Abstract
Objectives
Celiac disease (CeD) has been associated with a low response to hepatitis B (HBV) vaccination, but guidelines for testing and revaccination among individuals with CeD are sparse. We examined the risk of future HBV among individuals with CeD in a population-based Swedish cohort. Furthermore, we examined the rate of prior HBV infection in CeD patients.
Methods
All individuals in Sweden diagnosed with biopsy-verified CeD between 1990 and 2017 were identified through the ESPRESSO cohort. Each individual with CeD was matched by age, sex, calendar year, and birth country (Nordic vs. other) with up to 5 reference individuals.
Results
We identified 44,721 CeD and 222,238 reference individuals. The incidence rates of diagnosed HBV were 2.3 and 2.9 per 100,000 person-years, respectively. This represented no association with CeD (HR 0.77 (0.45–1.30)). This null association was similar for those with a Nordic (HR 0.80 (0.40–1.60)) and non-Nordic ((HR 0.31 (0.09–1.08)) country of birth. Rates of prior HBV infection were low (CeD 0.08%, controls 0.06%). This corresponded to a small but insignificant increase among individuals with CeD (odds ratio, OR 1.41 (0.97–2.05).
Conclusion
In a population-based Swedish cohort, there was no increased risk of developing HBV in individuals with CeD. This finding does not support current practices of testing and revaccination for HBV. Additional studies should be completed in areas with higher endemic rates of HBV. Slightly higher rates of prior HBV infection in CeD may be secondary to increased testing in those seeking medical care for another disease process.
Supplementary Information
The online version contains supplementary material available at 10.1007/s10620-025-08878-3.
Keywords: celiac, coeliac, cohort, gluten, liver disease, hepatitis B virus, Sweden
Introduction
Celiac disease (CeD) is an autoimmune enteropathy triggered by the ingestion of gluten in genetically susceptible individuals [1]. Over the past 15 years, there has been an increasing recognition of the connection between CeD and both viral and bacterial infections, especially pneumococcal infections associated with hyposplenism [2–4]. With 1% of the population estimated to have CeD, understanding the clinical implications of these increased infectious risks is critically important for medical practitioners.
The WHO has established a goal to eliminate viral hepatitis by 2030 with a strategy involving increased access to testing and treatment, improved awareness, and a reduction in new cases [5]. Vaccinations have played a crucial role in significantly reducing infections and their associated morbidity and mortality. Widespread vaccination programs throughout the world have successfully controlled or eradicated many diseases. Hepatitis B (HBV) vaccination protocols, however, are varied throughout the world with many countries instituting universal vaccination in childhood and others targeting vaccination to only at-risk groups. Studies have demonstrated that individuals with CeD have a lower response to HBV vaccination [6–8]. The underlying etiology for this inadequate response has been attributed to CeD-associated genetics including HLA-DQ2, malnutrition, generalized inflammation, and compounded by active gluten ingestion [8, 9]. Based on these data, common practice in many countries is to test for HBV immunity in CeD patients and to revaccinate those who lack protective titers [10, 11].
Despite widespread practice of vaccinations, there is a paucity of evidence-based guidelines for clinical management of screening and revaccination for HBV in individuals with CeD. There is furthermore, little research into actual rates of HBV in the CeD population.
The objective of this study was to examine the risk of individuals with CeD subsequently developing HBV, as well as the rate of acute or chronic HBV infection prior to CeD diagnosis in a large population-based cohort in Sweden.
Methods
Celiac Disease
We performed a population-based, retrospective cohort study. All individuals (including children) in Sweden diagnosed with biopsy-verified CeD between 1990 and 2017 were identified utilizing Swedish SnoMed classification codes in the Epidemiology Strengthened by Histopathology Reports in Sweden (ESPRESSO) cohort [12] (see appendix for SnoMed codes). ESPRESSO contains histopathology information obtained from all 28 pathology departments in Sweden starting in the year 1965. Prior to 2012, small intestinal biopsy with Marsh 3 villus atrophy was considered the gold standard for CeD diagnosis across Sweden. In 2012 the European Society for Pediatric Gastroenterology, Hepatology, and Nutrition (ESPGHAN) published guidelines for non-biopsy diagnosis of CeD in selected children [13]. Children diagnosed without biopsy after 2012 were not included in this analysis. In assessing development of HBV in established individuals with CeD, we excluded those with a diagnosis of viral hepatitis or liver transplant prior to their CeD diagnosis, or the corresponding date for the reference population (see appendix for relevant international classification of disease (ICD codes). Individuals with an established diagnosis of HBV were included in the analysis of subsequent CeD diagnosis.
