Abstract
Nanoparticles of blanched green banana peels (BGBP) were prepared using physical technique (by grinding) in order to avoid any harm effect on human health that could occurred when metals were used for preparing nanoparticles size (NPs) of banana peels. This work was designed to study the preparation of nano scale (70–135 nm for TEM) (243.4–933.9 nm for SEM) and normal size (0.12–0.25 µm for TEM) (1.150 µm for SEM) from BGBP after milling and evaluate the activities of their extracts as antioxidant, antimicrobial and anticancer agents. The size and shape of nanoparticles were analyzed using Scanning Electron Microscope (SEM) and it cleared the appearance of particle aggregation was attributed to mechanical pressure and friction resulting from the interaction between the abrasive balls and the pulverizing vessel’s inner surface. Also, Transmission Electron Microscope (TEM) shows presence of different spherical shapes ranging between 70 and 135 nm, along with the emergence of slender fibrillary shapes., on the other hand, Fourier transform IR (FT-IR) cleared that the higher extraction yields of phenolic compounds and greater antioxidant activities were achieved due to the increased surface area of nano-scale samples following milling. In addition, X-ray diffraction (XRD) determined the materials crystalline structure. Bioactive compounds (mainly phenolic compounds) were recovered by extracting banana peels weather from normal size or (NPs). The extracted bioactive compounds were subjected to evolution as antioxidant, antimicrobial and anticancer agents. Dealing with this study, it was concluded that bioactive compounds extracted from NPS of BGBP showed antioxidant, antimicrobial and anticancer activities higher than those extracted from the normal size ones. So, it is strongly recommended to use NPs of BGBP for producing these bioactive compounds since these compounds are important to protect humans against a lot of dangerous diseases. Finally, the potential applications of these compounds in the pharmaceutical or food industries would be beneficial.
Keywords: Banana peels (Musa acuminata), Superfine grinding (ball milling), Bioactive compounds, Antioxidant, Antimicrobial, Anticancer
Subject terms: Biotechnology, Cancer, Cell biology, Microbiology, Structural biology, Environmental sciences, Diseases, Nanoscience and technology
Introduction
Banana is the largest herbaceous plant globally, ranks among the top ten vital crops, occupying the fourth spot in global production with over 7 million tons harvested through traditional farming methods. Roughly1. In addition, approximately 90 million tons of bananas are grown in tropical regions like Africa (13%), South and Central America (28%), and South Eastern Asia (47%). However, 40% of this yield is discarded, mostly in the form of banana peels2. On the other hand, the average consumption of bananas per person is 12 kg per month, making it the leading food crop in the world after rice, wheat and corn. Consequently, here has been a significant rise in worldwide banana production over the last two decades, reaching approximately 117 million tons in 2019 from around 70 million tons in 19993.
Innovative approaches are needed to develop safe and powerful new and eco-friendly technologies, aiming for the advancement of green technology that plays a major role in environmental sustainability by producing nanostructures safely for both human health and the environment4–6. Nanotechnology refers to the designing, synthesis, and use of particles ranging from 1 to 100 nm in size, leading to a variety of nano-scale materials that improve solubility and bioavailability, reduce toxicity, enhance tissue macrophage circulation, and protect against physical and chemical degradation5. Nanoparticles possess a notable benefit because of their elevated ratio of surface area to volume, which enhances their surface reactivity and changes the physical and biological properties of nanomaterial’s6,7.
There are many physical methods that are widely used to produce nanomaterials, among these methods are inert gas condensation (IGC), physical evaporation, electric arc discharge, sputtering, and laser methods. Many characterization analysis techniques of nanomaterials, including ultraviolet–visible (UV–V) spectroscopy, XRD (X-ray diffraction), BET (Brunauere emmette teller), FESEM (Field emission scanning electron microscopy), FTIRS (Fourier transform infrared spectroscopy), TEM (Transmission electron microscopy) and Zeta size analysis. The unique properties that distinguish nanomaterials, allows them to penetrate many applications that directly serve the world. Nanomaterials have been utilized in various applications in the environment, agriculture, food industries, medical industries, chemical processing, and military industries8.
Banana peels are rich in their content of dietary fiber (50% on a dry matter basis), proteins (7% dry weight), and essential amino acids9. Furthermore, the significant amount of organic material found in banana peels, including fats, proteins, and carbohydrates, indicates that they are a valuable source of fiber and carbohydrates. Nevertheless, the considerable amount of fiber found in banana skins can aid in treating constipation and enhancing one’s overall health and well-being10. Also, Banana peels contain high levels of phytochemical compounds, particularly antioxidants. The concentration of phenolic compounds in banana peel varies between 0.90 and 3.0 g per 100 g of dry weight11. The peel was particularly high in phenolic, with a concentration of 907 mg/100 g dry weight. It additionally has antioxidant substances like polyphenols, catecholamines, and carotenoids12. These phytochemicals have been shown to exert important biological effects (antibacterial, antihypertensive, antdiabetic, anti-inflammatory activities and anticancer). Thus, the presence of these important and biologically active substances in banana peels indicates that the peels possess valuable medicinal potential that should be explored and utilized13.
In addition, bananas contain various antimicrobial compounds such as dopamine, gentisic acid, ferulic acid, lupeol, and 3-carene, which give them natural antioxidant and antimicrobial properties. It provides a simple and environmentally friendly alternative to chemical antibacterial substances and fungicides14. Interestingly, some fruits, such as banana peels, have shown higher antimicrobial activity in their seeds and peels compared to the pulp15. The use of peels as antimicrobial agents can help restore current environmental waste problems and at the same time bring enormous benefits to humanity16. Also, various compounds produced by banana peel such as flavonoids and polyphenols have shown anti-cancer activity. Flavonoids are a class of compounds known to have antioxidant and anti-cancer activity. Polyphenols can prevent carcinogenesis by affecting molecular pathways in the initiation, promotion, progression, and metastasis stages17.
This study was initially conducted to investigate if it is feasible to produce banana peel nanoparticles through a physical method (grinding) as opposed to a chemical method, which may have negative effects on human health with prolonged usage. The second aim of this research is to determine how altering the particle size of banana peels powder from normal to NPs impacts the antioxidant, antimicrobial, and anticancer properties of their bioactive extracts. The size and shape of nanoparticles were analyzed using Scanning Electron Microscope (SEM), Transmission Electron Microscope (TEM), Fourier transform IR (FT-IR) and X-ray diffraction (XRD).
Materials and methods
Materials
Plant material
Bananas (Musa acuminata) were purchased in their second stage of maturity, when they were light green without the use of ethylene, from the Faculty of Agriculture farm at Kafr El-sheikh University, located in Kafr El-sheikh Governorate, Egypt.
