Abstract
1. The membrane potential of rods in the isolated toad retina was recorded while changing the ionic composition of the extracellular medium.
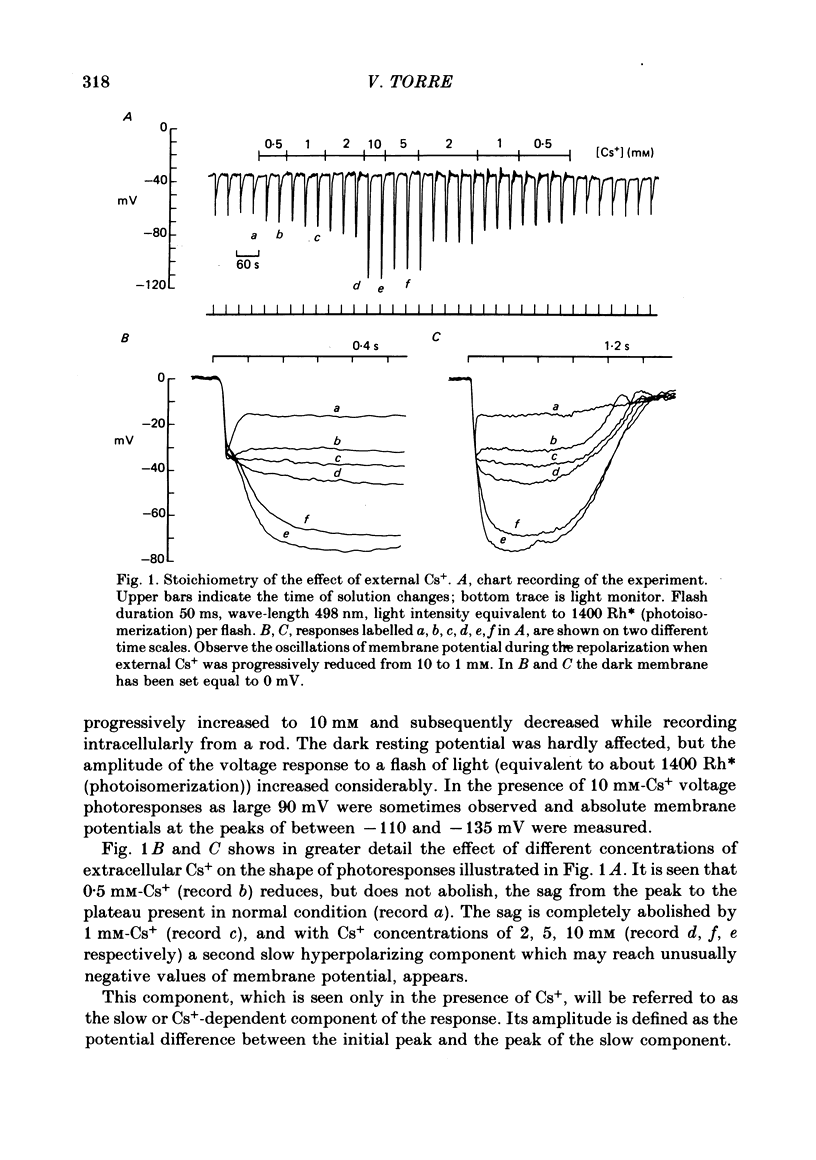
2. Caesium (Cs+) at a concentration of 1 mM was sufficient to completely block the sag from the peak to the plateau in the bright-flash voltage response.
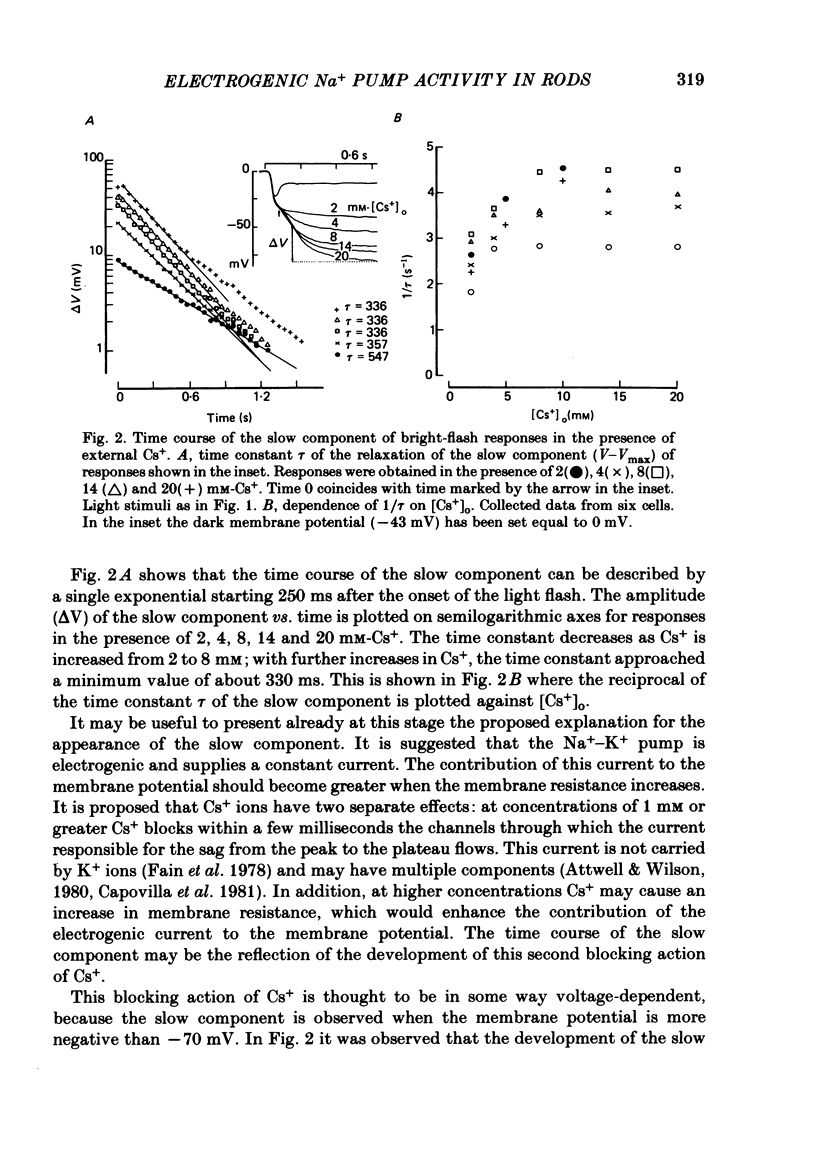
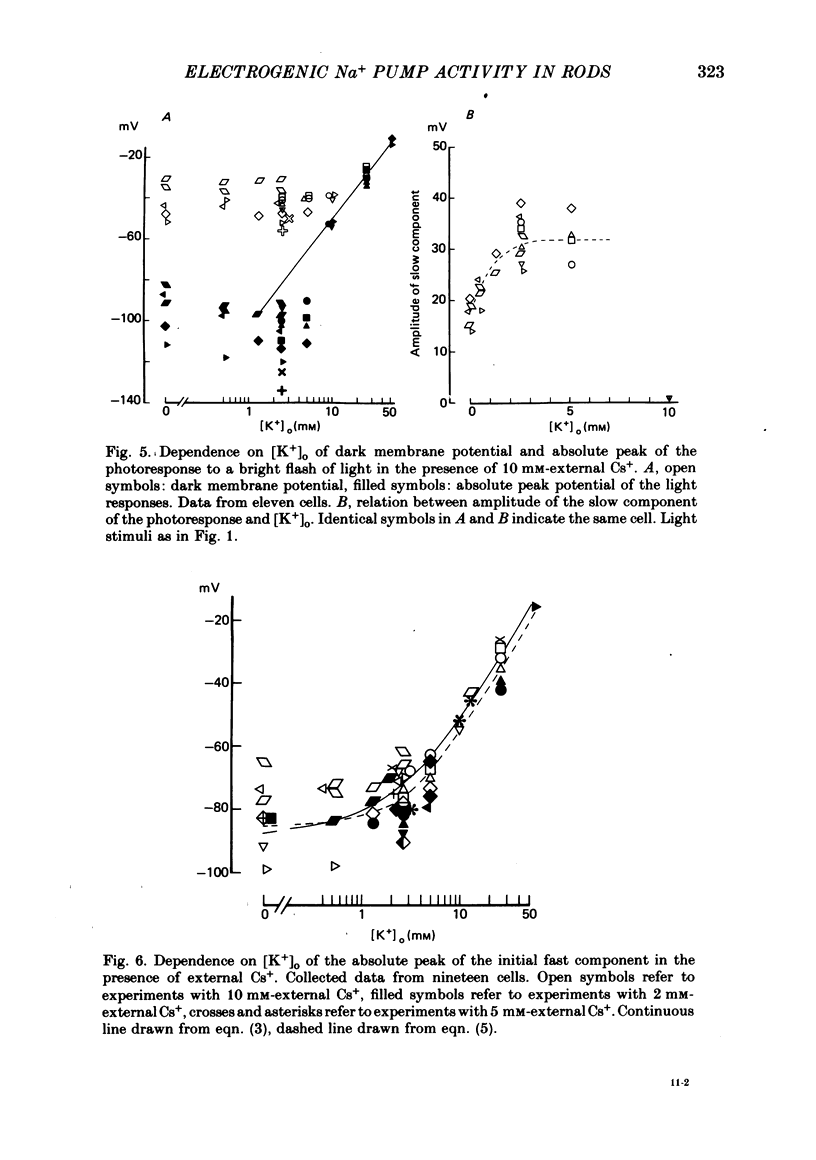
3. In the presence of 10 mM-Cs+ the bright-flash response increased in amplitude to about 90 