Reference Population
Information on age, sex, migration status, and family relationships, is maintained by a government agency (Statistics Sweden) in the Total Population Register [14]. The Total Population Register was used to match CeD individuals with up to 5 reference comparators from the general population. Matching was based upon age, sex, county of residence, calendar year, and country of birth (Nordic vs. other) (definitions in Supplementary Tables 1, 2).
We also excluded any study participant for whom there was formal emigration or migration within 3 years before the index diagnosis date.
HBV was introduced to the Swedish National Patient Register with defining ICD-9 codes in 1987 [15]. The incidence of HBV was determined through collection of data from this register (Supplementary Table 3) with the exclusion of information prior to 1987. Robust national healthcare systems in Sweden and the surrounding Nordic countries have promoted HBV screening, vaccination of high-risk groups, and public health education resulting in very low endemic rates of HBV [16]. Most cases of HBV among Swedish residents are found in immigrants from highly endemic areas, and so to best characterize the population with HBV in Sweden we stratified data by place of birth (native Nordic residents and immigrants from non-Nordic countries) [17].
Rates of HBV vaccination were not available for this analysis as the National Patient Register does not contain this specific information. The public health agency of Sweden first introduced HBV vaccination in 1996, with restricted recommendation for only high-risk groups. With the advent of combination vaccines, babies that were not considered at risk subsequently began receiving routine HBV vaccination, and as of 2016 it has been offered routinely[18]. There is no specific guidance regarding vaccination in those with CeD in Sweden. Our follow-up ended in 2021, so it can be assumed that the majority of individuals in this population did not receive HBV vaccination. Vaccination data for non-Nordic individuals were also not available.
Outcome
The primary outcome was the risk of developing acute or chronic HBV infection as identified using ICD10 codes (Supplementary Table 3). An additional analysis looked at individuals with CeD and risk of prior HBV infection. Follow-up started the day of biopsy confirmation of CeD diagnosis.
Education Data as a Covariate
Previous studies have established relationships between HBV infection and HBV outcomes with education level [19–21]. We used three strata of schooling (≤ 9 years compulsory school; 10–12 years upper secondary school; > 12 years college or university) to define education level in the population. For children who were diagnosed at under 18 years old, the furthest educational attainment of the two parents was used.
Statistics
We used Cox proportional hazards to measure the Hazard Ratios (HRs) for HBV infection, comparing CeD patients to the reference population. Initial analysis was conditioned upon age, sex, country, and calendar year of biopsy with subsequent analyses also adjusting for maternal HBV infection, maternal education, and the following time dependent comorbidities: type 1 Diabetes, autoimmune thyroid disease, rheumatoid arthritis, inflammatory bowel disease, psoriasis, HIV, hepatobiliary disease, and ascites. Unless otherwise noted, HRs are presented with the 95% confidence interval (CI) and conditioned upon this full list of covariates. Follow-up began on the day of the diagnostic CeD biopsy or corresponding matching date in comparators, and ended with diagnosis of HBV, death, or emigration. Statistical analysis was completed using SAS v9.4.
Additional Analyses
Siblings
So as to test the robustness of our findings, we identified siblings of CeD patients to serve as a second set of controls. Individuals with CeD who have full siblings in Sweden were identified using the Swedish Total Population Register and additional analyses were conducted using those siblings that were alive and without CeD at the time of the index patient’s diagnosis. Utilizing siblings decreases confounding related to genetics and environment. For this study specifically, it allowed for more precise matching of personal demographics including country of birth, maternal education, and maternal HBV status which were likely to impact the risk of HBV.
Risk of HBV Infection Prior to CeD
So as to determine whether individuals with undiagnosed (and untreated) CeD are at increased risk of HBV, we performed a case–control study comparing the incidence of CeD in individuals with a history of acute or chronic HBV infection with their matched controls. For this analysis, we calculated Odds Ratios (ORs) and their corresponding 95% confidence intervals.
This study was approved by the Research Ethics Committee of Karolinska Institutet, Stockholm Sweden and personal consent was waived due to the register-based nature of the study [22].
Results
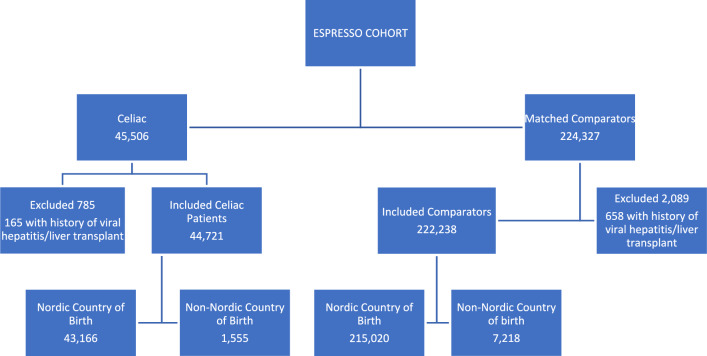
We identified 45,506 individuals with CeD, of which 44,721 unique individuals were included after application of the exclusion criteria, those with other data irregularities, or those for whom there was no matched comparator, and we included 222,238 matched comparators (Fig. 1; Table 1). The median follow-up was 15.2 (9.9–21.4) years.
