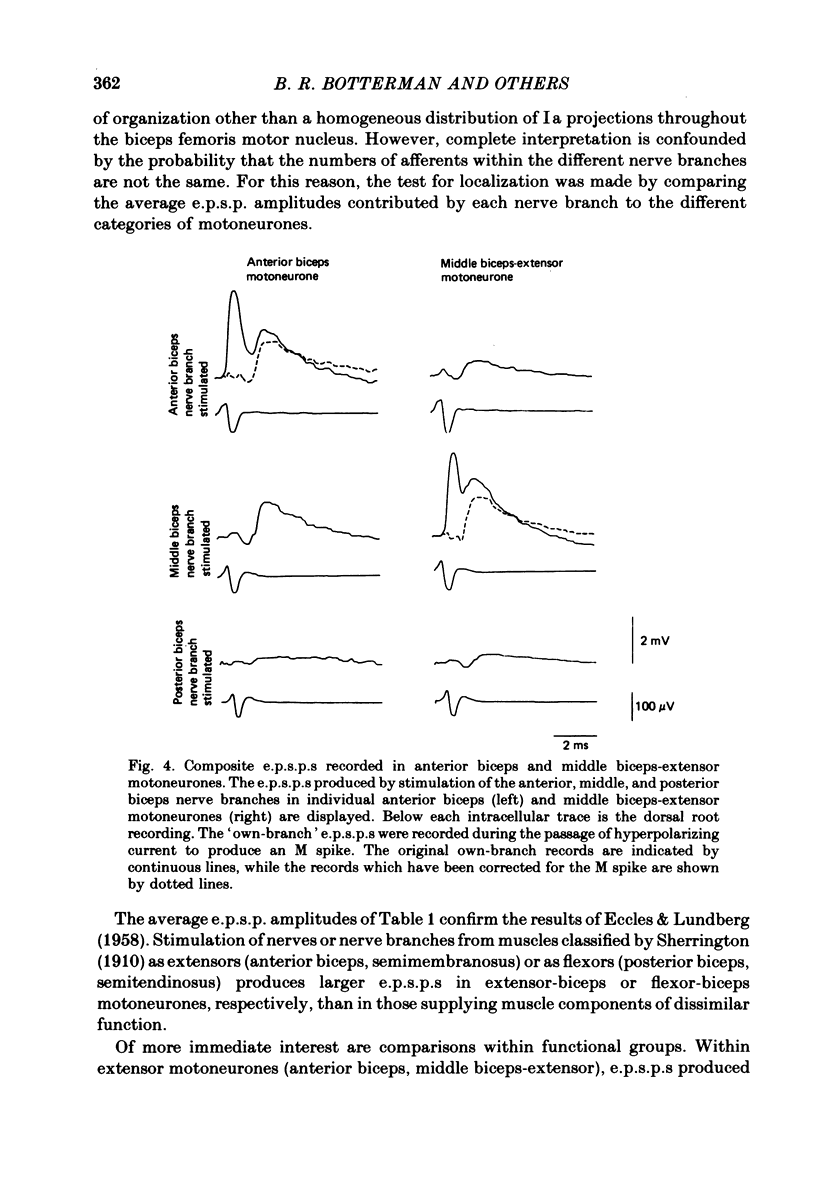



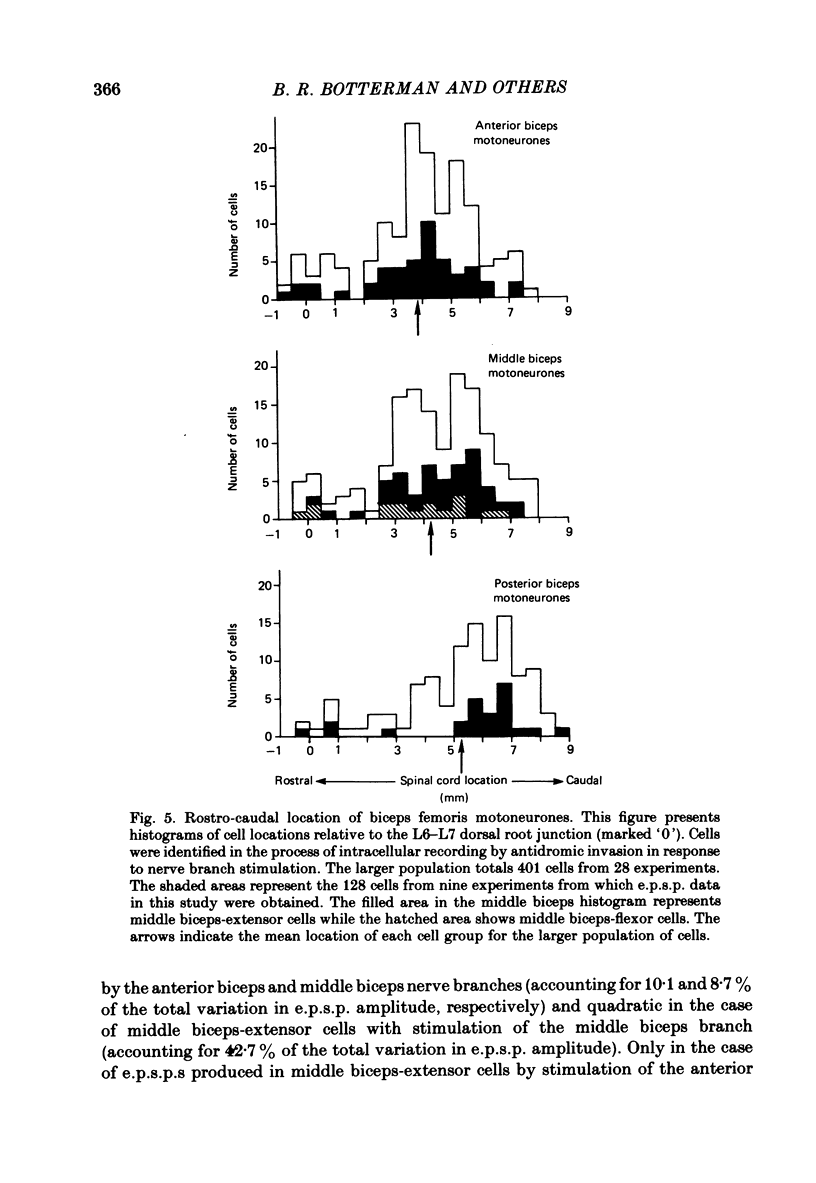











Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barrett J. N., Crill W. E. Specific membrane properties of cat motoneurones. J Physiol. 1974 Jun;239(2):301–324. doi: 10.1113/jphysiol.1974.sp010570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilotto G., Schor R. H., Uchino Y., Wilson V. J. Localization of proprioceptive reflexes in the splenius muscle of the cat. Brain Res. 1982 Apr 22;238(1):217–221. doi: 10.1016/0006-8993(82)90786-7. [DOI] [PubMed] [Google Scholar]
- Binder M. D., Kroin J. S., Moore G. P., Stauffer E. K., Stuart D. G. Correlation analysis of muscle spindle responses to single motor unit contractions. J Physiol. 1976 May;257(2):325–336. doi: 10.1113/jphysiol.1976.sp011371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder M. D. Topographic organization of monosynaptic reflexes in the cat spinal cord. Neurosci Lett. 1980 Nov;20(2):121–124. doi: 10.1016/0304-3940(80)90132-9. [DOI] [PubMed] [Google Scholar]
- Botterman B. R., Hamm T. M., Reinking R. M., Stuart D. G. Distribution of monosynaptic Ia excitatory post-synaptic potentials in the motor nucleus of the cat semitendinosus muscle. J Physiol. 1983 May;338:379–393. doi: 10.1113/jphysiol.1983.sp014678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botterman B. R., Hamm T. M., Reinking R. M., Stuart D. G. Intramuscular localization of monosynaptic Ia reflex effects in the cat biceps femoris muscle. Neurosci Lett. 1981 Jun 12;24(1):35–41. doi: 10.1016/0304-3940(81)90355-4. [DOI] [PubMed] [Google Scholar]
- Brink E. E., Jinnai K., Wilson V. J. Pattern of segmental monosynaptic input to cat dorsal neck motoneurons. J Neurophysiol. 1981 Sep;46(3):496–505. doi: 10.1152/jn.1981.46.3.496. [DOI] [PubMed] [Google Scholar]
- Brown K. T., Flaming D. G. Technique for precision beveling of relatively large micropipettes. J Neurosci Methods. 1979 Mar;1(1):25–34. doi: 10.1016/0165-0270(79)90004-9. [DOI] [PubMed] [Google Scholar]
- Burke R. E. Group Ia synaptic input to fast and slow twitch motor units of cat triceps surae. J Physiol. 1968 Jun;196(3):605–630. doi: 10.1113/jphysiol.1968.sp008526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke R. E., Levine D. N., Tsairis P., Zajac F. E., 3rd Physiological types and histochemical profiles in motor units of the cat gastrocnemius. J Physiol. 1973 Nov;234(3):723–748. doi: 10.1113/jphysiol.1973.sp010369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke R. E., Rymer W. Z. Relative strength of synaptic input from short-latency pathways to motor units of defined type in cat medial gastrocnemius. J Neurophysiol. 1976 May;39(3):447–458. doi: 10.1152/jn.1976.39.3.447. [DOI] [PubMed] [Google Scholar]
