Abstract
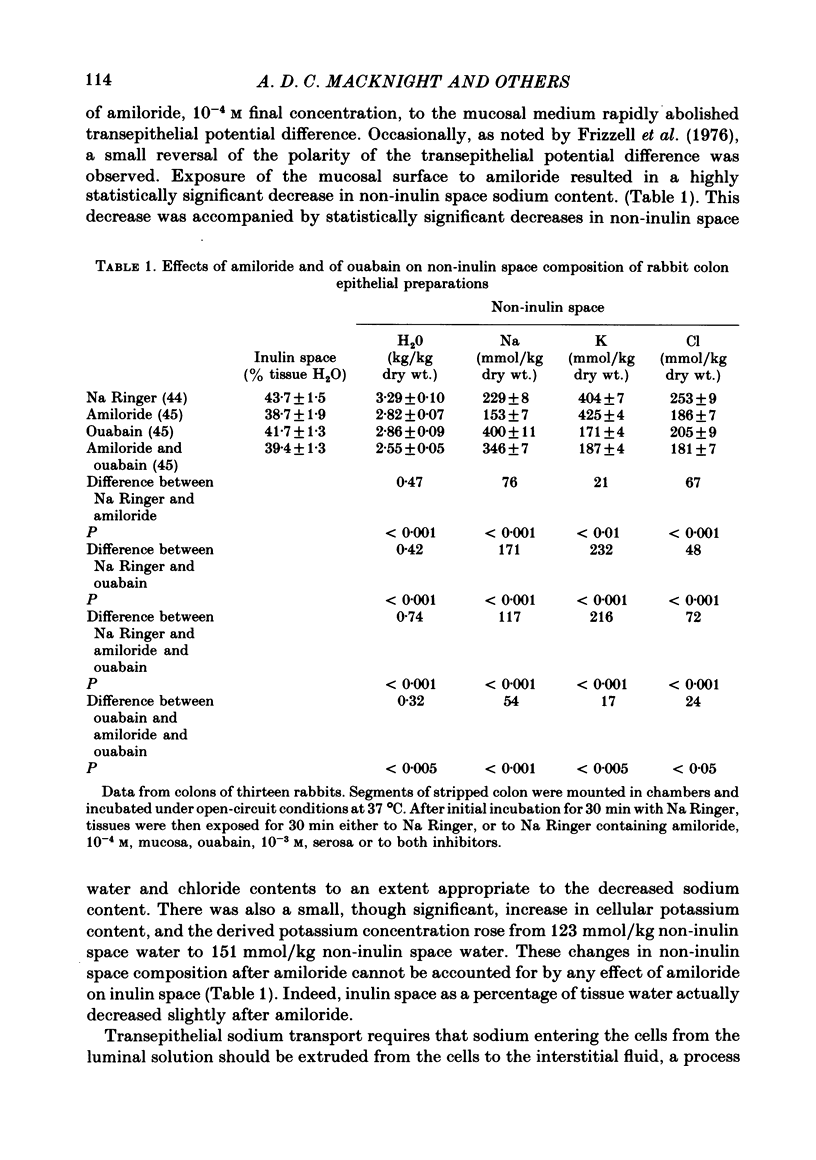
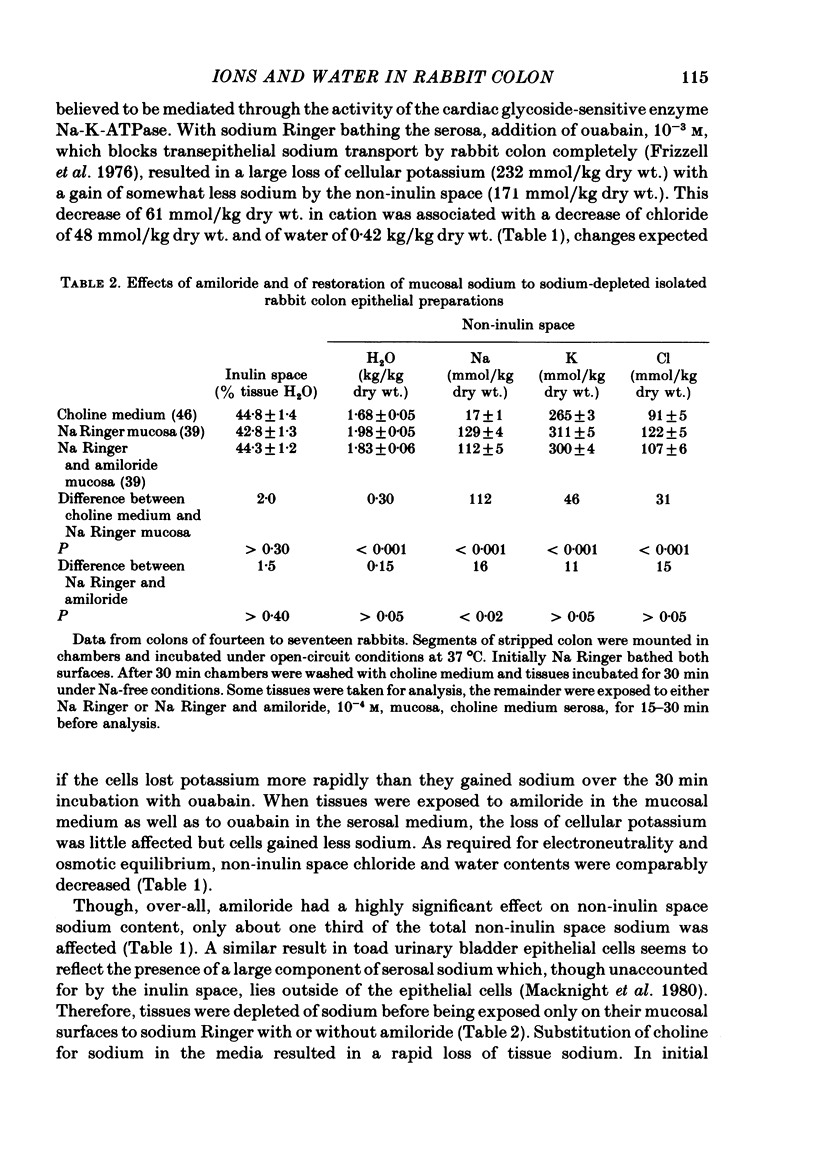
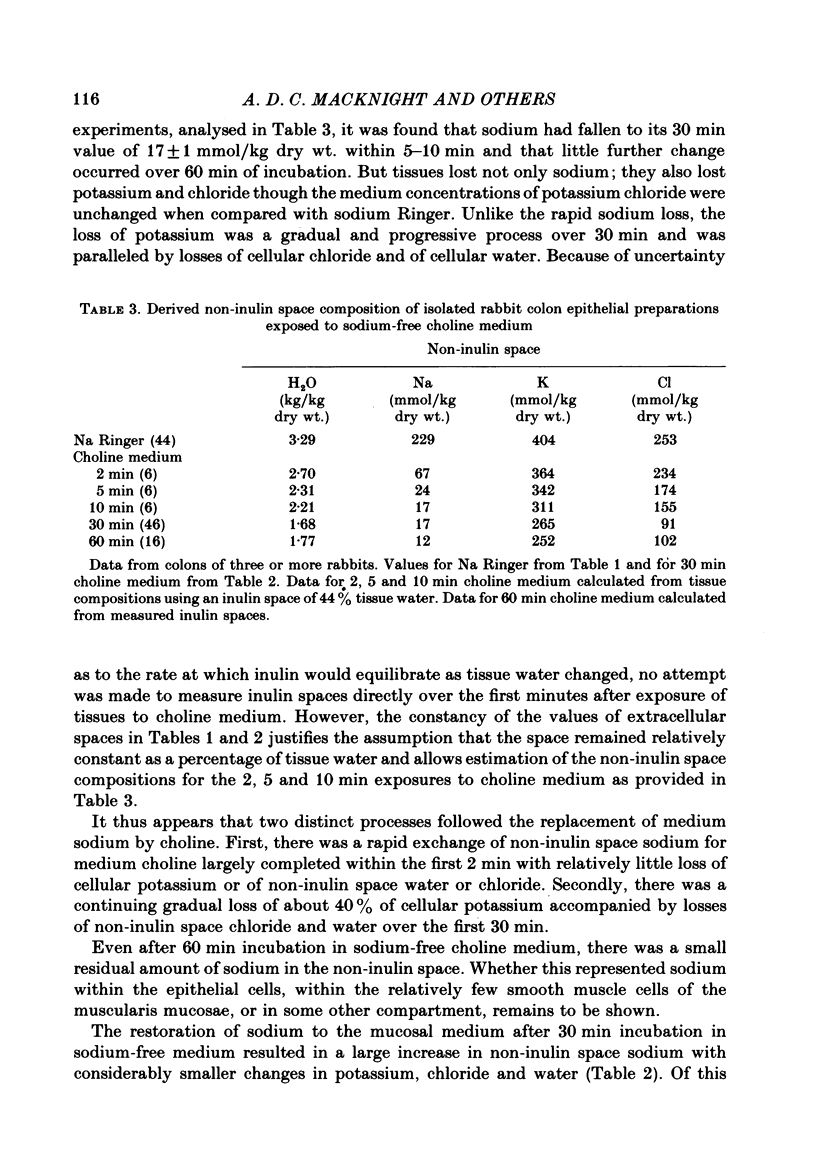
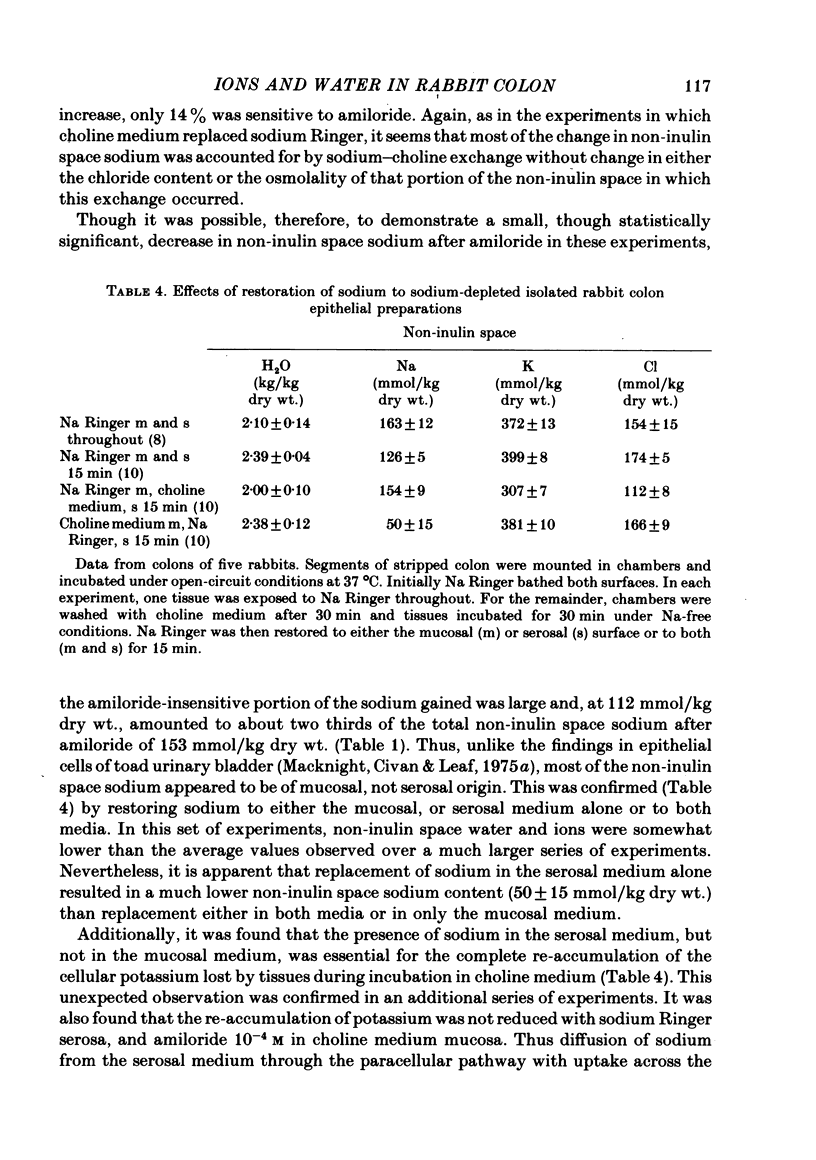
1. Isolated sheets of rabbit descending colon epithelial cells stripped from their underlying muscle coats were incubated in chambers at 37 degrees C with oxygenated media, and their non-inulin space water, sodium, potassium and chloride contents were subsequently determined. 2. With sodium Ringer bathing both surfaces, amiloride, 10(-4) M, decreased non-inulin space sodium content by 76 mmol/kg dry wt. Ouabain, 10(-3) M, caused loss of non-inulin space potassium which was not completely compensated for by uptake of sodium over 30 min incubation. Chloride and water, therefore, decreased. Amiloride, 10(-4) M, inhibited but did not prevent this uptake of sodium after ouabain. 3. Tissues exposed to sodium-free choline Ringer rapidly exchanged non-inulin space sodium for choline and, more slowly, lost potassium, chloride and water. The equilibration of sodium in the non-inulin space when sodium Ringer was restored to the mucosal medium alone was largely amiloride-insensitive. For restoration of non-inulin space potassium to normal levels, sodium was required in the serosal but not the mucosal medium. 4. Neither the absence of glucose nor the absence of chloride from the mucosal medium affected the non-inulin space sodium content when sodium was restored to the mucosal medium bathing sodium-depleted tissues. 