Fig. 1.
Flowchart of Study Participants
Table 1.
Characteristics of patients with celiac disease and their matched comparators
| Characteristic | Overall | |
|---|---|---|
| Celiac disease (n = 44,721) |
Matched comparators (n = 222,238) |
|
| Female, no. (%) | 27 990 (62.6%) | 139 113 (62.6%) |
| Male, no (%) | 16 731 (37.4%) | 83 125 (37.4%) |
| Age | ||
| Mean (SD) | 32.6 (25.3) | 32.6 (25.3) |
| Median (IQR) | 28.5 (9.1–54.1) | 28.4 (9.1–54.1) |
| Range, min–max | 0.0–95.4 | 0.0–95.8 |
| Categories, no. (%) | ||
| < 18y | 17 494 (39.1%) | 87 104 (39.2%) |
| 18y–< 40y | 9 857 (22.0%) | 48 816 (22.0%) |
| 40y–< 60y | 8 764 (19.6%) | 43 449 (19.6%) |
| ≥ 60y | 8 606 (19.2%) | 42 869 (19.3%) |
| Level of education, no. (%) | ||
| ≤ 9 years | 8 497 (19.0%) | 43 941 (19.8%) |
| 10–12 years | 11 676 (26.1%) | 58 967 (26.5%) |
| > 12 years | 7 843 (17.5%) | 37 348 (16.8%) |
| Missing | 16 705 (37.4%) | 81 982 (36.9%) |
| Country of birth | ||
| Nordic | 43 166 (96.5%) | 215 020 (96.7%) |
| Non-Nordic | 1 555 (3.5%) | 7 218 (3.3%) |
| Level of education using highest level of education in parents when missing, no. (%) | ||
| ≤ 9 years | 8 930 (20.0%) | 46 885 (21.1%) |
| 10–12 years | 18 461 (41.3%) | 92 981 (41.8%) |
| > 12 years | 16 782 (37.5%) | 80 397 (36.2%) |
| Missing | 548 (1.2%) | 1 975 (0.9%) |
| Start year of follow-up, no. (%) | ||
| 1990–1999 | 13 457 (30.1%) | 66 975 (30.1%) |
| 2000–2009 | 19 594 (43.8%) | 97 376 (43.8%) |
| 2010–2017 | 11 670 (26.1%) | 57 887 (26.0%) |
| Disease history ever before start of follow-up, no. (%) | ||
| Type 1 diabetes | 1 488 (3.3%) | 692 (0.3%) |
| Autoimmune thyroid disease | 1 068 (2.4%) | 1 906 (0.9%) |
| Rheumatoid arthritis | 380 (0.8%) | 1 231 (0.6%) |
| Inflammatory bowel disease | 1 161 (2.6%) | 781 (0.4%) |
| Psoriasis | 433 (1.0%) | 1 261 (0.6%) |
| HIV | 13 (0.0%) | 45 (0.0%) |
| Hepatobiliary disease | 1 764 (3.9%) | 6 036 (2.7%) |
| Ascites | 55 (0.1%) | 66 (0.0%) |
| Maternal Hepatitis B (before birth of index individual), no. (%) | ||
| Yes | 18 (0.0%) | 95 (0.0%) |
| No | 38 834 (86.8%) | 193 120 (86.9%) |
| Missing (missing PNR in mother) | 5 869 (13.1%) | 29 023 (13.1%) |
| Follow-up, years | ||
| Mean (SD) | 15.8 (7.6) | 16.1 (7.5) |
| Median (IQR) | 15.2 (9.9–21.4) | 15.5 (10.1–21.7) |
| Range, min–max | 0.0–32.0 | 0.0–32.0 |
Risk of Hepatitis B Infection in Celiac Disease
The incidence of HBV infection was 2.3 (1.2–3.4) per 100,000 person-years in CeD versus 2.9 (2.3–3.4) per 100,000 person-years in comparators. Kaplan–Meier curves of the proportion of CeD patients and comparators without a diagnosis of HBV are shown in Fig. 2. When conditioned on the original matching set (age, sex, county, country of birth, and calendar year of biopsy) this resulted in an HR of 0.77 (0.45–1.30). Further adjustment to account for maternal education, maternal HBV status, and the medical comorbidities described above, yielded a similar HR of 0.68 (0.38–1.19). On stratified analyses, HBV risk was not significantly impacted by duration of CeD, sex, or education level (Table 2).
