Abstract
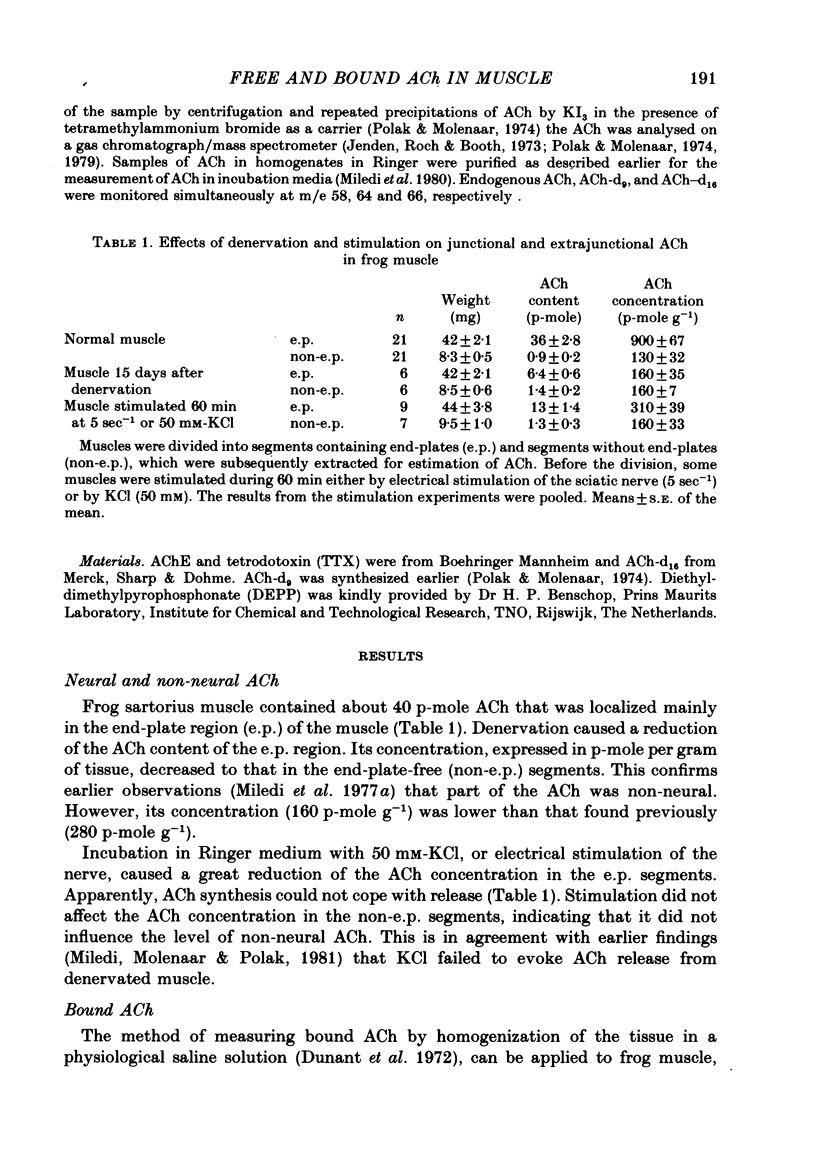
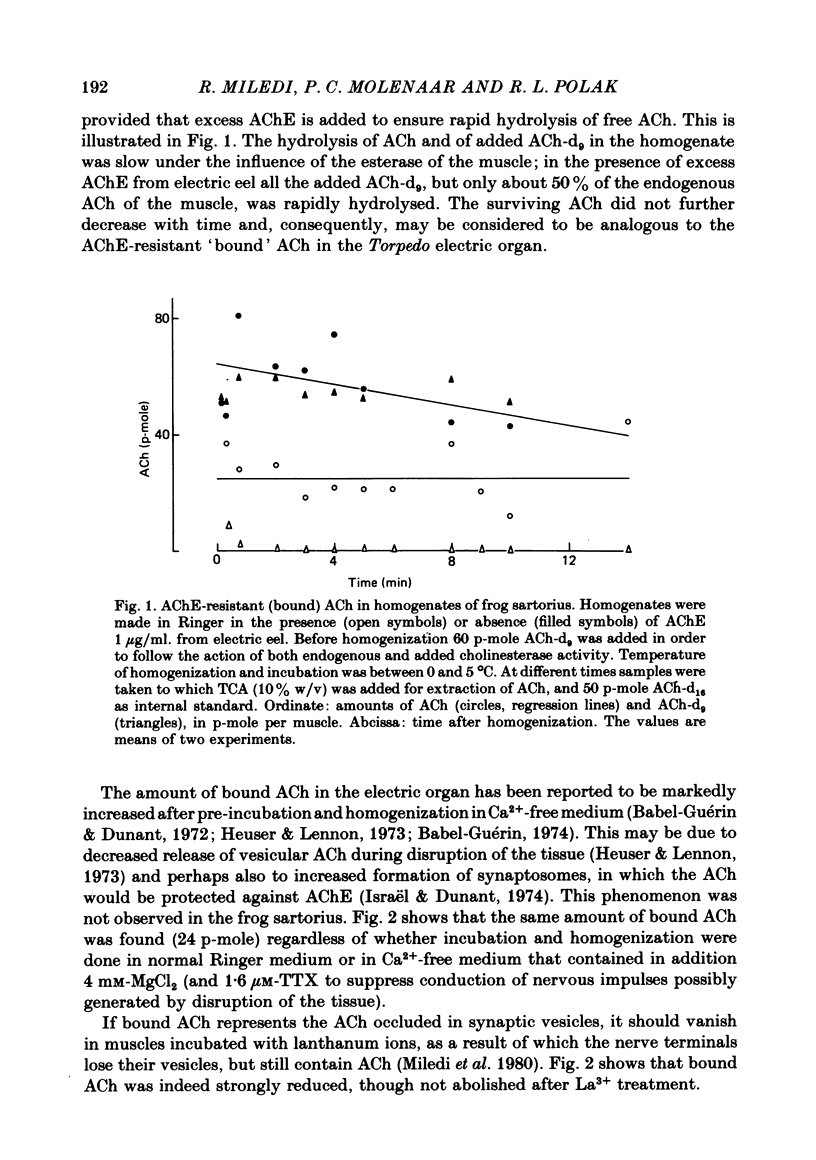
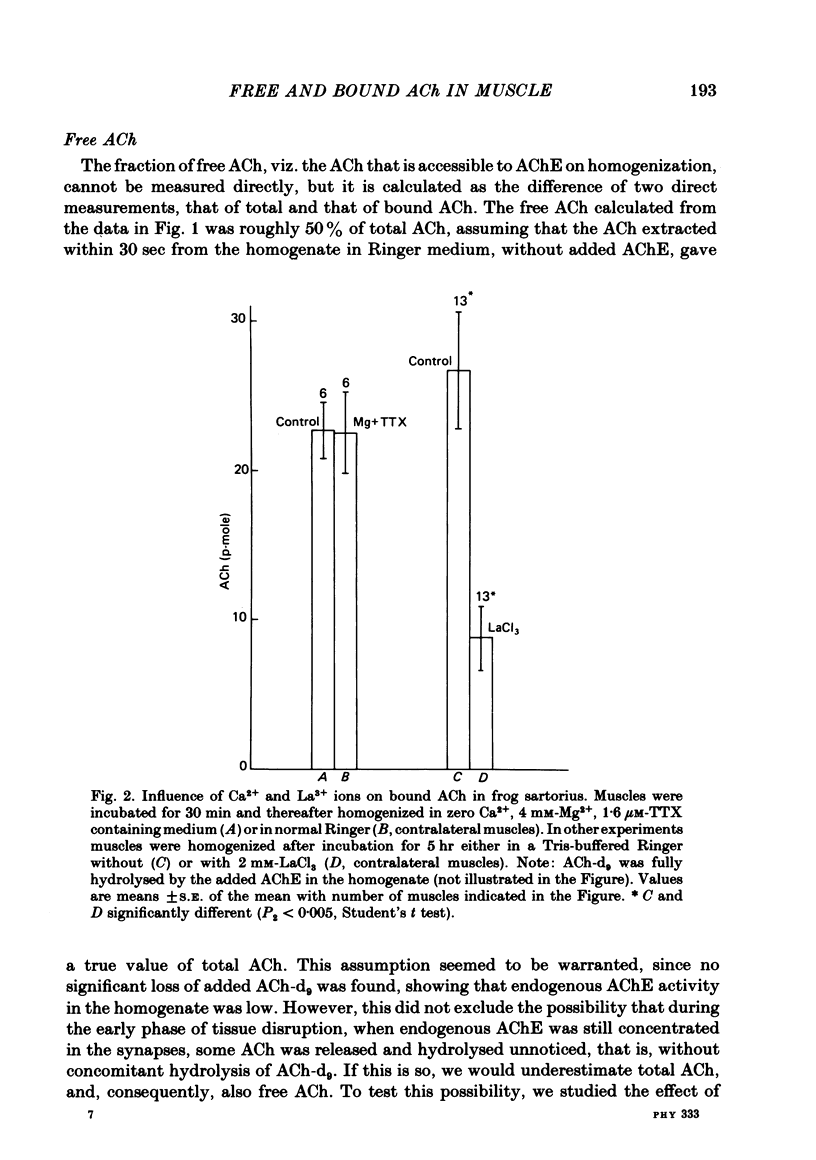
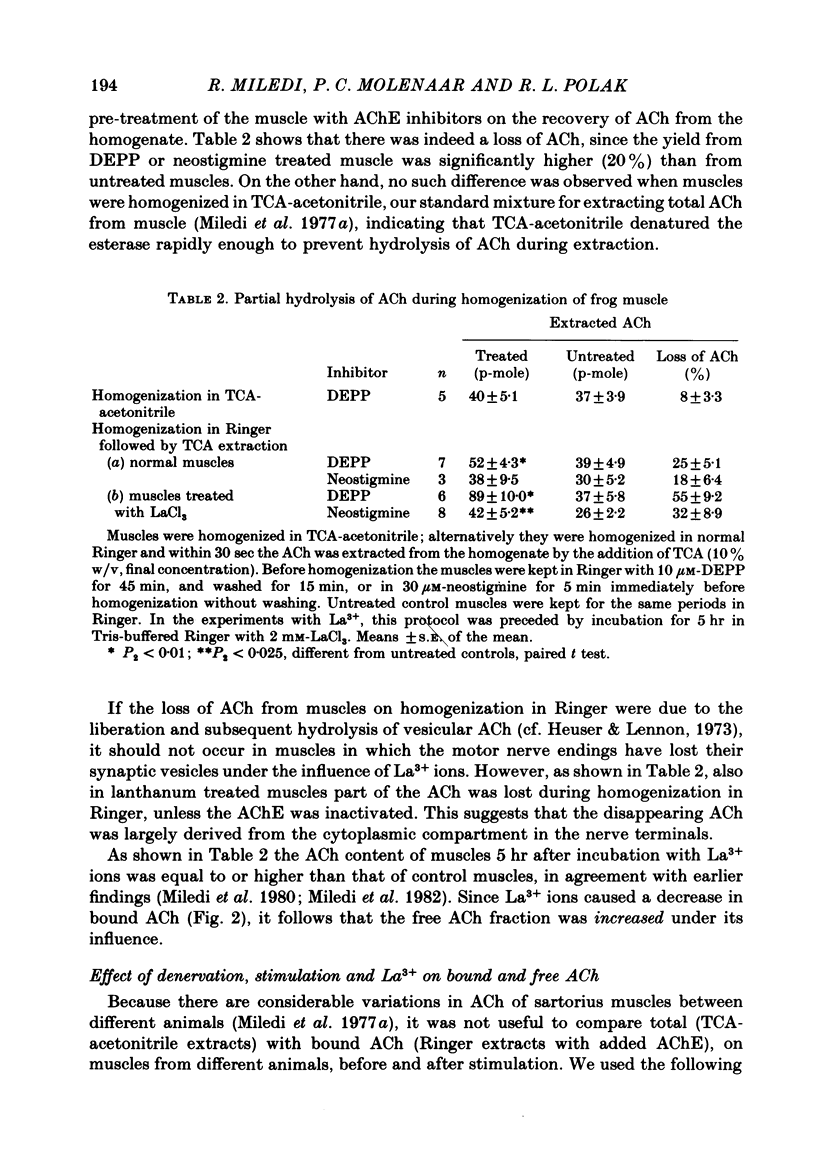
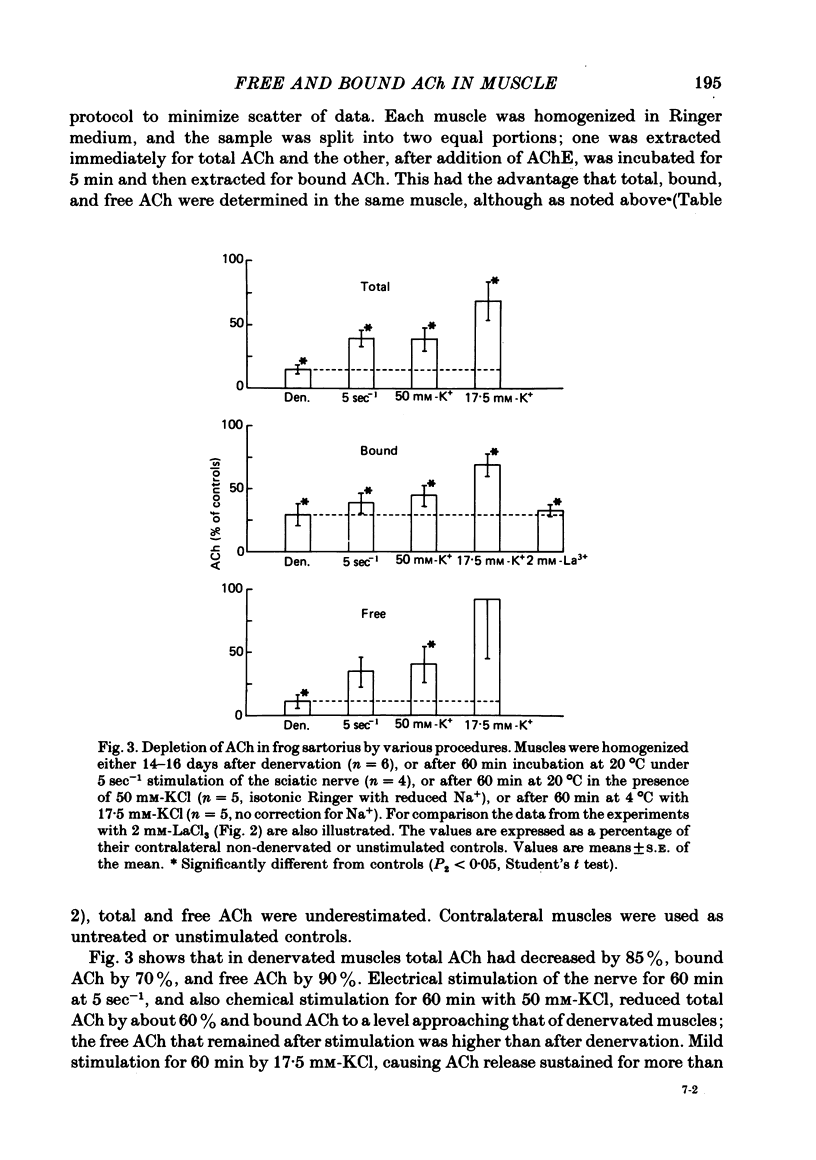
1. Frog sartorius muscles were divided into end-plate containing (e.p.) and end-plate-free (non-e.p.) segments or homogenized in Ringer solution at 0 degrees C in the presence or absence of added acetylcholinesterase from electric eel. ACh was extracted from the tissue or from the homogenates and measured by mass fragmentography. 2. The concentration of ACh in non-e.p. segments was about six times lower than that in e.p. segments. 3. Homogenization of muscles in Ringer caused the hydrolysis of a small fraction ('free-1') of total ACh; addition of extra acetylcholinesterase caused hydrolysis of another, greater, fraction ('free-2' ACh). The esterase-resistant ('bound') ACh was stable at 0 degrees C up to 15 min of incubation. 4. Denervation for 15 days, which caused the disappearance of the nerve terminals, did not influence ACh in non-e.p. segments, but reduced total and bound ACh by about 75%, and free-2 ACh by 90%. 5. Treatment with La3+ ions, which caused the disappearance of synaptic vesicles, did not influence total ACh, but reduced bound ACh by 75%, whereas free-1 and free-2 ACh were increased. 