Abstract
Introduction
Herpes zoster is an acute condition caused by the reactivation of varicella zoster virus, often affecting the skin and mucosa. When varicella zoster virus invades the trigeminal ganglion, it can lead to trigeminal herpes zoster, with postherpetic neuralgia as the most common complication. Postherpetic neuralgia, characterized by persistent pain for over 3 months after skin lesions heal, significantly impacts quality of life, including sleep, mood, and social interactions. Current treatments, including antiviral drugs, analgesics, and neurotrophic agents, are often insufficient, and many patients still suffer from refractory postherpetic neuralgia. This highlights the need for alternative treatments. Trigeminal ganglion electrical stimulation has shown potential in managing refractory trigeminal herpes zoster and preventing postherpetic neuralgia. However, reports on its effectiveness remain scarce. This article presents two rare cases where trigeminal ganglion electrical stimulation was successfully used to treat refractory trigeminal herpes zoster and prevent postherpetic neuralgia.
Case presentation
This study reports two cases of trigeminal herpes zoster that were refractory to pharmacological treatment and successfully alleviated using trigeminal ganglion electrical stimulation, which also effectively prevented postherpetic neuralgia. Both patients, a 62-year-old Chinese female and a 65-year-old Chinese male, presented with severe pain, itching, sensory disturbances, and extensive vesicular lesions on the left side of the face, involving the ophthalmic (V1) and maxillary (V2) branches. Despite receiving antiviral drugs, analgesics, and neurotrophic agents, their symptoms remained inadequately controlled, with pain scores ranging from 8 to 10. After undergoing trigeminal ganglion electrical stimulation, the pain score of both patients dropped to 2–3 on the first day post-treatment, with significant improvement in the herpes zoster blisters and pain. During follow-up, the pain continued to improve, with marked reduction in the pain and itching in the affected areas, and sleep quality also improved. At 1, 3, and 6 months of follow-up, neither patient had developed postherpetic neuralgia. These cases suggest that trigeminal ganglion electrical stimulation may be an effective method for treating refractory trigeminal herpes zoster and preventing postherpetic neuralgia, significantly improving herpes symptoms and alleviating pain.
Conclusions
These cases demonstrate that trigeminal ganglion electrical stimulation can provide rapid and sustained pain relief while preventing postherpetic neuralgia in refractory trigeminal herpes zoster patients. This highlights the potential of trigeminal ganglion electrical stimulation as a novel therapeutic approach, particularly for patients who do not respond to conventional treatments. Its success in these cases suggests a promising direction for further research and clinical application.
Keywords: Trigeminal ganglion, Electrical stimulation, Herpes zoster, Postherpetic neuralgia, Trigeminal postherpetic neuralgia
Introduction
Herpes zoster (HZ) is an acute skin and mucous membrane disease caused by the reactivation of varicella zoster virus (VZV) infection in the ganglion, characterized by vesicles and severe neuralgia along the ganglion distribution [1, 2]. The incidence of HZ is positively correlated with age, with an annual incidence of 3.9–42/100,000, and about half of them occur in people over 60 years old [3]. When VZV invades the trigeminal ganglion (TG; also known as Gasserian ganglion [GG]), the reactivation and replication of the virus cause edema and necrosis of the neurons and nerves of the ganglion and nerve, resulting in trigeminal herpes zoster (THZ) [4]. The most common complication of THZ is postherpetic neuralgia (PHN) of trigeminal nerve, which is neuralgia that persists for more than 3 months after the healing of THZ skin lesions. The incidence of PHN is related to factors such as age, the severity of vesicles, and the location of skin lesions, accounting for about 15–20% of patients with HZ [5, 6]. PHN causes a serious decline in the quality of life of patients, affecting sleep, mood, and social interaction, and even leading to depression and suicide [7, 8]. At present, the main treatment of THZ includes antiviral drugs, analgesics, nerve nutrition drugs, and so on, aiming to control virus replication, relieve pain, promote skin lesion healing, and prevent or reduce the occurrence of PHN [9, 10]. However, these drug treatments are not ideal, and some patients still have PHN, and it is difficult to relieve. This indicates that, for intractable herpes zoster, the limitations of pharmacotherapy alone remain significant. Studies have shown that antiviral drugs such as acyclovir can inhibit VZV replication, accelerate rash healing, relieve pain, and reduce the risk of PHN [11]. Corticosteroids cannot reduce the incidence of PHN, but when combined with antiviral drugs, they can accelerate rash healing and relieve pain [12, 13]. In addition, sometimes a combination of multiple drugs is needed for analgesia, such as anticonvulsants pregabalin, opioids tramadol, and antidepressants such as duloxetine [11]. Many patients do not respond to oral drugs, and some adverse events, such as nausea, vomiting, and dizziness, are intolerable for elderly patients [14, 15]. Therefore, finding new and effective treatment methods is a clinical problem for the treatment of refractory THZ and the prevention of PHN.