Fig. 2.
Risk of hepatitis B in patients with celiac disease vs. comparators
Table 2.
Risk of Hepatitis B in patients with Celiac disease and matched general population comparators
| Group | N | N events | Incidence rate (95% CI) per 100,000 PY | HR* (95%CI) |
HR** (95%CI) |
|||
|---|---|---|---|---|---|---|---|---|
| Celiac disease | Comparators | Celiac disease | Comparators | Celiac disease | Comparators | |||
| Overall | 44 721 (100%) | 222 238 (100%) | 16 (0.04%) | 103 (0.05%) | 2.3 (1.2–3.4) | 2.9 (2.3–3.4) | 0.77 (0.45–1.30) | 0.68 (0.38–1.19) |
| Follow-up | ||||||||
| 0–< 1y | 44 721 (100%) | 222 238 (100%) | 1 (0.00%) | 4 (0.00%) | 2.3 (0.0–6.7) | 1.8 (0.0–3.6) | 1.25 (0.14–11.18) | 1.08 (0.11–10.85) |
| 1–< 5y | 43 996 (98.4%) | 220 321 (99.1%) | 8 (0.02%) | 29 (0.01%) | 4.6 (1.4–7.8) | 3.3 (2.1–4.6) | 1.34 (0.61–2.95) | 1.09 (0.42–2.84) |
| 5–< 10y | 42 268 (94.5%) | 212 314 (95.5%) | 3 (0.01%) | 34 (0.02%) | 1.6 (0.0–3.3) | 3.5 (2.3–4.7) | 0.41 (0.12–1.33) | 0.27 (0.07–1.02) |
| ≥ 10y | 33 266 (74.4%) | 168 137 (75.7%) | 4 (0.01%) | 36 (0.02%) | 1.3 (0.0–2.6) | 2.4 (1.6–3.1) | 0.59 (0.21–1.67) | 0.55 (0.15–1.97) |
| ≥ 1y | 43 996 (98.4%) | 220 321 (99.1%) | 15 (0.03%) | 99 (0.04%) | 2.3 (1.1–3.4) | 2.9 (2.4–3.5) | 0.75 (0.43–1.29) | 0.63 (0.35–1.14) |
| Sex | ||||||||
| Females | 27 990 (62.6%) | 139 113 (62.6%) | 5 (0.02%) | 74 (0.05%) | 1.1 (0.1–2.1) | 3.3 (2.5–4.0) | 0.32 (0.13–0.80) | 0.28 (0.11–0.74) |
| Males | 16 731 (37.4%) | 83 125 (37.4%) | 11 (0.07%) | 29 (0.03%) | 4.3 (1.7–6.8) | 2.2 (1.4–3.0) | 1.99 (0.98–4.03) | 1.51 (0.64–3.55) |
| Age | ||||||||
| < 18y | 17 494 (39.1%) | 87 104 (39.2%) | 3 (0.02%) | 29 (0.03%) | 1.0 (0.0–2.1) | 1.9 (1.2–2.6) | 0.49 (0.15–1.61) | 0.32 (0.07–1.42) |
| 18y–< 40y | 9 857 (22.0%) | 48 816 (22.0%) | 8 (0.08%) | 36 (0.07%) | 5.1 (1.6–8.6) | 4.6 (3.1–6.1) | 1.01 (0.47–2.18) | 0.98 (0.41–2.33) |
| 40y–< 60y | 8 764 (19.6%) | 43 449 (19.6%) | 3 (0.03%) | 28 (0.06%) | 2.0 (0.0–4.3) | 3.7 (2.4–5.1) | 0.58 (0.18–1.94) | 0.39 (0.10–1.52) |
| ≥ 60y | 8 606 (19.2%) | 42 869 (19.3%) | 2 (0.02%) | 10 (0.02%) | 2.1 (0.0–5.1) | 2.0 (0.8–3.3) | 1.19 (0.25–5.62) | 0.66 (0.05–9.49) |
| Year | ||||||||
| 1990–1999 | 13 457 (30.1%) | 66 975 (30.1%) | 6 (0.04%) | 52 (0.08%) | 2.0 (0.4–3.5) | 3.3 (2.4–4.2) | 0.55 (0.24–1.29) | 0.38 (0.14–1.02) |
| 2000–2009 | 19 594 (43.8%) | 97 376 (43.8%) | 9 (0.05%) | 46 (0.05%) | 3.0 (1.0–4.9) | 3.0 (2.2–3.9) | 0.98 (0.48–2.01) | 1.00 (0.47–2.14) |
| 2010–2017 | 11 670 (26.1%) | 57 887 (26.0%) | 1 (0.01%) | 5 (0.01%) | 1.0 (0.0–3.0) | 1.0 (0.1–1.9) | 1.19 (0.13–10.69) | 4.11 (0.22–78.17) |
| Level of education | ||||||||
| ≤ 9 years | 8 930 (20.0%) | 46 885 (21.1%) | 4 (0.04%) | 42 (0.09%) | 3.1 (0.1–6.2) | 6.0 (4.2–7.9) | 0.30 (0.07–1.33) | 0.24 (0.04–1.28) |
| 10–12 years | 18 461 (41.3%) | 92 981 (41.8%) | 9 (0.05%) | 37 (0.04%) | 2.9 (1.0–4.8) | 2.4 (1.6–3.1) | 1.53 (0.65–3.59) | 1.71 (0.62–4.72) |
| > 12 years | 16 782 (37.5%) | 80 397 (36.2%) | 3 (0.02%) | 19 (0.02%) | 1.1 (0.0–2.4) | 1.5 (0.8–2.1) | 0.45 (0.05–4.25) | 0.43 (0.04–4.34) |
*Conditioned on matching set