mV, thus reaching an absolute membrane potential of between -110 and -135 mV. These responses consisted of an initial fast component of about 35 mV followed by a much slower component which could be as large as 50 mV.
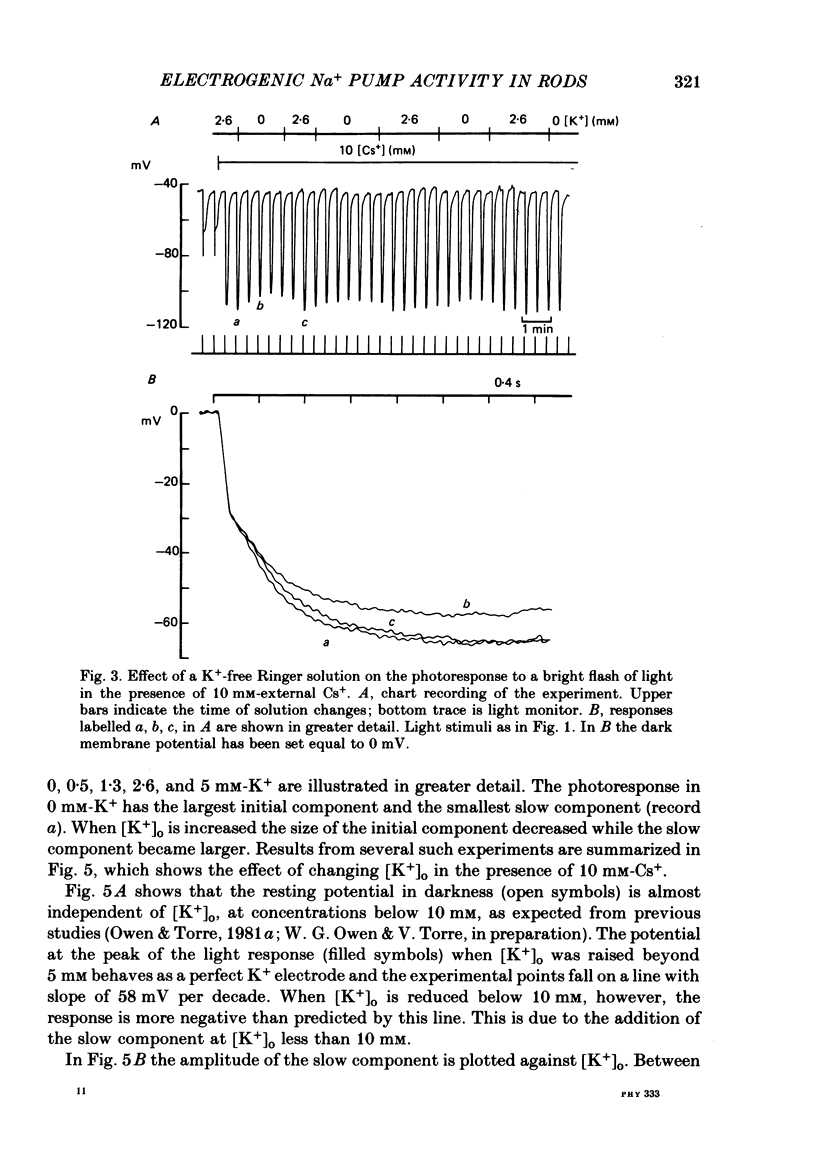
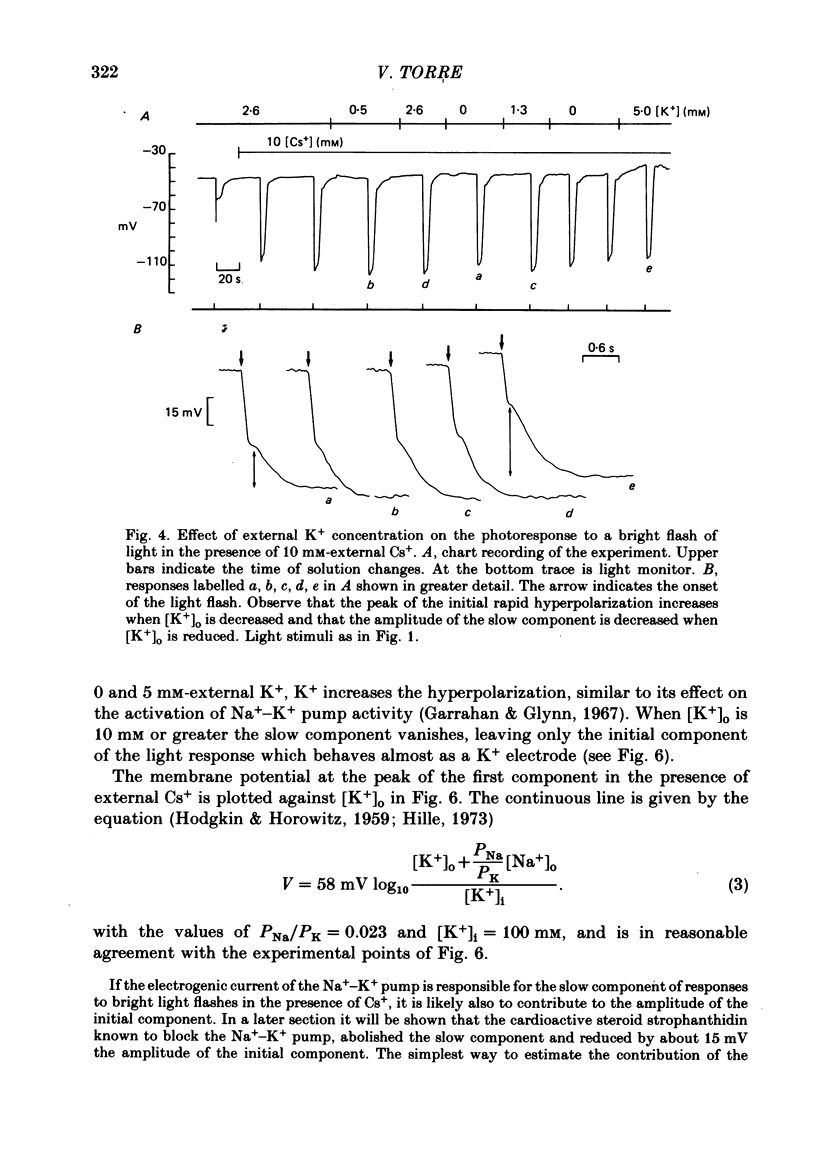
4. At the peak of the initial fast component the rod membrane conformed closely to the behaviour of a K+ electrode with a PNa/PK ratio of 0·023. On average the amplitude of the slow component was about 35 mV in the presence of 2·6 mM-K+ and was reduced to about 25 mV in a K+-free Ringer.