Chemicals and solvents
All chemicals were pure (1, 1-diphenyl-2-picrylhydrazyl (DPPH), acetic acid (0.5%), acetonitrile (99.5%) and ascorbic acid and solvents used in this study purchased from El-Gomhoria Company for Chemicals and Drugs at Tanta City, Egypt.
Microorganisms
All strains of the microorganisms were kindly provided by the Department of Plant Pathology at Kafrelsheikh University Faculty of Agriculture. We examined these microbes to ensure their authenticity and purity. In addition to gram negative Escherichia coli (ATCC 8739) and K. pneumonia (ATCC13883), a bacterial strains representing gram positive Bacillus Subtilis (ATCC 6633) and Staph.aureus (ATCC 6538). Aspergillus niger was used to represent molds in this experiment, C. albicans (ATCC 10221) as a representation of yeasts, the original cultures were stored in 10% glucose and new cultures were regularly created every two months. The cultures were stored in a refrigerator at 5°C until used.
Methods
Preparation of sample
Bananas “Grande Nain” cultivar (Musa acuminata), were obtained from the farm of the Faculty of Agriculture, Kafr El-sheikh University, Egypt. Fruits were selected according to uniformity and level of maturity (stage 2—light green). The fruits were weighed, washed with distilled water, then peeled and the peels were cut into homogeneous parts. They were immersing in water at 100 °C for 6 min. Then immediately immersed in a 0.5 percent solution of sodium metbisulfite for 15 min to prevent browning. The peels were placed in separate stainless steel trays and air-dried at 50 °C in an air oven until a constant weight was obtained (about 17 h). The resulting dried material is crushed, ground, and sift using 100 mesh screen sieve, then stored in polyethylene plastic bags at a temperature of 25 °C until used.
Preparation of BGBP and NPs extracts
The dried of BGBP and NPs samples (1 g) were individually placed in 10 ml of distilled water, 70% methanol, 70% ethanol, and 70% acetone for 24 h at room temperature. The extraction process involved filtering every mixture with Whatman No. 1 filter paper three times. The filtrate was evaporated to dryness at 40 °C using a rotary evaporator. The unrefined extracts were kept in a fridge until they were analyzed.
Gross chemical composition
Moisture, ash, ether extract, crude fiber and crude protein were performed according to the methods given18. The total carbohydrates amount was determined by subtracting protein, ether extract and ash from the 100% total mass, as reported19. The aforementioned tests were all conducted three times, and the findings were reported as an average based on the weight of the samples after they were dried.
Total phenolic content
The total phenol (TP) content was measured using the Folin Ciocalteu reagent assay and gallic acid was used as the standard20. 9 ml of distilled water was placed in a 25 ml volumetric flask along with 1 ml each of BGBP and NPs extracts. After the addition of one milliliter of Folin Ciocalteu’s phenol reagent, the mixture was shaken. After five minutes. The blend was mixed with 10 ml of Na2CO3 solution containing 7% concentration (w/v). The solution was thinned out and then combined with distilled water to reach a total volume of 25 ml. After 90 min of incubation. The spectrophotometer (Unicum UV 300) was used to measure absorbance at 750 nm with the blank set as the detector at room temperature. The amount of phenolic content in samples was measured in milligrams of gallic acid equivalents (GAE/g dry weight). Samples were analyzed in triplicates.
Total flavonoid content
Total flavonoid (TF) was determined by the aluminum chloride method using quercetin as a standard21. 4 ml of distilled water was first added to a 10 ml volumetric flask, and then 1 ml of BGBP and NPs of BGBP extracts were added. 0.3 ml of 5% NaNO2 was added to the flask, followed by the addition of 0.3 ml of 10% AlCl3 after 5 min. 6 min later, 2 ml of 1 M NaOH was introduced and the volume was topped up to 10 ml with distilled water. The mixtures were fully mixed and the level of absorption was measured at 510 nm using a spectrophotometer (Unicum UV 300) compared to a reagent blank that had been prepared. The concentration of flavonoids in the sample was indicated in milligrams of quercetin equivalents (QE) per gram of dry weight. Samples were analyzed in triplicates.
Total tannins content
Total tannin (TT) was measured using the Folin-Ciocalteu reagent22. 7.5 ml of distilled water was mixed with 1 ml of BGBP and NPs of BGBP extracts, then 0.5 ml of Folin reagent and 1 ml of 35% sodium carbonate solution were added. The diluted solution was mixed with distilled water to reach a volume of 10 ml, and then the absorbance was taken at 775 nm using a Unicom UV 300 spectrophotometer while comparing it to a blank reagent. The amount of tannins in the sample was determined in milligrams of tannic acid equivalent (TE) per gram of dry weight. Samples were analyzed in triplicates.
Identification of phenolic compounds by HPLC
Phenolic compounds in BGBP and NPs of BGBP acetone extracts were identified using HPLC23. The HPLC system includes an Agilent 1100 series with a DAD detector (G1315B) and DEGASSER (G1322A). 5 μl sample injections were carried out using an Agilent 1100 series auto-sampler. The column employed for the chromatographic separations was the ZORBAX-EclipseXDB-C18 (4.6 × 250 mm, particle size 5 μm). A steady 1 ml/min flow rate was employed with the following mobile phases: (A) distilled water with 0.5% acetic acid and pH 2.65; and solvent (B) 99.5% acetonitrile with 0.5% acetic acid. The DAD detector was set at wavelength 280 nm for 50 min, eluting linearly from A to B. The identification of phenolic compounds in acetone extracts of BGBP and NPs of BGBP was done by comparing their retention times with a standard mixture chromatogram. Peak area measurements were used to determine the concentration of a single compound.
Converting BGBP particles from normal size (70–135 nm) and nano-scale (1–100 nm) using ball milling
Ball milling (BM) is a green technology for the preparation and functionalization of nano derivatives24. BM was carried out employing the Planetary Micro Mill (Pulverisette 7 classic line, FritschGmbH, Germany). BGBP was placed in a stainless-steel container with Zirconium dioxide grinding balls, which were 5–10 mm in diameter. The BM process was carried out in an argon gas environment to avoid the impact of milling heat, with a powder mass to ball mass ratio of 1:7 by weight. The high-speed milling operated at a speed of 500 revolutions per minute (rpm) for 5 h25.
Scanning electron microscopy (SEM)
The physical structure and shape of banana peel powder were characterized using a JSM-7500F high-vacuum Scanning Electron Microscope (SEM) (Jeol Ltd, Japan). The specimen was placed on the metallic mount and gilded, enabling the samples to be prepared and moved into the microscope chamber in a vacuum. Following this, SEM was utilized to take images of the powder with a 15 kV acceleration voltage26.