**Conditioned on matching set and adjusted for education, maternal Hepatitis B, and time dependent medical comorbidities
Endemic rates of HBV around the world are extremely variable. To better characterize the native Nordic population and how it may differ from an aggregate of non-Nordic countries, we performed a sub-analysis of the risk of developing HBV in CeD patients dependent upon country of birth. There were 1555 CeD and 7218 comparators of non-Nordic heritage. Incidence rates of HBV were higher in both CeD patients and general population comparators in the non-Nordic subgroup, 19.1 (0.4–37.8) per 100,000 PY in CeD and 37.1 (25.0–49.2) per 100,000 PY in general population controls. In this population, there was no increased risk associated with CeD (HR 0.31 (0.09–1.08). Among those born in Nordic countries, the incidence of HBV developing in CeD patients was 1.7 (0.8–2.7) per 100,000 PY versus 1.9 (1.5–2.4) per 100,000 PY in controls. This corresponds to an HR of 0.80 (0.40–1.60) in the native Nordic population.
When we repeated the analysis, now utilizing siblings as comparators, 29,686 CeD patients had a total of 50,586 full siblings identified from the Total Population Register (Supplementary Table 4). The incidence of HBV was 1.9 (0.6–3.1) per 100,000 PY in CeD and 3.6 (2.3–4.9) per 100,000 PY in siblings (HR of 0.83 (0.28–2.40)). Similar to our findings using general population comparators, there was no significantly increased risk of HBV in those with CeD.
Risk of Celiac Disease in Hepatitis B Infection
As a secondary analysis we assessed the risk of CeD in individuals with a history of acute or chronic HBV infection. Overall, there were 37 (0.08%) CeD patients and 129 (0.06%) comparators with a history of HBV infection. There was a trend toward an increase of prior HBV infection in CeD patients compared with controls (OR 1.41 (0.97–2.05)) that did not reach statistical significance. This trend was driven more by those of Nordic country of birth (OR 2.05 (1.28–3.28)) than of non-Nordic country of birth (OR 0.87 (0.45–1.70)).
Discussion
In this population-based study, we did not observe an increased risk of HBV infection in individuals with CeD compared to the reference population. This null finding was present across age strata, sex, calendar period, place of birth, and remained robust after adjusting for maternal education, maternal HBV, and comorbidities previously described. This finding was similarly null when we used siblings as an alternative reference population. We did observe a trend toward an increase of prior HBV infection in CeD patients. This possible association may be secondary to increased interface between the HBV patient and the healthcare system. Even for those born outside a Nordic country who have an increased risk of HBV, those with CeD were not at increased risk of acquiring HBV infection.
Hepatitis B is a small, bloodborne DNA virus that can cause liver disease ranging from mild illness to a serious, chronic condition resulting in liver failure and cancer. It can be transmitted either horizontally via percutaneous and mucosal exposure or vertically from mother to baby. According to the World Health Organization (WHO), an estimated 257 million people are living with chronic HBV infection worldwide, but the prevalence varies greatly by geography. Prevalence is highest in Sub-Saharan Africa and East Asia where an estimated 6.1 and 6.2%, respectively, are living with chronic HBV infection. Significantly lower prevalence rates (between 0.1 and 1%) are seen in Europe and North America [23].