6. Electrical stimulation of the nerve at 5 sec-1 or incubation with 50 mM-KCl did not affect ACh in the non-e.p. segments, but reduced by roughly 60% total, bound, and free ACh. 7. It is concluded that about 75% of bound ACh derives from synaptic vesicles, corresponding to 11,000 molecules per vesicle, and 25% from non-neural ACh; that free-1 and free-2 ACh derive mainly from the nerve terminal cytoplasm, although they may be contaminated by vesicular ACh.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BIRKS R., KATZ B., MILEDI R. Physiological and structural changes at the amphibian myoneural junction, in the course of nerve degeneration. J Physiol. 1960 Jan;150:145–168. doi: 10.1113/jphysiol.1960.sp006379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babel-Guérin E., Dunant Y. Entrée de calcium et libération d'actylcholine dans l'organe électrique de la torpille. C R Acad Sci Hebd Seances Acad Sci D. 1972 Dec 18;275(25):2961–2964. [PubMed] [Google Scholar]
- Babel-Guérin E. Metabolisme du calcium et liberation de l'acetylcholine dans l'organe electrique de la Torpille. J Neurochem. 1974 Sep;23(3):525–532. doi: 10.1111/j.1471-4159.1974.tb06055.x. [DOI] [PubMed] [Google Scholar]
