Abstract
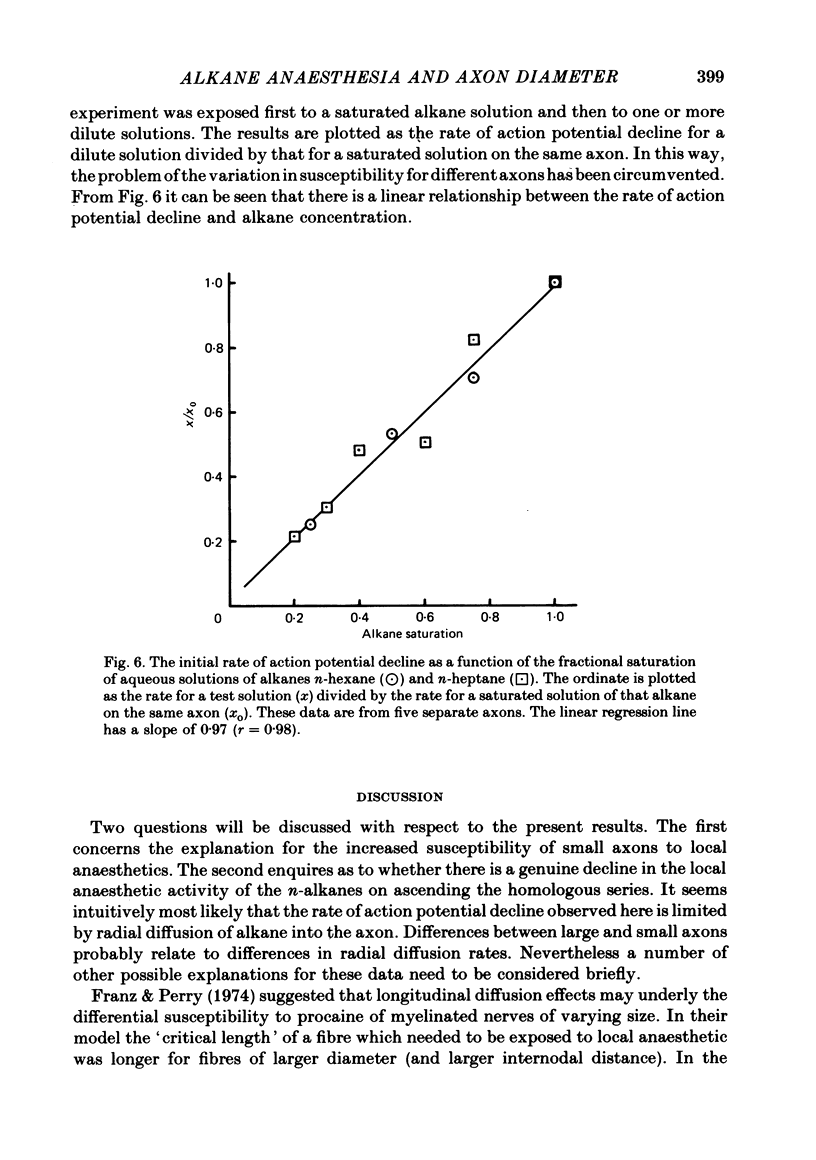
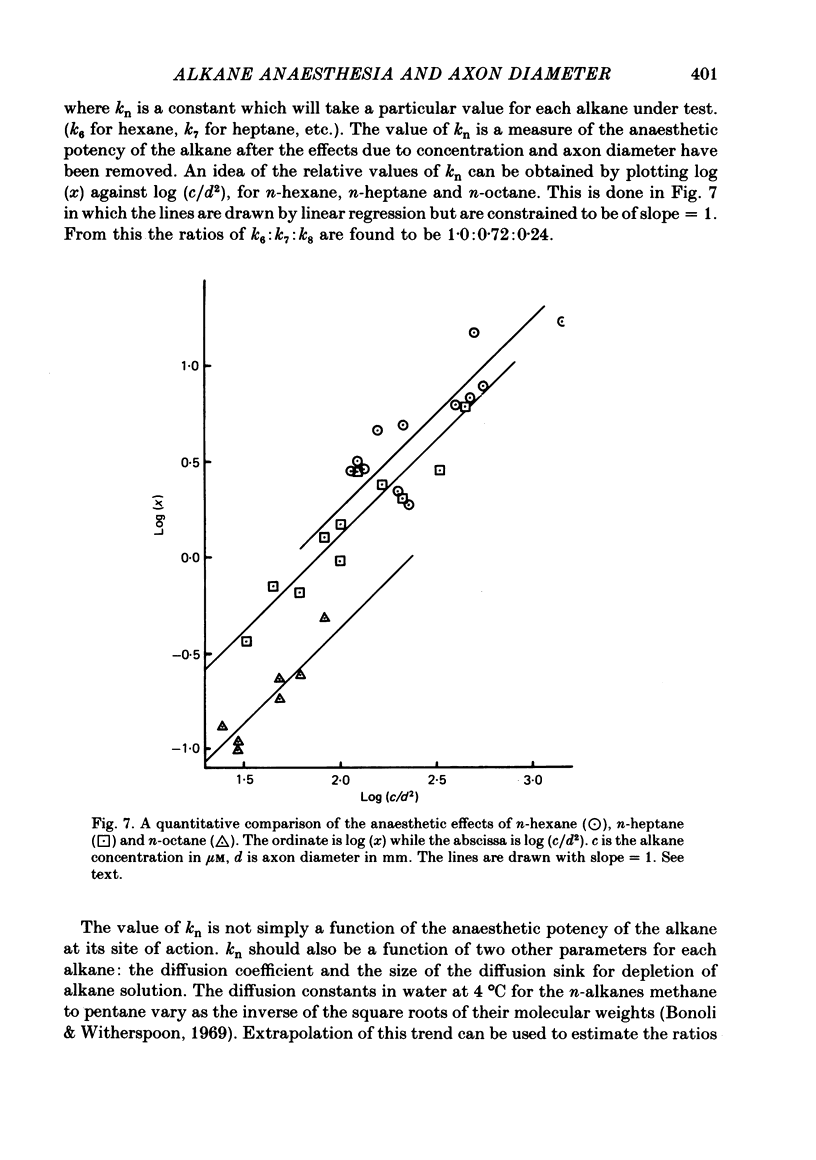
1. The anaesthetic effects of aqueous solutions of the n-alkanes pentane to nonane on the propagated action potential of squid axons have been investigated for a range of axon diameters. 2. By the use of small axons (approx. 200 microns diameter) to minimize effects due to long diffusion times and alkane depletion it was found that n-pentane and n-hexane caused a rapid reversible inhibition of the impulse, while higher homologues had progressively less effect, n-nonane being apparently inert. 3. The rate of action potential decline due to the n-alkanes was found to be strongly dependent on axon diameter. For n-hexane, n-heptane and n-octane the rate of decline was inversely proportional to the square of the axon diameter. 4. The mechanisms which may underly the increased sensitivity of small axons to impulse blockade by n-alkanes are discussed. A quantitative comparison is made between the effects of n-hexane, n-heptane and n-octane on the action potential. It is argued that this supports the idea of a real decline in anaesthetic potency on ascending the homologous series, rather than an effect due to long diffusion times and solution depletion.