Transmission electron microscope (TEM)
In TEM, a small amount of BGBP and NPs of BGBP in water was placed on a copper grid with carbon coating, then left to dry at room temperature. The images were captured using a TEM (JEOL-JEM-1200 EX, Japan) at 80 kV. The pattern of electron diffraction was also documented for a specific area. The PSS- NICOMP 380-ZLS particle sizing spectrophotometer, located in St. Barbara, USA, was used to determine the mean particle size and particle size distribution27.
Fourier transform IR analysis (FT-IR)
FT-IR spectral analysis was conducted with a Bruker FT-IR spectrometer (Invenio S, Germany). The IR spectra were collected using a wavenumber range of 400–4000 cm−1, with a spectral resolution of 4 cm−1 and 64 scans28.
X-ray diffraction (XRD) measurement
The XRD diffractometer used to analyze XRD patterns of fibers, cellulose, and CNCs was the PANalytical Xpert pro MRD diffractometer in Amsterdam, Netherlands, operated at 40 kV and 30 mA with Cu Ka radiation at 0.15406 Å wavelength and a nickel monochromator filtering wave. The specimens were scanned at room temperature with a scanning rate of 0.4°/min across the range of 20 = 5–40°. The equation used to determine the crystallinity index (CI%) was calculated as follows29.
where I200 represents the intensity at level 200, while Iam is the lowest intensity between levels 110 and 200, specifically at the peak of 20 to 21 and 9. 1200 is made up of both the crystalline and amorphous sections, whereas Iam specifically denotes the amorphous portion. The Scherer equation was utilized to determine the crystallite size of BGBP and NPs30.
 |
where K is the Scherrer constant (0.94), λ is the X-ray wavelength (λ = 0.15406 Å), β1/2 is the full width at the half maximum (FWHM) of the XRD peak, and θ is the Bragg’s angle.
The size of the crystallite was determined by analyzing the diffractograms and removing the impact of the amorphous peak (Iam) from the peak height of 200 planes.
Determination of antioxidant activity by DPPH of BGBP in normal size (70–135 nm) and nano-scale (1–100 nm)
The antioxidant capacity of various banana peel extracts was assessed using 1, 1-diphenyl-2-picryl hydrazyl (DPPH) as the indicator. To summarize, a solution of 0.1 mM DPPH in ethanol was made. 1 ml of this solution was mixed with 3 ml of various ethanol extracts at varying concentrations (3.9, 7.8, 15.62, 31.25, 62.5, 125, 250, 500, 1000 µg/ml). Only the ethanol-soluble extracts were utilized, and different concentrations were made through dilution. The blend was vigorously shaken and left at room temperature for 30 min before measuring absorbance at 517 nm. Through the utilization of a spectrophotometer (specifically the UV–VIS Milton Roy model). The experiment was performed three times using ascorbic acid as the reference standard compound. The concentration of the sample needed to inhibit 50% of the DPPH free radical was determined by calculating the IC 50 value with a Log dose inhibition curve. Higher free radical activity was suggested by lower absorbance in the reaction mixture31. The percent DPPH scavenging effect was calculated by using following Equation:
where A0 was the Absorbance of control reaction and Al was the Absorbance.
in presence of test or standard sample.
Determination of antimicrobial activity of BGBP in normal size (70–135 nm) and nano-scale (1–100 nm)
The antimicrobial activity of different samples of normal and NPs of BGBP powder were measured using Disk or well Diffusion Approaches32. The agar well diffusion method is commonly employed for assessing the antimicrobial properties of plant or microbial extracts. Just like in the disk-diffusion method, the agar plate surface is inoculated by spreading the microbial inoculum across the entire agar surface. Next, a sterile cork borer or tip is used to punch a hole with a diameter of 6–8 mm aseptically, following which (10mg/ml) of the antimicrobial agent or sample extract or extract solution added to the well. Next, agar plates are placed in an incubator with conditions specific to the type of microorganism being tested. The antimicrobial substance spreads through the agar medium and hinders the growth of the tested microbial strain.
Determination of Anticancer activity of BGBP of in normal size and NPS
The cytotoxicity of all the extracts was determined by a tetrazolium (MTT) assay33. HepG2 cells were seeded onto 96 well plates at a density of 2 × 105 cells/ml per well in 100 μl of RPMI 1640 using a Bioklenz laminar air flow hood, and incubated for 24 h at 37°C and 5% CO2 in a CO2 incubator (New Brunswick Galaxy 170S). Following that, the medium was exchanged with a fresh one that had varying concentrations of the sample (31.25, 62.5, 125, 250, 500, and 1000 μg/ml) for an additional 48-h incubation period. Next, each well received 20 μl of MTT stock solution (5 mg/ml in phosphate-buffered solution) and was incubated for 5 h. The solution was eliminated, and 200 μl of DMSO was introduced into each well to dissolve the MTT metabolic product. The ELISA reader measured the absorbance at 560 nm. The formula was used to determine the percentage viability of the samples.
Statistical analysis
The received data were statistically analyzed34. All tests were repeated triplicate. The data was assessed for statistical significance using one-way analysis of variance (ANOVA) followed by Duncan’s multiple-range test, with a significance level of p < 0.05. Statistical analysis was conducted with SPSS version 23.0 for all data.
Results and discussion
Proximate chemical composition of BGBP
Table 1 showcased the nutritional content of BGBP agro-wastes in this research.
Table 1.
Proximate chemical composition of BGBP powder (Percentage were calculated on dry weight basis).
| Components | Concentration (%) |
|---|---|
| Moisture** | 91.21 ± 0.04 |
| Ash | 13.42 ± 0.73 |
| Ether extract | 3.98 ± 0.11 |
| Crude fibers | 28.74 ± 0.29 |
| Crude Protein | 9.06 ± 0.06 |
| *Total carbohydrates | 73.54 ± 0.9 |
All values in Table are means of triplicate determinations ± standard deviation (SD).
* Total carbohydrates were calculated by difference. **: On wet weight basis.
Proximate analysis provides important data on the nutritional content and aids in evaluating the sample’s quality. It gives details about the levels of moisture, protein, ether extract, ash, fiber, and carbohydrate. Research has indicated that fruit wastes, including peels and seeds, contain a significant amount of essential nutrients such as carbohydrates, proteins, ether extract, fibers, and phytochemicals35. It should be noted from Table 1 that moisture content of the sample was relatively high (91.21%) and close enough to those found by36. In addition, it should be noted also that banana peels were rich in carbohydrates. Furthermore they have moderate amounts of crude fiber, ash and crude protein. Meanwhile, they seem to have a low level of ether extract. Banana peels are rich in their content of dietary fiber (50% on a dry matter basis), proteins (7% dry weight), and essential amino acids9. Also, the results obtained align with previous findings9,37,38.