- Clark A. W., Hurlbut W. P., Mauro A. Changes in the fine structure of the neuromuscular junction of the frog caused by black widow spider venom. J Cell Biol. 1972 Jan;52(1):1–14. doi: 10.1083/jcb.52.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunant Y., Gautron J., Israël M., Lesbats B., Manaranche R. Les compartiments d'acetylcholine de l'organe electrique de la torpille et leurs modifications par la stimulation. J Neurochem. 1972 Aug;19(8):1987–2002. doi: 10.1111/j.1471-4159.1972.tb01488.x. [DOI] [PubMed] [Google Scholar]
- Dunant Y., Israël M., Lesbats B., Manaranche R. Oscillation of acetylcholine during nerve activity in the Torpedo electric organ. Brain Res. 1977 Apr 8;125(1):123–140. doi: 10.1016/0006-8993(77)90364-x. [DOI] [PubMed] [Google Scholar]
- Fonnum F. Is choline acetyltransferase present in synaptic vesicles? Biochem Pharmacol. 1966 Oct;15(10):1641–1643. doi: 10.1016/0006-2952(66)90216-4. [DOI] [PubMed] [Google Scholar]
- Gorio A., Hurlbut W. P., Ceccarelli B. Acetylcholine compartments in mouse diaphragm. Comparison of the effects of black widow spider venom, electrical stimulation, and high concentrations of potassium. J Cell Biol. 1978 Sep;78(3):716–733. doi: 10.1083/jcb.78.3.716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heuser J., Miledi R. Effects of lanthanum ions on function and structure of frog neuromuscular junctions. Proc R Soc Lond B Biol Sci. 1971 Dec 14;179(1056):247–260. doi: 10.1098/rspb.1971.0096. [DOI] [PubMed] [Google Scholar]