TG is the largest cranial nerve ganglion, located at the trigeminal impression of the temporal bone apex. It is crescent-shaped and composed of typical pseudounipolar neurons. The periphery of the cell body protrudes into three nerves, namely the ophthalmic nerve, the maxillary nerve, and the mandibular nerve. TG is responsible for transmitting the general somatic sensation of the ipsilateral face [16]. TG is the best treatment target for trigeminal neuralgia [17], and stimulating TG can relieve the pain in the facial area innervated by the trigeminal nerve [18]. In recent years, studies have shown that TGES technology can effectively relieve trigeminal neuropathic pain (TNP) [17, 19, 20]. TGES is a minimally invasive nerve stimulation technique that inhibits the transmission of pain signals by implanting electrodes to stimulate TG. The principle of TGES is to use the sensory masking effect of electrical stimulation, that is, to produce a slight numbness or tingling sensation in the pain area, masking or reducing the original pain sensation [21–23]. Lise Kustermans et al. [17] reported that 22 patients with refractory TNP underwent TGES treatment and were followed up with for 24 months. The results showed that the patients’ pain level, medication dosage, quality of life, and satisfaction were significantly improved, and no serious complications occurred. However, there are very few reports of TGES applied to refractory THZ and successfully preventing PHN. This article reports two rare cases of using TGES to treat refractory THZ and successfully prevent PHN, respectively for right V1 and V2 HZ. The patients received TGES treatment within 2 months after the onset of THZ, with continuous electrical stimulation for 1 week. The patients had pain relief, skin lesion healing, no obvious adverse reactions, and no recurrence and PHN occurrence during the 6-month follow-up. This article discusses the feasibility and effectiveness of TGES treatment of THZ and prevention of PHN, providing a new treatment option for clinical practice.
Case presentation
Case report 1
A 62-year-old Chinese female patient presented with severe pain, itching, and paresthesia on the left side of her face for over 5 days. She had large vesicular lesions on her face with marked tenderness, which severely impaired her work, life, and sleep quality. At another hospital, the patient was diagnosed with trigeminal herpes zoster (THZ) involving the ophthalmic (V1) and maxillary (V2) branches. The patient had taken oral antiviral drugs, analgesics, intravenous infusion of nerve nutrition drugs, and other treatments, but the pain relief effect was poor. The patient was very sensitive to opioids, and she had severe vomiting after taking tramadol sustained-release capsules. The patient had a history of hypertension, which was well controlled, and no history of diabetes, surgery, cancer in the family, smoking, or drinking. After the patient was transferred to our pain department, the outpatient assessment used the numeric rating scale (NRS), and the NRS score was 8–10. Considering that drug treatment was ineffective in the patient, we planned to perform electrostimulation of the trigeminal ganglion through the foramen ovale (TGES) under the guidance of digital subtraction angiography (DSA) to relieve the patient’s pain. Before the operation, the patient did not have any obvious signs of