Total phenols (TP), flavonoids (TF) and tannins (TT) of normal size (70–135 nm) and nano-scale (1–100 nm)
Of BGBP extracts:
Phenolic compounds are important secondary metabolites and are found in higher levels in banana peels compared to other fruits39. Table 2 displays the outcomes of TP, TF, and TT in aqueous, 70% methanol, 70% acetone, and 70% ethanol extracts. The information in Table 2 demonstrated that the acetone extract from normal-sized and nano-sized banana peels has the highest concentrations of TP (30.02 and 50.3 mg/g dry weight), TF (31.56 and 55.4 mg/g dry weight) and TT (22.95 and 36.05 mg/g dry weight), respectively. While the aqueous extract showed the lowest values for TP (15.97 and 27.37 mg/g dry weight), TF (18.23 and 28.1 mg/g dry weight), and TT (19.34 and 30.39 mg/g dry weight) respectively. The results obtained indicate that banana acetone extract possesses a high content of phenolic, flavonoids and tannins. Banana showed high content in phenolic compounds, especially vanillic and ferulic acids. Banana acetone extracts found to have considerable antioxidant and antimicrobial activities. Banana leaves acetone extract also presented vigorous activity against breast and liver hepatocellular tumor cell lines40,41.
Table 2.
Total phenols (TP), flavonoids (TF) and tannins (TT) of normal size and nano-scale of BGBP extracts.
| Sample | Extracts | TP(mg GAE/g DW) | TF(mg QE/g DW) | TT (mg TE/g DW) |
|---|---|---|---|---|
| Normal size (70–135 nm) | Aqueous | 15.97c ± 0.10 | 18.23c ± 0.34 | 19.34b ± 0.23 |
| Methanol 70% | 27.28b ± 0.19 | 29.14b ± 0.25 | 19.35b ± 0.11 | |
| Acetone 70% | 30.02a ± 0.08 | 31.56a ± 0.3 | 22.95a ± 0.08 | |
| Ethanol 70% | 27.86b ± 0.15 | 29.39b ± 0.21 | 21.30 ± 0.06 | |
| Nano-scale (1–100 nm) | Aqueous | 27.37c ± 0.40 | 28.1d ± 0.08 | 30.39c ± 0.15 |
| Methanol 70% | 42.15b ± 0.34 | 43.69c ± 0.17 | 31.80 ± 0.76 | |
| Acetone 70% | 50.3a ± 0.28 | 55.4a ± 0.89 | 36.05a ± 0.11 | |
| Ethanol 70% | 44.28b ± 0.33 | 46.58b ± 0.28 | 33.18b ± 0.18 |
All values represented as mean ± S.D Means with different letters are significantly different (p < 0.05).
Nevertheless, ethanol yielded good outcomes for extracting BGBP and NPs, with TP content measuring (27.86 and 44.28 mg/g DW), flavonoids content measuring (29.39 and 46.58 mg/g DW), and tannins measuring (21.30 and 33.18 mg/g DW), respectively. The methanol extract in this study showed phenolic content at 27.28 and 42.15 mg/g DW. These results exceed the ones he achieved42,43.
Identification and quantification of phenolic compounds of normal size (70–135 nm) and nano-scale (1–100 nm)
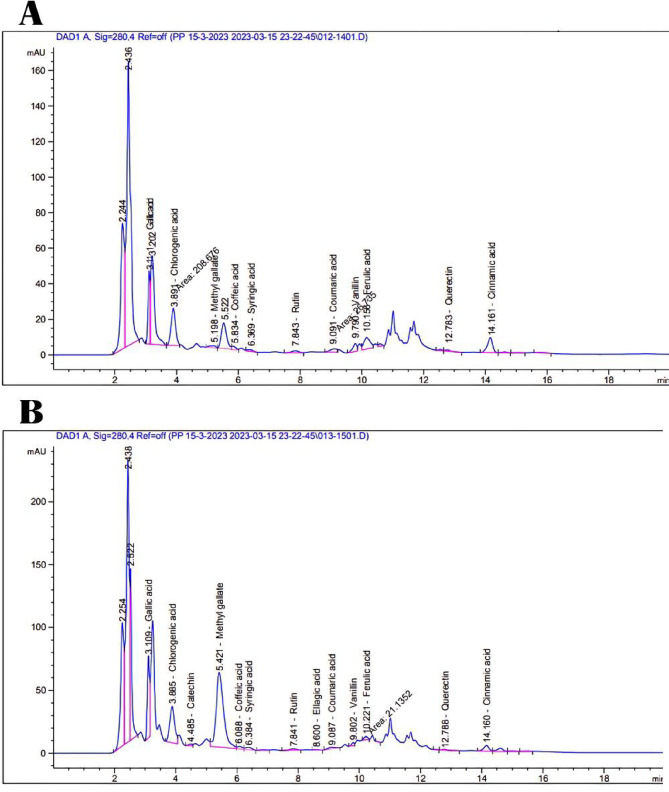
Data in Fig. 1 and Table 3 indicate that the levels of specific phenolic compounds were extracted using a 70% acetone extract. Variations in diversity, environmental factors, and farming methods have a significant impact on the composition and levels of phenolic compounds. Yet, the drying procedure could impact the composition of the matrix components and their ability to promote biological activity. The separation and identification of phenolic compounds in normal size and NPs of banana peels were analyzed by high-performance liquid chromatography (HPLC). 11 phenolic compounds identified in banana peels both in normal size and NPs extracted using acetone include gallic acid, chlorogenic acid, methyl gallate, coffeic, syringic acid, rutin, coumaric acid, vanillin, ferulic acid, querectin and cinnamic acid. Ellagic acid and catechin are found in NPs banana peels, but are not present in normal size banana peels this is a result of the ball grinding process. The ultra-fine grinding led to an increase in the concentrations of Gallic acid, chlorogenic acid, methyl gallate, querectin, coffeic acid, syringic and rutin. Nevertheless, the levels of coumaric acid, vanillin, ferulic acid, and cinnamic acid decreased as a result of the grinding procedure. The results obtained align with previous findings44–47.
Fig. 1.
HPLC chromatogram of BGBP in normal size (A) and nano-scale (B).
Table 3.