- Israel M., Dunant Y., Manaranche R. The present status of the vesicular hypothesis. Prog Neurobiol. 1979;13(3):237–275. doi: 10.1016/0301-0082(79)90017-0. [DOI] [PubMed] [Google Scholar]
- Israël M., Dunant Y. On the mechanism of acetylcholine release. Prog Brain Res. 1979;49:125–139. doi: 10.1016/S0079-6123(08)64627-0. [DOI] [PubMed] [Google Scholar]
- Jenden D. J., Roch M., Booth R. A. Simultaneous measurement of endogenous and deuterium-labeled tracer variants of choline and acetylcholine in subpicomole quantities by gas chromatography-mass spectrometry. Anal Biochem. 1973 Oct;55(2):438–448. doi: 10.1016/0003-2697(73)90134-6. [DOI] [PubMed] [Google Scholar]
- MILEDI R. The acetylcholine sensitivity of frog muscle fibres after complete or partial devervation. J Physiol. 1960 Apr;151:1–23. [PMC free article] [PubMed] [Google Scholar]
- Marchbanks R. M., Israël M. Aspects of acetylcholine metabolism in the electric organ of Torpedo marmorata. J Neurochem. 1971 Mar;18(3):439–448. doi: 10.1111/j.1471-4159.1971.tb11971.x. [DOI] [PubMed] [Google Scholar]
- Marchbanks R. M., Israël M. The heterogeneity of bound acetylcholine and synaptic vesicles. Biochem J. 1972 Oct;129(5):1049–1061. doi: 10.1042/bj1291049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miledi R., Molenaar P. C., Polak R. L. An analysis of acetylcholine in frog muscle by mass fragmentography. Proc R Soc Lond B Biol Sci. 1977 Jun 15;197(1128):285–297. doi: 10.1098/rspb.1977.0071. [DOI] [PubMed] [Google Scholar]