The relationship between CeD and HBV has been examined mostly with respect to vaccination response. Multiple studies have documented a higher non-response in individuals with CeD (35–50% non-response) [9] compared to the general population (4–10% non-response). The reasons for this variation have not been fully elucidated, but there is data to support the role of celiac permissive HLA genotypes [24], gluten exposure, and active inflammation [8, 25, 26]. The interpretation of these findings is further complicated by the challenge of quantifying the anamnestic response to vaccination [6]. This refers to the immune system’s robust reaction characterized by a rapid production of specific antibodies and memory T-cells upon re-exposure to an antigen previously encountered through vaccination. Prior to re-exposure, antibodies to the particular antigen may or may not be detectable in the bloodstream.
There are no formal guidelines on the monitoring and treatment of HBV immunity in CeD, however, as a result of this documented suboptimal response, clinical practice in many centers has been to check antibody titers and revaccinate when non-immunity is found. To this point there has been little data documenting if there are related clinical consequences to this lower immunity. In a recent study by Habash et al., the authors utilized the National Health and Nutrition Examination Survey (NHANES) database between 2009 and 2014 to investigate incidence of HBV in CeD [27]. They found among 93 patients with CeD, there were no cases of HBV infection. These same authors conducted an analysis of patients at the Mayo Clinic (a large CeD referral center) which yielded an HBV prevalence in CeD lower than the national average (0.11% versus 0.33%). The nation-wide cases of HBV were primarily found in Asian Americans and Pacific Islanders (50% of HBV cases) [28] which is a minority population in Olmstead County. This highlights the importance of evaluating this question in multiple populations across the globe. Moreover, this study found no differences in HBV immunity between individuals with CeD and control subjects, challenging previous findings of an inadequate vaccine response in CeD.
The population-based nature of our study allows for examination of a much larger dataset than what has been previously published. Similar to the smaller US studies, we found no increased risk of HBV in previously diagnosed CeD patients. There were marginal differences in outcomes when full siblings were used as comparators.
Significantly less research has been conducted into the risk of developing CeD in those with a history of HBV infection. The exact mechanism for why a genetically susceptible individual develops CeD is unknown and likely represents a combination of genetic, environmental, and immunological factors. The development of other autoimmune diseases following infection has been well described in systemic lupus erythematosus, type 1 diabetes, and rheumatoid arthritis [29–31]. While exact mechanisms are not known, hypotheses around molecular mimicry, epitope spreading, and bystander effect have been proposed [32]. In attempts to unlock the mystery of loss of gluten tolerance in CeD, studies have also looked at the possibility of viral infections as a trigger for disease pathogenesis. Enteroviral infections have been found to be significantly more frequent in children who developed CeD autoimmunity (CeDA) compared to control children (OR 6.3 (1.8–22.3) [33]. Additional studies have examined relationships with reovirus [34], rotavirus [35], and adenovirus [36].
Like other viruses, HBV has been investigated as an infectious spark for autoimmune disease. Although evidence is sparse, there is evidence supporting a causal relationship between HBV and polyarteritis nodosa and glomerulonephritis [32]. There is little data on any possible role of HBV in development of CeD. One small study found a 9% prevalence of CeD autoimmunity (CeDA) in a group of 88 patients with chronic HBV infection [37]. This is a percentage that is clearly above that of the baseline population. Another study of 50 individuals with HBV infection found histologic changes consistent with CeD in 10% [38]. In contrast, a small Italian study did not find an increase in CeDA among a group of HBV carrier patients [39]. It is worth mentioning that in all these studies, patients with a history of HBV were screened for CeD and CeDA. As such, it is important to note that a true temporal relationship cannot be ascertained due to the nature of the study design and the limited data available. Our data potentially show a small increased risk of CeD among individuals with a prior history of HBV, but also cannot establish any causal relationship. Therefore, it is prudent to exercise caution in drawing conclusions based solely on this limited data.
The population-based nature of our study allows examination of a much larger dataset than what has been previously studied, however, several limitations are worth noting. We are missing maternal information on many non-Nordic individuals as some of these mothers were not part of the Swedish population register. The Swedish National Patient Register also does not collect routine vaccination data or antibody titers, however, given the age of most individuals in the patient registry, it should be assumed that the majority did not receive routine HBV vaccination. This dataset lacks information on risk factors, such as intravenous drug use, high-risk sexual behaviors, and high-risk professions that may impact HBV exposure and transmission. The absence of a universal HBV vaccination program to newborns and the historically limited nationwide implementation of needle exchange programs until recently, have resulted in an annual reporting of approximately 100 new cases of acute HBV to the Public Health Agency of Sweden. Among the limitations is the lack of data on children diagnosed with CeD without small intestinal biopsy.