abnormality, such as cough, fever, or respiratory distress. Routine biological tests such as blood routine examination, blood biochemistry examination, and coagulation examination also did not find any obvious abnormalities. The intervention involved percutaneous puncture of the foramen ovale with a 14-gauge sheath needle under DSA guidance, advancing it into Meckel’s cave, and implanting a temporary electrode of the trigeminal ganglion through the sheath needle (Fig. 1A, B). The electrode had eight contacts, each with a length of 3 mm and a spacing of 4 mm. The electrical stimulation test parameters were a pulse width of 200 microseconds, a frequency of 60 Hz, and a voltage of 0.5 V. The patient reported numbness in the V1 + V2 branch area, which corresponded to the distribution of the pain. We confirmed that there were no adverse effects, such as facial twitching, diplopia, or dysarthria. After fixing the end of the electrode to the corner of the mouth with a suture, we connected it to an external pulse generator. The operation was successfully completed. On the first day after the operation, the patient’s oral pain score dropped to 2–3. After appropriately increasing the voltage to 0.8 V, the patient reported numbness in the V1 + V2 branch area, and the pain was further relieved. We suggested that the patient stop taking tramadol sustained-release capsules and continue to take oral pregabalin 75 mg twice a day, flupirtine maleate meloxicam tablets 0.5 mg twice a day, and mecobalamin 0.5 mg three times a day. On the third day after the operation, the patient’s oral pain was further relieved, and there was no NRS score greater than 3, there was no breakthrough pain, the area of pain and itching in the herpes area became smaller, and the patient’s sleep condition had improved. On the seventh day after the operation, the patient’s oral pain in the forehead, face, and wing of the nose improved significantly, and only occasional needle-like discomfort was reported; the NRS score was 2–3. We removed the temporary electrode after routine disinfection in the invasive treatment room. On the same day, the patient continued oral drug treatment. On the eighth day, the patient was discharged smoothly. No pain or postherpetic neuralgia (PHN) was found during follow-up at 1, 3, and 6 months after discharge.
Fig. 1.
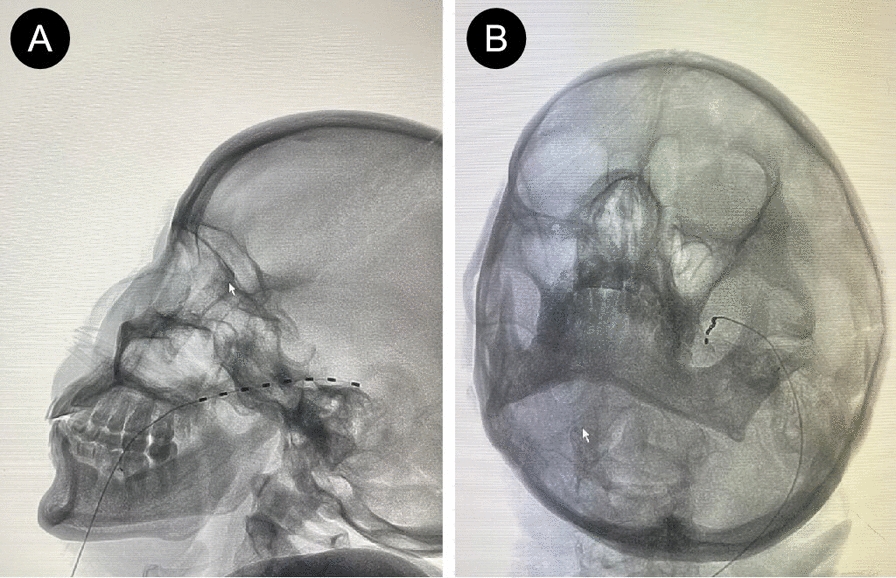
A, B Under local anesthesia, a sheath needle was inserted into the foramen ovale under the guidance of digital subtraction angiography, and a temporary electrode of the trigeminal ganglion was implanted. The position of the electrode tip was confirmed by X-ray
Case report 2
A 65-year-old Chinese male patient suffered from severe pain, itching, and paresthesia in the left facial region with shingles for 35 days. He was diagnosed with trigeminal herpes zoster (THZ) affecting the ophthalmic (V1) and maxillary (V2) branches at another hospital and was referred to the pain department of the Affiliated Hospital of Zunyi Medical University for treatment. His pain was characterized by persistent burning and electric-shock-like pain, ranging from moderate to severe, with paroxysmal attacks every 30–45 minutes, and the NRS score was 9–10. Before admission, the patient had received antiviral drugs, traditional Chinese medicine, and analgesics at the local hospital. Although the herpes zoster lesions gradually formed crusts, the