Abstract
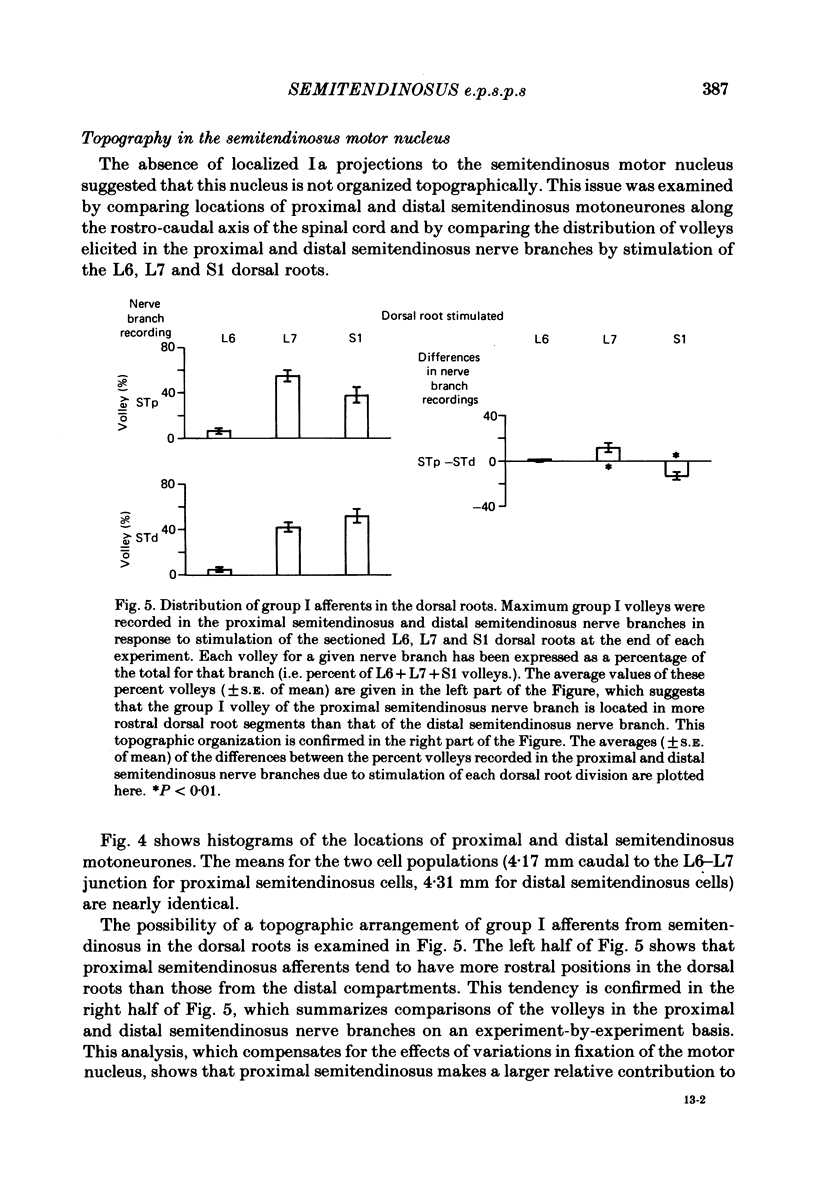
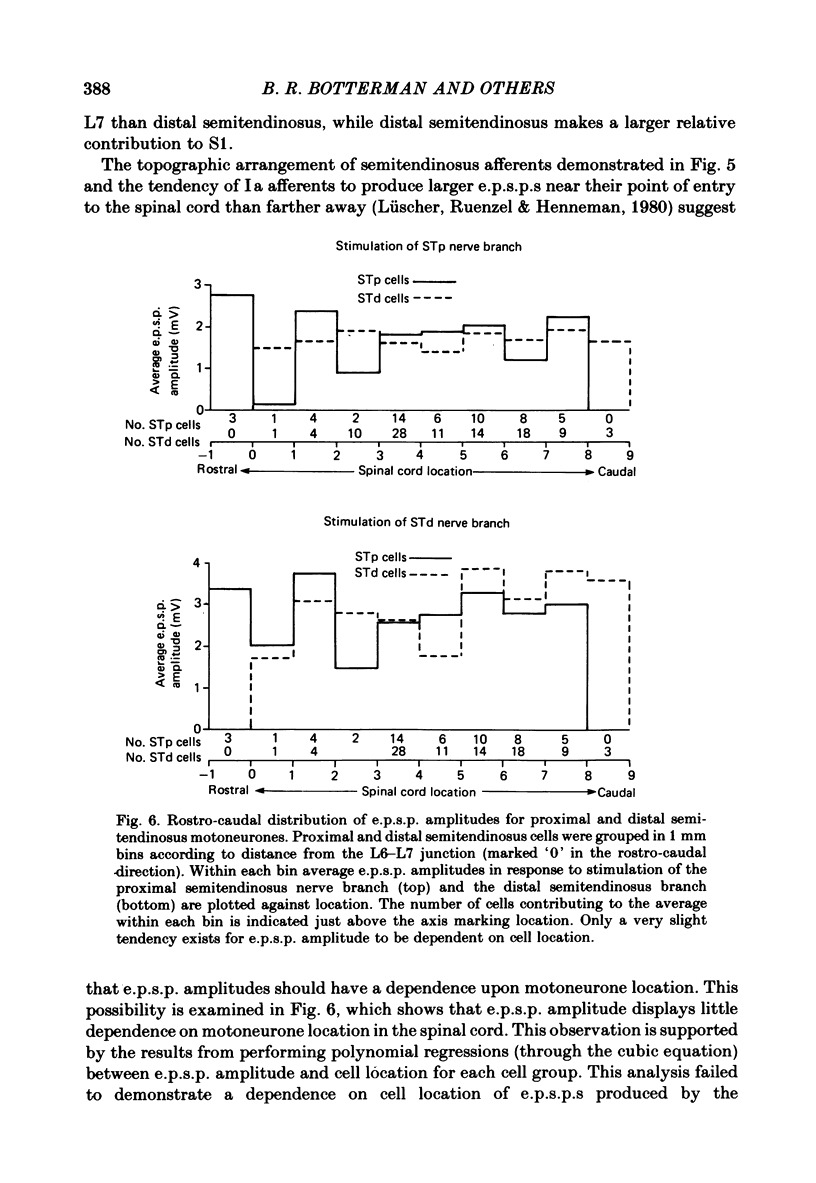
Evidence is presented for a lack of localization of monosynaptic Ia excitatory post-synaptic potentials (e.p.s.p.s) in the motor nucleus supplying the atypical cat hind limb muscle semitendinosus, which has anatomically distinct in-series compartments. Recordings were made from dorsal root filaments containing functionally isolated Ia, spindle group II and Ib axons from the proximal and distal compartments of semitendinosus. Twitch of either of these in-series compartments resulted in accelerated discharge of Ia and spindle group II fibres in the other compartment. Ib fibres of either compartment showed an in-series response to twitch of a single compartment which was weaker than twitch of the whole muscle, a finding which was consistent with the diminished force produced by twitch of either compartment alone. In addition, intracellular recordings were made from semitendinosus motoneurones in anaesthetized low-spinal cats during electrical stimulation of the nerve branches to proximal semitendinosus and distal semitendinosus. Comparison of proximal semitendinosus and distal semitendinosus motoneurones failed to reveal any difference between the two cell groups with respect to the average Ia e.p.s.p. amplitude produced by either the proximal or distal semitendinosus nerve branch. However, e.p.s.p.s due to stimulation of distal semitendinosus were approximately 65% larger, on average, than those due to stimulation of proximal semitendinosus in either motoneurone group. Analysis of cell location along the rostro-caudal axis of the spinal cord indicated that the proximal and distal semitendinosus cell groups are largely co-extensive. Recordings of volleys in the proximal and distal semitendinosus nerve branches in response to stimulation of the L6, L7 and S1 dorsal roots showed that group I afferents from the proximal semitendinosus compartment tend to have a more rostral entry point to the spinal cord than do distal semitendinosus afferents. E.p.s.p. amplitude in either cell group due to stimulation of either nerve branch showed little dependence on cell location in the spinal cord. The results are discussed with respect to the relation between muscle function and the distribution of monosynaptic Ia connexions.