Full text
PDF

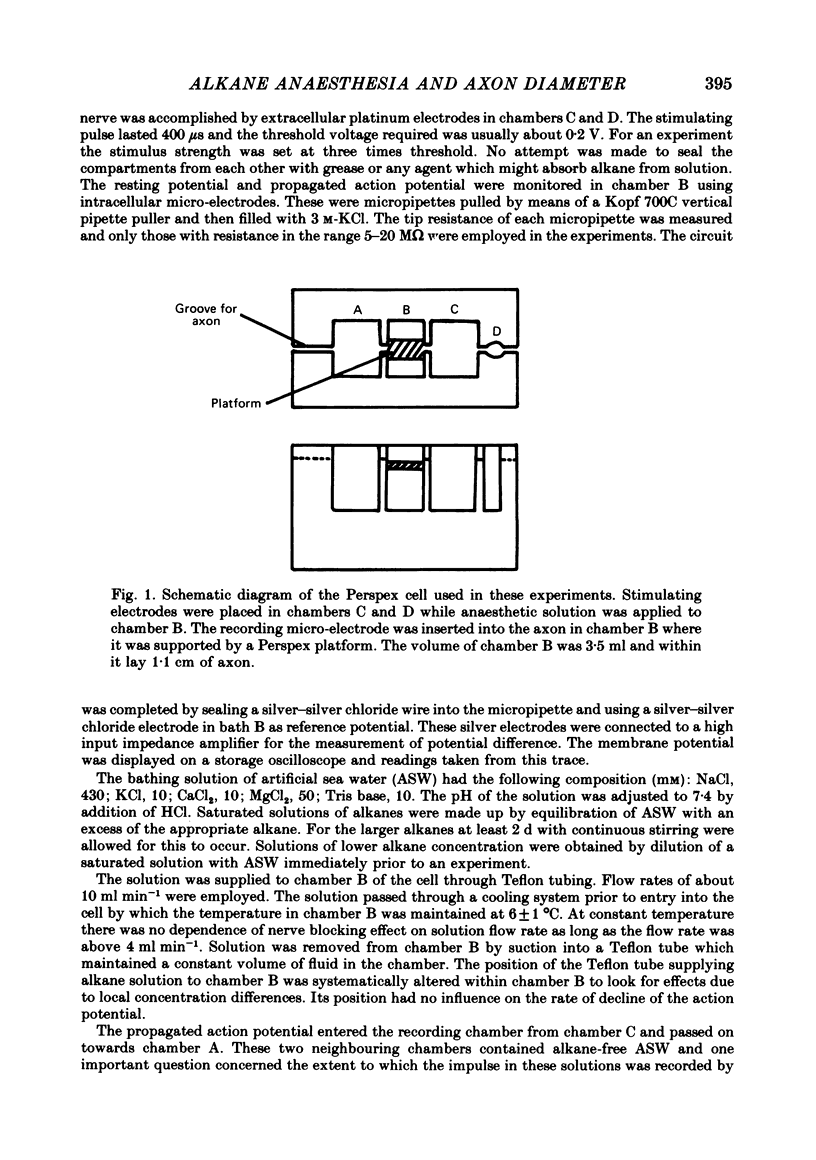
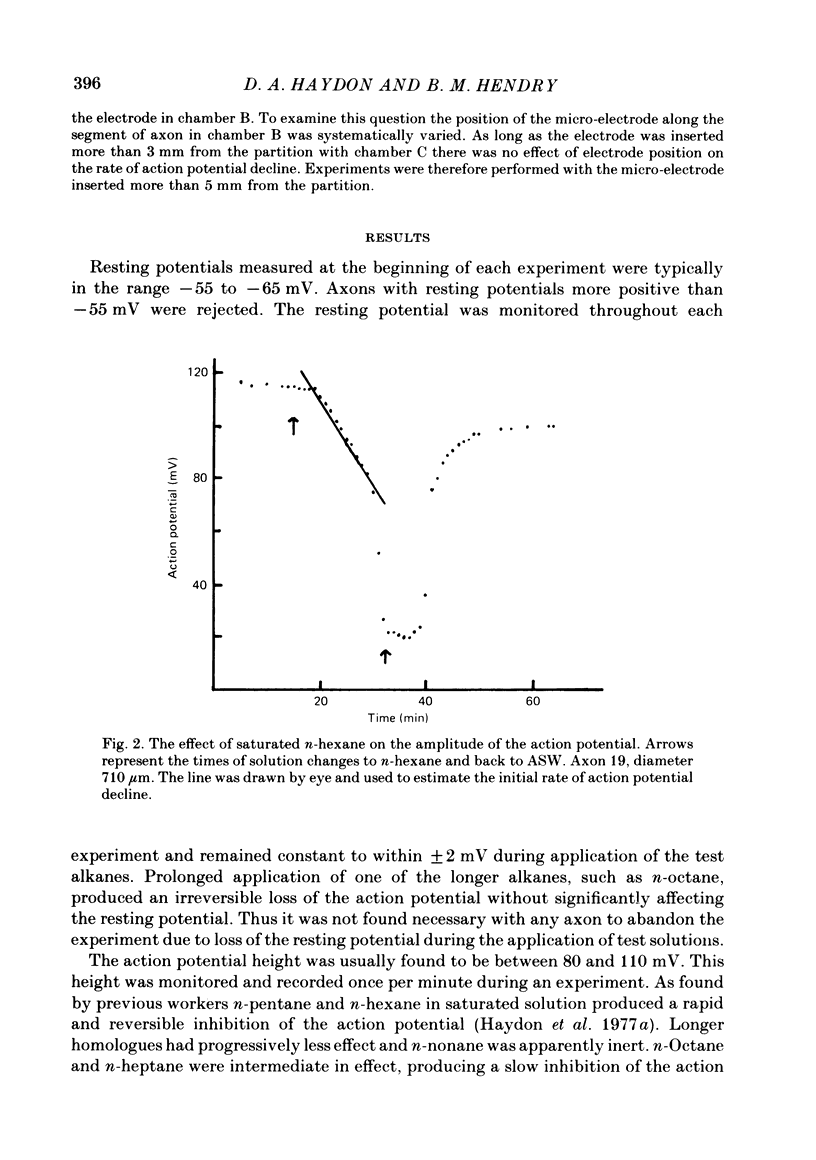
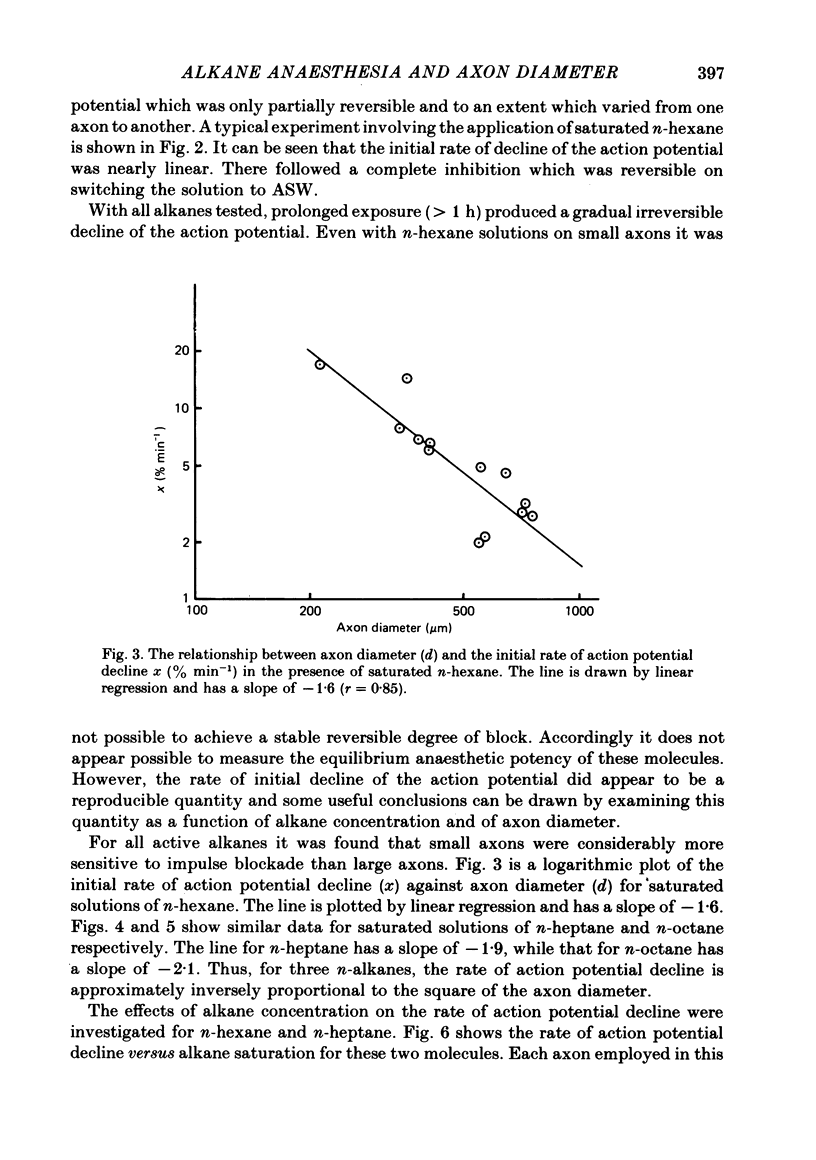
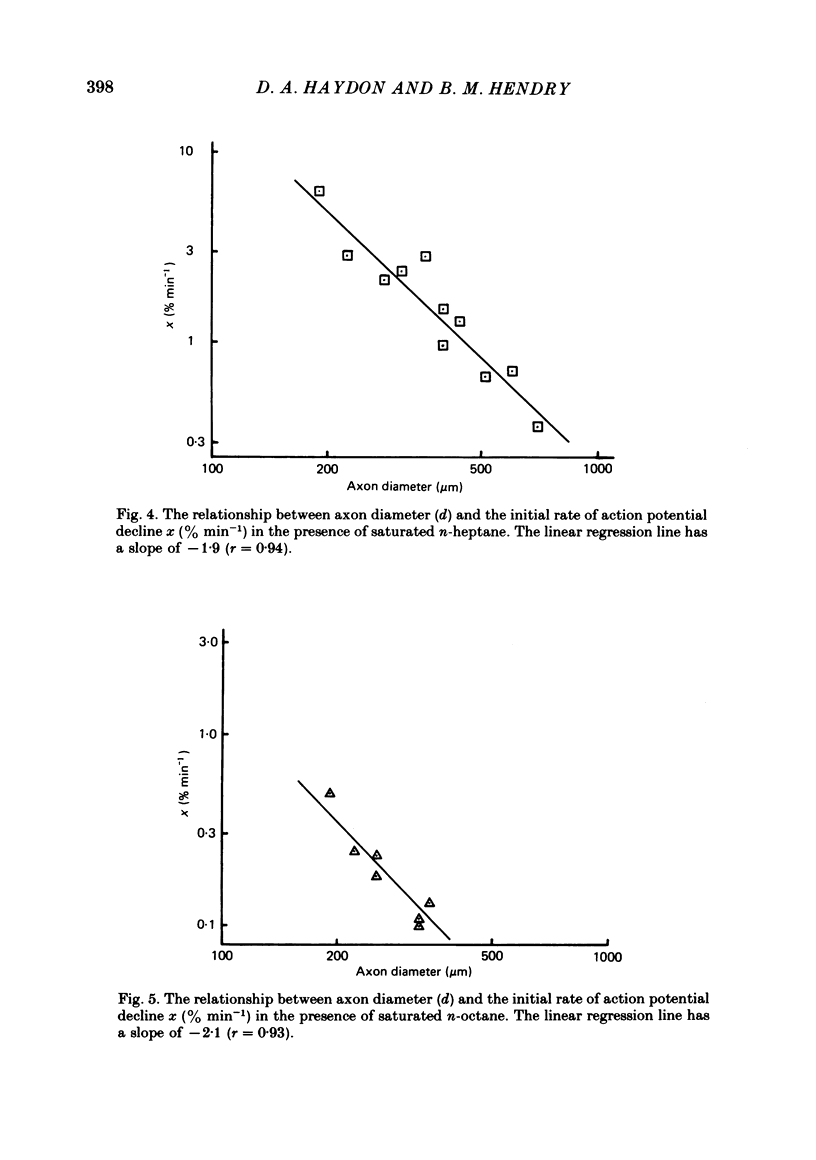



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adelman W. J., Jr, Moses J., Rive R. V. An anatomical basis for the resistance and capacitance in series with excitable membrane of the squid giant axon. J Neurocytol. 1977 Dec;6(6):621–646. doi: 10.1007/BF01176377. [DOI] [PubMed] [Google Scholar]
- Franz D. N., Perry R. S. Mechanisms for differential block among single myelinated and non-myelinated axons by procaine. J Physiol. 1974 Jan;236(1):193–210. doi: 10.1113/jphysiol.1974.sp010430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haydon D. A., Hendry B. M., Levinson S. R., Requena J. Anaesthesia by