- COHEN L. A. Localization of stretch reflex. J Neurophysiol. 1953 May;16(3):272–285. doi: 10.1152/jn.1953.16.3.272. [DOI] [PubMed] [Google Scholar]
- COHEN L. A. Organization of stretch reflex into two types of direct spinal arcs. J Neurophysiol. 1954 Sep;17(5):443–453. doi: 10.1152/jn.1954.17.5.443. [DOI] [PubMed] [Google Scholar]
- Cameron W. E., Binder M. D., Botterman B. R., Reinking R. M., Stuart D. G. "Sensory partitioning" of cat medial gastrocnemius muscle by its muscle spindles and tendon organs. J Neurophysiol. 1981 Jul;46(1):32–47. doi: 10.1152/jn.1981.46.1.32. [DOI] [PubMed] [Google Scholar]
- Cameron W. E., Binder M. D., Botterman B. R., Reinking R. M., Stuart D. G. Motor unit--muscle spindle interactions in active muscles of decerebrate cats. Neurosci Lett. 1980 Aug;19(1):55–60. doi: 10.1016/0304-3940(80)90255-4. [DOI] [PubMed] [Google Scholar]
- Dum R. P., Burke R. E., O'Donovan M. J., Toop J., Hodgson J. A. Motor-unit organization in flexor digitorum longus muscle of the cat. J Neurophysiol. 1982 Jun;47(6):1108–1125. doi: 10.1152/jn.1982.47.6.1108. [DOI] [PubMed] [Google Scholar]
- ECCLES J. C., ECCLES R. M., LUNDBERG A. The convergence of monosynaptic excitatory afferents on to many different species of alpha motoneurones. J Physiol. 1957 Jun 18;137(1):22–50. doi: 10.1113/jphysiol.1957.sp005794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ECCLES J. C., ECCLES R. M., SHEALY C. N. An investigation into the effect of degenerating primary afferent fibers on the monosynpatic innervation of motoneurons. J Neurophysiol. 1962 Jul;25:544–558. doi: 10.1152/jn.1962.25.4.544. [DOI] [PubMed] [Google Scholar]
- ECCLES R. M., LUNDBERG A. Integrative pattern of Ia synaptic actions on motoneurones of hip and knee muscles. J Physiol. 1958 Dec 4;144(2):271–298. doi: 10.1113/jphysiol.1958.sp006101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engberg I., Lundberg A. An electromyographic analysis of muscular activity in the hindlimb of the cat during unrestrained locomotion. Acta Physiol Scand. 1969 Apr;75(4):614–630. doi: 10.1111/j.1748-1716.1969.tb04415.x. [DOI] [PubMed] [Google Scholar]
- English A. W. Interlimb coordination during stepping in the cat: an electromyographic analysis. J Neurophysiol. 1979 Jan;42(1 Pt 1):229–243. doi: 10.1152/jn.1979.42.1.229. [DOI] [PubMed] [Google Scholar]
