Abstract
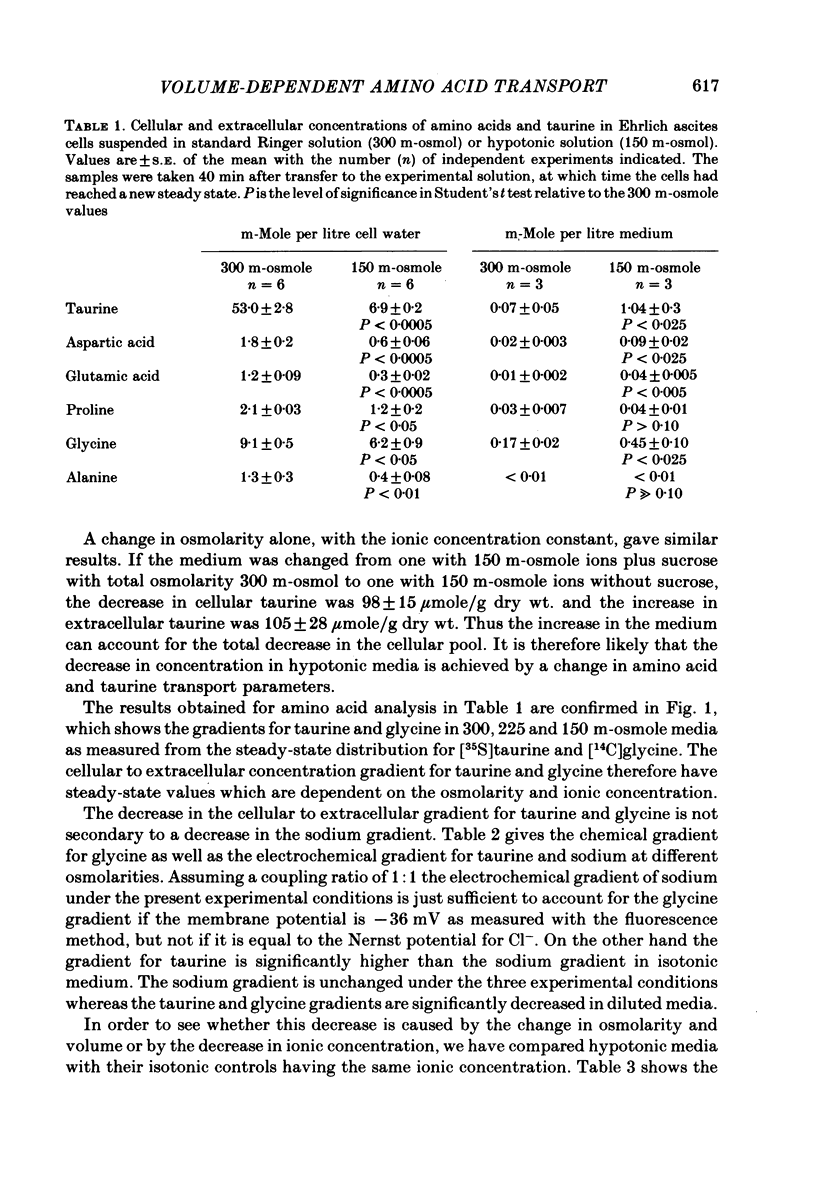
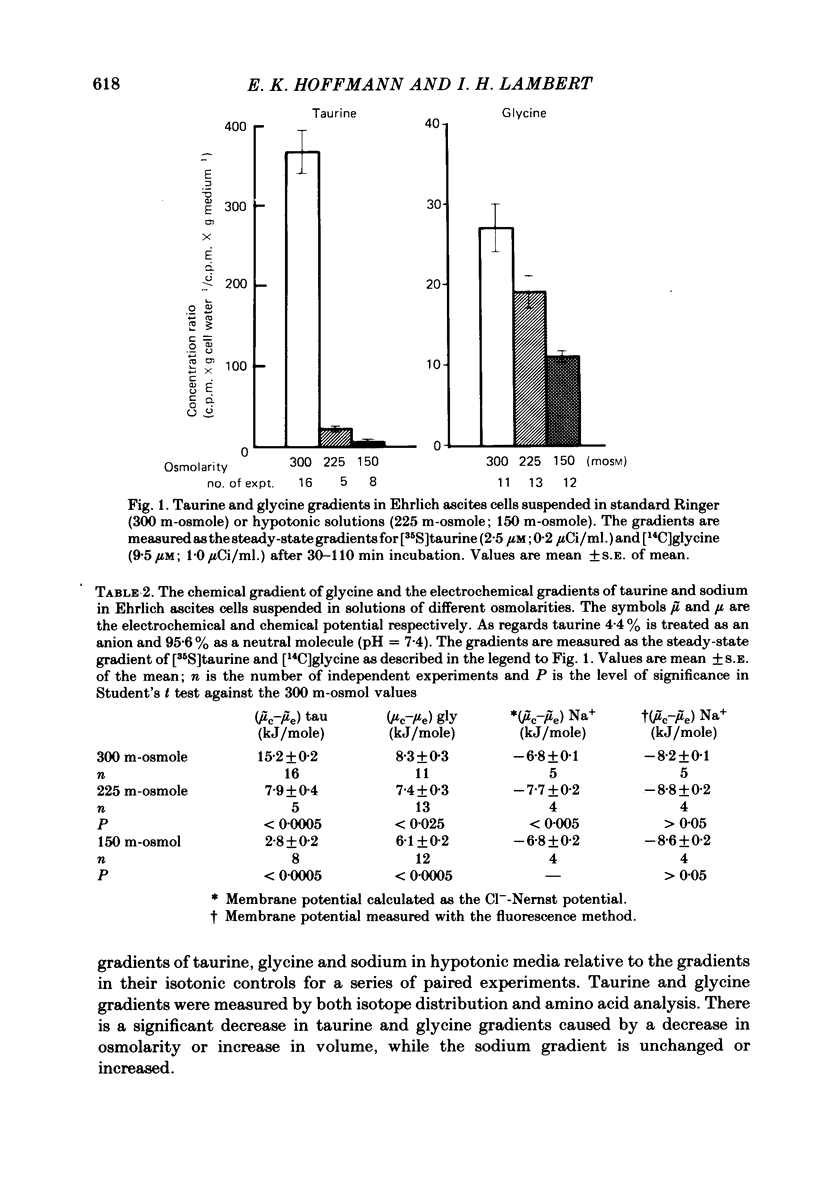
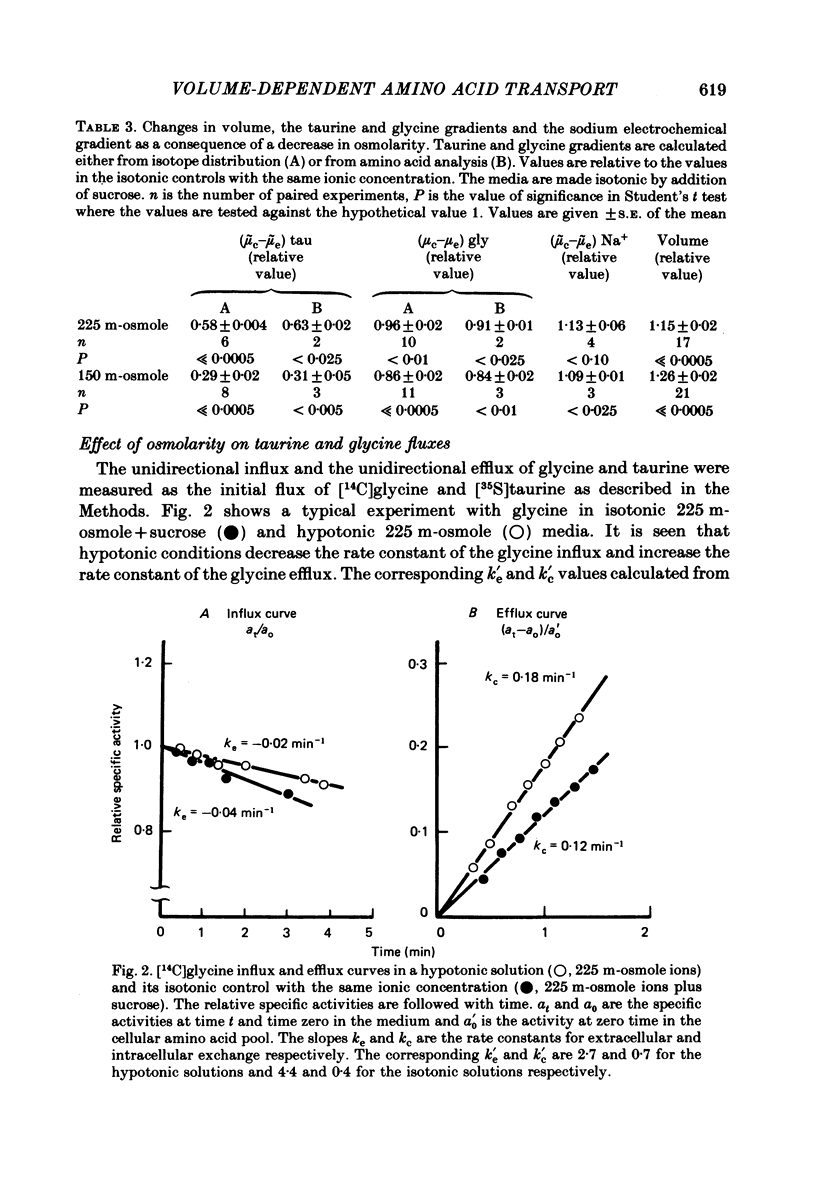
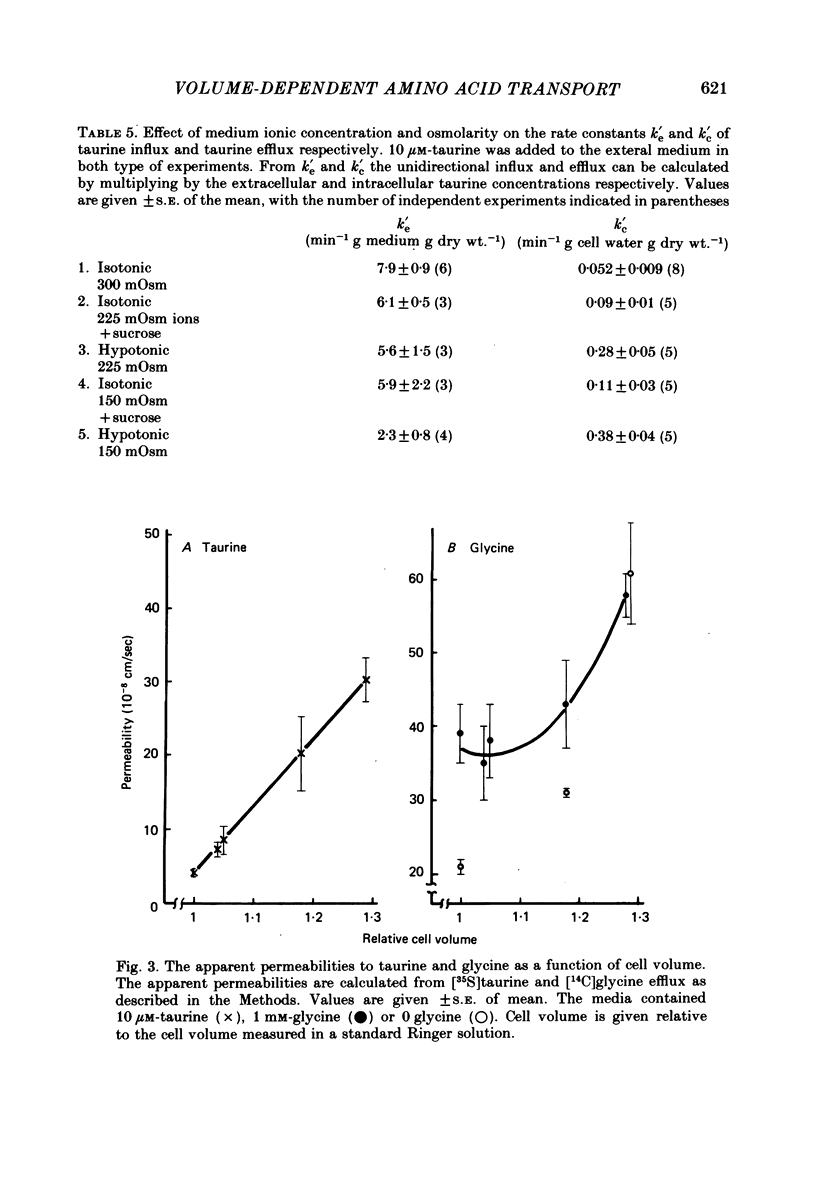
Cellular and extracellular concentrations of amino acids were measured in Ehrlich ascites cells by amino acid analysis and by distribution of radioactive amino acids between cells and medium. Dilution of the medium results in a reduction in the cellular concentration of non-essential amino acids and taurine and an equivalent increase in the extracellular content of these amino acids. The membrane potential and the electrochemical gradient of sodium were measured. The decrease in the cellular to extracellular gradient of taurine and glycine is not a consequence of a decrease in the sodium gradient but is caused by the changes in osmolarity and cell volume. The unidirectional influx and efflux of glycine and taurine were measured as the initial influx of [14C]glycine and [35S]taurine, using a rapid filter technique. For both amino acids the rate constants for influx are