pain persisted, which seriously affected his daily life and sleep. He was transferred to our hospital, hoping to relieve the pain. The patient had no history of hypertension, diabetes, surgery, cancer in the family, smoking, or drinking. After admission, the doctor gave him oral acetaminophen hydrocodone 325 mg three times a day and pregabalin 75 mg twice a day. But he had obvious symptoms of dizziness, nausea, and vomiting after taking pregabalin, so we considered performing TGES under the guidance of DSA to relieve his severe pain. Before the operation, the patient did not have any obvious signs of abnormality, such as cough, fever, and respiratory distress. Routine biological tests such as blood routine examination, blood biochemistry examination, and coagulation examination also did not find any obvious abnormalities. We used the same trigeminal ganglion electrostimulation intervention for the second patient as for the first patient, to relieve his chronic head and face pain (Fig. 2A, B). On the first day after the operation, the patient’s pain score was 1–2; after appropriately increasing the voltage to 1.2 V, the patient reported numbness in the V1 and V2 areas, and the pain was relieved. He continued to take oral pregabalin 75 mg twice a day, anfen dihydrocodeine 0.5 g three times a day, flupirtine maleate meloxicam tablets 0.5 mg twice a day, and mecobalamin 0.5 mg three times a day. On the third day after the operation, the patient reported that the pain was relieved; there was no NRS score greater than 3, the area of pain and itching in the herpes area became smaller, and the sleep condition improved significantly. On the seventh day after the operation, the patient reported that the pain in the forehead, face, and wing of the nose was significantly relieved, only needle-like discomfort was reported, and the NRS score was 1–2. Then, the electrode was removed after routine disinfection in the invasive treatment room, and he continued oral drug treatment on the same day. On the eighth day, the patient was discharged smoothly. During the whole treatment process, the patient’s pain was significantly relieved, his sleep condition was improved, the operation was successfully completed, and no postoperative infection or other adverse reactions occurred. No pain or PHN was found during follow-up at 1, 3, and 6 months after discharge.
Fig. 2.

A, B Under local anesthesia, a sheath needle was inserted into the foramen ovale under the guidance of digital subtraction angiography, and a temporary electrode of the trigeminal ganglion was implanted. The position of the electrode tip was confirmed by X-ray
Discussion
HZ is caused by the reactivation of VZV. It usually manifests as burning pain and unilateral vesicular lesions, affecting up to 60% and 20% of the thoracic and cervical regions, respectively [24, 25]. Up to 20% of cases involve the trigeminal nerve [25]. Previous studies have shown that THZ is more difficult to treat than herpes zoster in other regions from the cervical to the sacral level. It is likely to progress to PHN if effective pain relief and anti-inflammatory treatment are not obtained in its acute and subacute phases [3]. PHN and its refractory treatment cause serious economic burden to patients and society. Therefore, for THZ, pain physicians should explore an effective method to alleviate THZ and prevent the occurrence of PHN.