- FRANK K., FUORTES M. G. Stimulation of spinal motoneurones with intracellular electrodes. J Physiol. 1956 Nov 28;134(2):451–470. doi: 10.1113/jphysiol.1956.sp005657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleshman J. W., Munson J. B., Sypert G. W., Friedman W. A. Rheobase, input resistance, and motor-unit type in medial gastrocnemius motoneurons in the cat. J Neurophysiol. 1981 Dec;46(6):1326–1338. doi: 10.1152/jn.1981.46.6.1326. [DOI] [PubMed] [Google Scholar]
- Fleshman J. W., Munson J. B., Sypert G. W. Homonymous projection of individual group Ia-fibers to physiologically characterized medial gastrocnemius motoneurons in the cat. J Neurophysiol. 1981 Dec;46(6):1339–1348. doi: 10.1152/jn.1981.46.6.1339. [DOI] [PubMed] [Google Scholar]
- Lederer W. J., Spindler A. J., Eisner D. A. Thick slurry bevelling: a new technique for bevelling extremely fine microelectrodes and micropipettes. Pflugers Arch. 1979 Sep;381(3):287–288. doi: 10.1007/BF00583261. [DOI] [PubMed] [Google Scholar]
- Lüscher H. R., Ruenzel P., Fetz E., Henneman E. Postsynatpic population potentials recorded from ventral roots perfused with isotonic sucrose: connections of groups Ia and II spindle afferent fibers with large populations of motoneurons. J Neurophysiol. 1979 Jul;42(4):1146–1164. doi: 10.1152/jn.1979.42.4.1146. [DOI] [PubMed] [Google Scholar]
- Lüscher H. R., Ruenzel P., Henneman E. Topographic distribution of terminals of Ia and group II fibers in spinal cord, as revealed by postsynaptic population potentials. J Neurophysiol. 1980 Apr;43(4):968–985. doi: 10.1152/jn.1980.43.4.968. [DOI] [PubMed] [Google Scholar]
- Mendell L. M., Henneman E. Terminals of single Ia fibers: location, density, and distribution within a pool of 300 homonymous motoneurons. J Neurophysiol. 1971 Jan;34(1):171–187. doi: 10.1152/jn.1971.34.1.171. [DOI] [PubMed] [Google Scholar]
- Nelson S. G., Mendell L. M. Projection of single knee flexor Ia fibers to homonymous and heteronymous motoneurons. J Neurophysiol. 1978 May;41(3):778–787. doi: 10.1152/jn.1978.41.3.778. [DOI] [PubMed] [Google Scholar]
- O'Donovan M. J., Pinter M. J., Dum R. P., Burke R. E. Actions of FDL and FHL muscles in intact cats: functional dissociation between anatomical synergists. J Neurophysiol. 1982 Jun;47(6):1126–1143. doi: 10.1152/jn.1982.47.6.1126. [DOI] [PubMed] [Google Scholar]
- Ogden T. E., Citron M. C., Pierantoni R. The jet stream microbeveler: an inexpensive way to bevel ultrafine glass micropipettes. Science. 1978 Aug 4;201(4354):469–470. doi: 10.1126/science.663670. [DOI] [PubMed] [Google Scholar]
- Perret C., Cabelguen J. M. Central and reflex participation in the timing of locomotor activations of a bifunctional muscle, the semi-tendinosus, in the cat. Brain Res. 1976 Apr 23;106(2):390–395. doi: 10.1016/0006-8993(76)91035-0. [DOI] [PubMed] [Google Scholar]