Quantification of the main phenolic compounds present in acetone extracts (ug/ml crude extract) by HPLC.
| Compounds | Normal size (70–135 nm) | Nano-scale (1–100 nm) |
|---|---|---|
| Gallic acid | 28.58 | 54.21 |
| Chlorogenic acid | 15.3 | 27.39 |
| Catechin | – | 2.70 |
| Methyl gallate | 0.83 | 44.38 |
| Coffeic acid | 1.38 | 1.65 |
| Syringic acid | 0.92 | 1.54 |
| Rutin | 2.24 | 2.50 |
| Ellagic acid | - | 1.10 |
| Coumaric acid | 0.84 | 0.69 |
| Vanillin | 1.73 | 0.62 |
| Ferulic acid | 6.38 | 1.44 |
| Querectin | 1.20 | 1.09 |
| Cinnamic acid | 1.85 | 0.99 |
Characterization of nano banana peel particles
SEM characterization
SEM micrographs of the prepared particles and originals images are shown in Fig. 2C, D. The SEM images of NPs of BGBP powders revealed that the particles underwent fragmentation and developed sharp edges in consequence of the mechanical forces applied during BM. It also showed that the particles had a spherical shape, although some particles showed irregular shapes48. The appearance of particle aggregation was attributed to mechanical pressure and friction resulting from the interaction between the abrasive balls and the pulverizing vessel’s inner surface. Compared with pre-milling and after milling originals images as shown in Fig. 2A, B. There are some findings suggest that BM induced ultrafine grinding can modify original surface structures, which, in turn, may affect the physicochemical characteristics of powdery materials49. The results50 support our findings.
Fig. 2.
Original images (A, B), SEM images (C, D) and TEM images (E, F) of BGBP pre and after milling.
TEM’s characterization
The TEM analysis of banana peel powder is depicted in Fig. 2E, F, both before and after grinding with a BM. In Fig. 2E, the typical BGBP size ranges from 0.12 to 0.25 uM. Figure 2F shows clear presence of different spherical shapes ranging between 70 and 135 nm, along with the emergence of slender fibrillary shapes. Compared with pre-milling and after milling originals images as shown in Fig. 2A, B. They vary from extremely small nanofibers to elongated nanofibers. Due to their rounded shape and magnetic characteristics, these nano-sized particles show significant potential for use in areas like medicine, food, and nanotechnology51. Take note of the significance the properties and behavior of nanoparticles or nanofibers are significantly affected by their size and shape52.
Fourier transform IR analysis (FT-IR)
Figure 3A displays the findings of FT-IR analysis conducted on both normal and nano-sized BGBP extract. Fourier-transform infrared spectroscopy was used to identify alterations in the functional metabolites in both regular and nano-sized BGBP by examining characteristic IR peaks. Both samples exhibited peaks in their spectra at around 3296.35 and 3302.03 for regular size and nano-scale, respectively. Typically, the bands from 3500 to 3200 cm−1 are associated with OH stretching vibrations of phenolic structures53. Peaks in the range of 2918–2851 cm−1 are likely caused by stretching vibrations of CH and CH2. The peak observed at 1638 cm−1 is indicative of alkene C=C stretching vibrations. The band observed at 1034 cm−1 was attributed to the stretching vibrations of C–O in lignin and cellulose26. The nano-scale sample at 3302.03 cm−1 showed a slight increase in its peak spectrum when using ball-milling technique compared to the other spectrum, possibly caused by the aggregation of phenolic metabolites due to cellulose structure degradation. Moreover, certain peak transmittances like the peaks at 2087.22, 2916.23, and 2856.93 cm−1 associated with CH, CH2, and OH stretching vibrations were observed and altered following the milling process. This could be the result of milling processes improving the breakdown of hydroxyl groups in cellulose and hemicellulose, leading to the formation of soluble metabolite aggregates and expanding the porosity and external surface area54. The higher extraction yields of phenolic compounds and greater antioxidant activities were achieved due to the increased surface area of nano-scale samples following milling. The peak observed at 1012 cm−1 was identified as originating from the C–O stretching vibrations of lignin and cellulose. When comparing the spectra of normal and nano-scale, the absorption peaks at 1188, 1248, 1322 and 1625 cm−1 were altered due to smaller particle size. The results obtained align with previous findings26.
Fig. 3.

FT-IR (A), XRD (B) and DPPH (C) radical scavenging activity of BGBP in normal size and nano scale.
Measurement of X-ray diffraction (XRD)
The crystalline structure of BGBP was examined through XRD testing before and after grinding, and the results can be seen in Fig. 3B. Clear differences were observed in the crystalline structure of the powder pre and post grinding. A broad diffraction pattern was found at 23.03° for the NPs, distinct peaks were evident in the powder’s BM 2θ at around 64.56° and 78.04°, showing a crystalline section that matches cellulose’s usual crystalline pattern55.
Antioxidant activity of normal size (70–135 nm) and nano-scale (1–100 nm) of blanched green banana peels extract
Results of antioxidant activity of normal and NPS of BGBP extract are shown in Fig. 3C. It should be noted that antioxidant activity of normal and nano BGBP samples extract increased with increasing the concentration of samples extract, Concentrations ranging from 0 to 1000 μg/mL have been utilized. This in turn helped to increase the yield of the bioactive compounds extracted from the peels. These results were supported by those of56, who reported that banana peels are rich in antioxidant compounds such as vitamin C, vitamin E, β -carotene and anthocyanin.
Apparent also from the same figure that nanoparticles of banana peels extract had antioxidant activity higher than that of normal particles extract and V.C, The IC50 percentage was 2.09, 5.99 and 2.45 ug/ml respectively57, who reported that Nanoparticles exhibit superior DPPH radical scavenging abilities compared to the extract from larger particles, indicating that decreasing the size of the extract enhances its antioxidant properties.
Antimicrobial activity of normal size (70–135 nm) and nano-scale (1–100 nm) of BGBP extract
The antibacterial activity of starting materials (PPE of BGBP and NPs of BGBP) toward Gram-positive and Gram-negative bacteria were assessed Fig. 4A–C. Compared with the antibiotic Gentamycin was used as a bacterial standard. The results revealed that BGBP nanoparticles at a concentration of 10 mg/mL showed promising antibacterial properties against B. Subtitles, S. aureus, E. coli and K. pneumoniae where the inhibition zones were 36, 40, 30 and 32 mm respectively. Moreover the lethal effect on gram positive (B. Subtitles and S. aureus) was higher than that of gram negative (E. coli and K. pneumoniae). S. aureus showed the highest sensitivity to NPs of BGBP, whereas E. coli displayed the lowest sensitivity. These results were in agreement with those of58, and were inconsistent with59.
Fig. 4.
Antimicrobial activity (A, B), Zone of inhibition of bioactive compounds extracted from normal and NPS of BGBP against various pathogenic bacteria, C. albicans and A. niger (C, D). Where: P: Normal size of BGBP. B: Blank (solvent). N: Nano particles size of BGBP. Con: In the case of bacteria, the antibiotic is gentamycin, and in the case of fungi, the antibiotic is flucazole.
The mechanism of action of NPs of BGBP can be attributed to electrostatic interaction that damages cell membranes, deactivates proteins and enzymes, generates ROS and oxidative stress, binds to proteins causing disruption in homeostasis (electron transport chain), affects signal transduction and Restraint, DNA damage60,61. Furthermore, the effectiveness of damaging the cell wall by the nanoparticles is attributed to the roughness of their outer surface. This leads to nanoparticles penetrating the plasma membrane and inducing toxicity in bacteria., as observed by62. The result63,64 supports our findings.