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BROWN M. C., MATTHEWS P. B. An investigation into the possible existence of polyneuronal innervation of individual skeletal muscle fibres in certain hind-limb muscles of the cat. J Physiol. 1960 Jun;151:436–457. doi: 10.1113/jphysiol.1960.sp006450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilotto G., Schor R. H., Uchino Y., Wilson V. J. Localization of proprioceptive reflexes in the splenius muscle of the cat. Brain Res. 1982 Apr 22;238(1):217–221. doi: 10.1016/0006-8993(82)90786-7. [DOI] [PubMed] [Google Scholar]
- Binder M. D. Further evidence that the Golgi tendon organ monitors the activity of a discrete set of motor units within a muscle. Exp Brain Res. 1981;43(2):186–192. doi: 10.1007/BF00237762. [DOI] [PubMed] [Google Scholar]
- Binder M. D., Kroin J. S., Moore G. P., Stauffer E. K., Stuart D. G. Correlation analysis of muscle spindle responses to single motor unit contractions. J Physiol. 1976 May;257(2):325–336. doi: 10.1113/jphysiol.1976.sp011371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder M. D., Stuart D. G. Responses of Ia and spindle group II afferents to single motor-unit contractions. J Neurophysiol. 1980 Mar;43(3):621–629. doi: 10.1152/jn.1980.43.3.621. [DOI] [PubMed] [Google Scholar]
- Bodine S. C., Roy R. R., Meadows D. A., Zernicke R. F., Sacks R. D., Fournier M., Edgerton V. R. Architectural, histochemical, and contractile characteristics of a unique biarticular muscle: the cat semitendinosus. J Neurophysiol. 1982 Jul;48(1):192–201. doi: 10.1152/jn.1982.48.1.192. [DOI] [PubMed] [Google Scholar]
- Botterman B. R., Hamm T. M., Reinking R. M., Stuart D. G. Localization of monosynaptic Ia excitatory post-synaptic potentials in the motor nucleus of the cat biceps femoris muscle. J Physiol. 1983 May;338:355–377. doi: 10.1113/jphysiol.1983.sp014677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brink E. E., Jinnai K., Wilson V. J. Pattern of segmental monosynaptic input to cat dorsal neck motoneurons. J Neurophysiol. 1981 Sep;46(3):496–505. doi: 10.1152/jn.1981.46.3.496. [DOI] [PubMed] [Google Scholar]