5. It is argued that, whereas non-inulin space potassium and water contents are synonymous with their cellular values, only about one third of non-inulin space sodium is cellular when sodium Ringer bathes both surfaces, and the concentration of the sodium within the cellular transport pool approximated 20 mmol/kg H2O, consistent with estimates obtained from other techniques.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beck F., Bauer R., Bauer U., Mason J., Dörge A., Rick R., Thurau K. Electron microprobe analysis of intracellular elements in the rat kidney. Kidney Int. 1980 Jun;17(6):756–763. doi: 10.1038/ki.1980.88. [DOI] [PubMed] [Google Scholar]
- COTLOVE E., TRANTHAM H. V., BOWMAN R. L. An instrument and method for automatic, rapid, accurate, and sensitive titration of chloride in biologic samples. J Lab Clin Med. 1958 Mar;51(3):461–468. [PubMed] [Google Scholar]
- Dawson D. C. Na and Cl transport across the isolated turtle colon: parallel pathways for transmural ion movement. J Membr Biol. 1977 Dec 15;37(3-4):213–233. doi: 10.1007/BF01940933. [DOI] [PubMed] [Google Scholar]
- Erlij D., Smith M. W. Sodium uptake by frog skin and its modification by inhibitors of transepithelial sodium transport. J Physiol. 1973 Jan;228(1):221–239. doi: 10.1113/jphysiol.1973.sp010083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRAZIER H. S., DEMPSEY E. F., LEAF A. Movement of sodium across the mucosal surface of the isolated toad bladder and its modification by vasopressin. J Gen Physiol. 1962 Jan;45:529–543. doi: 10.1085/jgp.45.3.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frizzell R. A., Koch M. J., Schultz S. G. Ion transport by rabbit colon. I. Active and passive components. J Membr Biol. 1976;27(3):297–316. doi: 10.1007/BF01869142. [DOI] [PubMed] [Google Scholar]