- Miledi R., Molenaar P. C., Polak R. L. The effect of lanthanum ions on acetylcholine in frog muscle. J Physiol. 1980 Dec;309:199–214. doi: 10.1113/jphysiol.1980.sp013504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molenaar P. C., Polak R. L. Acetylcholine synthesizing enzymes in frog skeletal muscle. J Neurochem. 1980 Nov;35(5):1021–1025. doi: 10.1111/j.1471-4159.1980.tb07855.x. [DOI] [PubMed] [Google Scholar]
- Molenaar P. C., Polak R. L. Analysis of the preferential release of newly synthesized acetylcholine by cortical slices from rat brain with the aid of two different labelled precursors. J Neurochem. 1976 Jan;26(1):95–99. [PubMed] [Google Scholar]
- Polak R. L., Molenaar P. C. A method for determination of acetylcholine by slow pyrolysis combined with mass fragmentography on a packed capillary column. J Neurochem. 1979 Feb;32(2):407–412. doi: 10.1111/j.1471-4159.1979.tb00364.x. [DOI] [PubMed] [Google Scholar]
- Polak R. L., Molenaar P. C. Pitfalls in determination of acetylcholine from brain by pyrolysis-gas chromatography/mass spectrometry. J Neurochem. 1974 Dec;23(6):1295–1297. doi: 10.1111/j.1471-4159.1974.tb12230.x. [DOI] [PubMed] [Google Scholar]