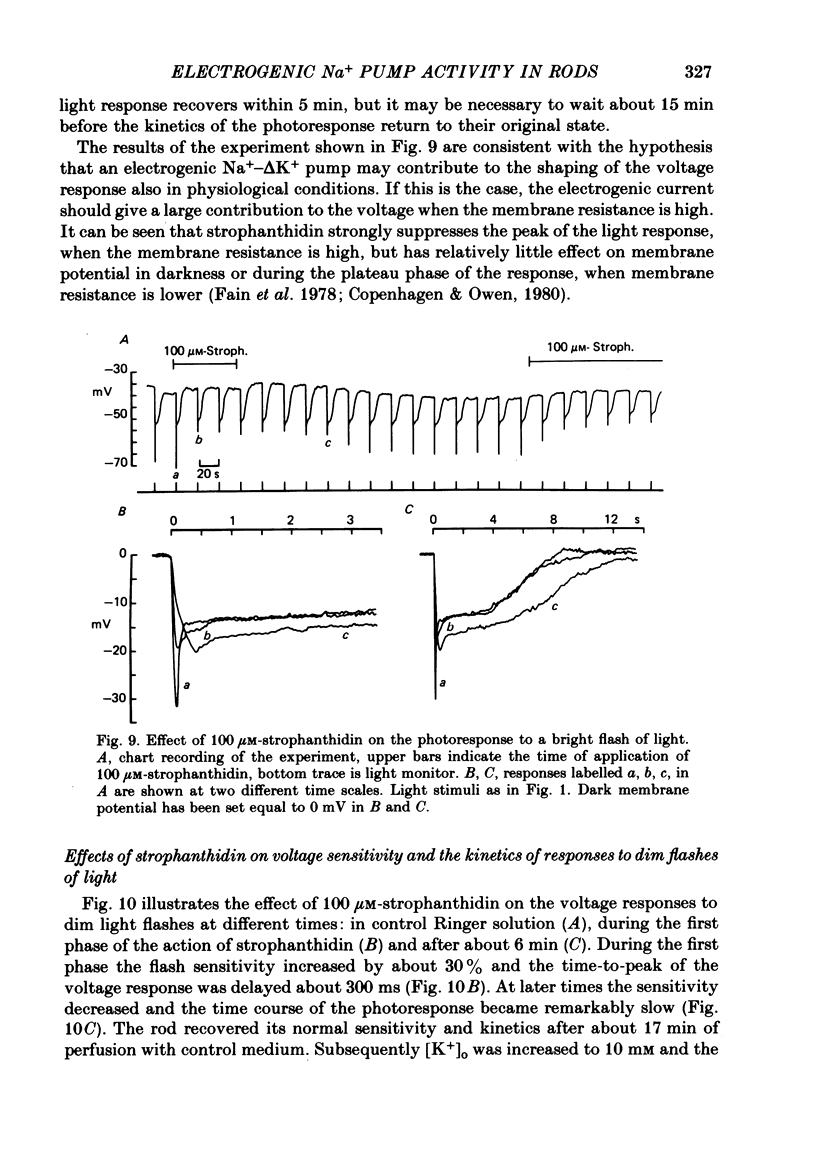
5. Addition of 100 μM-strophanthidin to the perfusate induced several reversible changes in the electrical activity of rods. The dark resting membrane potential depolarized by about 5 mV and the kinetics of the voltage response to dim flashes of light slowed down. The voltage sensitivity initially increased by about 30%, but the peak of the response to a bright flash of light was reduced by about 13 mV.