- COHEN L. A. Localization of stretch reflex. J Neurophysiol. 1953 May;16(3):272–285. doi: 10.1152/jn.1953.16.3.272. [DOI] [PubMed] [Google Scholar]
- COHEN L. A. Organization of stretch reflex into two types of direct spinal arcs. J Neurophysiol. 1954 Sep;17(5):443–453. doi: 10.1152/jn.1954.17.5.443. [DOI] [PubMed] [Google Scholar]
- Cameron W. E., Binder M. D., Botterman B. R., Reinking R. M., Stuart D. G. "Sensory partitioning" of cat medial gastrocnemius muscle by its muscle spindles and tendon organs. J Neurophysiol. 1981 Jul;46(1):32–47. doi: 10.1152/jn.1981.46.1.32. [DOI] [PubMed] [Google Scholar]
- ECCLES J. C., ECCLES R. M., LUNDBERG A. The convergence of monosynaptic excitatory afferents on to many different species of alpha motoneurones. J Physiol. 1957 Jun 18;137(1):22–50. doi: 10.1113/jphysiol.1957.sp005794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ECCLES R. M., LUNDBERG A. Integrative pattern of Ia synaptic actions on motoneurones of hip and knee muscles. J Physiol. 1958 Dec 4;144(2):271–298. doi: 10.1113/jphysiol.1958.sp006101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRANK K., FUORTES M. G. Stimulation of spinal motoneurones with intracellular electrodes. J Physiol. 1956 Nov 28;134(2):451–470. doi: 10.1113/jphysiol.1956.sp005657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankowska E., Odutola A. Crosses and uncrossed synaptic actions on motoneurones of back muscles in the cat. Brain Res. 1980 Jul 21;194(1):65–78. doi: 10.1016/0006-8993(80)91319-0. [DOI] [PubMed] [Google Scholar]
- Lüscher H. R., Ruenzel P., Henneman E. Topographic distribution of terminals of Ia and group II fibers in spinal cord, as revealed by postsynaptic population potentials. J Neurophysiol. 1980 Apr;43(4):968–985. doi: 10.1152/jn.1980.43.4.968. [DOI] [PubMed] [Google Scholar]
- Nelson S. G., Mendell L. M. Projection of single knee flexor Ia fibers to homonymous and heteronymous motoneurons. J Neurophysiol. 1978 May;41(3):778–787. doi: 10.1152/jn.1978.41.3.778. [DOI] [PubMed] [Google Scholar]