- Frizzell R. A., Schultz S. G. Effect of aldosterone on ion transport by rabbit colon in vitro. J Membr Biol. 1978 Feb 6;39(1):1–26. doi: 10.1007/BF01872752. [DOI] [PubMed] [Google Scholar]
- Frizzell R. A., Turnheim K. Ion transport by rabbit colon: II. Unidirectional sodium influx and the effects of amphotericin B and amiloride. J Membr Biol. 1978 May 3;40(3):193–211. doi: 10.1007/BF02002968. [DOI] [PubMed] [Google Scholar]
- Garcia-Diaz J. F., Armstrong W. M. The steady-state relationship between sodium and chloride transmembrane electrochemical potential differences in Necturus gallbladder. J Membr Biol. 1980 Aug 7;55(3):213–222. doi: 10.1007/BF01869462. [DOI] [PubMed] [Google Scholar]
- Graf J., Giebisch G. Intracellular sodium activity and sodium transport in necturus gallbladder epithelium. J Membr Biol. 1979 Jun 7;47(4):327–355. doi: 10.1007/BF01869743. [DOI] [PubMed] [Google Scholar]
- Hughes P. M., Macknight A. D. Effects of replacing medium sodium by choline, caesium, or rubidium, on water and ion contents of renal cortical slices. J Physiol. 1977 May;267(1):113–136. doi: 10.1113/jphysiol.1977.sp011804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jørgensen P. L. Sodium and potassium ion pump in kidney tubules. Physiol Rev. 1980 Jul;60(3):864–917. doi: 10.1152/physrev.1980.60.3.864. [DOI] [PubMed] [Google Scholar]