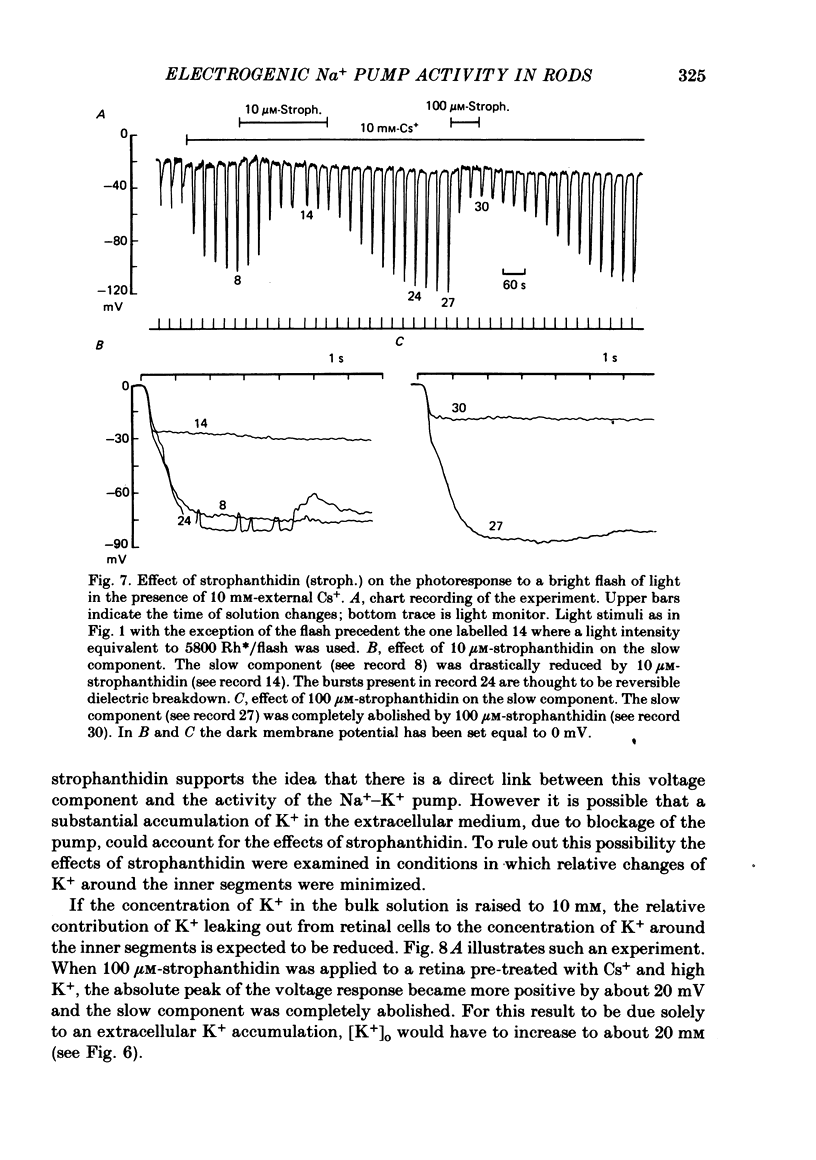
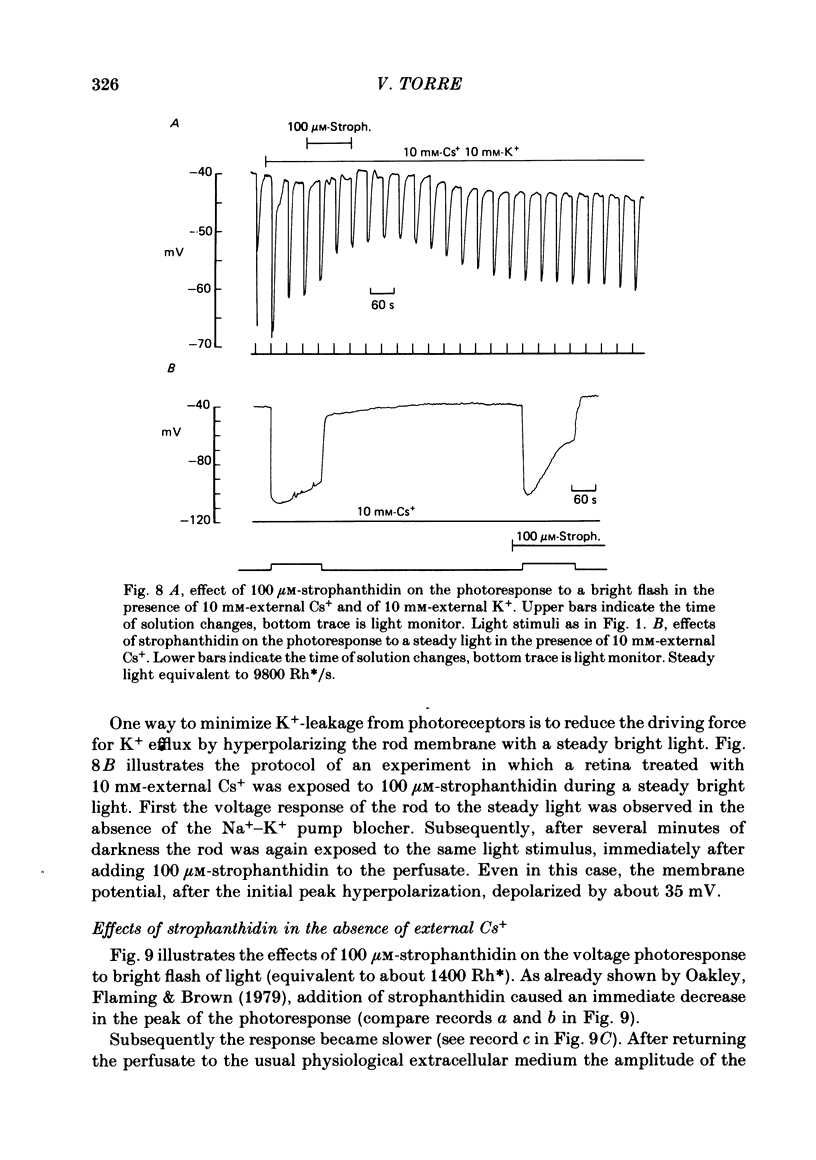
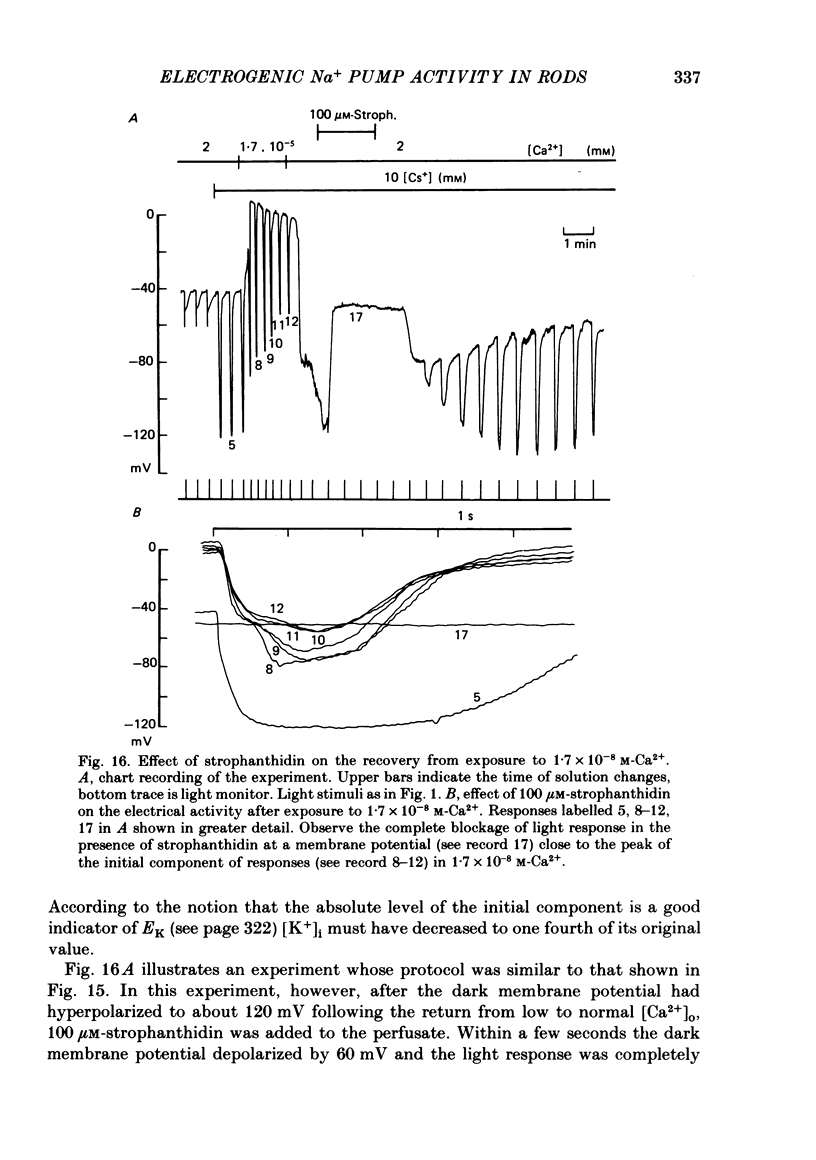
6. In rods treated with 10 mM-Cs+ the slow component present in the bright flash response was abolished by