Consistent with the treatment of herpes zoster in other parts of the body, the main treatment method for THZ is pharmacotherapy. A combination of drugs is usually required to achieve good clinical results. This includes a combination of topical and systemic drugs, relaxation, psychological intervention, and social support. Currently, local treatment recommends the use of a lidocaine patch as the first-line treatment option and an 8% capsaicin patch as the second-line treatment option. A randomized controlled trial showed that the lidocaine patch can significantly relieve acute herpes zoster pain and prevent PHN [26]. Systemic drugs include antiviral drugs (such as acyclovir), anticonvulsants (such as pregabalin-like drugs), opioids (tramadol), antidepressants (such as norepinephrine and serotonin reuptake inhibitors, such as duloxetine), and so on [27–30]. However, pharmacotherapy is slow to take effect, some patients have poor treatment results, and side effects such as nausea, vomiting, constipation, drowsiness, confusion, sedation, urinary retention, and cardiac toxicity are obvious [31]. Therefore, although pharmacotherapy is the routine treatment for THZ, its effectiveness is limited. There are many side effects, and it cannot meet the needs of all patients. In this situation, neuromodulation techniques, as a new emerging treatment modality, such as electrical stimulation, pulsed radiofrequency, and nerve block, become another important supplement. In many retrospective studies, patients with THZ and PHN received electrical stimulation, pulsed radiofrequency, and nerve block treatment, and the results showed that they could effectively relieve pain, reduce drug dosage, improve sleep quality, alleviate anxiety and depression, and effectively prevent the occurrence of PHN. However, reports on trigeminal ganglion electrical stimulation to prevent PHN are rare [5, 32]. This indicates that neuromodulation technology is a safe and effective treatment method that can reduce the progression of neuropathological changes and provide a new option for the treatment of THZ. However, the application of nerve block treatment may cause neurological sequelae, such as aseptic meningitis, headache, and arachnoiditis. The risk of complications is high [33]. Electrical stimulation and pulsed radiofrequency are gradually increasing in application due to their significant effects, low operation difficulty, and low risk [33].
Pulsed radiofrequency (PRF), developed as an improvement over traditional radiofrequency thermocoagulation, is a minimally invasive technique widely used in clinical practice for managing chronic pain. Compared with the older thermocoagulation method, PRF offers the advantage of delivering therapeutic effects without causing significant tissue damage, making it a safer and more refined alternative. It uses pulsed current to act on the nerve before the needle tip, forming a high-voltage field around the nerve tissue. This indirectly activates the superficial neurons of the spinal dorsal horn, changing the function of nerve myelin cells. It also inhibits the electrophysiology of nerve fiber conduction and interferes with the transmission of nerve impulses, thereby alleviating the patient’s pain symptoms [34]. Studies have shown that trigeminal ganglion (TG) PRF can effectively relieve pain, reduce drug dosage,and effectively reduce the occurrence of PHN [5, 35]. In addition, another study showed that electrical stimulation has a lower incidence of complications, shorter operation time, and better safety and patient compliance than PRF therapy [36–38]. However, the application of PRF also has some problems, such as complex operation, the need for professional equipment, difficulty in ensuring the accuracy of needle tip position, and possible nerve damage. Consequently, we further explored a simpler, safer, and more effective neuromodulation technique—TGES.
TGES is a promising technique for the treatment of trigeminal neuralgia that was first proposed by Meyerson and Hakansson in 1976 and has been widely applied and studied in the following decades. Among 14 patients, 11 reported significant analgesia after more than 4 years of follow-up [39]. Mehrkens and Steude conducted a 5-year follow-up study on 235 patients from 1980 to 2005, reporting that 52% of patients considered TGES to reduce their pain by half [22]. Mengzhen Xu et al. [40] reported six cases of short-term TGES intervention, followed up with for about 6 months after discharge. The results showed that the patients’ pain, sleep quality, and quality of life were further improved. These results further confirmed the feasibility and effectiveness of TGES, indicating that temporary TGES intervention can bring different degrees of pain alleviation to patients, or even completely eliminate pain, and there is no pain aggravation. The specific analgesic mechanism of TGES is not clear; it involves peripheral, spinal, and brain neurons and circuits. R Melzack et al. [22] found that TGES activates Aβ fibers, produces a gate effect, and inhibits the input of pain signals. TGES also activates the descending inhibitory system, increases endogenous opioids in the spinal dorsal horn, and inhibits the output of pain signals [21]. In addition, TGES regulates the brain’s pain modulation network, including the dorsolateral prefrontal cortex, insula, anterior cingulate gyrus, thalamus, brainstem, and other regions, affecting the cognitive and emotional dimensions of pain [23]. However, research into its use in THZ therapy and PHN prevention is limited. Internationally, most cases involve long-term electrical stimulation implants. However, considering the specific circumstances of our patients, we opted for a short-term method of trigeminal ganglion electrical stimulation implantation. During the electrode implantation process, we placed three contact points within the foramen ovale to ensure extensive coverage of the patient’s pain area and effectively prevent a reduction in coverage due to electrode displacement. In these two cases, patients experienced a pain relief rate of over 50%, and there were no incidents of electrode dislocation prior to removal. Furthermore, we conducted regular follow-ups to assess postoperative pain improvement. In both patients, we observed the herpes zoster blisters healing and crusting over, with a significant reduction in pain and NRS scores around 1–2. In the two patients mentioned above, this method effectively relieved pain and successfully prevented the occurrence of PHN, proving that, for those patients with refractory THZ, TGES is an alternative option. However, as this article presents only two representative cases, its findings should be interpreted with caution. Future research involving a larger number of cases and the inclusion of a control group will be necessary to further validate the efficacy and safety of TGES in treating intractable herpes zoster and preventing postherpetic neuralgia.