the n-alkanes. A comparative study of nerve impulse blockage and the properties of black lipid bilayer membranes. Biochim Biophys Acta. 1977 Oct 3;470(1):17–34. doi: 10.1016/0005-2736(77)90058-x. [DOI] [PubMed] [Google Scholar]
- Haydon D. A., Hendry B. M., Levinson S. R., Requena J. The molecular mechanisms of anaesthesia. Nature. 1977 Jul 28;268(5618):356–358. doi: 10.1038/268356a0. [DOI] [PubMed] [Google Scholar]
- Haydon D. A., Kimura J. E. Some effects of n-pentane on the sodium and potassium currents of the squid giant axon. J Physiol. 1981 Mar;312:57–70. doi: 10.1113/jphysiol.1981.sp013615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haydon D. A., Requena J., Urban B. W. Some effects of aliphatic hydrocarbons on the electrical capacity and ionic currents of the squid giant axon membrane. J Physiol. 1980 Dec;309:229–245. doi: 10.1113/jphysiol.1980.sp013506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keynes R. D., Bezanilla F., Taylor R. E., Rojas E. The rate of action of tetrodotoxin on sodium conductance in the squid giant axon. Philos Trans R Soc Lond B Biol Sci. 1975 Jun 10;270(908):365–375. doi: 10.1098/rstb.1975.0016. [DOI] [PubMed] [Google Scholar]
- NATHAN P. W., SEARS T. A. THE SUSCEPTIBILITY OF NERVE FIBRES TO ANALGESICS. Anaesthesia. 1963 Oct;18:467–476. doi: 10.1111/j.1365-2044.1963.tb13570.x. [DOI] [PubMed] [Google Scholar]
- RUD J. Local anesthetics. An electrophysiological investigation of local anesthesia of peripheral nerves, with special reference to xylocaine. Acta Physiol Scand Suppl. 1961;51(178):1–171. [PubMed] [Google Scholar]
- Seeman P. The membrane actions of anesthetics and tranquilizers. Pharmacol Rev. 1972 Dec;24(4):583–655. [PubMed] [Google Scholar]
- Staiman A., Seeman P. Conduction-blocking concentrations of anesthetics increase with nerve axon diameter: studies with alcohol, lidocaine and tetrodotoxin on single myelinated fibers. J Pharmacol Exp Ther. 1977 May;201(2):340–349. [PubMed] [Google Scholar]
- Stein R. B., Pearson K. G. Predicted amplitude and form of action potentials recorded from unmyelinated nerve fibres. J Theor Biol. 1971 Sep;32(3):539–558. doi: 10.1016/0022-5193(71)90155-x. [DOI] [PubMed] [Google Scholar]
- Wishnia A. Substrate specificity at the alkane binding sites of hemoglobin and myoglobin. Biochemistry. 1969 Dec;8(12):5064–5070. doi: 10.1021/bi00840a058. [DOI] [PubMed] [Google Scholar]