strophanthidin with an apparent Km of 3 μM.
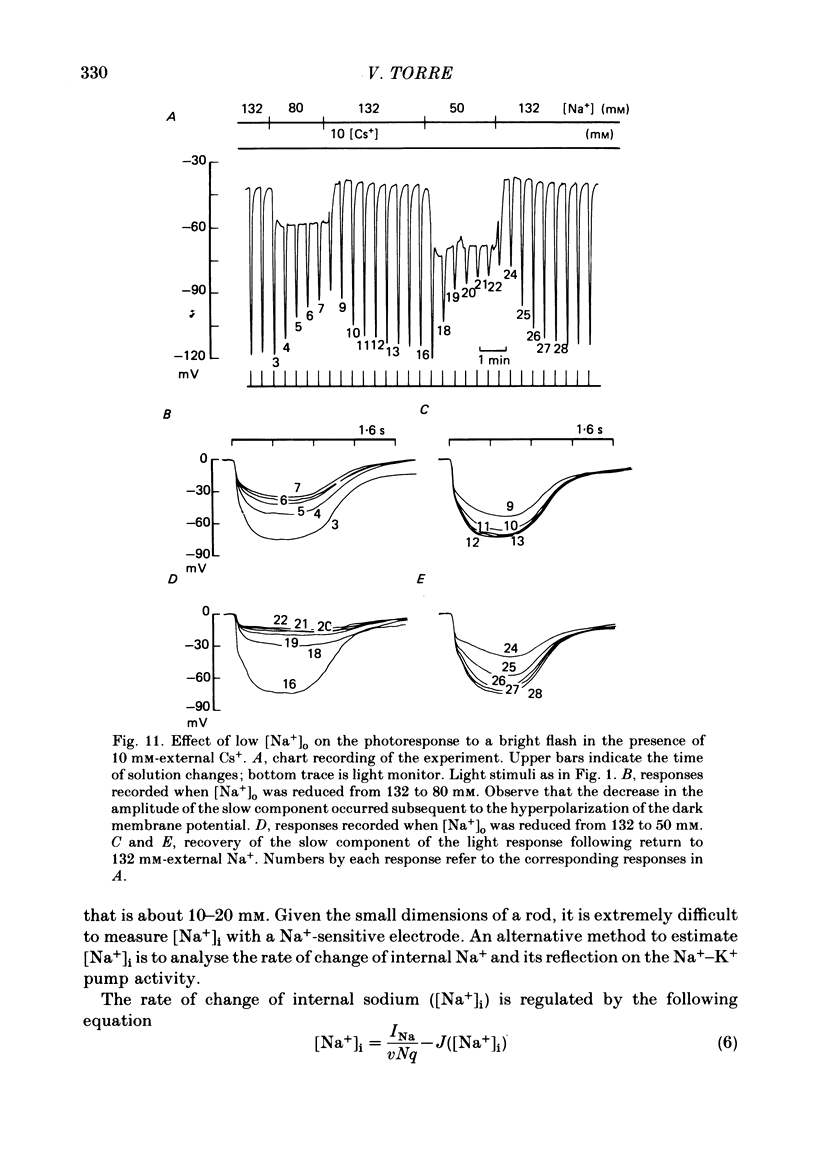
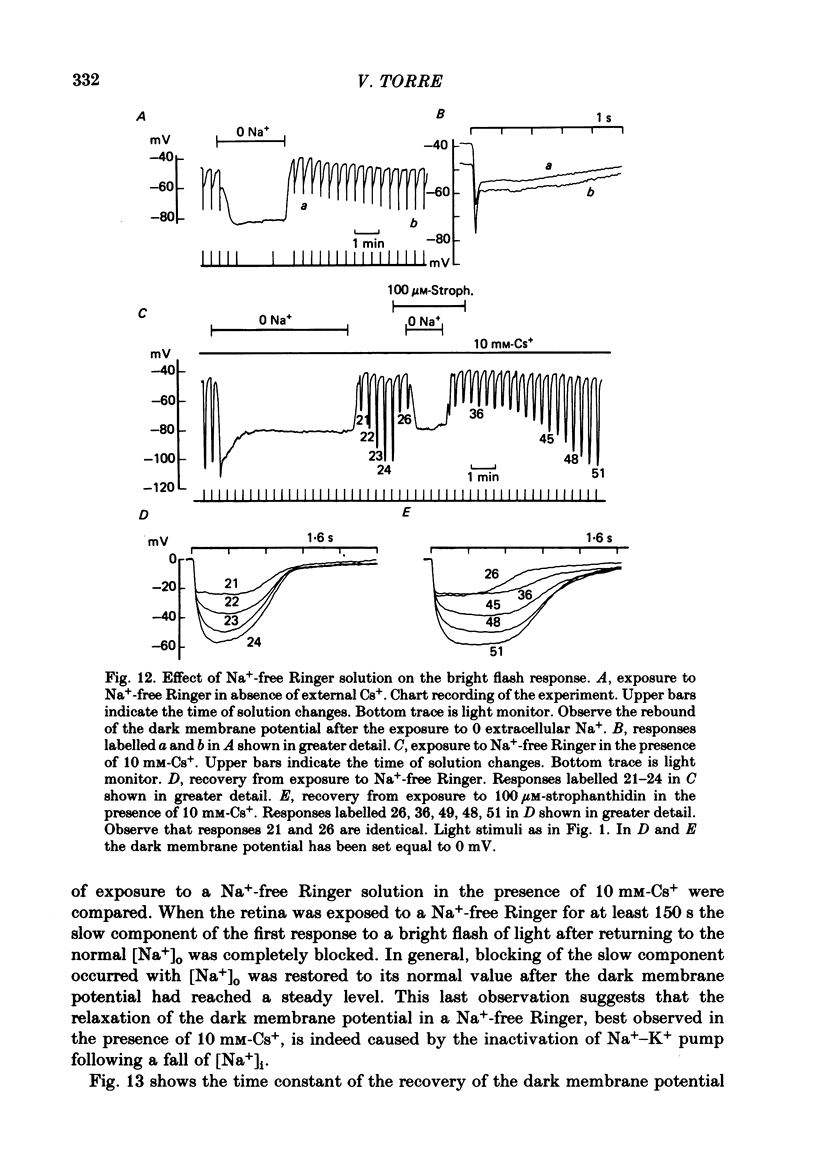
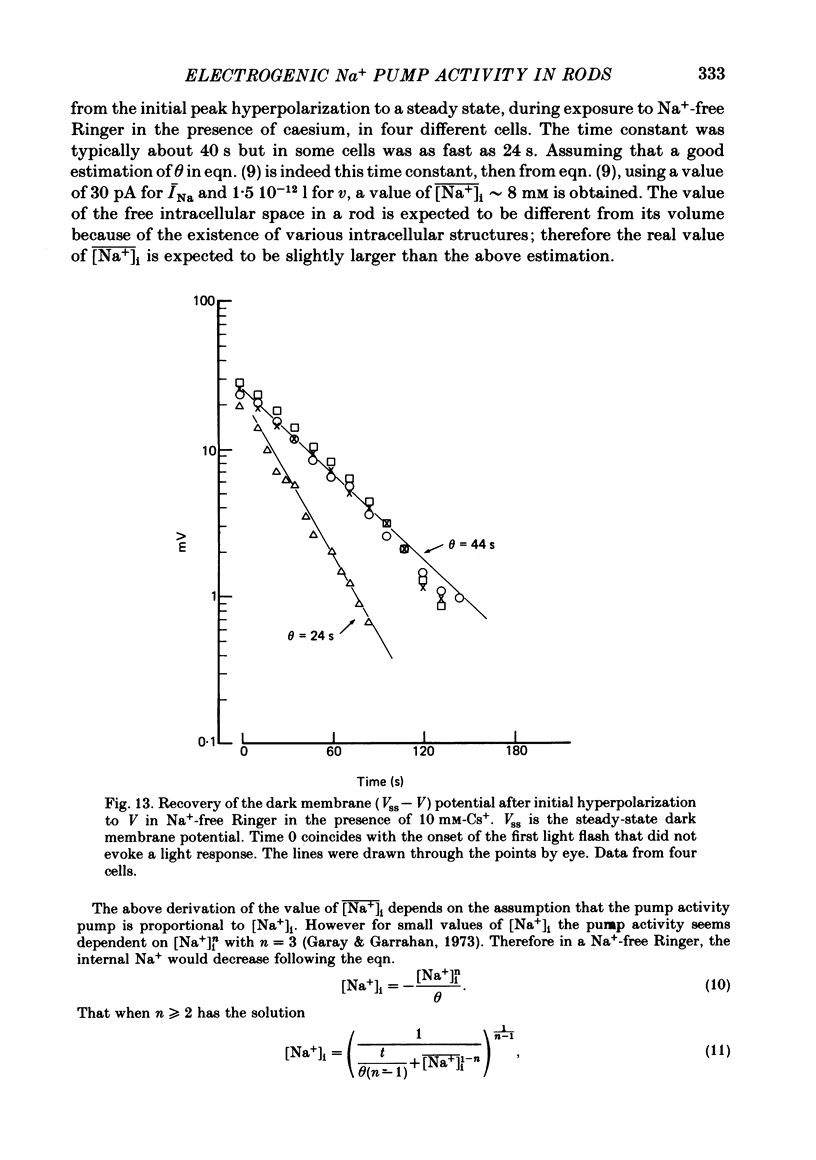
7. The amplitude of the slow component decreased with a time lag of about 2 min when external Na+ was reduced. A previous exposure of the retina to a Na+-free Ringer solution for at least 3 min modified the voltage photoresponse in a way similar to that observed in the presence of 100 μM-strophanthidin.