Conclusions
On the basis of the two cases presented, we propose that TGES may be an effective therapeutic approach for patients with refractory THZ, offering a potential method to prevent the development of PHN. TGES represents a novel direction for managing this challenging condition, providing hope for patients who do not respond to conventional treatments. However, this case report is inherently limited by the lack of a control group, short follow-up period, and reliance on only two cases. Further studies with larger patient cohorts, longer follow-up durations, and controlled designs are needed to comprehensively evaluate the efficacy and safety of TGES in treating refractory THZ.
Image Privacy
To ensure the protection of the patient’s privacy, identifying features in the accompanying images, such as facial features, have been blurred or removed. These modifications were made in accordance with ethical guidelines for case report publications.
Acknowledgements
Not applicable.
Author contributions
WHW was the main contributor to the writing and revision of this manuscript; BYQ and JY conceived the project, provided financial support, contributed to the study design, and edited the manuscript. SWW, QA, JHL, and DXZ were involved in drafting the manuscript. All authors read and approved the final version of the manuscript.
Funding
This case report is funded by the National Natural Science Foundation of China (82160683, 81960660).
Availability of data and materials
Not applicable.
Declarations
Ethics approval and consent to participate
As this is a descriptive case report of clinical observations, ethics approval was waived by the Ethics Committee of the Affiliated Hospital of Zunyi Medical University. Written informed consent was obtained from the patient for the publication of this case report, including the use of any accompanying images. The patient has reviewed the case details and has consented to their submission for publication in this journal.
Consent for publication
Written informed consent was obtained from the patient for publication of this case report and any accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Bangyong Qin, Email: qbyzy@163.com.
Jie Yuan, Email: yuanjie@zmu.edu.cn.