- KIRSCHNER L. B. On the mechanism of active sodium transport across the frog skin. J Cell Physiol. 1955 Feb;45(1):61–87. doi: 10.1002/jcp.1030450106. [DOI] [PubMed] [Google Scholar]
- LITTLE J. R. DETERMINATION OF WATER AND ELECTROLYTES IN TISSUE SLICES. Anal Biochem. 1964 Jan;7:87–95. doi: 10.1016/0003-2697(64)90122-8. [DOI] [PubMed] [Google Scholar]
- Leaf A. Transepithelial transport and its hormonal control in toad bladder. Ergeb Physiol. 1965;56:216–263. [PubMed] [Google Scholar]
- Lewis S. A., Eaton D. C., Diamond J. M. The mechanism of Na+ transport by rabbit urinary bladder. J Membr Biol. 1976 Aug 27;28(1):41–70. doi: 10.1007/BF01869690. [DOI] [PubMed] [Google Scholar]
- MACROBBIE E. A., USSING H. H. Osmotic behaviour of the epithelial cells of frog skin. Acta Physiol Scand. 1961 Nov-Dec;53:348–365. doi: 10.1111/j.1748-1716.1961.tb02293.x. [DOI] [PubMed] [Google Scholar]
- Macknight A. D., Civan M. M., Leaf A. Some effects of ouabain on cellular ions and water in epithelial cells of toad urinary bladder. J Membr Biol. 1975;20(3-4):387–401. doi: 10.1007/BF01870645. [DOI] [PubMed] [Google Scholar]
- Macknight A. D., Civan M. M., Leaf A. The sodium transport pool in toad urinary bladder epithelial cells. J Membr Biol. 1975;20(3-4):365–367. doi: 10.1007/BF01870644. [DOI] [PubMed] [Google Scholar]
- Macknight A. D. Comparison of analytic techniques: chemical, isotopic, and microprobe analyses. Fed Proc. 1980 Sep;39(11):2881–2887. [PubMed] [Google Scholar]
