Abstract
1. Voltage-sensitive membrane-bound dyes and a matrix of 100 photodetectors were used to detect the spread of evoked electrical activity at the CA1 region of rat hippocampus slices. A display processor was designed in order to visualize the spread of electrical activity in slow motion.
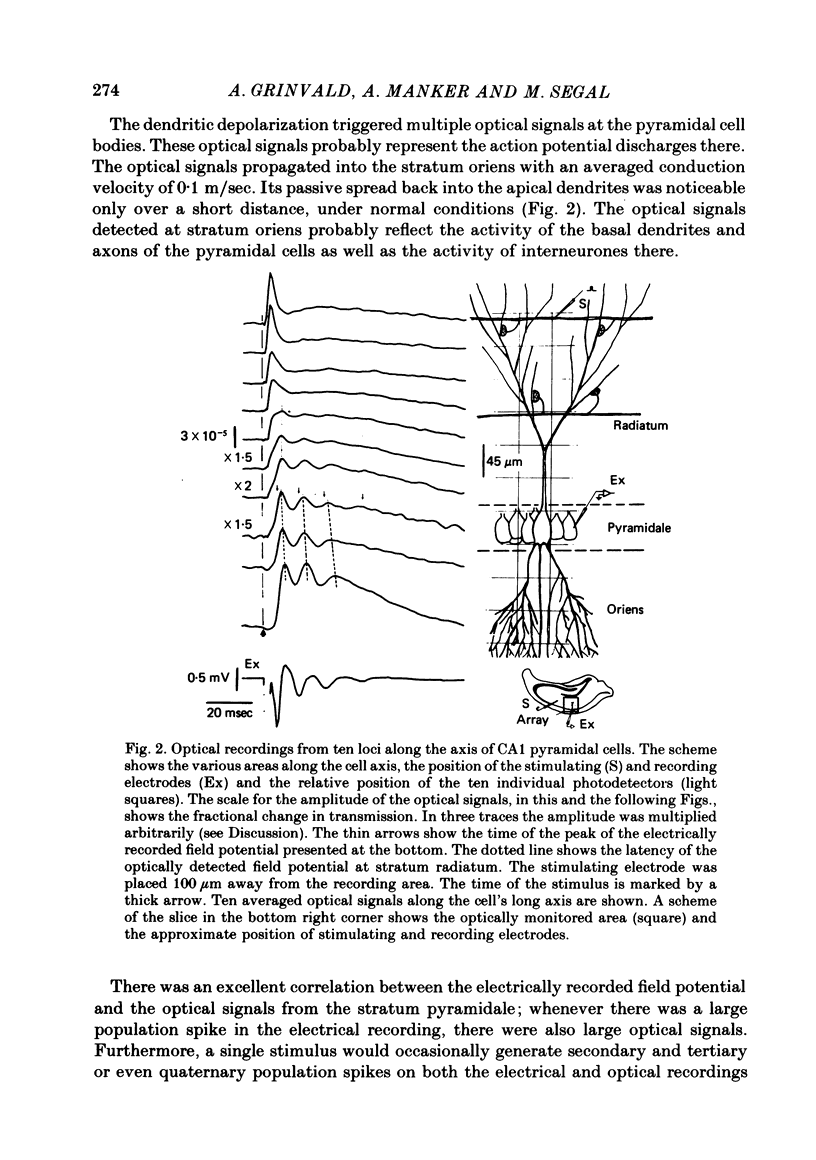
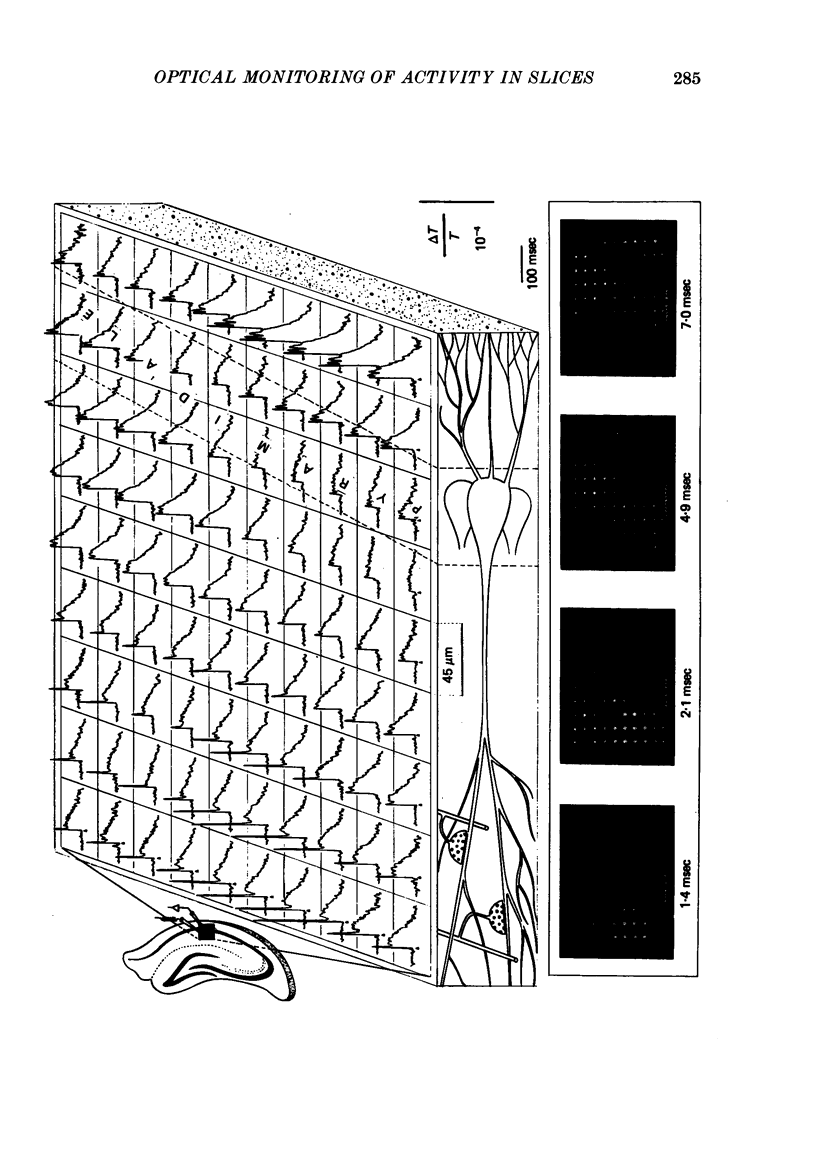
2. The stimulation of the Schaffer collateral-commissural path in the stratum radiatum evoked short latency (2-4 msec) fast optical signals, followed by longer latency (4-15 msec) slow signals which decayed within 20-50 msec. Multiple fast signals were frequently detected at the stratum pyramidale; they propagated toward the stratum oriens with an approximate conduction velocity of 0.1 m/sec.
3. The fast signals were unaltered in a low Ca2+ high Mg2+ medium but were blocked by tetrodotoxin. These signals probably represent action potentials in the Schaffer collateral axons. Their conduction velocity was about 0.2 m/sec and their refractory period about 3-4 msec.
4. The slow signals were absent in a low Ca2+ medium and probably represent excitatory post-synaptic potentials (e.p.s.p.s) generated in the apical dendrites of the pyramidal cells. They were generated in the stratum radiatum, where the presynaptic signals were seen, and spread into somata and basal dendrites (the stratum pyramidale and oriens, respectively).
5. The timing of the signals with fast rise-time, which were detected at the statum pyramidale, approximately coincided with the timing of the extracellularly recorded field potentials. These multiple discharges probably represent action potentials of the pyramidal cells. They spread back into the apical dendrites but with significant attenuation of the amplitudes of the high frequency components of the pyramidal action potentials.
6. Hyperpolarizing potentials could be detected when strong stimuli were applied to the stratum radiatum or alveus. The net hyperpolarizations were detected only in the stratum pyramidale and the border region between the stratum pyramidale and radiatum. Frequently the inhibition was masked by the large e.p.s.p.s. However, its existence could be demonstrated by treatment of the slice with picrotoxin or a low Cl- medium. Under these conditions a long-lasting depolarization of the apical dedrites was evoked by the stimulation. This was associated with an increase of the multiple discharges in the stratum pyramidale and oriens.
7. These studies illustrate the usefulness of voltage-sensitive dyes in the analysis of passive and active electrical properties, pharmacological properties and synaptic connexions in mammalian brain slices, at the level both of small neuronal elements (dendrites, axons) and of synchronously active neuronal populations.