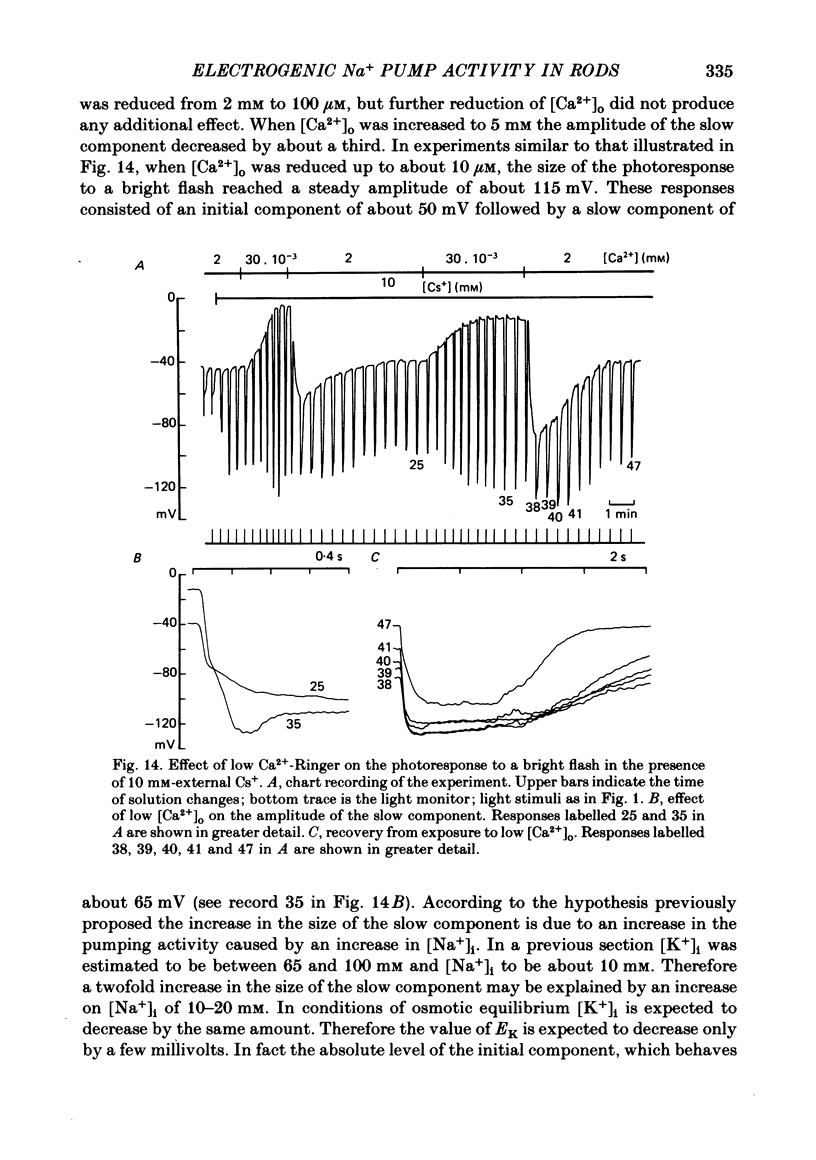
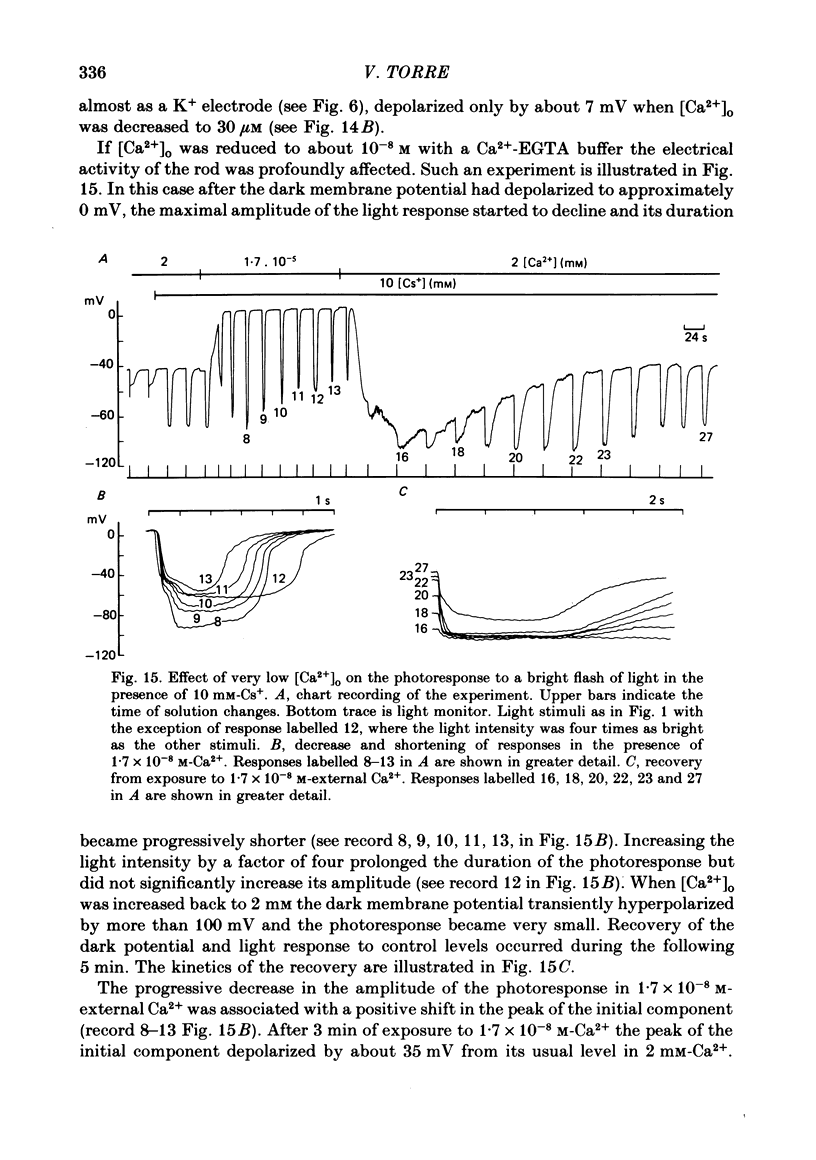
8. When external Ca2+ concentration ([Ca2+]o) was increased from 2 to 5 mM the slow component decreased by about 30%. When [Ca2+]o was reduced the slow component increased. A twofold increase was observed when [Ca2+]o was lower than 10-4 M.
9. It is suggested that the slow component of the voltage response in the presence of external Cs+ is caused by an electrogenic current driven by the Na+—K+ transport system, during a voltage-dependent block of external Cs+ of some K+ channels.