Implications for Screening and Revaccination
Our data contributes to a growing body of literature questioning the necessity of assessing HBV immunity and subsequent revaccination in CeD. Studies are needed in populations with higher endemic rates of HBV in order to assess if risk remains at or below population norms. As is the case in individuals without CeD, personalized discussions with patients should be encouraged to assess risk factors including frequent travel to endemic areas, intravenous drug use, or a sexual partner with HBV infection. In these cases, HBV screening and revaccination may be encouraged, whereas low-risk individuals may forego screening altogether.
Supplementary Information
Below is the link to the electronic supplementary material.
Author Contributions
JJ and JFL wrote and revised the first draft JS completed the statistical analysis PHRG and JFL supervised the project All authors contributed to the conceptualization and reading of the manuscript and approved the final version for publication.
Funding
Open access funding provided by Karolinska Institute. No funding from any agency in the public, commercial, or not-for-profit sector has been provided for the research. Dr Ludvigsson has coordinated an unrelated study on behalf of the Swedish IBD quality register (SWIBREG). That study received funding from Janssen corporation. Dr Ludvigsson has also received financial support from MSD developing a paper reviewing national healthcare registers in China. Dr Ludvigsson is currently discussing potential research collaboration with Takeda. Hannes Hagström’s institutions have received research funding from Astra Zeneca, EchoSens, Gilead, Intercept, MSD, Novo Nordisk and Pfizer. He has served as consultant or on advisory boards for Astra Zeneca, Bristol Myers-Squibb, MSD and Novo Nordisk and has been part of hepatic events adjudication committees for KOWA and GW Pharma. Ann-Sofi Duberg has received honorary for expert meeting and lectures from Gilead and MSD. Rajani Sharma has served as a consultant for Takeda and Volv. Soo Aleman has received honoraria for lectures and educational events from Gilead, AbbVie, MSD and Biogen, and reports grants from Gilead and AbbVie. There are no relevant disclosures for Jacqueline Jossen, Benjamin Lebwohl, Jonas Söderling or Peter HR Green.
Data Availability
No datasets were generated or analyzed during the current study.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Green PH, Cellier C. Celiac disease. N Engl J Med 2007;357:1731–1743. [DOI] [PubMed] [Google Scholar]
- 2.Röckert Tjernberg A et al. Celiac disease and serious infections: a nationwide cohort study from 2002 to 2017. Off J Am Coll Gastroenterol ACG 2022;117:1675–1683. [DOI] [PubMed] [Google Scholar]
- 3.Ludvigsson JF et al. Coeliac disease and risk of sepsis. Gut 2008;57:1074–1080. [DOI] [PubMed] [Google Scholar]
- 4.Lebwohl B et al. Risk of clostridium difficile infection in patients with celiac disease: a population-based study. Am J Gastroenterol 2017;112:1878–1884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.World Health, O., Global health sector strategy on viral hepatitis 2016–2021. Towards ending viral hepatitis. 2016, World Health Organization: Geneva.
- 6.Zingone F et al. Long-term antibody persistence and immune memory to hepatitis B virus in adult celiac patients vaccinated as adolescents. Vaccine 2011;29:1005–1008. [DOI] [PubMed] [Google Scholar]
- 7.Vitaliti G et al. Hepatitis B vaccine in celiac disease: yesterday, today and tomorrow. World J Gastroenterol 2013;19:838–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nemes E et al. Gluten intake interferes with the humoral immune response to recombinant hepatitis B vaccine in patients with celiac disease. Pediatrics 2008;121:e1570–e1576. [DOI] [PubMed] [Google Scholar]
- 9.Opri R et al. Immune response to Hepatitis B vaccine in patients with celiac disease: a systematic review and meta-analysis. Hum Vaccin Immunother 2015;11:2800–2805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rousseff T et al. Hepatitis B virus vaccination and revaccination response in children diagnosed with coeliac disease: a multicentre prospective study. Acta Gastroenterol Belg 2019;82:27–30. [PubMed] [Google Scholar]
- 11.Vajro P, Paolella G, Nobili V. Children unresponsive to hepatitis B virus vaccination also need celiac disease testing. J Pediatr Gastroenterol Nutr 2012;55:e131. [DOI] [PubMed] [Google Scholar]
- 12.Ludvigsson JF, Lashkariani M. Cohort profile: ESPRESSO (epidemiology strengthened by histoPathology reports in Sweden). Clin Epidemiol 2019;11:101–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Husby S et al. European society for pediatric gastroenterology, hepatology, and nutrition guidelines for the diagnosis of coeliac disease. J Pediatr Gastroenterol Nutr 2012;54:136–160. [DOI] [PubMed] [Google Scholar]
- 14.Ludvigsson JF et al. Registers of the Swedish total population and their use in medical research. Eur J Epidemiol 2016;31:125–136. [DOI] [PubMed] [Google Scholar]
- 15.Ludvigsson JF et al. External review and validation of the Swedish national inpatient register. BMC Public Health 2011;11:450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Colombe S et al. Monitoring the progress towards the elimination of hepatitis B and C in Sweden: estimation of core indicators for 2015 and 2018. BMC Infect Dis 2022;22:885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Duberg AS et al. Chronic hepatitis B virus infection and the risk of hepatocellular carcinoma by age and country of origin in people living in Sweden: a national register study. Hepatol Commun 2022;6:2418–2430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Public Health Agency of Sweden. Vaccination programmes and recommendations. 2022 [cited 2023 10/5/2023]; Available from: https://www.folkhalsomyndigheten.se/the-public-health-agency-of-sweden/communicable-disease-control/vaccinations/vaccination-programmes/.