- Macknight A. D., DiBona D. R., Leaf A. Sodium transport across toad urinary bladder: a model "tight" epithelium. Physiol Rev. 1980 Jul;60(3):615–715. doi: 10.1152/physrev.1980.60.3.615. [DOI] [PubMed] [Google Scholar]
- Macknight A. D., Leaf A. Regulation of cellular volume. Physiol Rev. 1977 Jul;57(3):510–573. doi: 10.1152/physrev.1977.57.3.510. [DOI] [PubMed] [Google Scholar]
- McIver D. J., Macknight A. D. Extracellular space in some isolated tissues. J Physiol. 1974 May;239(1):31–49. doi: 10.1113/jphysiol.1974.sp010554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Doherty J., Garcia-Diaz J. F., Armstrong W. M. Sodium-selective liquid ion-exchanger microelectrodes for intracellular measurements. Science. 1979 Mar 30;203(4387):1349–1351. doi: 10.1126/science.424756. [DOI] [PubMed] [Google Scholar]
- Rick R., Dörge A., Macknight A. D., Leaf A., Thurau K. Electron microprobe analysis of the different epithelial cells of toad urinary bladder. Electrolyte concentrations at different functional states of transepithelial sodium transport. J Membr Biol. 1978 Mar 10;39(2-3):257–271. doi: 10.1007/BF01870334. [DOI] [PubMed] [Google Scholar]
- Rick R., Dörge A., von Arnim E., Thurau K. Electron microprobe analysis of frog skin epithelium: evidence for a syncytial sodium transport compartment. J Membr Biol. 1978 Mar 20;39(4):313–331. doi: 10.1007/BF01869897. [DOI] [PubMed] [Google Scholar]
- Rose R. C., Schultz S. G. Studies on the electrical potential profile across rabbit ileum. Effects of sugars and amino acids on transmural and transmucosal electrical potential differences. J Gen Physiol. 1971 Jun;57(6):639–663. doi: 10.1085/jgp.57.6.