- Perret C., Cabelguen J. M. Main characteristics of the hindlimb locomotor cycle in the decorticate cat with special reference to bifunctional muscles. Brain Res. 1980 Apr 14;187(2):333–352. doi: 10.1016/0006-8993(80)90207-3. [DOI] [PubMed] [Google Scholar]
- ROMANES G. J. The motor cell columns of the lumbo-sacral spinal cord of the cat. J Comp Neurol. 1951 Apr;94(2):313–363. doi: 10.1002/cne.900940209. [DOI] [PubMed] [Google Scholar]
- Rasmussen S., Chan A. K., Goslow G. E., Jr The cat step cycle: electromyographic patterns for hindlimb muscles during posture and unrestrained locomotion. J Morphol. 1978 Mar;155(3):253–269. doi: 10.1002/jmor.1051550302. [DOI] [PubMed] [Google Scholar]
- Scott J. G., Mendell L. M. Individual EPSPs produced by single triceps surae Ia afferent fibers in homonymous and heteronymous motoneurons. J Neurophysiol. 1976 Jul;39(4):679–692. doi: 10.1152/jn.1976.39.4.679. [DOI] [PubMed] [Google Scholar]
- Sherrington C. S. Flexion-reflex of the limb, crossed extension-reflex, and reflex stepping and standing. J Physiol. 1910 Apr 26;40(1-2):28–121. doi: 10.1113/jphysiol.1910.sp001362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watt D. G., Stauffer E. K., Taylor A., Reinking R. M., Stuart D. G. Analysis of muscle receptor connections by spike-triggered averaging. 1. Spindle primary and tendon organ afferents. J Neurophysiol. 1976 Nov;39(6):1375–1392. doi: 10.1152/jn.1976.39.6.1375. [DOI] [PubMed] [Google Scholar]
- Willis W. D., Tate G. W., Ashworth R. D., Willis J. C. Monosynaptic excitation of motoneurons of individual forelimb muscles. J Neurophysiol. 1966 May;29(3):410–424. doi: 10.1152/jn.1966.29.3.410. [DOI] [PubMed] [Google Scholar]
- Windhorst U. A possible partitioning of segmental muscle stretch reflex into incompletely de-coupled parallel loops. Biol Cybern. 1979 Oct 3;34(4):205–215. doi: 10.1007/BF00337427. [DOI] [PubMed] [Google Scholar]
- Windhorst U. Auxiliary spinal networks for signal focussing in the segmental stretch reflex system. Biol Cybern. 1979 Oct;34(3):125–135. doi: 10.1007/BF00336964. [DOI] [PubMed] [Google Scholar]
- Windhorst U. Considerations on mechanisms of focussed signal transmission in the multi-channel muscle stretch reflex system. Biol Cybern. 1978 Nov 24;31(2):81–90. doi: 10.1007/BF00344238. [DOI] [PubMed] [Google Scholar]
- Windhorst U. Origin and nature of correlations in the Ia feedback pathway of the muscle control system. Biol Cybern. 1978 Nov 24;31(2):71–79. doi: 10.1007/BF00344237. [DOI] [PubMed] [Google Scholar]