- 19.Zhang L et al. Status of HBsAg seroprevalence in 15 million rural couples in China: a cross-sectional study. Sci Rep 2017;7:42822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ataei B et al. A case-control study of risk factors for hepatitis B infection: a regional report among Isfahanian adults. J Res Med Sci 2019;24:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Peng S et al. Maternal age and educational level modify the association between chronic hepatitis B infection and preterm labor. BMC Pregnancy Childbirth 2020;20:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ludvigsson JF et al. Ethical aspects of registry-based research in the Nordic countries. Clin Epidemiol 2015;7:491–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.World Health Organization. Hepatitis B. 2022, June 24. [cited 2023 6–1–2023]; Available from: https://www.who.int/news-room/fact-sheets/detail/hepatitis-b.
- 24.Li ZK et al. The effect of HLA on immunological response to hepatitis B vaccine in healthy people: a meta-analysis. Vaccine 2013;31:4355–4361. [DOI] [PubMed] [Google Scholar]
- 25.Zingone F et al. Role of gluten intake at the time of hepatitis B virus vaccination in the immune response of celiac patients. Clin Vaccine Immunol 2013;20:660–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ertekin V, Tosun MS, Selimoglu MA. Is there need for a new hepatitıs B vaccine schedule for children with celiac disease? Hepat Mon 2011;11:634–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Habash N et al. Celiac disease: risk of Hepatitis B infection. J Pediatric Gastroenterol Nutr 2022;74:328–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.US Department of Health and Human Services. Viral Hepatitis in the United States: Data and Trends. 2018 [cited 2023 5/15/2023]; Available from: https://www.hhs.gov/hepatitis/learn-about-viral-hepatitis/data-and-trends/index.html#:~:text=850%2C000%20people%20in%20the%20U.S.,or%20as%20low%20as%20730%2C000.&text=More%20than%20half%20of%20persons,that%20they%20have%20the%20virus.
- 29.Quaglia M et al. Viral infections and systemic lupus erythematosus: new players in an old story. Viruses 2021;13:277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Arango MT, S.Y., Cervera R, et al., Infection and autoimmune diseases. Autoimmunity: From Bench to Bedside ed. 2013: El Rosario University Press.
- 31.Nekoua MP, Alidjinou EK, Hober D. Persistent coxsackievirus B infection and pathogenesis of type 1 diabetes mellitus. Nat Rev Endocrinol 2022;18:503–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Maya R, Gershwin ME, Shoenfeld Y. Hepatitis B virus (HBV) and autoimmune disease. Clin Rev Allergy Immunol 2008;34:85–102. [DOI] [PubMed] [Google Scholar]
- 33.Oikarinen M et al. Enterovirus infections are associated with the development of celiac disease in a birth cohort study. Front Immunol 2020;11:604529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brown JJ, Jabri B, Dermody TS. A viral trigger for celiac disease. PLoS Pathog 2018;14:e1007181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stene LC et al. Rotavirus infection frequency and risk of celiac disease autoimmunity in early childhood: a longitudinal study. Am J Gastroenterol 2006;101:2333–2340. [DOI] [PubMed] [Google Scholar]
- 36.Størdal K et al. Review article: exposure to microbes and risk of coeliac disease. Aliment Pharmacol Ther 2021;53:43–62. [DOI] [PubMed] [Google Scholar]
- 37.Hamidreza S et al. The prevalence of celiac autoantibodies in hepatitis patients. Iran J Allergy, Asthma Immunol 1970;9:157. [PubMed] [Google Scholar]
- 38.Nau AL et al. Prevalence and clinical features of celiac disease in patients with hepatitis B virus infection in Southern Brazil. Rev Soc Bras Med Trop 2013;46:397–402. [DOI] [PubMed] [Google Scholar]
- 39.Leonardi S, La Rosa M. Are hepatitis B virus and celiac disease linked? Hepat Mon 2010;10:173–175. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No datasets were generated or analyzed during the current study.