Apparent also from the same figure that the extracted bioactive compounds that yielded from the nano banana peels had effect on gram positive and negative bacteria higher than those extracted from the normal ones this is shown in Fig. 4A–C. This could be attributed to the high yield of bioactive compounds extracted from the NPS compared to those extracted from the normal ones, which in turn could be related to the high surface area of the NPS. These results were in agreement27.
The antifungal activity of BGBP and NPs towards C. albicans and A. niger was shown in Fig. 4B–D. Compared with the Flucazole as fungi standard. The results showed that BGBP and NPs showed antifungal activity against C. albicans, as the inhibition zones were 23 and 32 mm respectively. These results were in agreement64, while no effectiveness was shown on A. niger. The results obtained align with previous findings59.
Anticancer effect of normal size (70–135 nm) and nano-scale (1–100 nm) of BGBP extract
Data shown in Fig. 5 indicated that the blanched normal and NPS banana peels extract illustrated high inhibition effect on the hepatocellular carcinoma cells (HepG2). Cytotoxicity of different concentrations of normal and nano banana peels (31.25–1000 µg/ml) were evaluated. Results indicated that the IC50 of bioactive compounds extracted from normal peels was 283.55 µg/ml and nano peels was 189.69 µg/ml. Moreover, the inhibition effect increased with increasing the concentration of banana peels extract, whither from normal or nano peels. In addition the effect of nano banana peels extract was higher than that of normal one. This could be attributed to the high yield of bioactive compounds extracted from blanched green nano banana peels were higher than those of normal one, hence the inhibition effect of the nanoparticles extract was higher than that of the normal one. The results showed that 70% ethanol extract of fruit peel (PBEE) and heart (HBEE) of Raja Bulu banana had median inhibition concentration (IC50) for DPPH scavenging activity at 115.32 µg/mL and 162.52 µg/mL respectively. Overall, the diverse essential micronutrients and phytochemicals found in fruits are largely responsible for their biological activities. These bioactive compounds can potentially stop cancer by functioning as antioxidants, slowing cell growth, triggering cell death, preventing cell invasion, and impacting signaling pathways within cells65,66. Nanoparticles with enhanced surface characteristics can diffuse more easily inside tumor cells, thus delivering an optimal concentration of drugs at tumor site while reducing the toxicity. Cancer cells can be targeted with greater affinity by utilizing NMs with tumor specific constituents. Furthermore, it bypasses the bottlenecks of indiscriminate biodistribution of the antitumor agent and high administration dosage. Here, we focus on the recent advances on the use of various nanomaterials for cancer treatment, including targeting cancer cell surfaces, tumor microenvironment (TME), organelles, and their mechanism of action. The paradigm shift in cancer management is achieved through the implementation of anticancer drug delivery using nano routes41.
Fig. 5.
Anticancer activity of BGBP in normal size (A, C, E) and nano-scale (B, D, E). (A, B) inhibition of cells, (C, D) IC50 (%), (E) cell viability (%).
There are many different methods for preparing nanomaterial, nanomaterial are used in many applications in various fields such as the environment, agriculture, industry, medicine, military defense systems, electronics, and energy storage. Regarding future trends, it is the use of nanomaterial in energy storage devices, electronics, environmental protection, and renewable energy production. For example, the use of nanomaterial in developing solar cell systems. Nanomaterial will be used to improve the efficiency of electrodes in energy storage batteries, hydrogen production as a renewable energy source, delivering medicines and treating chronic diseases such as cancer. From the above, the future of advanced technology is closely linked to progress in the field of nanomaterial technology engineering.
Conclusions
Banana peels that subjected to blanched treatment were treated with ultrafine grinding treatment to change the particles size from normal to nanoparticles size (physical grinding). This technique was used instead of the chemical technique so, the peels will be safe for human consumption changing the particles size from normal to nanoparticles size helped to increase the level of the phenolic compounds extracted from the nano peels, hence increase the activity of nano banana peels as antioxidant, antimicrobial and anticancer agents.
Acknowledgements
The authors are very grateful to Kafrelsheikh University and Agricultural Research Center, Food Technology Research Institute, Giza, Egypt for funding the scientific research entitled “Nanoparticles of banana peels as a potential source of bioactive compounds and their activities on HepG2”.
Author contributions
W. and D. wrote methodology and the manuscript text and prepared Figs. 1–5. On the other hand, S. and A. prepared original draft and writing—review and editing. All authors reviewed the manuscript.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
This work was funded by Kafrelsheikh University and was enhanced by a project funded by Agricultural Research Center, Food Technology Research Institute, Giza, Egypt entitled “Nanoparticles of banana peels as a potential source of bioactive compounds and their activities on HepG2”.
Data availability
The data used to support the findings of this study are available from the corresponding author upon request by the same email: walid.metwali@agr.kfs.edu.eg.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Bezerra, C. V., Rodrigues, A. M. D. C., Amante, E. R. & Silva, L. H. M. D. Nutritional potential of green banana flour obtained by drying in spouted bed. Rev. Bras. Frutic.35, 1140–1146 (2013). [Google Scholar]
- 2.Emaga, T. H. et al. Ripening influences banana and plantain peels composition and energy content. Trop. Anim. Health Prod.43, 171–177 (2011). [DOI] [PubMed] [Google Scholar]
- 3.Zou, F., Tan, C., Zhang, B., Wu, W. & Shang, N. The valorization of banana by-products: Nutritional composition, bioactivities, applications, and future development. Foods.11(20), 3170 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Velusamy, P., Kumar, G. V., Jeyanthi, V., Das, J. & Pachaiappan, R. Bio-inspired green nanoparticles: Synthesis, mechanism, and antibacterial application. Toxicol. Res.32(2), 95–102 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Soni, M., Mehta, P., Soni, A. & Goswami, G. K. Green nanoparticles: Synthesis and applications. IOSR J. Biotechnol. Biochem.4(3), 78–83 (2018). [Google Scholar]
- 6.Verma, A., Gautam, S. P., Bansal, K. K., Prabhakar, N. & Rosenholm, J. M. Green nanotechnology: Advancement in phytoformulation research. Medicines6(1), 39 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang, X., Wang, Y., Chen, Z. G. & Shin, D. M. Advances of cancer therapy by nanotechnology. Cancer Res. Treat.41(1), 1–11 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Al-Harbi, N. & Abd-Elrahman, N. K. Physical methods for preparation of nanomaterials, their characterization and applications: A review. J. Umm Al-Qura Univ. Appl. Sci. 1–22 (2024).