Full text
PDF




















Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANDERSEN P., ECCLES J. C., LOYNING Y. PATHWAY OF POSTSYNAPTIC INHIBITION IN THE HIPPOCAMPUS. J Neurophysiol. 1964 Jul;27:608–619. doi: 10.1152/jn.1964.27.4.608. [DOI] [PubMed] [Google Scholar]
- Andersen P., Silfvenius H., Sundberg S. H., Sveen O., Wigström H. Functional characteristics of unmyelinated fibres in the hippocampal cortex. Brain Res. 1978 Apr 7;144(1):11–18. doi: 10.1016/0006-8993(78)90431-6. [DOI] [PubMed] [Google Scholar]
- Andersen P., Sundberg S. H., Sveen O., Wigström H. Specific long-lasting potentiation of synaptic transmission in hippocampal slices. Nature. 1977 Apr 21;266(5604):736–737. doi: 10.1038/266736a0. [DOI] [PubMed] [Google Scholar]
- Anderson P., Lomo T. Mode of activation of hippocampal pyramidal cells by excitatory synapses on dendrites. Exp Brain Res. 1966;2(3):247–260. [PubMed] [Google Scholar]
- Brown T. H., Fricke R. A., Perkel D. H. Passive electrical constants in three classes of hippocampal neurons. J Neurophysiol. 1981 Oct;46(4):812–827. doi: 10.1152/jn.1981.46.4.812. [DOI] [PubMed] [Google Scholar]
- Cohen L. B., Keynes R. D. Changes in light scattering associated with the action potential in crab nerves. J Physiol. 1971 Jan;212(1):259–275. doi: 10.1113/jphysiol.1971.sp009321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen L. B., Keynes R. D., Hille B. Light scattering and birefringence changes during nerve activity. Nature. 1968 May 4;218(5140):438–441. doi: 10.1038/218438a0. [DOI] [PubMed] [Google Scholar]
- Cohen L. B., Keynes R. D., Landowne D. Changes in axon light scattering that accompany the action potential: current-dependent components. J Physiol. 1972 Aug;224(3):727–752. doi: 10.1113/jphysiol.1972.sp009920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen L. B., Salzberg B. M., Davila H. V., Ross W. N., Landowne D., Waggoner A. S., Wang C. H. Changes in axon fluorescence during activity: molecular probes of membrane potential. J Membr Biol. 1974;19(1):1–36. doi: 10.1007/BF01869968. [DOI] [PubMed] [Google Scholar]
- Cohen L. B., Salzberg B. M., Grinvald A. Optical methods for monitoring neuron activity. Annu Rev Neurosci. 1978;1:171–182. doi: 10.1146/annurev.ne.01.030178.001131. [DOI] [PubMed] [Google Scholar]
- Cohen L. B., Salzberg B. M. Optical measurement of membrane potential. Rev Physiol Biochem Pharmacol. 1978;83:35–88. doi: 10.1007/3-540-08907-1_2. [DOI] [PubMed] [Google Scholar]
- Fujii S., Hirota A., Kamino K. Optical indications of pace-maker potential and rhythm generation in early embryonic chick heart. J Physiol. 1981 Mar;312:253–263. doi: 10.1113/jphysiol.1981.sp013627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujii S., Hirota A., Kamino K. Optical recording of development of electrical activity in embryonic chick heart during early phases of cardiogenesis. J Physiol. 1981 Feb;311:147–160. doi: 10.1113/jphysiol.1981.sp013578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grinvald A., Cohen L. B., Lesher S., Boyle M. B. Simultaneous optical monitoring of activity of many neurons in invertebrate ganglia using a 124-element photodiode array. J Neurophysiol. 1981 May;45(5):829–840. doi: 10.1152/jn.1981.45.5.829. [DOI] [PubMed] [Google Scholar]
- Grinvald A., Farber I. C. Optical recording of calcium action potentials from growth cones of cultured neurons with a laser microbeam. Science. 1981 Jun 5;212(4499):1164–1167. doi: 10.1126/science.7233210. [DOI] [PubMed] [Google Scholar]
- Grinvald A., Ross W. N., Farber I. Simultaneous optical measurements of electrical activity from multiple sites on processes of cultured neurons. Proc Natl Acad Sci U S A. 1981 May;78(5):3245–3249. doi: 10.1073/pnas.78.5.3245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grinvald A., Salzberg B. M., Cohen L. B. Simultaneous recording from several neurones in an invertebrate central nervous system. Nature. 1977 Jul 14;268(5616):140–142. doi: 10.1038/268140a0. [DOI] [PubMed] [Google Scholar]