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz S. G., Curran P. F. Coupled transport of sodium and organic solutes. Physiol Rev. 1970 Oct;50(4):637–718. doi: 10.1152/physrev.1970.50.4.637. [DOI] [PubMed] [Google Scholar]
- Schultz S. G., Frizzell R. A., Nellans H. N. Active sodium transport and the electrophysiology of rabbit colon. J Membr Biol. 1977 May 12;33(3-4):351–384. doi: 10.1007/BF01869524. [DOI] [PubMed] [Google Scholar]
- Skou J. C. The (Na++K+) activated enzyme system and its relationship to transport of sodium and potassium. Q Rev Biophys. 1974 Jul;7(3):401–434. doi: 10.1017/s0033583500001475. [DOI] [PubMed] [Google Scholar]
- Turnheim K., Frizzell R. A., Schultz S. G. Interaction between cell sodium and the amiloride-sensitive sodium entry step in rabbit colon. J Membr Biol. 1978 Mar 10;39(2-3):233–256. doi: 10.1007/BF01870333. [DOI] [PubMed] [Google Scholar]
- Wills N. K., Lewis S. A., Eaton D. C. Active and passive properties of rabbit descending colon: a microelectrode and nystatin study. J Membr Biol. 1979 Mar 28;45(1-2):81–108. doi: 10.1007/BF01869296. [DOI] [PubMed] [Google Scholar]
- Wills N. K., Lewis S. A. Intracellular Na+ activity as a function of Na+ transport rate across a tight epithelium. Biophys J. 1980 Apr;30(1):181–186. doi: 10.1016/S0006-3495(80)85086-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yorio T., Bentley P. J. Permeability of the rabbit colon in vitro. Am J Physiol. 1977 Jan;232(1):F5–F9. doi: 10.1152/ajprenal.1977.232.1.F5. [DOI] [PubMed] [Google Scholar]