Full text
PDF












Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Attwell D., Wilson M. Behaviour of the rod network in the tiger salamander retina mediated by membrane properties of individual rods. J Physiol. 1980 Dec;309:287–315. doi: 10.1113/jphysiol.1980.sp013509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bader C. R., Macleish P. R., Schwartz E. A. A voltage-clamp study of the light response in solitary rods of the tiger salamander. J Physiol. 1979 Nov;296:1–26. doi: 10.1113/jphysiol.1979.sp012988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylor D. A., Hodgkin A. L. Detection and resolution of visual stimuli by turtle photoreceptors. J Physiol. 1973 Oct;234(1):163–198. doi: 10.1113/jphysiol.1973.sp010340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylor D. A., Lamb T. D., Yau K. W. The membrane current of single rod outer segments. J Physiol. 1979 Mar;288:589–611. [PMC free article] [PubMed] [Google Scholar]
- Benz R., Conti F. Reversible electrical breakdown of squid giant axon membrane. Biochim Biophys Acta. 1981 Jul 6;645(1):115–123. doi: 10.1016/0005-2736(81)90518-6. [DOI] [PubMed] [Google Scholar]
- Bezanilla F., Armstrong C. M. Negative conductance caused by entry of sodium and cesium ions into the potassium channels of squid axons. J Gen Physiol. 1972 Nov;60(5):588–608. doi: 10.1085/jgp.60.5.588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown J. E., Pinto L. H. Ionic mechanism for the photoreceptor potential of the retina of Bufo marinus. J Physiol. 1974 Feb;236(3):575–591. doi: 10.1113/jphysiol.1974.sp010453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capovilla M., Cervetto L., Pasino E., Torre V. The sodium current underlying the responses of toad rods to light. J Physiol. 1981 Aug;317:223–242. doi: 10.1113/jphysiol.1981.sp013822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capovilla M., Cervetto L., Torre V. Effects of changing external potassium and chloride concentrations on the photoresponses of Bufo bufo rods. J Physiol. 1980 Oct;307:529–551. doi: 10.1113/jphysiol.1980.sp013452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cervetto L., Pasino E., Torre V. Electrical responses of rods in the retina of Bufo marinus. J Physiol. 1977 May;267(1):17–51. doi: 10.1113/jphysiol.1977.sp011799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciani S., Krasne S., Hagiwara S. A model for the effects of potential and external K+ concentration on the Cs+ blocking of inward rectification. Biophys J. 1980 Apr;30(1):199–204. doi: 10.1016/S0006-3495(80)85089-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copenhagen D. R., Owen W. G. Current-voltage relations in the rod photoreceptor network of the turtle retina. J Physiol. 1980 Nov;308:159–184. doi: 10.1113/jphysiol.1980.sp013466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Detwiler P. B., Hodgkin A. L., McNaughton P. A. Temporal and spatial characteristics of the voltage response of rods in the retina of the snapping turtle. J Physiol. 1980 Mar;300:213–250. doi: 10.1113/jphysiol.1980.sp013159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisner D. A., Lederer W. J. Characterization of the electrogenic sodium pump in cardiac Purkinje fibres. J Physiol. 1980 Jun;303:441–474. doi: 10.1113/jphysiol.1980.sp013298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fain G. L., Quandt F. N., Bastian B. L., Gerschenfeld H. M. Contribution of a caesium-sensitive conductance increase to the rod photoresponse. Nature. 1978 Mar 30;272(5652):466–469. doi: 10.1038/272467a0. [DOI] [PubMed] [Google Scholar]
- Garay R. P., Garrahan P. J. The interaction of sodium and potassium with the sodium pump in red cells. J Physiol. 1973 Jun;231(2):297–325. doi: 10.1113/jphysiol.1973.sp010234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrahan P. J., Glynn I. M. The sensitivity of the sodium pump to external sodium. J Physiol. 1967 Sep;192(1):175–188. doi: 10.1113/jphysiol.1967.sp008295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glynn I. M., Karlish S. J. The sodium pump. Annu Rev Physiol. 1975;37:13–55. doi: 10.1146/annurev.ph.37.030175.000305. [DOI] [PubMed] [Google Scholar]
- HODGKIN A. L., HOROWICZ P. The influence of potassium and chloride ions on the membrane potential of single muscle fibres. J Physiol. 1959 Oct;148:127–160. doi: 10.1113/jphysiol.1959.sp006278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagins W. A., Yoshikami S. Ionic mechanisms in excitation of photoreceptors. Ann N Y Acad Sci. 1975 Dec 30;264:314–325. doi: 10.1111/j.1749-6632.1975.tb31492.x. [DOI] [PubMed] [Google Scholar]
- Hagiwara S., Miyazaki S., Rosenthal N. P. Potassium current and the effect of cesium on this current during anomalous rectification of the egg cell membrane of a starfish. J Gen Physiol. 1976 Jun;67(6):621–638. doi: 10.1085/jgp.67.6.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hille B. Potassium channels in myelinated nerve. Selective permeability to small cations. J Gen Physiol. 1973 Jun;61(6):669–686. doi: 10.1085/jgp.61.6.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korenbrot J. I., Cone R. A. Dark ionic flux and the effects of light in isolated rod outer segments. J Gen Physiol. 1972 Jul;60(1):20–45. doi: 10.1085/jgp.60.1.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MULLINS L. J., NODA K. THE INFLUENCE OF SODIUM-FREE SOLUTIONS ON THE MEMBRANE POTENTIAL OF FROG MUSCLE FIBERS. J Gen Physiol. 1963 Sep;47:117–132. doi: 10.1085/jgp.47.1.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oakley B., 2nd, Flaming D. G., Brown K. T. Effects of the rod receptor potential upon retinal extracellular potassium concentration. J Gen Physiol. 1979 Dec;74(6):713–737. doi: 10.1085/jgp.74.6.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Repke K., Est M., Portius H. J. Uber die Ursache der Speciesunterschiede in der Digitalisempfindlichkeit. Biochem Pharmacol. 1965 Dec;14(12):1785–1802. doi: 10.1016/0006-2952(65)90269-8. [DOI] [PubMed] [Google Scholar]
- Robinson J. D., Flashner M. S. The (Na+ + K+)-activated ATPase. Enzymatic and transport properties. Biochim Biophys Acta. 1979 Aug 17;549(2):145–176. doi: 10.1016/0304-4173(79)90013-2. [DOI] [PubMed] [Google Scholar]
- Robinson J. W. The difference in sensitivity to cardiac steroids of (Na++K+)-stimulated ATPase and amino acid transport in the intestinal mucosa of the rat and other species. J Physiol. 1970 Jan;206(1):41–60. doi: 10.1113/jphysiol.1970.sp008996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachs J. R. Interaction of external K, Na, and cardioactive steroids with the Na-K pump of the human red blood cell. J Gen Physiol. 1974 Feb;63(2):123–143. doi: 10.1085/jgp.63.2.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sillman A. J., Ito H., Tomita T. Studies on the mass receptor potential of the isolated frog retina. II. On the basis of the ionic mechanism. Vision Res. 1969 Dec;9(12):1443–1451. doi: 10.1016/0042-6989(69)90060-1. [DOI] [PubMed] [Google Scholar]
- Thomas R. C. Electrogenic sodium pump in nerve and muscle cells. Physiol Rev. 1972 Jul;52(3):563–594. doi: 10.1152/physrev.1972.52.3.563. [DOI] [PubMed] [Google Scholar]
- Yau K. W., McNaughton P. A., Hodgkin A. L. Effect of ions on the light-sensitive current in retinal rods. Nature. 1981 Aug 6;292(5823):502–505. doi: 10.1038/292502a0. [DOI] [PubMed] [Google Scholar]
- Zuckerman R. Ionic analysis of photoreceptor membrane currents. J Physiol. 1973 Dec;235(2):333–354. doi: 10.1113/jphysiol.1973.sp010390. [DOI] [PMC free article] [PubMed] [Google Scholar]