- Gupta R. K., Salzberg B. M., Grinvald A., Cohen L. B., Kamino K., Lesher S., Boyle M. B., Waggoner A. S., Wang C. H. Improvements in optical methods for measuring rapid changes in membrane potential. J Membr Biol. 1981 Feb 15;58(2):123–137. doi: 10.1007/BF01870975. [DOI] [PubMed] [Google Scholar]
- Jefferys J. G. Initiation and spread of action potentials in granule cells maintained in vitro in slices of guinea-pig hippocampus. J Physiol. 1979 Apr;289:375–388. doi: 10.1113/jphysiol.1979.sp012742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowles W. D., Schwartzkroin P. A. Axonal ramifications of hippocampal Ca1 pyramidal cells. J Neurosci. 1981 Nov;1(11):1236–1241. doi: 10.1523/JNEUROSCI.01-11-01236.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipton P. Effects of membrane depolarization on light scattering by cerebral cortical slices. J Physiol. 1973 Jun;231(2):365–383. doi: 10.1113/jphysiol.1973.sp010238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llinás R., Sugimori M. Electrophysiological properties of in vitro Purkinje cell dendrites in mammalian cerebellar slices. J Physiol. 1980 Aug;305:197–213. doi: 10.1113/jphysiol.1980.sp013358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacVicar B. A., Dudek F. E. Electrotonic coupling between pyramidal cells: a direct demonstration in rat hippocampal slices. Science. 1981 Aug 14;213(4509):782–785. doi: 10.1126/science.6266013. [DOI] [PubMed] [Google Scholar]
- Ross W. N., Reichardt L. F. Species-specific effects on the optical signals of voltage-sensitive dyes. J Membr Biol. 1979 Aug;48(4):343–356. doi: 10.1007/BF01869445. [DOI] [PubMed] [Google Scholar]
- Ross W. N., Salzberg B. M., Cohen L. B., Grinvald A., Davila H. V., Waggoner A. S., Wang C. H. Changes in absorption, fluorescence, dichroism, and Birefringence in stained giant axons: : optical measurement of membrane potential. J Membr Biol. 1977 May 6;33(1-2):141–183. doi: 10.1007/BF01869514. [DOI] [PubMed] [Google Scholar]
- Salzberg B. M., Davila H. V., Cohen L. B. Optical recording of impulses in individual neurones of an invertebrate central nervous system. Nature. 1973 Dec 21;246(5434):508–509. doi: 10.1038/246508a0. [DOI] [PubMed] [Google Scholar]
- Salzberg B. M., Grinvald A., Cohen L. B., Davila H. V., Ross W. N. Optical recording of neuronal activity in an invertebrate central nervous system: simultaneous monitoring of several neurons. J Neurophysiol. 1977 Nov;40(6):1281–1291. doi: 10.1152/jn.1977.40.6.1281. [DOI] [PubMed] [Google Scholar]
- Schwartzkroin P. A. Characteristics of CA1 neurons recorded intracellularly in the hippocampal in vitro slice preparation. Brain Res. 1975 Mar 7;85(3):423–436. doi: 10.1016/0006-8993(75)90817-3. [DOI] [PubMed] [Google Scholar]
- Schwartzkroin P. A., Prince D. A. Effects of TEA on hippocampal neurons. Brain Res. 1980 Mar 3;185(1):169–181. doi: 10.1016/0006-8993(80)90680-0. [DOI] [PubMed] [Google Scholar]
- Schwartzkroin P. A., Slawsky M. Probable calcium spikes in hippocampal neurons. Brain Res. 1977 Oct 21;135(1):157–161. doi: 10.1016/0006-8993(77)91060-5. [DOI] [PubMed] [Google Scholar]
- Segal M. The action of serotonin in the rat hippocampal slice preparation. J Physiol. 1980 Jun;303:423–439. doi: 10.1113/jphysiol.1980.sp013297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner D. A., Schwartzkroin P. A. Steady-state electrotonic analysis of intracellularly stained hippocampal neurons. J Neurophysiol. 1980 Jul;44(1):184–199. doi: 10.1152/jn.1980.44.1.184. [DOI] [PubMed] [Google Scholar]
- Waggoner A. S., Grinvald A. Mechanisms of rapid optical changes of potential sensitive dyes. Ann N Y Acad Sci. 1977 Dec 30;303:217–241. [PubMed] [Google Scholar]
- Wong R. K., Prince D. A., Basbaum A. I. Intradendritic recordings from hippocampal neurons. Proc Natl Acad Sci U S A. 1979 Feb;76(2):986–990. doi: 10.1073/pnas.76.2.986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto C. Intracellular study of seizure-like afterdischarges elicited in thin hippocampal sections in vitro. Exp Neurol. 1972 Apr;35(1):154–164. doi: 10.1016/0014-4886(72)90066-0. [DOI] [PubMed] [Google Scholar]