- 9.Emaga, T. H., Andrianaivo, R. H., Wathelet, B., Tchango, J. T. & Paquot, M. Effects of the stage of maturation and varieties on the chemical composition of banana and plantain peels. Food Chem.103(2), 590–600 (2007). [Google Scholar]
- 10.Anjum, S., Sundaram, S. & Rai, G. Nutraceutical application and value addition of banana (Musa paradisica L. Variety, “bhusawal keli”) peel: A review. Int. J. Pharm. Pharm. Sci.6(10), 81–85 (2014). [Google Scholar]
- 11.Nguyen, T. B. T., Ketsa, S. & Van Doorn, W. G. Relationship between browning and the activities of polyphenoloxidase and phenylalanine ammonia lyase in banana peel during low temperature storage. Postharvest Biol. Technol.30(2), 187–193 (2003). [Google Scholar]
- 12.Someya, S., Yoshiki, Y. & Okubo, K. Antioxidant compounds from bananas (Musa Cavendish). Food Chem.79(3), 351–354 (2002). [Google Scholar]
- 13.Ighodaroro, O., Mairiga, J. & Adeyi, A. Reducing and anti-proxidant profiles of flavonoids in Ocimum gratissimum. Int. J. Chem. Sci.2(1), 85–89 (2009). [Google Scholar]
- 14.Mostafa, H. S. Banana plant as a source of valuable antimicrobial compounds and its current applications in the food sector. J. Food Sci.86(9), 3778–3797 (2021). [DOI] [PubMed] [Google Scholar]
- 15.Jain, P., Bhuiyan, M. H., Hossain, K. R. & Bachar, S. C. Antibacterial and antioxidant activities of local seeded banana fruits. Afr. J. Pharm. Pharmacol.5(11), 1398–1403 (2011). [Google Scholar]
- 16.Chanda, S., Baravalia, Y., Kaneria, M. & Rakholiya, K. Fruit and vegetable peels–strong natural source of antimicrobics. Curr. Res. Technol. Educ. Top. Appl. Microbiol. Microb. Biotechnol.1, 444–450 (2010). [Google Scholar]
- 17.Ferguson, P. J., Kurowska, E., Freeman, D. J., Chambers, A. F. & Koropatnick, D. J. A flavonoid fraction from cranberry extract inhibits proliferation of human tumor cell lines. J. Nutr.134(6), 1529–1535 (2004). [DOI] [PubMed] [Google Scholar]
- 18.AC A. Association of official analytical chemists. Official methods of analysis of AOAC International (1990).
- 19.Tadrus, M. Chemical and Biological Studies on Some Baby Food (Cairo University Publ, 1989). [Google Scholar]
- 20.Singleton, V. L. & Rossi, J. A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic.16(3), 144–158 (1965). [Google Scholar]
- 21.Zhishen, J., Mengcheng, T. & Jianming, W. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem.64(4), 555–559 (1999). [Google Scholar]
- 22.Polshettiwar, S., Ganjiwale, R., Wadher, S. & Yeole, P. Spectrophotometric estimation of total tannins in some ayurvedic eye drops. Indian J. Pharm. Sci. 69(4) (2007).
- 23.Ben-Hammouda, M., Kremer, R. J., Minor, H. C. & Sarwar, M. A chemical basis for differential allelopathic potential of sorghum hybrids on wheat. J. Chem. Ecol.21, 775–786 (1995). [DOI] [PubMed] [Google Scholar]
- 24.Moosakazemi, F., Mohammadi, M. T., Mohseni, M., Karamoozian, M. & Zakeri, M. Effect of design and operational parameters on particle morphology in ball mills. Int. J. Min. Process.165, 41–49 (2017). [Google Scholar]
- 25.Gorrasi, G. & Sorrentino, A. Mechanical milling as a technology to produce structural and functional bio-nanocomposites. Green Chem.17(5), 2610–2625 (2015). [Google Scholar]
- 26.Hong, S. J., Das, P. R. & Eun, J. B. Effects of superfine grinding using ball-milling on the physical properties, chemical composition, and antioxidant properties of Quercus salicina (Blume) leaf powders. J. Sci. Food Agric.101(8), 3123–3131 (2021). [DOI] [PubMed] [Google Scholar]
- 27.Ibrahim, H. M. Green synthesis and characterization of silver nanoparticles using banana peel extract and their antimicrobial activity against representative microorganisms. J. Radiat. Res. Appl. Sci.8(3), 265–275 (2015). [Google Scholar]
- 28.El-Haddad, M. N. Chitosan as a green inhibitor for copper corrosion in acidic medium. Int. J. Biol. Macromol.55, 142–149 (2013). [DOI] [PubMed] [Google Scholar]
- 29.Park, S., Baker, J. O., Himmel, M. E., Parilla, P. A. & Johnson, D. K. Cellulose crystallinity index: Measurement techniques and their impact on interpreting cellulase performance. Biotechnol. Biofuels3, 1–10 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nazir, M. S., Wahjoedi, B. A., Yussof, A. W. & Abdullah, M. A. Eco-friendly extraction and characterization of cellulose from oil palm empty fruit bunches. BioResources8(2), 2161–2172 (2013). [Google Scholar]
- 31.Brand-Williams, W., Cuvelier, M.-E. & Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT-Food Sci. Technol.28(1), 25–30 (1995). [Google Scholar]
- 32.Magaldi, S. et al. Well diffusion for antifungal susceptibility testing. Int. J. Infect. Dis.8(1), 39–45 (2004). [DOI] [PubMed] [Google Scholar]
- 33.Li, F. et al. Antiproliferative activity of peels, pulps and seeds of 61 fruits. J. Funct. Foods5(3), 1298–1309 (2013). [Google Scholar]
- 34.Steel, R. G. D. & Torrie, J. H. Principles and Procedures of Statistics (1960).
- 35.Pyar, H. & Peh, K. Chemical compositions of banana peels (Musa sapientum) fruits cultivated in Malaysia using proximate analysis. Res. J. Chem. Environ.22(2), 108–111 (2018). [Google Scholar]
- 36.Nagarajaiah, S. B. & Jamuna Prakash, J. P. Chemical composition and antioxidant potential of peels from three varieties of banana (2011).