References
- 1.Kennedy P. The spectrum of neurological manifestations of varicella-zoster virus reactivation. Viruses. 2023;15(8):1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang C, Dou Z, Yan M, Wang B. Efficacy and safety of pulsed radiofrequency in herpes zoster related trigeminal neuralgia: a systematic review and meta-analysis. J Pain Res. 2023;16:341–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sampathkumar P, Drage LA, Martin DP. Herpes zoster (shingles) and postherpetic neuralgia. Mayo Clin Proc. 2009;84(3):274–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Iancu GM, Stănilă DM, Cipăian RC, Rotaru M. Ophthalmic herpes zoster with severe complications in an immunocompromised patient: a case report and review of the literature. Exp Ther Med. 2022;23(3):214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wan CF, Song T. Comparison of two different pulsed radiofrequency modes for prevention of postherpetic neuralgia in elderly patients with acute/subacute trigeminal herpes zoster. Neuromodulation. 2022;25(8):1364–71. [DOI] [PubMed] [Google Scholar]
- 6.Gan EY, Tian EA, Tey HL. Management of herpes zoster and postherpetic neuralgia. Am J Clin Dermatol. 2013;14(2):77–85. [DOI] [PubMed] [Google Scholar]
- 7.Zheng B, Song L, Liu H. Gasserian ganglion injected with adriamycin successfully relieves intractable trigeminal nerve postherpetic neuralgia for an elderly patient: a case report. Medicine. 2018;97(38):e12388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Philip A, Thakur R. Post herpetic neuralgia. J Palliat Med. 2011;14(6):765–73. [DOI] [PubMed] [Google Scholar]
- 9.Kanbayashi Y, Hosokawa T. Vaccination against and treatment of acute herpes zoster for prevention of postherpetic neuralgia. Curr Pain Headache Rep. 2013;17(10):371. [DOI] [PubMed] [Google Scholar]
- 10.Ehrenstein B. Diagnosis, treatment and prophylaxis of herpes zoster. Z Rheumatol. 2020;79(10):1009–17. [DOI] [PubMed] [Google Scholar]
- 11.Saguil A, Kane S, Mercado M, Lauters R. Herpes zoster and postherpetic neuralgia: prevention and management. Am Fam Phys. 2017;96(10):656–63. [PubMed] [Google Scholar]
- 12.Chen N, Li Q, Yang J, Zhou M, Zhou D, He L. Antiviral treatment for preventing postherpetic neuralgia. Cochrane Database Syst Rev. 2014. 10.1002/14651858.CD006866.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wood MJ, Johnson RW, McKendrick MW, Taylor J, Mandal BK, Crooks J. A randomized trial of acyclovir for 7 days or 21 days with and without prednisolone for treatment of acute herpes zoster. N Engl J Med. 1994;330(13):896–900. [DOI] [PubMed] [Google Scholar]
- 14.Moore RA, Derry S, Aldington D, Cole P, Wiffen PJ. Amitriptyline for neuropathic pain in adults. Cochrane Database Syst Rev. 2015;7:008242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Szok D, Tajti J, Nyári A, Vécsei L. Therapeutic approaches for peripheral and central neuropathic pain. Behav Neurol. 2019;2019:8685954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Messlinger K, Russo AF. Current understanding of trigeminal ganglion structure and function in headache. Cephalalgia. 2019;39(13):1661–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kustermans L, Van Buyten JP, Smet I, Coucke W, Politis C. Stimulation of the Gasserian ganglion in the treatment of refractory trigeminal neuropathy. J Craniomaxillofac Surg. 2017;45(1):39–46. [DOI] [PubMed] [Google Scholar]
- 18.Iyengar S, Johnson KW, Ossipov MH, Aurora SK. CGRP and the trigeminal system in migraine. Headache. 2019;59(5):659–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Taub E, Munz M, Tasker RR. Chronic electrical stimulation of the Gasserian ganglion for the relief of pain in a series of 34 patients. J Neurosurg. 1997;86(2):197–202. [DOI] [PubMed] [Google Scholar]
- 20.Klein J, Sandi-Gahun S, Schackert G, Juratli TA. Peripheral nerve field stimulation for trigeminal neuralgia, trigeminal neuropathic pain, and persistent idiopathic facial pain. Cephalalgia. 2016;36(5):445–53. [DOI] [PubMed] [Google Scholar]