decreased and the rate constants for efflux increased following a reduction in osmolarity. The taurine and glycine fluxes were analysed as a simple pump and leak system. Reduction in osmolarity increases the leak permeability to both taurine and glycine. The results for glycine are also discussed in relation to a Na+-glycine co-transport model, where reduction in osmolarity increases the leak permeability to glycine. The cellular permeability to taurine and glycine increases as a function of increasing cell volume. The taurine permeability increases 7-fold and the glycine permeability 1.5-fold with an increase in cell volume of 30%. The absolute increase in permeability is equal for both taurine and glycine.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Eagle H. Buffer combinations for mammalian cell culture. Science. 1971 Oct 29;174(4008):500–503. doi: 10.1126/science.174.4008.500. [DOI] [PubMed] [Google Scholar]
- Eddy A. A. The amino acid pumps of living cells. Sci Prog. 1981 Summer;67(266):245–270. [PubMed] [Google Scholar]
- Fugelli K. Gamma-aminobutyric acid (GABA) in fish erythrocytes. Experientia. 1970 Apr 15;26(4):361–361. doi: 10.1007/BF01896886. [DOI] [PubMed] [Google Scholar]
- Geck P., Pietrzyk C., Burckhardt B. C., Pfeiffer B., Heinz E. Electrically silent cotransport on Na+, K+ and Cl- in Ehrlich cells. Biochim Biophys Acta. 1980 Aug 4;600(2):432–447. doi: 10.1016/0005-2736(80)90446-0. [DOI] [PubMed] [Google Scholar]
- Heinz E., Geck P., Pfeiffer B. Energetic problems of the transport of amino acids in Ehrlich cells. J Membr Biol. 1980 Dec 15;57(2):91–94. doi: 10.1007/BF01868995. [DOI] [PubMed] [Google Scholar]
- Heinz E., Geck P., Pietrzyk C., Burckhardt G., Pfeiffer B. Energy sources for amino acid transport in animal cells. J Supramol Struct. 1977;6(1):125–133. doi: 10.1002/jss.400060110. [DOI] [PubMed] [Google Scholar]
- Hendil K. B., Hoffmann E. K. Cell volume regulation in Ehrlich ascites tumor cells. J Cell Physiol. 1974 Aug;84(1):115–125. doi: 10.1002/jcp.1040840113. [DOI] [PubMed] [Google Scholar]
- Hoffmann E. K., Simonsen L. O., Sjøholm C. Membrane potential, chloride exchange, and chloride conductance in Ehrlich mouse ascites tumour cells. J Physiol. 1979 Nov;296:61–84. doi: 10.1113/jphysiol.1979.sp012991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Philo R. D., Eddy A. A. Equilibrium and steady-state models of the coupling between the amino acid gradient and the sodium electrochemical gradient in mouse ascites- tumour cells. Biochem J. 1978 Sep 15;174(3):811–817. doi: 10.1042/bj1740811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philo R. D., Eddy A. A. The membrane potential of mouse ascites-tumour cells studied with the fluorescent probe 3,3'-dipropyloxadicarbocyanine. Amplitude of the depolarization caused by amino acids. Biochem J. 1978 Sep 15;174(3):801–810. doi: 10.1042/bj1740801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remtulla M. A., Katz S., Applegarth D. A. Effect of taurine on passive ion transport in rat brain synaptosomes. Life Sci. 1979 May 14;24(20):1885–1892. doi: 10.1016/0024-3205(79)90240-6. [DOI] [PubMed] [Google Scholar]