- Reinking R. M., Stephens J. A. Interface unit for on-line measurements of motor unit properties with a small laboratory computer. Am J Phys Med. 1975 Aug;54(4):186–193. [PubMed] [Google Scholar]
- Richmond F. J., Abrahams V. C. Morphology and enzyme histochemistry of dorsal muscles of the cat neck. J Neurophysiol. 1975 Nov;38(6):1312–1321. doi: 10.1152/jn.1975.38.6.1312. [DOI] [PubMed] [Google Scholar]
- Schwestka R., Windhorst U., Schaumberg R. Patterns of parallel signal transmission between multiple alpha efferents and multiple Ia afferents in the cat semitendinosus muscle. Exp Brain Res. 1981;43(1):34–46. doi: 10.1007/BF00238807. [DOI] [PubMed] [Google Scholar]
- Stephens J. A., Reinking R. M., Stuart D. G. Tendon organs of cat medial gastrocnemius: responses to active and passive forces as a function of muscle length. J Neurophysiol. 1975 Sep;38(5):1217–1231. doi: 10.1152/jn.1975.38.5.1217. [DOI] [PubMed] [Google Scholar]
- Stuart D. G., Goslow G. E., Mosher C. G., Reinking R. M. Stretch responsiveness of Golgi tendon organs. Exp Brain Res. 1970 Jun 25;10(5):463–476. doi: 10.1007/BF00234263. [DOI] [PubMed] [Google Scholar]
- Stuart D. G., Mosher C. G., Gerlach R. L., Reinking R. M. Selective activation of Ia afferents by transient muscle stretch. Exp Brain Res. 1970 Jun 25;10(5):477–487. doi: 10.1007/BF00234264. [DOI] [PubMed] [Google Scholar]
- Sturart D. G., Mosher C. G., Gerlach R. I., Reinking R. M. Mechanical arrangement and transducing properties of Golgi tendon organs. Exp Brain Res. 1972;14(3):274–292. doi: 10.1007/BF00816163. [DOI] [PubMed] [Google Scholar]
- Windhorst U., Meyer-Lohmann J. The influence of extrafusal muscle activity on discharge patterns of primary muscle spindle endings. Pflugers Arch. 1977 Dec 12;372(2):131–138. doi: 10.1007/BF00585326. [DOI] [PubMed] [Google Scholar]
- Windhorst U. Origin and nature of correlations in the Ia feedback pathway of the muscle control system. Biol Cybern. 1978 Nov 24;31(2):71–79. doi: 10.1007/BF00344237. [DOI] [PubMed] [Google Scholar]