- Suszkiw J. B., Zimmermann H., Whittaker V. P. Vesicular storage and release of acetylcholine in Torpedo electroplaque synapses. J Neurochem. 1978 Jun;30(6):1269–1280. doi: 10.1111/j.1471-4159.1978.tb10455.x. [DOI] [PubMed] [Google Scholar]
- Tauc L. Are vesicles necessary for release of acetylcholine at cholinergic synapses? Biochem Pharmacol. 1979 Dec 15;28(24):3493–3498. doi: 10.1016/0006-2952(79)90389-7. [DOI] [PubMed] [Google Scholar]
- Tucek S., Zelená J., Ge I., Vyskocil F. Choline acetyltransferase in transected nerves, denervated muscles and Schwann cells of the frog: correlation of biochemical electron microscopical and electrophysiological observations. Neuroscience. 1978;3(8):709–724. doi: 10.1016/0306-4522(78)90067-2. [DOI] [PubMed] [Google Scholar]
- Welsch F., Schmidt D. E., Dettbarn W. D. Acetylcholine, choline acetyltransferase and cholinesterases in motor and sensory nerves of the bull frog. Biochem Pharmacol. 1972 Mar 15;21(6):847–856. doi: 10.1016/0006-2952(72)90128-1. [DOI] [PubMed] [Google Scholar]
- Zimmermann H. Vesicle recycling and transmitter release. Neuroscience. 1979;4(12):1773–1804. doi: 10.1016/0306-4522(79)90058-7. [DOI] [PubMed] [Google Scholar]
- von Wedel R. J., Carlson S. S., Kelly R. B. Transfer of synaptic vesicle antigens to the presynaptic plasma membrane during exocytosis. Proc Natl Acad Sci U S A. 1981 Feb;78(2):1014–1018. doi: 10.1073/pnas.78.2.1014. [DOI] [PMC free article] [PubMed] [Google Scholar]