- 21.Sandkühler J. Models and mechanisms of hyperalgesia and allodynia. Physiol Rev. 2009;89(2):707–58. [DOI] [PubMed] [Google Scholar]
- 22.Melzack R, Wall PD. Pain mechanisms: a new theory. Science. 1965;150(3699):971–9. [DOI] [PubMed] [Google Scholar]
- 23.Mokhtari T, Ren Q, Li N, Wang F, Bi Y, Hu L. Transcutaneous electrical nerve stimulation in relieving neuropathic pain: basic mechanisms and clinical applications. Curr Pain Headache Rep. 2020;24(4):14. [DOI] [PubMed] [Google Scholar]
- 24.Harpaz R, Ortega-Sanchez IR, Seward JF. Prevention of herpes zoster: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2008. 10.1186/s12895-020-00110-1. [PubMed] [Google Scholar]
- 25.Pelloni LS, Pelloni R, Borradori L. Herpes zoster of the trigeminal nerve with multi-dermatomal involvement: a case report of an unusual presentation. BMC Dermatol. 2020;20(1):12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bianchi L, Piergiovanni C, Marietti R, et al. Effectiveness and safety of lidocaine patch 5% to treat herpes zoster acute neuralgia and to prevent postherpetic neuralgia. Dermatol Ther. 2021;34(1):e14590. [DOI] [PubMed] [Google Scholar]
- 27.Attal N, Cruccu G, Baron R, et al. EFNS guidelines on the pharmacological treatment of neuropathic pain: 2010 revision. Eur J Neurol. 2010;17(9):1113-e88. [DOI] [PubMed] [Google Scholar]
- 28.Dworkin RH, O’Connor AB, Backonja M, et al. Pharmacologic management of neuropathic pain: evidence-based recommendations. Pain. 2007;132(3):237–51. [DOI] [PubMed] [Google Scholar]
- 29.Hempenstall K, Nurmikko TJ, Johnson RW, A’Hern RP, Rice AS. Analgesic therapy in postherpetic neuralgia: a quantitative systematic review. PLoS Med. 2005;2(7):e164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Johnson RW, Rice AS. Clinical practice. Postherpetic neuralgia. N Engl J Med. 2014;371(16):1526–33. [DOI] [PubMed] [Google Scholar]
- 31.Hadley GR, Gayle JA, Ripoll J, et al. Postherpetic neuralgia: a review. Curr Pain Headache Rep. 2016;20(3):17. [DOI] [PubMed] [Google Scholar]
- 32.Kim ED, Bak HH, Jo DH, Park HJ. Clinical efficacy of transforaminal epidural injection for management of zoster-associated pain: a retrospective analysis. Skeletal Radiol. 2018;47(2):253–60. [DOI] [PubMed] [Google Scholar]
- 33.Maarbjerg S, Di Stefano G, Bendtsen L, Cruccu G. Trigeminal neuralgia—diagnosis and treatment. Cephalalgia. 2017;37(7):648–57. [DOI] [PubMed] [Google Scholar]
- 34.Li H, Ding Y, Zhu Y, Han Z, Yao P. Effective treatment of postherpetic neuralgia at the first branch of the trigeminal nerve by high-voltage pulsed radiofrequency. Front Neurol. 2021;12: 746035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wan C, Dong DS, Song T. High-voltage, long-duration pulsed radiofrequency on Gasserian ganglion improves acute/subacute zoster-related trigeminal neuralgia: a randomized, double-blinded controlled trial. Pain Phys. 2019;22(4):361–8. [PubMed] [Google Scholar]
- 36.Wan CF, Song T. Efficacy of pulsed radiofrequency or short-term spinal cord stimulation for acute/subacute zoster-related pain: a randomized, double-blinded controlled trial. Pain Phys. 2021;24(3):215–22. [PubMed] [Google Scholar]
- 37.Hamaguchi S, Shinozaki M. Complications associated with spinal cord stimulation, radiofrequency and pulsed radiofrequency. Masui. 2016;65(7):686–92. [PubMed] [Google Scholar]
- 38.Texakalidis P, Tora MS, Anthony CL, et al. Peripheral trigeminal branch stimulation for refractory facial pain: a single-center experience. Clin Neurol Neurosurg. 2020;194:105819. [DOI] [PubMed] [Google Scholar]
- 39.Meyerson BA, Håkansson S. Alleviation of atypical trigeminal pain by stimulation of the Gasserian ganglion via an implanted electrode. Acta Neurochir Suppl (Wien). 1980;30:303–9. [DOI] [PubMed] [Google Scholar]
- 40.Xu M, Liu J, Zhang H, Li R, Wei J. Trigeminal ganglion electrical stimulation for trigeminal nerve postherpetic neuralgia: a retrospective study. J Pain Res. 2023;16:3633–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.