- 37.Emaga, T. H., Robert, C., Ronkart, S. N., Wathelet, B. & Paquot, M. Dietary fibre components and pectin chemical features of peels during ripening in banana and plantain varieties. Bioresour. Technol.99(10), 4346–4354 (2008). [DOI] [PubMed] [Google Scholar]
- 38.Essien, J., Akpan, E. & Essien, E. Studies on mould growth and biomass production using waste banana peel. Bioresour. Technol.96(13), 1451–1456 (2005). [DOI] [PubMed] [Google Scholar]
- 39.Sidhu, J. S. & Zafar, T. A. Bioactive compounds in banana fruits and their health benefits. Food Qual. Saf.2(4), 183–188 (2018). [Google Scholar]
- 40.Salama, Z. A. et al. In-vitro antioxidant, antimicrobial and anticancer activities of banana leaves (Musa acuminata) and olive leaves (Olea europaea L.) as by-products. Res. J. Pharm. Technol.13(2), 687–696 (2020). [Google Scholar]
- 41.Das, C. A., Kumar, V. G., Dhas, T. S., Karthick, V. & Kumar, C. V. Nanomaterials in anticancer applications and their mechanism of action—A review. Nanomed. Nanotechnol. Biol. Med.47, 102613 (2023). [DOI] [PubMed] [Google Scholar]
- 42.Aboul-Enein, A. M. et al. Identification of phenolic compounds from banana peel (Musa paradaisica L.) as antioxidant and antimicrobial agents. J. Chem. Pharm. Res.8(4), 46–55 (2016). [Google Scholar]
- 43.Görgüç, A., Bircan, C. & Yılmaz, F. M. Sesame bran as an unexploited by-product: Effect of enzyme and ultrasound-assisted extraction on the recovery of protein and antioxidant compounds. Food Chem.283, 637–645 (2019). [DOI] [PubMed] [Google Scholar]
- 44.Das, P. R. & Eun, J.-B. A comparative study of ultra-sonication and agitation extraction techniques on bioactive metabolites of green tea extract. Food Chem.253, 22–29 (2018). [DOI] [PubMed] [Google Scholar]
- 45.Das, P. R., Kim, Y., Hong, S.-J. & Eun, J.-B. Profiling of volatile and non-phenolic metabolites—Amino acids, organic acids, and sugars of green tea extracts obtained by different extraction techniques. Food Chem.296, 69–77 (2019). [DOI] [PubMed] [Google Scholar]
- 46.Fernandes, A., Mateus, N., Cabral, M. & de Freitas, V. Analysis of phenolic compounds in cork from Quercus suber L. by HPLCâ DAD/ESIâ MS (2011).
- 47.Khawas, P., Das, A., Sit, N., Badwaik, L. & Deka, S. Nutritional composition of culinary Musa ABB at different stages of development. Am. J. Food Sci. Technol.2(3), 80–87 (2014). [Google Scholar]
- 48.Mulenos, M. R., Lujan, H., Pitts, L. R. & Sayes, C. M. Silver nanoparticles agglomerate intracellularly depending on the stabilizing agent: Implications for nanomedicine efficacy. Nanomaterials10(10), 1953 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhao, X., Zhu, H., Zhang, G. & Tang, W. Effect of superfine grinding on the physicochemical properties and antioxidant activity of red grape pomace powders. Powder Technol.286, 838–844 (2015). [Google Scholar]
- 50.Ocker, B., Wurster, R. & Seiler, H. Investigation of nanoparticles in high resolution scanning electron microscopy (SEM) and low voltage SEM by digital image-analysis. Scanning Microsc.9(1), 3 (1995). [Google Scholar]
- 51.Park, B., Kim, B. H. & Yu, T. Synthesis of spherical and cubic magnetic iron oxide nanocrystals at low temperature in air. J. Colloid Interface Sci.518, 27–33 (2018). [DOI] [PubMed] [Google Scholar]
- 52.Avolio, M. et al. Elongated magnetic nanoparticles with high-aspect ratio: A nuclear relaxation and specific absorption rate investigation. Phys. Chem. Chem. Phys.21(34), 18741–18752 (2019). [DOI] [PubMed] [Google Scholar]
- 53.Baeza, J. & Freer, J. Chemical characterization of wood and its components. Wood Cellul. Chem.2(1), 275–384 (2000). [Google Scholar]
- 54.Zhao, X.-Y. et al. Application of superfine pulverization technology in biomaterial industry. J. Taiwan Inst. Chem. Eng.40(3), 337–343 (2009). [Google Scholar]
- 55.Xie, Z., Guan, W., Ji, F., Song, Z. & Zhao, Y. Production of biologically activated carbon from orange peel and landfill leachate subsequent treatment technology. J. Chem.2014(1), 491912 (2014). [Google Scholar]
- 56.Kanazawa, K. & Sakakibara, H. High content of dopamine, a strong antioxidant, in cavendish banana. J. Agric. Food Chem.48(3), 844–848 (2000). [DOI] [PubMed] [Google Scholar]
- 57.Zorzi, G. K., Caregnato, F., Moreira, J. C. F., Teixeira, H. F. & Carvalho, E. L. S. Antioxidant effect of nanoemulsions containing extract of Achyrocline satureioides (Lam) DC—Asteraceae. AAPS PharmSciTech.17, 844–850 (2016). [DOI] [PubMed] [Google Scholar]
- 58.Sumathy, N. & Sumathy, J. Antibacterial and antifungal activity of musa fruit peels against skin and gastrointestinal tract diseases. Herb. Tech. Ind.2, 9–11 (2011). [Google Scholar]
- 59.Susan, K., Jesteena, J. & Ragunathan, R. Eco-friendly synthesis of silver nanoparticles using banana (Musa acuminate colla) peel, its phytochemical, antimicrobial and anticancer activity. IJ Rec. Sci. Res.8(10), 21098–21106 (2017). [Google Scholar]
- 60.Baptista, P. V. et al. Nano-strategies to fight multidrug resistant bacteria—“A battle of the titans”. Front. Microbiol.9, 1441 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Arora, N., Thangavelu, K. & Karanikolos, G. N. Bimetallic nanoparticles for antimicrobial applications. Front. Chem.8, 412 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhang, L., Jiang, Y., Ding, Y., Povey, M. & York, D. Investigation into the antibacterial behaviour of suspensions of ZnO nanoparticles (ZnO nanofluids). J. Nanoparticle Res.9, 479–489 (2007). [Google Scholar]
- 63.Chabuck, Z. A. G., Al-Charrakh, A. H., Hindi, N. K. K. & Hindi, S. K. K. Antimicrobial effect of aqueous banana peel extract, Iraq. Res. Gate Pharm. Sci.1, 73–75 (2013). [Google Scholar]
- 64.Hashem, A. H. & El-Sayyad, G. S. Antimicrobial and anticancer activities of biosynthesized bimetallic silver-zinc oxide nanoparticles (Ag-ZnO NPs) using pomegranate peel extract. Biomass Convers. Biorefinery 1–13 (2023).
- 65.Liu, R. H. Potential synergy of phytochemicals in cancer prevention: Mechanism of action. J. Nutr.134(12), 3479S-S3485 (2004). [DOI] [PubMed] [Google Scholar]
- 66.Nichenametla, S. N., Taruscio, T. G., Barney, D. L. & Exon, J. H. A review of the effects and mechanisms of polyphenolics in cancer. Crit. Rev. Food Sci. Nutr.46(2), 161–183 (2006). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used to support the findings of this study are available from the corresponding author upon request by the same email: walid.metwali@agr.kfs.edu.eg.